Page 1

suspension for suction leads
height-adjustable
telescopic arm
internal cable
feed
300° pivot range
Art.-Nr.: 360 188 Ver.: c
Trolley optional (standard version w/o trolley)
Operating Manual
pump module:
small, quiet, powerful suction
Page 2
ergo vac
User guide
Sales and Service Information
mbnet Engineering GmbH has a network of customer service, sales and advisory centres.
Contact your nearest mbnet Engineering GmbH subsidiary to obtain the address of your
local distributor.
You can find a full list of all subsidiaries and distributors on our website: www.mbnet.de
Sales information can also be obtained from: info@mbnet.de
Ergo vac bears the mark (IEC 60601-1, Class I, Type BF without defibrillation protection), indicating its compliance
with the essential requirements regarding safety, functionality and labelling of Annex I of the Medical Device Directive
93/42/EEC. The requirements apply to patients, users and third persons who come into contact with this device within
the scope of its intended use.
Date of issue: 30.05.2019
mbnet Engineering GmbH
Kirschauer Straße 37a
D-02681 Callenberg
Telephone +49 (0)3592 34 83 0 u. 54 25 47
Fax +49 (0)3592 34 34 4 u. 54 25 49
E-Mail info@mbnet.de
Internet www.mbnet.de
Art.-no.: 360 188 Ver.: c
Date of issue:: 30.05.2019
Original
Page 3

Table of Contents
User guide
ergo vac
1 Safety Notes ................................................................... 4
1.1 Responsibility of the User .............................................. 4
1.2 Organisational Measures ................................................. 4
1.3 Indications for Use .......................................................... 4
1.4 Contra-indication ............................................................ 4
1.5 Safety-conscious Operation .......................................... 5
1.6 Safe Use with Electronics .............................................. 5
1.7 Operation with other Devices .......................................... 5
1.8 Maintenance ................................................................... 5
1.9 Terms of Warranty .......................................................... 6
1.10 Symbols and Pictograms ................................................ 6
1.10.1 Symbols Used in this Document ...................................... 6
1.10.2 Symbols Used on the Device ............................................ 7
2 Introduction .................................................................... 8
2.1 Elements of the Suction Device ...................................... 8
2.1.1 Overview ......................................................................... 8
2.1.2 Scope of Delivery ............................................................. 9
2.2 Control Box with Control Panel ...................................... 9
2.3 Suction Electrode Leads ................................................ 9
2.4 Cable Arm ..................................................................... 10
2.5 Pump Module ............................................................... 10
2.6 Fixation Pump Module ................................................. 11
2.7 Serial Number .............................................................. 11
2.8 Supplied accessories ................................................... 11
3 Operation ..................................................................... 12
6 Application .................................................................... 19
6.1 Operating Conditions ................................................... 19
6.2 Starting a Recording .................................................... 19
7 Maintenance and Care ................................................. 20
7.1 Visual Inspection ........................................................... 20
7.2 Cleaning the Housing and Cables ................................. 21
7.2.1 Cleaning and Storing the Electrodes ............................... 22
7.2.2 Recommended Cleaning and
Disinfection Methods for Electrodes ........................ 23 - 27
7.2.3 Cleaning the Suction Hoses ........................................... 28
7.2.4 Cleaning Connection Cables .......................................... 28
7.2.5 Admissible Disinfectants ................................................. 28
7.2.6 Non-admissible Detergents ............................................ 28
7.3 Disinfection.................................................................... 29
7.3.1 Admissible Disinfectants ................................................. 29
7.3.2 Non-admissible Disinfectants ......................................... 29
7.4 Battery .......................................................................... 29
7.4.1 Charging the Battery ...................................................... 29
7.4.2 Battery Disposal ............................................................. 30
7.5 Inspection Report ......................................................... 30
Replacement of Parts with a Limited Life,
every 3 – 5 years ............................................................ 31
7.6 Accessories and consumables ....................................... 31
7.7 Replacement of ECG Leads ........................................... 32
8 Troubleshooting ............................................................ 32
3.1 Getting Started ............................................................. 12
3.2 On / Off .......................................................................... 12
3.3 Power Supply ................................................................ 13
3.3.1 Displays for Mains or Battery Power .............................. 13
3.3.2 Isolation from Power Grid .............................................. 13
4 Controls ........................................................................ 13
4.1 Suction Levels .............................................................. 14
4.2 Blowing out Air ............................................................. 14
4.3 Cleaning ....................................................................... 14
5 ECG Recording ............................................................14
5.1 Placement of Electrodes ......................................... 14/15
5.2 Possible Sources of Errors with the ECG Recording ... 15
5.2.1 Preparation ..................................................................... 15
5.2.2 Application of Electrodes ................................................ 15
5.2.3 Before the Recording ..................................................... 16
5.2.4 During the Recording ..................................................... 16
5.2.5 Removal of Electrodes from the Skin ............................. 16
5.3 Electrode Identification and Colour Code ................... 16
5.4 Resting ECG with 10-lead Patient Cable
Electrode Placement for Standard Leads .................... 17
5.5 Right Precordial (C4r) ................................................... 18
8.1 Possible Errors ............................................................. 32
8.2 Preventing Electromagnetic Interference ................ 32/33
8.3 Warranty ....................................................................... 34
8.4 Accessories and Disposables ...................................... 34
9 Technical Data .............................................................. 34
9.1 Pump Module ............................................................... 34
9.2 Preventing Electromagnetic Interference ..................... 35
9.3 System Cable ............................................................... 35
9.4 Electrodes .................................................................... 35
9.5 Cable Arm .................................................................... 35
9.6 Safety Standards .......................................................... 35
10 EMC information ........................................................... 36
10.1 Table1: Immunity (all devices):
electromagnetic emissions ........................................... 37
10.2 Table 2: Immunity: electromagnetic immunity ............... 38
10.3 Table 3: electromagnetic immunity ............................... 39
Page 4
ergo vac
User Guide
1 Safety Notes
1.1 Responsibility of the User
The device must be used only by qualified physicians or trained medical professionals.
The responsibilities of the staff for operating and maintaining the device must be speci-
fied by the operator.
Ensure that the staff have read and understood the user guide. This applies in particular
to this section Safety notes.
The device must not be stacked at any moment.
Damaged or missing parts must be replaced immediately.
The safety, reliability and performance of the device can only be guaranteed when the
maintenance intervals as stated in Chapter 5: “Maintenance and Care” are observed.
Warning
Do not modify this equipment without authorization of the manufacturer.
1.2 Organisational Measures
Before using the device, ensure that a medical product representative has
explained its functions as well as the safety requirements.
Keep this user guide in an accessible place for reference purposes.
Make sure that it is always complete and legible.
Observe the operating and maintenance instructions.
1.3 Indications for Use
The device is an ECG vacuum and is operated in combination with normal ECG
devices. The device is suitable for both recording resting as well as exercise ECG and
is used for patients of both genders as well as all ancestries and age groups (preferably
as of the age of seven, also dependent on body size).
The device is only to be operated in a professional healthcare environment.
The device is suitable for use inside hospitals, cardiology centres,
outpatient clinics and medical practices.
The device can safely be used with pacemaker patients.
Always operate the device in line with the technical data indicated.
The device is not intended for sterile use or use outdoors.
This is a device of type BF. It is not defibrillation protected. As a safety precaution,
remove the electrodes before defibrillation!
1.4 Contra-indication
The device is not intended for sterile use.
The device must not be used in potentially explosive areas or in the presence of flam-
mable gases such as anaesthetic agents.
The device is not for direct cardiac application.
The device is not for use in an MRI suite.
Seite 4
Page 5
1.5 Safety-conscious Operation
Make sure that the staff have read and understood the operating instructions, in par-
ticular this section Safety Notes.
Do not touch the housing of the device during defibrillation.
To ensure patient safety, none of the electrodes, including the neutral electrode, nor the
patient or any person with simultaneous patient contact, must come in contact with
conductive parts, even when these are earthed.
Immediately report any changes that impair safety (including operating behaviour) to
the responsible person.
Only use accessories and disposables recommended or supplied by mbnet
Engineering GmbH. The use of accessories or disposables from other manufacturers
may result in injury, inaccurate information and/or damage to the unit.
User guide
ergo vac
1.6 Safe Use with Electronics
Operating the device without the correctly rated fuse or with defective cables
constitutes a danger to the life and limb of the patient or the operator!
Therefore take note of the following:
diately.
original.
The device must not be used if the power cable is damaged or
suspected of being damaged.
Damaged cable connections and connectors must be replaced imme-
Electrical safety devices, such as fuses, must not be modified.
Fuses must only be replaced with the same type and rating as the
1.7 Operation with other Devices
If the device is part of a medical system, only the original suction hoses from mbnet
Engineering GmbH must be connected to the device.
Portable communication devices, HF radios and devices labelled with the symbol:
(non-ionic electromagnetic radiation) can affect the operation of this device.
1.8 Maintenance
Danger of electric shock - do not open the device! It contains no parts, which can be
repaired by the user. Servicing must only be performed by qualified technicians
authorised by mbnet Engineering GmbH.
Switch off the device before cleaning and disconnect it from the mains.
Do not use high-temperature sterilisation processes (such as autoclaving). Do not use
e-beam or gamma radiation sterilisation.
Do not use aggressive or abrasive cleaners.
Do not, under any circumstances, immerse the device or cable assemblies in cleaning
liquid.
Seite 5
Page 6
ergo vac
User Guide
1.9 Terms of Warranty
Your device is warranted against defects in material and manufacture, as stated in the
Terms and Conditions. Excluded from this warranty is damage resulting from negligence
or improper use. The warranty covers the free replacement of the defective part. Any liability for subsequent damage is excluded. The warranty is void if unauthorised or unqualified
persons attempt to make repairs.
In case the device is defective, send it to your local mbnet Engineering GmbH representative or directly to the manufacturer. The manufacturer can only be held responsible for the
safety, reliability and performance of the apparatus if:
assembly operations, extensions, readjustments or repairs are carried out by persons
authorized by the manufacturer, and
the device and approved attached equipment is used in accordance
with the manufacturer's instructions, and
the maintenance intervals as stated in Chapter 5: “Maintenance and Care” have been
complied with.
No further guarantees are assumed. mbnet Engineering GmbH makes no warranty of
merchantability or fitness for a particular purpose with respect to the product or parts
thereof.
1.10 Symbols and Pictograms
1.10.1 Symbols Used in this Document
The safety level is classified according to ISO 3864-2. The following overview shows the
safety symbols and pictograms used in this user guide.
For general safety notes as listed in this section.
For electrical hazards, warnings or precautionary measures when dealing with
electricity.
For possibly dangerous situations which could lead to damage to property or system
failure. Important or helpful user information.
Reference to other instructions.
DANGER
Warning
For a possibly dangerous situation which could lead to severe personal injury or to
death.
For a direct danger which could lead to severe personal injury or to death.
Seite 6
Caution
For a possibly dangerous situation which could lead to slight personal injuries. This symbol
is also used to indicate possible damage to property.
Page 7

1.10.2 Symbols Used on the Device
BF-symbol, no protection against defibrillation current.
Dispose of as electronic waste.
Attention: consult accompanying documents.
Manufacturer
User guide
ergo vac
Date of manufacture
CE label
Follow the manufacturer’s instructions
Seite 7
Page 8

ergo vac
User Guide
2 Introduction
The ergo vac is an ECG suction device for recording heart potentials during resting and
stress ECGs and sending the data to the ECG device. The built-in control panel allows for
easy operation and efficient configuration of the device.
2.1 Elements of the Suction Device
2.1.1 Overview
suspension for suction leads
suction electrode
leads with colour
code
height-adjustable
telescopic arm
internal cable
feed
fixation pump module
Seite 8
pump module:
small, quiet, powerful
suction
Trolley optional
(standard version w/o trolley)
Page 9

2.1.2 Scope of Delivery
Standard Model
Resting and stress ECGs
Mains operation
Options
Battery operation
Trolley (with grounding cable)
Fixation pump module on trolley
2.2 Control Box with Control Panel
What makes the control box stand out is its optimal user ergonomics. It consists of a
control panel, control electronics and defibrillation protection. The control panel features
white and green backlighting as well as push buttons. The control panel is easy to operate
and to clean.
User guide
ergo vac
ON / OFF
Stop ECG
Clean
2.3 Suction Electrode Leads
The ten shielded, interference-free electrode lines are trouble-free and stand out by virtue of
their low abrasion and high flexibility.
Control
Box
Suction Power
Caution
Please pay attention on the careful handling of suction leads (see 7.2.2 page 23)
Seite 9
Page 10
ergo vac
User Guide
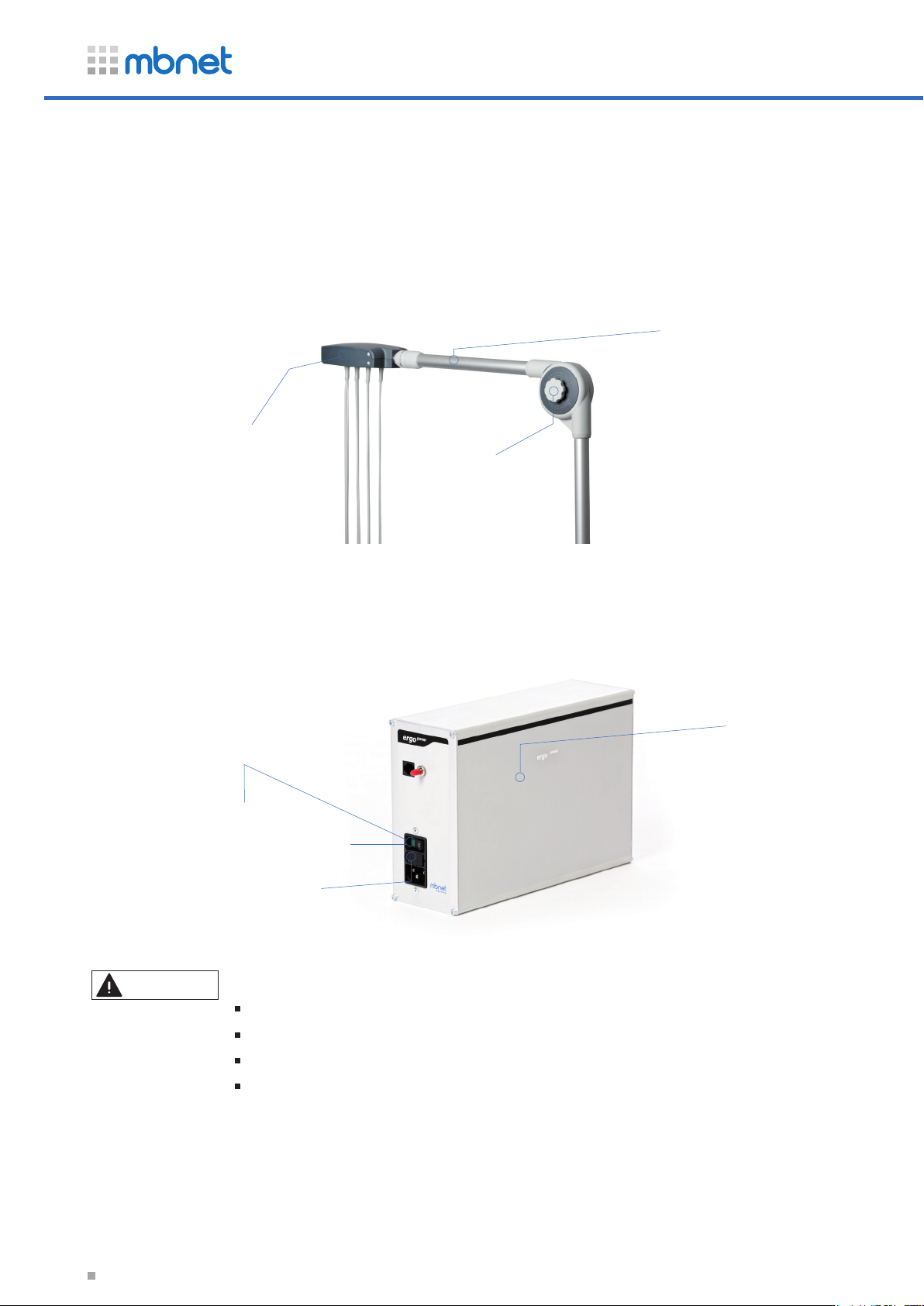
2.4 Cable Arm
The special feature of the cable arm is its hidden cable routing as well as its movable and
horizontal telescopic arm.
Control
height-adjustable
telescopic arm
Joint
2.5 Pump Module
Power
Power Switch
Fuse
Caution
The unit may only be connected to the following devices:
ECG devices that meet IEC 60601-1 standards.
ECG monitors that meet IEC 60601-1 standards.
The unit must not be connected to Class B ECG devices.
Any connection to unauthorized hardware is at your own risk.
It may also void the warranty.
pump module
Seite 10
Page 11
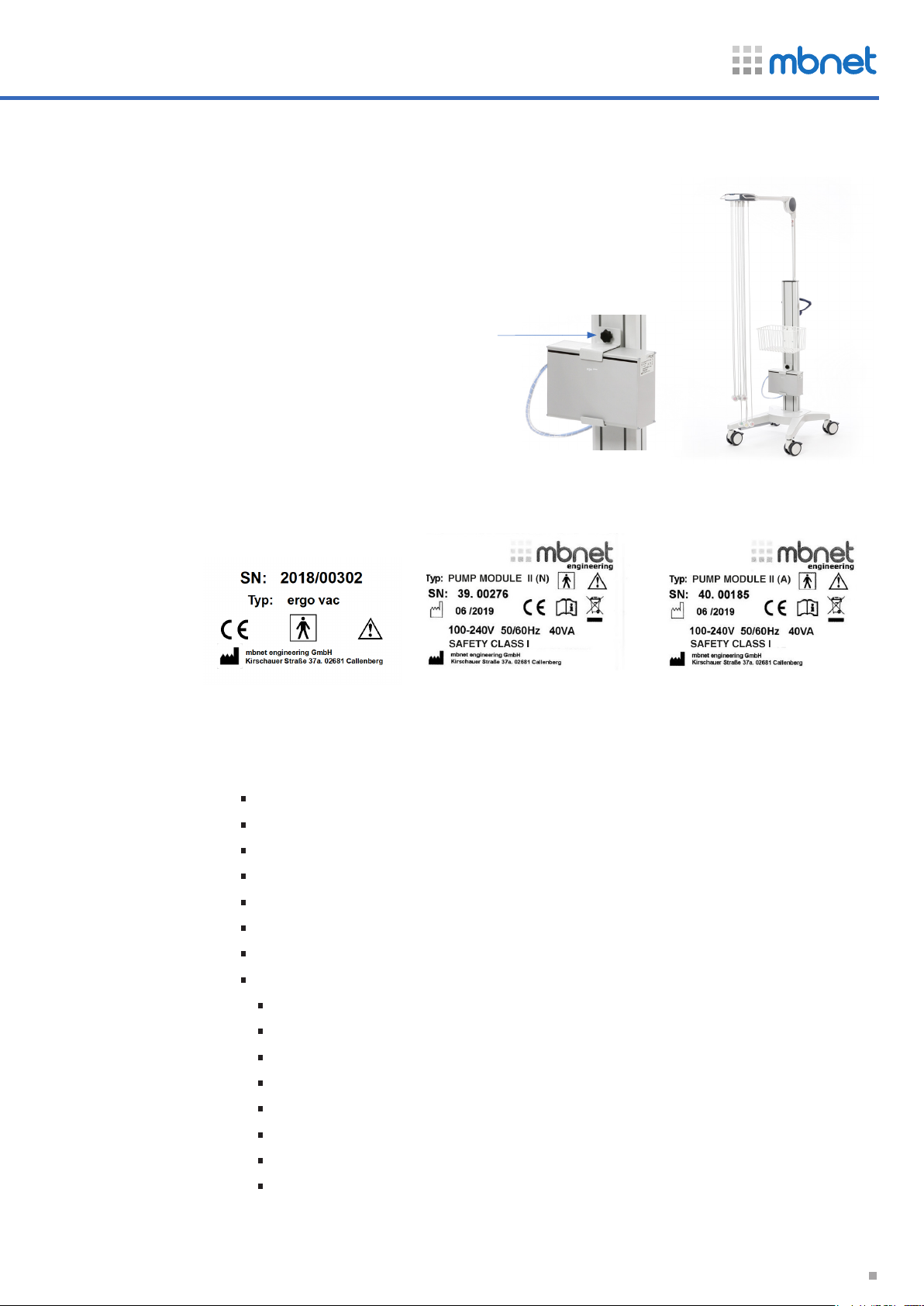
2.6 Fixation module pump
Bracket for pump module ergo vac with Trolley vexio-cart.
Comment: We have a large selection of brackets (bracket can be
created according to customer requirements), incl. table and wall
mounts
2.7 Serial Number
fixation
pump module
User guide
ergo vac
cable arm
pump module
pump module
2.8 Supplied accessories for ergo vac (mains) or (battery)
Cable arm with patient module
Electrode suction hose (6 x 1.30 m / 4 x 1.50 m)
Spacers (2 pcs with 3 rows / 2 pcs with 2 rows)
Pump unit, large
Power cable for mains connection
ECG Spray 250 ml
Standard bracket
Screw set consisting of:
2 x cable ties 200mm
1 x D-sub connection kit
1 x cable clip, small
1 x 5 mm and 1 x 2.5 mm Allen key
4 x each of M6x12 / M6x16 / M6x20 Allen screw
4 x each of M6 nut / M6 self-locking / M6 square nut
4 x 6.3 washer / 4 x 6.37 spring washer / 4 x rubber feet
Operation manual
with accumulator
Seite 11
Page 12
ergo vac
User Guide
3 Operation
3.1 Getting Started
HAZARD
Electric shock hazard. The device must not be used if it is not properly grounded or
the power cable is damaged or suspected to be damaged.
Location
The device must not be stored or operated in a wet, humid or dusty area.
It must also not be exposed to direct sunlight or heat from other sources.
The device must not come into contact with acids or acidic fumes.
The device should not be placed in the vicinity of X-ray, hf surgical equipment,
3.2 On / Off
The unit is switched on and off with the
the electrode can be sucked to the skin of the patient by applying light pressure with
the fingers.
diathermy units, large transformers or electric motors.
button. When pressing the ON button,
Warning
ON / OFF
Turn off the unit when not in use for a longer time.
Never apply suction when the electrodes are in a cleaning fluid.
When pressing the
5 seconds and then turns off automatically.
button, the suction pump creates a vacuum for about
Seite 12
Page 13

3.3 Power Supply
3.3.1 Displays for Mains or Battery Power
The unit can be connected to the mains or be supplied from the built-in battery (option).
In both modes the
Battery Life (Option):
continuous use.
Battery being charged:
(when power is off). If the device is switched on during charging, the
charging time may be longer.
The battery is charged when the device is connected to the mains. The
device can remain connected to the power grid without causing damage
to the device or battery.
The device can remain connected to the power grid without risk for
the battery.
Recharging Times:
not in use
button on the control panel will light up.
The internal battery provides power for up to two hours of
A completely depleted battery takes about 3.5 hours to fully charge
Battery recharging time if depleted: about 3.5 hours if the unit is
Charging time when in use: about 10 hours
User guide
ergo vac
3.3.2 Isolation from Power Grid
To isolate the device and the power supply from the mains, pull the power plug.
4 Controls
The controls are located on the front of the control box and control the entire
suction system.
clean / blow out air
suction levels
regulate suction power
Seite 13
Page 14

ergo vac
User Guide
4.1 Suction Levels
The system‘s suction lines can be individually adjusted for each patient using 5 suction
levels. When the unit is turned on, it activates an average default level (about -150 mbar).
The current suction level is indicated by the green LEDs on the control panel. The highest
level is about -220 mbar and should only be used in extreme cases (a lot of body hair).
The suction level must in each case be adjusted to the patient‘s skin type!
Warning
The device must not be used if the skin is broken. Strong suction or long-term
exposure to suction may result in hematomas! Particular caution is especially required
with older patients. The operator of the unit should ask the patient how he or she feels!
The electrodes should not be applied to the skin of the patient for more than
25 minutes.
4.2 Blowing out Air
When pressing the
about 5 seconds and then turned off automatically.
4.3 Cleaning
When the system is in standby mode, it can blow out the air from the suction lines by
pressing the cleaning button. The cleaning is stopped by pressing the
system then reverts back to standby mode.
Warning
Never apply suction when the electrodes are in a cleaning fluid.
button, the suction unit will be blown out with compressed air for
button. The
5 ECG Recording
Warning
5.1 Placement of Electrodes
Seite 14
Ensure that neither the patient nor the leading parts of the patient connection nor
the electrodes (including the neutral electrodes) come in contact with other persons or
conductive objects (even when these are earthed.).
Careful application of the electrodes and good electrode contact is important for a
good recording (see electrode positioning on pages 13 - 15).
A minimal resistance between skin and electrode is required to obtain the best ECG signal
and ensure the highest quality ECG recording. Therefore, please note the following points:
Page 15

5.1 Placement of Electrodes
1 Only use electrodes that are recommended by mbnet Engineering GmbH.
2 To increase the electrode's conductivity and adherence:
3 Check the electrode resistance.
4 If the electrode contact is higher than the acceptable level:
cleaning gel to remove the uppermost layer of epidermis.
5 Ensure that the patient is warm and relaxed before you start the recording.
6 After the recording, remove the electrodes by pressing on the cleaning button.
Clean the suction or vacuum electrodes according to the manufacturer's instructions.
Shave the areas where the electrodes are to be placed, if necessary.
Thoroughly clean the areas with alcohol or soapy water
(skin cream is often applied above all during the winter as this will
increase electrode resistance enormously(!) – Always COMPLETELY
remove skin cream at the application sites!)
Let the skin dry thoroughly before you apply the electrodes.
Remove the electrode and use an abrasive cleaning pad or abrasive
Apply the electrode.
User guide
ergo vac
*
Dedicated abrasive cleaning gel gives very good results in reducing the skin-electrode resistance.
5.2 Possible Sources of Errors with the ECG Recording
5.2.1 Preparation
If you are using new electrodes or those, which have not been used for a long time and have
therefore dried out, first stabilise the electrodes by placing them for at least three hours in a
1% salt solution (NaCl solution).
IMPORTANT: Use only pure NaCl and distilled or deionised water for this. No tap water!
Do not use physiological salt solution from a pharmacy! This contains additives, which can
damage the electrodes!
5.2.2 Application of Electrodes
The areas of skin to which the electrodes will be applied must be clean and dry. Use an
electrolyte ECG spray, which contains soluble chloride.
Do not use ECG gel! Only ECG spray!
Remove any skin cream!
Seite 15
Page 16

ergo vac
User Guide
5.2 Possible Sources of Errors with the ECG Recording
5.2.3 Before the Recording
Inform the patient about the procedure so that they are not frightened.
The patient must:
be lying down relaxed (attention: hands must be on the couch, not in the air)!
not be cold (above all for resting ECG recordings)!
No powerful devices must be in operation close by at the same time.
The couch should not be touching the walls!
5.2.4 During the Recording
Suction hoses must under no circumstances pull/tear/stretch the electrodes, but must hang
freely!
Wait with the recording of the ECG until you can see a good ECG recording on the screen.
Under no circumstances press the recording button beforehand!
Under no circumstances must the electrodes be applied on the patient's skin for longer than
25 minutes (risk of blisters forming)!
5.2.5 Removal of Electrodes from the Skin
Do not pull on the electrode cables, but touch the electrode carefully at the edge or activate
the blow-out function at the suction unit (
The electrodes will then fall off on their own
accord)!
5.3 Electrode Identification and Colour Code
The electrode colour codes in this section correspond to Code 1 (IEC).
Below you will find the corresponding colour codes in accordance with Code 2 (AHA).
IEC
label
IEC AHA
Colour
AHA
label
Colour
Seite 16
Extremity
Chest
according
to Wilson
Neutral
R
L
F
C1
C2
C3
C4
C5
C6
N black RL green
red
yellow
green
white / red
white / yellow
white / green
white / brown
white / black
white / purple
RA
LA
LL
V1
V2
V3
V4
V5
V6
white
black
red
brown / red
brown / yellow
brown / green
brown / brown
brown / black
brown / purple
Page 17

User guide
C2 yellow
C3 green
C4 brown
C5 black
C6 purple
5.4 Resting ECG with 10-lead Patient Cable
Electrode Placement for Standard Leads
ergo vac
C1 red
R red
N black
C2 yellow
C3 green
C4 brown
C5 black
C6 purple
L yellow
It is sometimes difficult
with a child to apply all
the electrodes. In this
case electrode C4 can be
placed on the right side
of the chest and the setting “Recording display”
programmed to V4r.
F green
IEC label AHA label
C1, red V1, red Fourth intercostal space at the right sternal border
C2, yellow V2, yellow Fourth intercostal space at the left sternal border
C3, green V3, green Midway between C2 and C4
C4, brown V4, blue Fifth intercostal space on the mid-clavicular line
C5, black V5, orange Anterior axillary line on the same horizontal level as C4
C6, purple V6, purple Mid-axillary line on the same horizontal level as C4
L, yellow LA, black Left arm (resting ECG)
R, red RA, white Right arm (resting ECG)
F, green LL, red Left foot (resting ECG)
N, black RL, green Right foot (resting ECG)
Connecting the ECG patient cable
Seite 17
Page 18

ergo vac
User Guide
5.5 Right Precordial (C4r)
The ACC/AHA guidelines recommend that in all patients with myocardial infarction with inferior ST elevation, an investigation into a possible RV ischemia or a RV infarction is carried
out; this investigation is undertaken with a right precordial C4r recording.
C1 red
C4 brown
R red
N black
F green
C2 yellow
C3 green
C5 black
C6 purple
L yellow
Explanations see
table on page 18
Seite 18
IEC label AHA label
C1, white / red V1, brown / red Fourth intercostal space at the right sternal border
C2, white / yellow V2, brown / yellow Fourth intercostal space at the left sternal border
C3, white / green V3, brown / green In the middle between C2 and C4
C4, white / brown V4, brown/ blue
C5, white / black V5, brown / orange Anterior axillary line on the same horizontal level as C4
C6, white / purple V6, brown / purple Mid-axillary line on the same horizontal level as C4
L, yellow LA, black Left arm
R, red RA, white Right arm
F, green LL, red Left foot
N, black RL, green Right foot
Connecting the ECG patient cable
Fifth intercostal space right on the mid-clavicular line
Page 19

6 Application
User guide
ergo vac
Caution
Do not take an ECG picture until you have read and understood the safety instructions at the
beginning of these instructions for use.
The device is a BF type device.
During ECG recording, make sure that neither the patient- nor the conductive parts of the
patient connection or the electrodes (including the neutral ones) come into contact with other
persons or conductive parts (even if they are grounded).
The device must not be used if the mains connection cable is damaged or there is a
suspicion of damage.
6.1 Operating Conditions
Caution
The device is not suitable for continuous operation; switch off again after use.
High-frequency fields and radiation can influence the quality of ECG leads..
The device can be operated under the following environmental conditions:
Ambient temperature: +10 °C and +40 °C
Relative humidity: between 30 % and 75 %
Air pressure: between 700 hPa and 1060 hPa
6.2 Starting a Recording
1 Preparing the patient
2 Switch on device and apply electrodes
3 Ask the patient about their well-being (note the suction strength of the electrodes)
4 Determine and adjust suction strength
The lower the pressure level, the better the skin tolerance!
Étape 1 & 2: for smooth skin Étape 4: for medium-hairy skin
Étape 3: for lightly-hairy skin Étape 5: for very hairy skin
5 Performing a measurement
6 Switch off the vacuum pump with the
7 The system switches off automatically after 10 seconds.
8 (Optional) After long periods of use and/or heavy perspiration by patients, the system can blow
out air by the pressing of the cleaning button.
9 Cleaning the electrodes (See. Chapter 7.2.1)
2
/
button, the electrodes detach from the patient
6, 8
4
Seite 19
Page 20

ergo vac
User Guide
7 Maintenance and Care
The device requires regular checks (Chapter 7.5). The test results must be recorded in
writing and compared to the values in the accompanying documents.
Maintenance work not described in this section may only be performed by a qualified,
authorised technician.
The following table indicates the intervals and responsibilities of the maintenance work
required. Local regulations in your country may stipulate additional or different inspection
intervals and tests.
Interval Maintenance step
Before every use
Every 6 months
Every 12 months
The useful life of the device electrodes is estimated to be 2 years.
7.1 Visual Inspection
Visually inspect the unit and cable assemblies for the following:
Device, housing and mains cable (not damaged or cracked)
Keypad (not damaged or cracked)
Electrode cable sheathing and connectors (undamaged)
No cracks, abrasion or wear in any cable assembly
Input/output connectors (not damaged or cracked)
Visual inspection of the device and ECG electrodes
Visual inspection of the device
(see page 21, 7.5 Inspection report)
- Cables and accessories
- Mains cable
Functional tests according to the instructions
(see page 30, 7.5 Inspection report)
Safety test according to § 11 MPBetreibV
Responsible
User
User
qualified staff
Seite 20
Warning
In addition to the visual inspection, the device should be switched on and the functions of
the operating field should also be checked. In this way, you can check that:
the device performs faultlessly
the display works
Defective units or damaged cables must be replaced immediately.
Page 21

7.2 Cleaning the Housing and Cables
User guide
ergo vac
Warning
Caution
Switch off the device before cleaning and disconnect it from the mains. Do not, under any
circumstances, immerse the device in cleaning liquid and do not sterilise it with hot water,
steam or air.
Do not autoclave the unit or any accessories.
Do not immerse the device in liquid.
The use of detergents with a high acid content or detergents that are otherwise
unsuitable can damage the device (i.e. cracks and wear of the plastic housing).
Always follow the dilution instructions provided by the manufacturer of the cleaning
solution.
Never use any of the following or similar cleaning products: Ethyl alcohol,
ethanol, acetone, hexane, abrasive or scouring powder or material, any cleaning
material that damages plastic.
The patient cable and other cable assemblies must not be exposed to excessive
mechanical stress. Whenever disconnecting the leads, hold the plugs and not the
cables. Store the leads in such a way as to prevent anyone stumbling over them
or any damage being caused by the wheels of medical equipment carts.
When cleaning, ensure that all labels and safety statements, whether etched,
printed or stuck to the device, remain in place and remain readable.
Thoroughly inspect the device and the accessories before cleaning.
Look for any signs of damage and make sure that the buttons and connectors work
correctly from a mechanical perspective.
Gently bend and flex cables, inspecting them for damage or extreme wear, exposed
wires and bent connectors.
Confirm that all connectors engage securely.
The housing of the device and the cable assemblies can be cleaned with a cloth slightly
moistened (not wet) on the surface only. If necessary, a domestic non-caustic cleaner or a
70 % alcohol solution can be used to remove grease stains and finger prints.
Wipe the equipment with a cloth slightly moistened (not wet) with one of the approved
cleaning solutions (see Chapter 5.2.4). Thoroughly wipe off any excess cleaning solution.
Do not let the cleaning solution run into or accumulate in connector openings, switches,
or gaps. If liquid gets into connectors, dry the area with warm air and then check that the
device operates properly.
Seite 21
Page 22

ergo vac
User Guide
7.2.1 Cleaning and Storing the Electrodes
Caution
NEVER use metallic or sharp items to clean the electrodes. This could damage them
irreparably.
Make absolutely sure that the suction unit is operating in cleaning mode when you
immerse the electrode into the cleaning liquid
Incorrect operation and vacuuming of cleaning liquid could mean that the device is
irreparably damaged.
Remove all impurities on the surface of the electrode immediately after use. You can
use a dry handkerchief or soft toothbrush for this (or the alcohol-free product
SaniCloth).
Do not let any impurities dry on the electrode!
Do not use any alcohol!
Do not use any tap or bottled drinking water!
Do not use any other soap solutions or abrasive cleaning products!
Light will cause a brown to black coating on the surface of the electrode as a result
of the oxidation of the silver. This can be wiped off with a mild ammonia solution or
by gentle rubbing with a microfibre cloth or an extremely fine sandpaper (at least 200
grain).
Store the electrodes somewhere dry and dark when they are not being used!
Do not expose the electrodes permanently to the light because otherwise they
will turn black!
Electrodes can be damaged or soiled with only small quantities of bromides, sulphides
and a few other metallic ions.
No contact with metals (bromides, sulphides etc.)!
.
Seite 22
Page 23

User guide
7.2.2 Recommended Cleaning and Disinfection Methods for Electrodes
Wiping disinfection / cleaning: to be carried out after every use
Intensive wiping disinfection / cleaning: 1x daily after the last use
Immersion disinfection, cleaning / drying: 1x daily after the last use
ergo vac
Caution
This cleaning method can damage the suction unit if not carried out correctly.
Instructions for Wiping Disinfection / Cleaning
1. Use only the disinfectants mentioned in Point 5.3.1.
2. Clean / disinfect all areas of the electrode, which have come into contact with the pa-
Electrode suction dome outside, grip area / suction area
Suction area
tient.
Cleaning of the
electrode contact surface
Electrode suction dome inside,
sealing lip, electrode body /
suction dome, suction area
Sealing lip
Diagram 1:
Suction dome cleaning
outside
Suction area
Diagram 2:
Clean contact surfaces
Attention: if the inside is cleaned incorrectly, particles (skin flakes, contact product
residues) can remain in the area of the sealing lip (see Diagram 3)
Diagram 3:
Suction dome cleaning inside
Seite 23
Page 24

ergo vac
User Guide
Diagram 4:
Impurities (electrode)
Diagram 5:
Check the position of the suction
dome on the electrode housing
After cleaning, check the optimal fit of the suction dome on the
electrode housing to ensure the suction electrode functions optimally.
Instructions: Intensive Wiping Disinfection / Cleaning
1. Use only the disinfectants mentioned in Point 5.3.1.
2. Clean / disinfect all areas of the electrode, which have come into contact with the pa-
tient.
Electrode suction dome outside, grip area / suction area
Cleaning of the
electrode contact surface
Suction area
Cleaning the inside of the electrode suction dome, sealing lip,
electrode body / suction dome /
suction area
Diagram 6:
Suction dome cleaning
outside
Seite 24
Diagram 7:
Clean contact area (electrode)
Sealing lip
Suction area
Diagram 8:
Suction dome cleaning inside
Page 25

User guide
Pull off the silicone suction dome from the electrode housing (in the direction of the arrow).
Then clean the inside of the suction dome and the electrode housing.
Pull off the
suction dome
Diagram 9:
Remove the suction dome
ergo vac
Replace the suction dome after it has been cleaned back on the electrode housing.
After cleaning, check the optimal fit of the suction dome on the
electrode housing to ensure the suction electrode functions optimally.
Diagram 10:
Check the position of the suction
dome on the electrode housing
Seite 25
Page 26

ergo vac
User Guide
Immersion Disinfection and Subsequent Cleaning and Drying
Caution
This cleaning method can damage the suction unit if not carried out correctly.
1. Switch off the suction unit.
2. Position the container for the cleaning liquid in such a way that no
medical equipment can be become wet from drops of liquid.
3. Pull off the silicone suction dome from the electrode housing.
Pull off the
suction dome
Diagram 11:
Remove the suction dome
4. ONLY immerse the electrode and the suction dome in a container with an admissible
disinfectant (Point 5.3.1)
Diagram 12:
Immersion disinfection
5. Prevent any cleaning liquid from dripping by taking suitable measures (cloth, container
to catch drips).
6. Activate the blow-out button on the suction unit.
Seite 26
Page 27

User guide
Activate this function twice in a row. If the blow-out function is not
activated correctly, it cannot be ruled out that cleaning liquid
will flow over the electrode suction hose into the suction device.
Diagram 13:
Blow-out/ cleaning key
7. Remove any cleaning liquid that has spilt with a suitable cloth.
ergo vac
If some cleaning liquid remains on the electrode, it can discolour the contact surface.
Diagram 14:
Error image -
discolouration of the electrode
8.
Replace the suction dome back onto the electrode housing after cleaning.
After cleaning, check the optimal fit of the suction dome on the
electrode housing to ensure the suction electrode functions optimally.
Diagram 15:
Check the position of the suction dome on the electrode
housing
Seite 27
Page 28

ergo vac
User Guide
7.2.3 Cleaning the Suction Hoses
UNDER NO CIRCUMSTANCES pull on the suction hoses during cleaning (risk of breaking the
cable)!
You MUST also instruct your temporary staff and the responsible cleaning staff on this matter!
Do not vacuum up any water into the suction hoses with the cleaning mode !
7.2.4 Cleaning Connection Cables
1 Check the cable before cleaning for any damage. Gently bend all the parts of the cable.
Inspect the cable insulation for cracks, damage or extreme wear, exposed wires and
bent connectors.
2 Wipe the equipment with a cloth slightly moistened (not wet) with one of the approved
cleaning solutions; the approved cleaning solutions are listed below.
3 Hold the cable with the cloth in the middle of the cable; wipe 20 cm of the cable at a
time with the cloth until the entire cable is clean. Never clean the cable along its entire
length at once as this can lead to damage to the cable insulation.
4 Thoroughly wipe off any excess cleaning solution. Do not let the cleaning solution run
into or accumulate in connector openings, switches, or gaps. If liquid still reaches the
connector openings, dry them with hot air.
7.2.5 Admissible Detergents
50% isopropanol (Isopropyl alcohol)
neutral, mild detergent (for example: “SaniCloth®“ and
“mikrozid universal wipers
all products designed for cleaning plastic.
7.2.6 Non-admissible Detergents
®
“)
Seite 28
Never use products containing the following:
Ethyl alcohol
Acetone
Hexane
Abrasive cleaning powder
Plastic-dissolving products
Page 29

7.3 Disinfection
Disinfection removes certain bacteria and viruses. Please refer to the manufacturer’s infor-
mation. Use commercially available disinfectants intended for clinics, hospitals and medical
practices.
Disinfect the device in the same way as described for cleaning the device in Chapter 5.2.
7.3.1 Admissible Disinfectants
User guide
ergo vac
Isopropanol (50%)
Propanol (35 %)
Aldehyde (2 – 4 %)
Ethanol (50 %)
7.3.2 Non-admissible Disinfectants
Organic solvents
Ammonia-based detergent
Abrasive cleaning agents
100% alcohol, Virex, Sani-Master
HB Quat
Conventional detergents (e.g. Fantastic®, Tilex® etc.)
Conductive solutions
Solutions or products containing the following ingredients:
®
Acetone
Ammonium chloride
Betadine
Chlorine, wax or wax compound
Ketone
Sodium salt
all products that are suitable for sensitive surfaces,
such as:
Bacillol® 30 foam / Bacillol® 30 Tissues
(10% Propanol-1, 15% Propanol-2, 20% Ethanol)
Mikrozid® AF (25% Ethanol, 35% 1Propanol-1)
7.4 Battery
No maintenance is required for the battery.
Depending on use, the battery should be replaced about every 4 years or if the battery
life falls significantly below 1 hour.
As long as the device is not in use, you should make sure that the battery is not com-
pletely depleted. Should the device not be used for more than three months, the battery
must be protected from complete discharge by recharging it.
7.4.1 Charging the Battery
A completely depleted battery takes about 3.5 hours to fully charge (when power is off).
A completely depleted battery takes about 3.5 hours to fully charge (when power is off).
If the device is switched on during charging, the charging time may be longer.
If the device is switched on during charging, the charging time may be longer.
The device can stay connected to the power grid without risk to the battery.
Connect the device to the power grid.
Seite 29
Page 30

ergo vac
User Guide
7.4.2 Battery Disposal
The battery must be brought to a recycling center in accordance with the relevant
regulations of a country or sent back to mbnet Engineering GmbH.
Warning
Danger! Risk of explosion! The battery must not be incinerated or treated as municipal waste.
Danger! Acid-burn risk! Do not open the battery under any circumstances.
7.5 Inspection Report
The user guide must be read before the inspection
Recommended inspection interval: every 6 months
Test
Serial number:
Visual inspection
(external condition)
Availability &
condition of accessories
Housing not damaged
Electrode connector port not damaged
ECG suction hoses
User guide
Results
Date
Mains cable
Functional test
Switch on device
Network cables
Suction strength control Functions properly
Remarks:
Recurrent test in line with the information from the ECG manufacturer
Inspection carried out by:
* In case of a defect, please contact the service department of your hospital, your mbnet Engineering GmbH representative or the local after-sales
service: (Name) (Telephone)
Seite 30
Page 31

User guide
Replacement of Parts with a Limited Life, every 3 – 5 years
ergo vac
Inspection
Internal battery
Replace the internal battery if its
operating time falls significantly
below 1 hour.
Device sent to mbnet Engineering GmbH to replace the
battery.
Replaced on:
The controller
Results Replacement
7.6 Accessories and consumables
Warning
Always use mbnet Engineering GmbH spare parts and disposables or products approved by mbnet Engineering GmbH. Failure to do so may invalidate the warranty.
Your local representative stocks all the disposables and accessories available for the
handy vaq. In case of difficulty, contact our head office. Our staff will be pleased to help
process your order or to provide information on all mbnet Engineering GmbH products.
Art. no.: Article
303 230 ECG Leads, Set of 10 leads (6 x 1.30 m / 4 x 1.50 m)
303 220 C1, 1.30 m
303 221 C2, 1.30 m
303 222 C5, 1.30 m
303 223 C4, 1.30 m
303 224 C5, 1.30 m
303 225 C6, 1.30 m
303 207 F, 1.30 m
303 208 L, 1.30 m
303 209 N, 1.30 m
303 210 R, 1.30 m
303 231 C1, 1.50 m
303 232 C2, 1.50 m
303 233 C3, 1.50 m
303 234 C4, 1.50 m
303 235 C5, 1.50 m
303 236 C6, 1.50 m
303 226 N, 1.50 m
303 227 L, 1.50 m
303 228 N, 1.50 m
303 229 R, 1.50 m
300 109 ECG Label for ECG Single Lead C1 - C6, F, N, L, R (set)
300 301 Spreader for ECG Single Leads (set)
300 400 ECG electrode contact spray
Seite 31
Page 32

ergo vac
User Guide
7.7 Replacement of ECG Leads
The ECG leads may be replaced as a whole (10 leads in total) or as single lead. When replacing the whole set of 10
ECG leads into the connector of the head the sequence of connecting the ECG leads (V1, V2, …., N, F, L, R) does
not matter.
8 Troubleshooting
8.1 Possible Errors
Error
Pump is not working
(no audible noise)
Pump is working,
but there is no suction
Weak suction
Pump is working, but there
is no or little suction, electrodes are falling off during
cardiac stress test
Battery is totally depleted
Battery is defective and / or
doesn‘t work
Possible Causes & Indications
Loose connecting plug at pump
No Power
Loose hose connection at pump or leaking hose
Hoses are kinked or pinched
Leaking hose connection at pump
Loose suction line in control box
Leaking suction line
Suction cups not seated properly on electrode element
Suction cup hose attachment twisted or rolled up
Dirty electrode elements or suction cups
Prolonged time of non-use
Battery life has been exceeded
Error Localization &
Troubleshooting
Firmly push in connecting plug
Check hose connection for tight fit
Eliminate cause
Check hose connection for tight fit
Eliminate cause
Firmly seat suction cup over
electrode element
Electrode elements and suction
cups
Device minimum: 3.5 hours
connected to power grid
Contact customer service
Should these tips be of no help in fixing the problem, please call your mbnet Engineering
GmbH dealer or directly contact mbnet Engineering GmbH.
Please have your model name and serial number ready. They are listed on the type label
on the pump housing.
8.2 Preventing Electromagnetic Interference
The user can help avoid electromagnetic disturbances by keeping the recommended minimum distance between portable and mobile HF telecommunication devices (transmitters)
and the unit. The distance depends on the output performance of the communication device as indicated in the table below.
*
“Non ionising electromagnetic radiation”
Seite 32
Page 33

User guide
ergo vac
HF source
Wireless
communications devices
Various radio services (TETRA 400)
Walkie-talkie (FRS)
Rescue service, police, fire brigade,
servicing (GMRS)
L TE band 13/17 704 – 707 710 / 745 / 780 0.2 0.3
GSM800 / 900
LTE band 5
Radio telephone (micro cellular) T1+,
CT2,CT3
GSM1800/1900
DECT (radio telephone)
LTE band 1/3/4/25
UMTS
Bluetooth, WLAN 802.11b/g/n
LTE band 7
RFID 2450 (active and passive
transponders and reading devices)
WLAN 802.11a / n 5100 – 5800 5240 / 5500 / 5785 2 0.3
Transmitter
frequency
(MHz)
380-390 385 1.8 0.3
430-470 450 2 0.3
800-960 810 / 870 / 930 2
1700 – 1990 1720 / 1845
2400 – 2570 2450 2 0.3
Testing
frequency
[MHz]
/ 1970 2 0.3
max. power
P (W)
Distance d
0.3
(m)
Caution
Portable HF telecommunication devices must not be used within a radius of 0.3 m
from the device and its cables.
Do not place the device on top of other electric/electronic devices - i.e. maintain a
sufficient distance to other devices (this includes the patient cables).
For permanent HF telecommunication devices (e.g. radio and TV), the recommended
distance can be calculated using the following formula:
d = 1.2 x
P
for 150 kHz up to 800 MHz and d = 2.3 x
P for 800 MHz up to 2.5
GHz
d = recommended minimum distance in metres
P = transmitting power in Watts
The user can take the following measures to prevent electromagnetic interference:
Increase the distance to sources of interference
Turn the device and thereby change the angle of the radiation
Connect a potential equalisation cable
Connect the device with another network connection
Use only original accessories
Near strong electromagnetic fields (MRT, radio, etc.) it is possible that the device will turn
off by itself or change the suction strength.
Then simply switch the device on again and / or regulate the suction strength again according to your needs.
Seite 33
Page 34

ergo vac
User Guide
8.3 Warranty
mbnet Engineering GmbH will assume the statutory warranty in accordance with its Terms
and Conditions of Sale, Delivery and Payment. Wear and tear parts and disposables are
excluded from the warranty.
The warranty will lapse in the following cases:
Damage resulting from incorrect operation and incorrect use.
In the event of defective installation, intervention by unauthorised persons or the use
of accessories, disposables or spare parts, which are not original parts of mbnet
Engineering GmbH.
If changes, extensions, repairs or other work was carried out by persons who are not
authorized by the manufacturer.
If the electrical facility in the room in which the device is connected, does not comply
with the requirements of VDE 0100-710.
If the instructions for use were not observed when the device was used.
8.4 Accessories and Disposables
Warning
Always use mbnet Engineering GmbH spare parts and disposables or products approved
by mbnet Engineering GmbH. Failure to do so may endanger life and/or invalidate the warranty.
Your local representative stocks all the disposables and accessories available for the device. A comprehensive list of all mbnet Engineering GmbH representatives can be found on
the mbnet Engineering GmbH website. In case of difficulty, contact our head office directly.
Our staff will be pleased to help with your concerns and any questions you may have.
9 Technical Data
9.1. Pump Module
Dimensions
Weight
Power Supply
Power Consumption
Fuse
250 x 170 x 85 mm
Modul pompe sans accumulateur: 2.5 kg
Modul pompe avec accumulateur: 4.0 kg
100 - 240 VAC
30 VA
2 x T1.6A/250V
Seite 34
Battery (Option)
Battery life
Charging time to 100 %
Charging time in use
Ambient conditions
Operating temperature
Relative humidity Air
pressure during use
Vacuum
Air flow
SLA (Sealed Lead Acid) Accu
approx. 800 charging cycles
approx. 3.5 hrs.
approx. 10 hrs.
10 to 50 °C, storage 10 to 40 °C
30 to 75 % (non-condensing)
700 to 1060 hPa
0 - 500 mbar
0- 5.5 l/min
Page 35

9.2 Patient Module
User guide
ergo vac
5 vacuum levels
9.3 System Cable
System cable
ECG connection
Control cable
Hose
9.4 Electrodes
Material
9.5 Cable Arm
Length
80, 120, 160, 200, 220 mbar
1.9 m
15-channel in accordance with IEC Standard
Norm
1.5 m
1.5 m
sintered Ag/AgCI, silicone suction cup
800 to 1100 mm
Range of height adjustment
Pivot range
1000 to 2000 mm
300°
9.6 Safety Standards
Safety standard
EMV
Compliance / Classification
Protection
IEC/EN 60601-1
IEC/EN 60601-1-2
CE/I in accordance with EC Directive 93/42
This device is not intended for outdoor use (IP X0)
Seite 35
Page 36

ergo vac
User Guide
10 EMC information
The unit meets the Collateral Standards of Electromagnetic compatibility – Requirements and tests
IEC/EN 60601-1-2 the limits and methods of measurement of electromagnetic disturbance characteristics of industrial, scientific and medical radio frequency equipment.
Medical electrical equipment is subject in regard to the electromagnetic compatibility (EMC) and its special precautionary measure. The unit must in reference to the mentioned EMC-hints in the accompanying
documents be installed and operated.
This medical device is intended for use in the electromagnetic environment specified in the following
tables. The user of this device should ensure that it is used in such an environment.
Guidance and manufacturer’s declaration – electromagnetic emissions
The ergo vac is intended for use in the electromagnetic environment specified below.
The customer or the user of the ergo vac should assure that it is used in such an environment.
Emission test
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations /
flicker emissions
IEC 61000-3-3
Compliance
Group 1
Class B
Complies
inherently
Complies
inherently
Electromagnetic environment - guidance
The ergo vac uses RF energy only for its internal function. Therefore, its
RF emissions are very low and are not likely to cause any interference in
nearby electronic equipment.
The ergo vac is suitable for use in all establishments, including domestic
establishments and those directly connected to the public low-voltage
power supply network that supplies buildings used for domestic purposes.
Seite 36
Page 37

User guide
10.1 Table 1:
Immunity (all devices): electromagnetic emissions
Guidance and manufacturer’s declaration – electromagnetic immunity
The ergo vac is intended for use in the electromagnetic environment specified below.
The customer or the user of s ergo vac hould assure that it is used in such an environment.
ergo vac
Immunity test
standard
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient / burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short interruptions and
voltage variations on power supply
lines
IEC 61000-4-11
Note: U
is the a.c. mains voltage prior to application of the test level.
T
Power frequency (50 / 60 Hz)
magnetic field
IEC 61000-4-8
IEC 60601
test level
± 8 kV contact
±15 kV air
± 2 kV for power
supply lines
± 1 kV for
input/output lines
± 1 kV line to line
± 2 kV line to earth
<5% U
(0,5 cycle)
T
40% UT (5 cycles)
70% UT (25 cycles)
<5% UT for 5 s
3 A / m 200 A / m Power frequency magnetic fields should
Compliance
level
± 8 kV contact
±15 kV air
± 2 kV for power
supply lines
± 1 kV line to line
± 2 kV line to earth
<5% UT (0,5 cycle)
40% UT (5 cycles)
70% UT (25 cycles)
<5% UT for 5 s
Electromagnetic
environment - guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30%
Mains power quality should be that of a
typical commercial or hospital environment.
Mains power quality should be that of a
typical commercial or hospital environment.
Mains power quality should be that of a
typical commercial or hospital environment.
be at levels characteristic of a typical
location in a typical commercial or hospital environment.
Seite 37
Page 38

ergo vac
P
P
P
User Guide
10.2 Table 2:
Immunity: electromagnetic immunity
Guidance and manufacturer’s declaration – electromagnetic immunity
The ergo vac is intended for use in the electromagnetic environment specified below.
The customer or the user of the ergo vac should assure that it is used in such an environment.
Electromagnetic environment - guidance
Portable and mobile RF communications equipment should be used no closer to any part of the handy vaq, including cables,
than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Immunity test
standard
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
Radiated RF
IEC 61000-4-3
Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,
should be less than the compliance level in each frequency range. b
Interference may occur in the vicinity of equipment marked with the following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the ergo vac is used exceeds the applicable RF compliance level above, the ergo vac should be observed to verify normal operation. If abnormal performance is observed,
additional measures may be necessary, such as re-orienting or relocating the ergo vac.
IEC 60601
test level
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 800 MHz
3 V/m
0.8 to 2.5 GHz
Compliance
level
V1 = 10 Vrms
150 kHz to 80 MHz
E1 = 10 V/m
80 MHz to 800 MHz
E2 = 10 V/m
800 MHz to 2.7 GHz
Recommended
separation distance
d = 0.35
d = 0.35
d = 0.7
a
150 kHz to 80 MHz
80 MHz to 800 MHz
0.8 to 2.7 GHz
c
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 10 V/m.
c Possible shorter distances outside ISM bands are not considered to have a better applicability of this table.
Seite 38
Page 39

User guide
P
P
P
10.3 Table 3:
electromagnetic immunity
Recommended separation distances
(not life-supporting devices)
Recommended separation distances between portable and mobile RF communications
equipment and the ergo vac
The ergo vac is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The
customer or the user of the ergo vac can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the ergo vac as recommended below, according to the
maximum output power of the communication equipment.
ergo vac
Rated maximum output
Separation distance according to frequency of transmitter
power of transmitter
m
W
150 kHz to 80 MHz
d = 0.35
0.01 0.04 m 0.04 m 0.07 m
0.1 0.12 m 0.12 m 0.22 m
1 0.35 m 0.35 m 0.7 m
10 1.2 m 1.2 m 2.2 m
100 3.5 m 3.5 m 7 m
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be
estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
Note 3: An additional factor of 10/3 is used in calculating the recommended separation distance to
decrease the likelihood that mobile/portable communications equipment could cause
interference if it is inadvertently brought into patient areas.
80 MHz to 800 MHz
d = 0.35
800 MHz to 2500 MHz
d = 0.35
Seite 39
Page 40

ergo vac
User Guide
Declation of Conformity
EC Declaration of Conformity
Directive 93/42/EEC Annex VII
Directive 2007/47/EEC Annex VII
The manufacturer mbnet Engineering GmbH
Kirschauer Strasse 37a
D-02681 Callenberg
hereby declares that the product
ergo vac (Class I)
is in compliance with the following:
Directive 93/42/EEC
Directive 2007/47/EEC
Applied Standards IEC 60601-1
IEC 60601-1-2
Seite 40
(Management)
Callenberg, 30.05.2019Manuel Bucher
Page 41

 Loading...
Loading...