Page 1
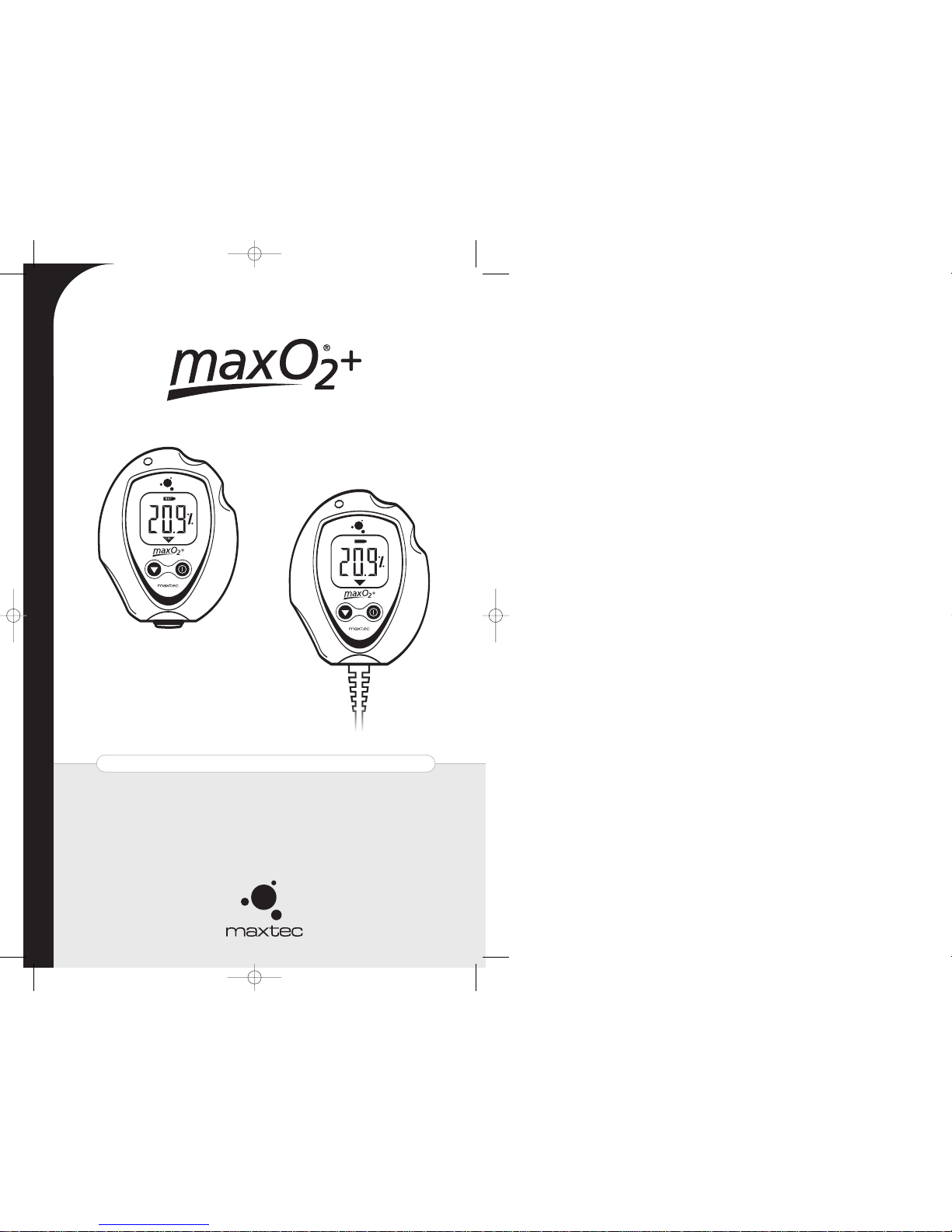
MAXO
2
®
+
A /AE USER’S GUIDE & OPERATING INSTRUCTIONS
BAT
CAL
MAXO
2
®
+
A
MAXO
2
®
+
AE
R217M40 Rev.D
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 1
Page 2
PREFACE
This manual describes the function, operation and maintenance of the MAXO
2
®
+
hand-held oxygen analyzer. A member of Maxtec®'s MAXO
2
®
analyzer line of
oxygen analyzers and monitors, the MAXO
2
®
+
utilizes the MAX-250+ oxygen sen-
sor and is engineered for long life, maximum reliability and stable performance.
The MAXO
2
®
+
is intended as a tool for use by qualified personnel to spot-check or
measure oxygen concentration of a delivered air /oxygen mixture. The
MAXO
2
®
+
is
not intended for use in continuous monitoring of oxygen delivery to a patient.
NOTE: In order to obtain optimum performance from your MAXO
2
®
+ analyzer, all operation and
maintenance must be performed in accordance with this manual. Please read the manual
thoroughly before using the analyzer and do not attempt any repair or procedure that is not
described herein. Maxtec
®
cannot warrant any damage resulting from misuse, unauthorized
repair or improper maintenance of the instrument.
Thank You
Thank you for your purchase of a Maxtec®MAXO
2
®
+
oxygen analyzer. We appreciate the time and energy you invest in selecting the equipment best suited to your
needs. In exchange, we are supplying you with a reliable, high-quality instrument
that, with proper care and operation, will provide you with years of exceptional
service. We also encourage your comments or suggestions as to how our equipment, in any way, can better serve your needs. Please feel free to write, FAX or email us at the address on page ii of this manual c/o the Maxtec
®
Marketing
Department. Please visit our website www.maxtecinc.com for more information on
our products and services.
WARNING:
Never install the sensor in a location that will expose the sensor to patient’s exhaled breath or
secretions, unless you intend to dispose of the sensor, flow diverter and tee adapter.
CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician.
CAUTION: NOT for use with inhalation agents. Operating the device in flammable or
explosive atmospheres may result in fire or explosion.
NOTE: Not for use in a MRI environment.
NOTE: Device specified for dry gas only.
NOTE: This product is latex free.
Never allow an excess length of tubing, lanyard or sensor cable near the patient’s
head or neck, which may result in strangulation.
ii iii
WWW.MAXTECINC.COM
DECLARATION OF CONFORMITY
Manufacturer's Name: Maxtec®, Inc.
Manufacturer's Address: Maxtec®, Inc.
6526 South Cottonwood Street
Salt Lake City, Utah 84107
USA
European Representative: Bio MS (Airox)
L'Echangeur, Parc d'activite Pau-Pyrenees
64000 Pau, France
Product: Oxygen Analyzer
Model(s): MAXO
2
®+ A, MAXO2®+ AE
Classification: IIa
Classification criteria: Clause 3.2 Rule 10 of Annex IX of MDD
We herewith declare that the above mentioned products meet the provisions of the following EC Council
Directives and Standards. All supporting documents are retained under the premises of the manufacturer and the notified body.
Directives: General application directives: Medical Device Directive,
COUNCIL DIRECTIVE 93/42/EEC of 14 June 1993 concerning
medical devices (MDD 93/42/EEC)
Standards: EN/IEC 601-1 1988, Amendment I 1991, Amendment II 1995
IEC 60601-1-2: 2001
EN 1041
IEC 878
EN 980
ISO 7767; 1997
EN 60601-1-4: 2000-04
Notified Body: TUV Product Service
RIDLERSTRASSE 65, D-80339 MUNICH, Germany
EC Certificate No.: G1 01 12 45041 001
Date CE mark was affixed: June 21, 2004
Name: Gordon R. Roth
Position: Manager, Quality System/ISO Representative
Maxtec
®
, Inc.
6526 South Cottonwood Street
Salt Lake City, UT 84107 U.S.A.
General Telephone: 801-266-5300
Toll Free U.S.A.: 800-748-5355
FAX: 801-270-5590
Web Site: www.maxtecinc.com
E-mai: info@maxtecinc.com
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 2
Page 3
Classification
Protection against electric shock: Internally powered equipment.
Protection against water: IPX1
Mode of Operation: Continuous
Sterilization: See section 7.0
Safety Labeling
The following symbols and safety labels are found on the MAXO
2
®
+
:
Attention, Consult ACCOMPANYING DOCUMENTS
Off/On
Calibration
Found to meet the requirements of U.S. and Canadian nationally
recognized codes and standards listed or classified by ETL.
v
WWW.MAXTECINC.COM
iv
MANUFACTURED BY MAXTEC, INC.
Before use, all individuals who will be using the MAXO
2
®
+
must become thoroughly
familiar with the information contained in this Operation Manual. Strict adherence
to the operating instructions is necessary for safe, effective product performance.
This product will perform only as designed if installed and operated in accordance
with the manufacturer’s operating instructions.
Use only genuine Maxtec
®
accessories and replacement parts. Failure to do so
may seriously impair the analyzer’s performance. Repair or alteration of the
MAXO
2
®
+
beyond the scope of the maintenance instructions, or by anyone other
than an authorized Maxtec®service person, could cause the product to fail to
perform as designed.
Calibrate the
MAXO
2
®
+
weekly when in operation, or if environmental conditions
change significantly. (ie, Elevation, Temperature, Pressure, Humidity — refer to
Section 3.0 of this manual).
Use of the
MAXO
2
®
+
near devices that generate electrical fields may cause erratic
readings.
If the
MAXO
2
®
+
is ever exposed to liquids (from spills or immersion) or to any
other physical abuse, turn the instrument OFF and then ON. This will allow the
unit to go through its self test to assure everything is operating correctly.
Never autoclave, immerse or expose the
MAXO
2
®
+
(including sensor) to high
temperatures (>70°C). Never expose the device to pressure, irradiation vacuum,
steam, or chemicals.
NOTE: Replace the batteries with recongnized high quality AA Alkaline or Lithium batteries.
NOTE: If the unit is going to be stored (not in use for 1 month), we recommend that you
remove the batteries to protect the unit from potential battery leakage.
FAILURE TO COMPLY WITH THESE WARNINGS AND CAUTIONS COULD RESULT IN
INSTRUMENT DAMAGE AND POSSIBLY JEOPARDIZE THE WELL BEING OF THE
PATIENT AND/OR HEALTH CARE PROFESSIONAL.
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 4
Page 4
1.0 SYSTEM OVERVIEW
1.1 Base Unit Description
The MAXO
2
®
+
analyzer provides unparalleled performance and reliability due
to an advanced design that includes the following features and operational
benefits.
• Extra-life oxygen sensor of approximately 900,000 O
2 percent hours
(2-year warranty)
• Durable, compact design that permits comfortable, hand-held operation
and easy to clean.
• Operation using only two AA Alkaline batteries (2 x 1.5 Volts) for approxi-
mately 5000 hours of performance with continuous use. For extra extended long life, two AA Lithium batteries may be used.
• Oxygen-specific, galvanic sensor that achieves 90% of final value in
approximately 15 seconds at room temperature.
• Large, easy-to-read, 3 1/2-digit LCD display for readings in the 0-100%
range.
• Simple operation and easy one-key calibration.
• Self-diagnostic check of analog and microprocessor circuitry.
• Low battery indication.
• Calibration reminder timer that alerts the operator, using a calibration icon
on the LCD display, to perform a unit calibration.
1
WWW.MAXTECINC.COM
vi
MANUFACTURED BY MAXTEC, INC.
TABLE OF CONTENTS
1.0 SYSTEM OVERVIEW . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.1
Base Unit Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
1.2
Component Identification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
1.3 Component Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
1.4 MAX-250+ Oxygen Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2.0
OPERATING INSTRUCTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2.1
Getting Started . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2.1.1
Protect Tape . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2.1.2 Remove the Battery Ribbon . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2.1.3 Automatic Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
2.2
Calibrating the MAXO
2
®
+
Oxygen Analyzer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4
2.2.1
In Line Calibration (Flow Diverter - Tee Adapter) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
2.2.2 Direct Flow Calibration (Barb) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
3.0 FACTORS INFLUENCING ACCURATE READINGS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
3.1
Elevation Changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
3.2 Temperature Effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
3.3 Pressure Effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6
3.4
Humidity Effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
4.0 CALIBRATION ERRORS AND ERROR CODES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
5.0 CHANGING THE BATTERIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
6.0
CHANGING THE OXYGEN SENSOR . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
6.1 MAXO
2
®
+
A Model . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
6.2 MAXO
2
®
+
AE Model . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
7.0 CLEANING AND MAINTENANCE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
8.0
SPECIFICATIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
8.1
Base Unit Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
8.2
Sensor Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
9.0 MAXO
2
®
+
SPARE PARTS AND ACCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
9.1
Included With Your Unit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12
9.2 Standard Replacement Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
9.3 Optional Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
9.3.1
Optional Adapters . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
9.3.2 Mounting Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
9.3.3 Carrying Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
10.0
WARRANTY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 6
Page 5

1.4 MAX-250+ Oxygen Sensor
The MAX-250+ oxygen sensor offers stability and extra life.
The MAX-250+ is a galvanic, partial pressure sensor that is specific to oxygen. It
consists of two electrodes (a cathode and an anode), a teflon membrane and an
electrolyte. Oxygen diffuses through the teflon membrane and immediately reacts
at a gold cathode. Concurrently, oxidation occurs electrochemically at the lead
anode, generating an electrical current and providing a voltage output. Electrodes
are immersed in a unique gelled weak acid electrolyte which is responsible for
the sensors long life and motion insensitive characteristic. Since the sensor is
specific to oxygen, the current generated is proportional to the amount of oxygen
present in the sample gas. When no oxygen is present, there is no electrochemical reaction and therefore, negligible current is produced. In this sense, the sensor is self-zeroing.
CAUTION: The Maxtec®MAX-250+ oxygen sensor is a sealed device containing a mild acid
electrolyte, lead (Pb), and lead acetate. Lead and lead acetate are hazardous waste
constituents and should be disposed of properly, or returned to Maxtec®for proper
disposal or recovery.
CAUTION: Do not use ethylene oxide sterilization. Do not immerse the sensor in any
cleaning solution, autoclave or expose the sensor to high temperatures.
CAUTION: Dropping sensor can adversely affect its performance.
2.0 OPERATING INSTRUCTIONS
2.1 Getting Started
2.1.1 Protect Tape
Prior to turning on the unit, a protective film covering the threaded sensor
face must be removed. After removing the film, wait approximately 20
minutes for the sensor to reach equilibrium.
2.1.2 Remove the Battery Ribbon
A ribbon has been inserted between the two case halves to prevent a battery
connection. Remove the ribbon by pulling it completely out of the case. To
energize the unit tighten all three screws with the included Phillips screwdriver.
NOTE: If you do not tighten all three screws, the unit may not turn on or it may erratically turn
on and off.
3
WWW.MAXTECINC.COM
2
MANUFACTURED BY MAXTEC, INC.
1.2 Component Identification
1.3 Component Description
1. 3 1/2-Digit Display - The 3 1/2 digit liquid crystal display (LCD) provides
direct readout of oxygen concentrations in the range of 0 – 105.0%
(100.1% to 105.0% used for calibration determination purposes). The
digits also display error codes and calibration codes as necessary.
2. Low Battery Indicator - The low battery indicator is located at the top of the
display and is only activated when the voltage on the batteries is below a
normal operating level.
3. “%” symbol - The “%” sign is located to the right of the concentration
number and is present during normal operation.
4. Calibration symbol - The calibration symbol is located at the bottom of the
display and is timed to activate when a calibration is necessary.
5. ON/OFF Key - This key is used to turn the device on or off.
6. Calibration Key - This key is used to calibrate the device. Holding the key
for more than three seconds will force the device to enter a calibration mode.
CAUTION: The device will assume a percent oxygen concentration when calibrating. Be
sure to apply 100% oxygen, or ambient air concentration to the device during calibration
or the device will not calibrate correctly.
Sample Inlet Connection
This is the port at which the device is connected to determine oxygen concentration.
FIGURE 1
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 8
Page 6

• If you are unsure about the displayed O2percentage.
(see Factors influencing accurate readings.)
A simple calibration may be made with the sensor open to static Ambient air. For
optimum accuracy Maxtec
®
recommends that the Sensor be placed in a closed
loop circuit where gas flow is moving across the sensor in a controlled manner.
2.2.1 In Line Calibration (Flow Diverter – Tee Adapter)
1. Attach the diverter to the MAXO
2
®
+
by threading it on to the bottom of the sensor.
2. Insert the MAXO
2
®
+
in the center position of the tee adapter. (figure 2, A)
3. Attach an open-ended reservoir to the end of the tee adapter. Then start
the calibration flow of oxygen at two liters per minute.
Six to 10 inches of corrugated tubing works well as a reservoir. A calibration oxygen flow to the
MAXO
2
®
+
of two liters per minute is recommended
to minimize the possibility of obtaining a "false" calibration value.
4. Allow the oxygen to saturate the sensor. Although a stable value is usually
observed within 30 seconds, allow at least two minutes to ensure that the
sensor is completely saturated with the calibration gas.
5. If the MAXO
2
®
+
is not already turned on, do so now by pressing the
analyzer
“ON” button.
6. Press the Cal button on the MAXO
2
®
+
until you read the word CAL on the
analyzer display. This can take approximately 3 seconds. The analyzer will
now look for a stable sensor signal and a good reading. When obtained,
the analyzer will display the calibration gas on the LCD.
NOTE: Analyzer will read “Cal Err St” if the sample gas has not stablized.
2.2.2 Direct Flow Calibration (Barb)
1. Attach the Barbed Adapter to the MAXO
2
®
+
by threading it on to the
bottom of the sensor.
2. Connect the Tygon tube to the barbed adapter. (figure 2, B)
3. Attach the other end of the clear sampling tube to a source of oxygen with
a known oxygen concentration value. Initiate flow of the calibration gas to
the unit. Two liters per minute is recommended.
5
WWW.MAXTECINC.COM
4
MANUFACTURED BY MAXTEC, INC.
2.1.3 Automatic Calibration
After the unit is turned on it will automatically calibrate to room air. The
display should be stable and reading 20.9%.
To check the oxygen concentration of a sample gas:
(after the unit has been calibrated)
1. Connect the Tygon tubing to the bottom of the
analyzer by threading the barbed adapter onto
the oxygen sensor. (figure 2, B)
2. Attach the other end of the sample hose to
the sample gas source and initiate flow of the
sample to the unit at a rate of 1-10 liters per
minute (2 liters per minute is recommended).
3. Using the "ON/OFF" key, make sure the unit
is in the power
"ON” mode.
4. Allow the oxygen reading to stabilize. This will normally take about 30
seconds or more.
2.2 Calibrating the MAXO2®+ Oxygen Analyzer
NOTE: We recommend use of medical grade oxygen at 100% when calibrating the MAXO
2
®
+
.
The MAXO
2
®
+
Analyzer should be calibrated upon initial power-up. Thereafter,
Maxtec
®
recommends calibration on a weekly basis. To serve as a reminder, a
one week timer is started with each new calibration. At the end of one week a
reminder icon “ ” will appear on the bottom of the LCD. Calibration is recommended if the user is unsure when the last calibration procedure was performed,
or if the measurement value is in question.
For hospital and home care a new calibration is required when
• The measured O2percentage in 100% O2is below 97.0% O
2
• The measured O2percentage in 100% O2is above 103.0% O
2
• The CAL reminder Icon is blinking at the bottom of the LCD
• If you are unsure about the displayed O2percentage. (see Factors
influencing accurate readings.)
For ID testing, (or optimum accuracy) a new calibration is required when
• The measured O2percentage in 100% O2is below 99.0% O
2
• The measured O2percentage in 100% O2is above 101.0% O
2
• The CAL reminder Icon is blinking at the bottom of the LCD
FIGURE 2
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 10
Page 7

• Calibrate the MAXO
2
®
+
at the same pressure as the sample gas.
• If sample gases flow through tubing, use the same apparatus and flow rates
when calibrating as when measuring.
3.4 Humidity Effects
Humidity (non-condensing) has no effect on the performance of the MAXO
2
®
+
other than diluting the gas, as long as there is no condensation. Depending on
the humidity, the gas may be diluted by as much as 4%, which proportionally
reduces the oxygen concentration. The device responds to the actual oxygen
concentration rather than the dry concentration. Environments where condensation may occur are to be avoided since moisture may obstruct passage of gas to
the sensing surface, resulting in erroneous readings and slower response time.
For this reason, the following is recommended:
• Avoid usage in environments greater than 95% relative humidity.
• When used in a breathing circuit, place the sensor upstream of the humidifier.
HELPFUL HINT: Dry sensor by lightly shaking moisture out, or flow a dry gas at two liters per
minute across the sensor membrane.
4.0 CALIBRATION ERRORS AND ERROR CODES
The MAXO
2
®
+
analyzers have a self test feature built into the software to detect
faulty calibrations, oxygen sensor failures, and low operating voltage. These are
listed below, and include possible actions to take, if an error code occurs.
E02: No sensor attached
MAXO
2
®
+
A: Open unit and disconnect and reconnect sensor. Unit should perform
an auto calibration and should read 20.9%. If not, contact Customer Service for
possible sensor replacement.
MAXO
2
®
+
AE: Disconnect and reconnect external sensor. Unit should perform an
auto calibration, and should read 20.9%. If not, contact Customer Service for
possible sensor replacement or cable replacement.
E03: No valid calibration data available
Make sure unit has reached thermal equilibrium. Press and hold the Calibration
Button for three seconds to manually force a new calibration.
E04: Battery below minimum operating voltage
Replace batteries.
7
WWW.MAXTECINC.COM
6
MANUFACTURED BY MAXTEC, INC.
4. Allow the oxygen to saturate the sensor. Although a stable value is usually
observed within 30 seconds, allow at least two minutes to ensure that the
sensor is completely saturated with the calibration gas.
5. If the MAXO
2
®
+
is not already turned on, do so now by pressing the
analyzer “ON” button.
6. Press the Cal button on the MAXO
2
®
+
until you read the word CAL on the
analyzer display. This can take approximately 3 seconds. The analyzer
will now look for a stable sensor signal and a good reading. When
obtained, the analyzer will display the calibration gas on the LCD.
3.0 FACTORS INFLUENCING ACCURATE READINGS
3.1 Elevation Changes
• Changes in elevation result in a reading error of approximately 1% of reading
per 250 feet.
• In general, calibration of the instrument should be performed when elevation at
which the product is being used changes by more than 500 feet.
3.2 Temperature Effects
The MAXO
2
®
+
will hold calibration and read correctly within ±3% when at thermal
equilibrium within the operating temperature range. The device must be thermally
stable when calibrated and allowed to thermally stabilize after experiencing temperature changes before readings are accurate. For these reasons, the following
is recommended:
• For best results, perform the calibration procedure at a temperature close to
the temperature where analysis will occur.
• Allow adequate time for the sensor to equilibrate to a new ambient temperature.
CAUTION: “CAL Err St” may result from a sensor that has not reached thermal equilibrium.
• When used in a breathing circuit, place the sensor upstream of the heater.
3.3 Pressure Effects
Readings from the MAXO
2
®
+
are proportional to the partial pressure of oxygen.
The partial pressure is equal to the concentration times the absolute pressure.
Thus, the readings are proportional to the concentration if the pressure is held
constant. Therefore, the following are recommended:
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 12
Page 8

FIGURE 4
Lock Lever
Carefully, bring the two halves of the case together while positioning the wires so
they are not pinched between the two case halves. The gasket separating the
halves will be captured on the back case half.
Reinsert the three screws and tighten until the screws are snug.
(figure 3)
The device will automatically perform a calibration and begin displaying % of oxygen.
HELPFUL HINT: If unit does not function, verify that the screws are tight to allow proper
electrical connection.
HELPFUL HINT (MAXO2+ AE): Before closing the two case halves together, verify that the keyed
slot on top of the coiled cable assembly is engaged on the small tab located on the back case.
This is designed to position the assembly in the correct orientation and prevent it from rotating. Improper positioning could hinder the case halves from closing and prevent operation
when tightening the screws.
6.0 CHANGING THE OXYGEN SENSOR
6.1 MAXO
2
®
+A Model
Should the oxygen sensor require changing, the device will indicate this by
presenting “Cal Err lo” on the display after initiating a calibration.
To change the oxygen sensor, begin by removing
the three screws from the back of the device.
A #1 phillips screwdriver is required to
remove these screws.
Once the screws are removed, gently separate
the two halves of the device.
Disconnect the oxygen sensor from the printed
circuit board by pressing the un-lock lever first and
then pulling the connector out of the receptacle.
The oxygen sensor can now be replaced from the back half of the case.
HELPFUL HINT: Be sure to orient the new sensor by aligning the red arrow on the sensor with
the arrow in the back case. A small tab is located on the back case that is designed to engage
the sensor and prevent it from rotating within the case. (figure 4)
NOTE: If the oxygen sensor is installed incorrectly, the case will not come back together and
the unit may be damaged when the screws are reinstalled.
8
MANUFACTURED BY MAXTEC, INC.
9
WWW.MAXTECINC.COM
CAL Err St: O2 Sensor reading not stable
Wait for displayed oxygen reading to stabilize, when calibrating the device at
100% oxygen.
Wait for unit to reach thermal equilibrium (Please note that this can take up to
one half hour, if the device is stored in temperatures outside the specified operating temperature range).
CAL Err lo: Sensor voltage too low
Press and hold the Calibration Button for three seconds to manually force a new
calibration. If unit repeats this error more than three times, contact Customer
Service for possible sensor replacement.
CAL Err hi: Sensor voltage too high
Press and hold the Calibration Button for three seconds to manually force a new
calibration. If unit repeats this error more than three times, contact Customer
Service for possible sensor replacement.
CAL Err Bat: Battery voltage too low to recalibrate
Replace batteries
5.0 CHANGING THE BATTERIES
Should the batteries require changing, the device will indicate this in one of two ways:
• The battery icon on the bottom of the display will begin to flash. This icon
will continue to flash until the batteries are changed. The unit will continue
to function normally for approx. 200 hours.
• If the device detects a very low battery level, an error code of “E04” will be present on the display, and the unit will not function until the batteries are changed.
To change the batteries, begin by removing the three
screws from the back of the device. A #1 Phillips
screwdriver is required to remove these screws.
Once the screws are removed, gently separate
the two halves of the device.
The batteries can now be replaced from the back half of the
case. Be sure to orient the new batteries as indicated in
the embossed polarity on the back case.
NOTE: If the batteries are installed incorrectly the
batteries will not make contact and the device will not operate.
FIGURE 3
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 14
Page 9

exhaled breath or secretions, unless you intend to dispose of the sensor, flow diverter
and tee adapter after use.
• Clean the sensor with a cloth moistened with isopropyl alcohol
(65% alcohol/water solution).
• Maxtec®does not recommend use of spray disinfectants because they can contain salt, which can accumulate in the sensor membrane and impair readings.
• The oxygen sensor is not intended for steam, ethylene oxide or radiation sterilization.
Accessories
• The flow diverter and tee adapter may be disinfected by washing them
with isopropyl alcohol or Cidex (per manufacturer's instructions). The parts
must be thoroughly dry before they are used.
• Sterilizing -The flow diverter and tee adapter may be sterilized using
Cidex, steam or ethylene oxide (per manufacturer's instructions). Due
to the varying conditions imposed on the materials during sterilization it
is not possible to determine the exact number of times the sterilization
processes can be carried out. Therefore, Maxtec
®
recommends that operators carefully examine the flow diverter and tee adapter after sterilization
and prior to use to verify that the item is fit for use. The operator should
verify that there are no cracks and the item does not show any indication
of material changes or physical damage that may compromise its effective
use. Both flow diverter and tee adapter should be free of any chemical
residue remaining from the sterilization process.
Because of the variability of the cleaning, disinfecting and sterilizing processes,
Maxtec
®
cannot provide specific sterilization instructions, nor can the sterility of
the item be ensured. Therefore, we highly recommend referring to the manufacturer's instructions on the details of method.
8.0 SPECIFICATIONS
8.1 Base Unit Specifications
Measurement Range: 0-100%
Resolution: 0.1%
Accuracy and Linearity: 1% of full scale at constant temperature, R.H. and
pressure when calibrated at full scale.
Total Accuracy: ±3% actual oxygen level over full operating
temperature range.
Response Time: 90% of final value in approximately 15 seconds at 23°C.
11
WWW.MAXTECINC.COM
10
MANUFACTURED BY MAXTEC, INC.
Reconnect the oxygen sensor to the connector on the printed circuit board.
Carefully bring the two halves of the case together while positioning the wires to
ensure they are not pinched between the two case halves. Make sure the sensor is
fully inserted and in the proper orientation.
Reinsert the three screws and tighten until the screws are snug. Verify the unit
operates properly.
The device will automatically perform a calibration and begin displaying % of oxygen.
6.2 MAXO
2
®
+AE Model
Should the oxygen sensor require changing, the device will indicate this by
presenting “Cal Err lo” on the display.
Unthread the sensor from the cable by rotating the thumbscrew connector
counterclockwise and pull the sensor from the connection.
Replace the new sensor by inserting the electrical plug from the coiled cord into
the receptacle on the oxygen sensor. Rotate the thumbscrew clockwise until snug.
The device will automatically perform a calibration and begin displaying % of oxygen.
7.0 CLEANING AND MAINTENANCE
Store the MAXO
2
®
+
analyzer in a temperature similar to its ambient environment of
daily use.
The instruction given below describes the methods to clean and disinfect the
instrument, sensor and its accessories (e.g. flow diverter, tee adapter):
Instrument
• When cleaning or disinfecting the exterior of the MAXO
2
®
+
analyzer, take
appropriate care to prevent any solution from entering the instrument. Do
not immerse unit in fluids.
• The
MAXO
2
®
+
analyzer surface may be cleaned using a mild detergent and
a moist cloth.
• The
MAXO
2
®
+
analyzer is not intended for steam, ethylene oxide or radiation
sterilization.
Oxygen Sensor
WARNING: Never install the sensor in a location that will expose the sensor to patient's
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 16
Page 10

9.2 Standard Replacement Parts
Part Number Item
R125P02-011 MAX-250+ Oxygen Sensor
R125P03-002 MAX-250E Oxygen Sensor
R115P85 MAX-250ESF Oxygen Sensor
R217P08 Gasket
RP06P25 #4-40 Pan Head Stainless Steel Screw
R217P16-001 Front Assembly
(Includes Board & LCD)
R217P11-002 Back Assembly
R217P24 Coiled Cable Assembly
R217P09-001 Overlay
9.3 Optional Accessories
9.3.1 Optional Adapters
Part Number Item
RP16P02 Blue Tee Adapter
R103P90 Perfusion Tee Adapter
RP16P12 Long-Neck Tee Adapter
RP16P05 Pediatric Tee Adapter
RP16P10 MAX-Quick Connect
R207P17 Threaded Adapter with Tygon Tubing
9.3.2 Mounting Options (requires dovetail)
Part Number Item
R206P75 Pole Clamp
R205P86 Wall Mount
R213P31 Swivel Mount
R100P10 Rail Mount
R217P23 Dovetail Assembly
9.3.3 Carrying Options
Part Number Item
RP77P01 Jump Bag
R217P22 Belt Clip and Pin
R213P02 Zipper Carrying Case with Shoulder Strap
R213P56 Deluxe Carrying Case, Water Tight
R217P32 Soft Case, Tight Fit Carrying Case
R213P90 Handi/MAXO
2
®
+
Water Tight Box
13
WWW.MAXTECINC.COM
12
MANUFACTURED BY MAXTEC, INC.
Warm-up Time: none required
Operating Temperature: 15°C - 40°C (59°F - 104°F)
Storage Temperature: -15°C - 50°C (5°F - 122°F)
Atmospheric Pressure 800-1013 mBars
Humidity: 0-95% (non-condensing)
Power Requirements: 2, AA Alkaline batteries (2 x 1.5 Volts)
Battery Life: approximately 5000 hours with continuous use
Low Battery Indication: "BAT" icon displayed on LCD
Sensor Type: Maxtec
®
MAX-250+ galvanic fuel cell
Expected Sensor Life: >900,000 O2percent hours minimum 2-years in
typical medical applications
A Model Dimensions: 3.0"(W) x 4.0"(H) x 1.5"(D) [76mm x 102mm x 38mm]
A Weight: 0.4 lbs. (170g)
AE Model Dimensions: 3.0"(W) x 36.0"(H) x 1.5"(D) [76mm x 914mm x 38mm]
Height includes external cable length (retracted).
AE Weight: 0.6 lbs. (285g)
8.2 Sensor Specifications
Type: Galvanic fuel sensor (0-100%)
Life: 2-years in typical applications
9.0 MAXO
2
+
SPARE PARTS AND ACCESSORIES
9.1 Included With Your Unit
Part Number Item
R217P43-002 MAXO2+A Medical Unit with Sensor
R217P44-002 MAXO2+AE Medical Unit with Sensor
R217M40 User’s Guide and Operating Instructions
R217P27 Quick Reference Calibration Card
RP76P06 Lanyard
R207P17 Threaded Adapter with Tygon Tubing
RP18P01 Screw Driver
R110P10 Flow Diverter
RP16P02 Blue Tee Adapter
R217P10-001 Quick Reference Guide
R217P15-001 Getting Started Guide
R217P10-002 Quick Reference Guide
R217P15-002 Getting Started Guide
X
X
XX
XX
XX
X
XX
XX
XX
X
X
X
X
BAT
CAL
A Model AE Model
X
X
X
XX
XX
XX
XX
X
XX
BAT
C
AL
A Model AE Model
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 18
Page 11

14
MANUFACTURED BY MAXTEC, INC.
10.0 WARRANTY
The MAXO
2
®
+
Analyzer is designed for medical oxygen delivery equipment and systems. Under normal
operating conditions, Maxtec®warrants the MAXO
2
®
+
Analyzer to be free from defects of workmanship or
materials for a period of 2-years from the date of shipment from Maxtec®, provided that the unit is properly operated and maintained in accordance with Maxtec®’s operating instructions. Based on Maxtec®’s
product evaluation, Maxtec
®
's sole obligation under the foregoing warranty is limited to making replacements, repairs, or issuing credit for equipment found to be defective. This warranty extends only to the
buyer purchasing the equipment directly from Maxtec®or through Maxtec®'s designated distributors and
agents as new equipment.
Maxtec
®
warrants the MAX-250+ oxygen sensor in the MAXO
2
®
+
Analyzer to be free from defects in mate-
rial and workmanship for a period of 2-years from Maxtec®'s date of shipment in a MAXO
2
®
+
unit. Should
a sensor fail prematurely, the replacement sensor is warranted for the remainder of the original sensor
warranty period.
Routine maintenance items, such as batteries, are excluded from warranty. Maxtec
®
and any other subsidiaries shall not be liable to the purchaser or other persons for incidental or consequential damages or
equipment that has been subject to abuse, misuse, mis-application, alteration, negligence or accident.
These warranties are exclusive and in lieu of all other warranties, expressed or implied, including warranty
of merchantability and fitness for a particular purpose.
15
WWW.MAXTECINC.COM
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 20
Page 12

17
WWW.MAXTECINC.COM
16
MANUFACTURED BY MAXTEC, INC.
medicalManual.RevD.qxd 9/9/04 4:18 PM Page 22
Page 13

medicalManual.RevD.qxd 9/9/04 4:18 PM Page 24
 Loading...
Loading...