Page 1

Radius-7
®
Wearable Pulse
CO-Oximeter
Operator's Manual
®
Page 2

Page 3

For Sale in the USA
These operating instructions provide the necessary information for proper operation of all
models of the Radius-7. There may be information provided in this manual that is not
relevant for your system. General knowledge of pulse oximetry and an understanding of the
features and functions of Radius-7 are prerequisites for its proper use. Do not operate
Radius-7 without completely reading and understanding these instructions.
Notice: Purchase or possession of this device does not carry any express or implied license to
use with replacement parts which would, alone or in combination with this device, fall within
the scope of one of the relating patents.
Note: Cleared Use Only: The device and related accessories are cleared by the Food and Drug
Administration (FDA) for noninvasive patient monitoring and may not be used for any
processes, procedures, experiments, or any other use for which the device is not intended or
cleared by the applicable regulatory authorities, or in any manner inconsistent with the
directions for use or labeling.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. See
instructions for use for full prescribing information, including indications, contraindications,
warnings and precautions.
For professional use. See instructions for use for full prescribing information, including
indications, contraindications, warnings, and precautions.
Wireless Radio:
Contains: FCC ID: VKF-MWM1 | Model: Radius-7 | Contains: IC: 7362A-MWM1 | IC Model:
MWM1
Masimo Corporation
52 Discovery
Irvine, CA 92618, USA
Tel.: 949-297-7000
Fax.: 949-297-7001
www.masimo.com
EU authorized representative for Masimo Corporation:
MDSS GmbH
Schiffgraben 41
D-30175 Hannover, Germany
Patents: www.masimo.com/patents.htm
APOD®, Discrete Saturation Transform®, DST®, FastSat®, FST®, PVi®, rainbow®, rainbow
Resposable®, RRa®, SET®, Signal Extraction Technology®, Signal IQ®, SpCO®, SpHb®,
SpMet® are federally registered trademarks of Masimo Corporation.
www.masimo.com 1 Masimo
WITH RESPECT TO ELECTRIC SHOCK, FIRE AND MECHANICAL HAZARDS ONLY
E357969
®, Masimo®, Pulse CO-Oximeter®, Radius-7®, Root®, Adaptive Probe Off Detection®,
related Collateral (ANSI/AAMI/IEC 60601-1-8:2006) Standards for which the
MEDICAL ELECTRICAL EQUIPMENT
IN ACCORDANCE WITH
ANSI/AAMI ES 60601-1:2005, CAN/CSA C22.2 No. 60601-1:2008, and
applicable Particular (IEC 60601-2-49:2011, EN/ISO 80601-2-61:2011) and
product has been found to comply by UL.
Page 4

rainbow Acoustic Monitoring™, RAM™, SpOC™, and X-Cal™ are trademarks of Masimo
Corporation. All other trademarks and registered trademarks are property of their respective
owners.
The use of the trademark Patient SafetyNet™ is under license from University HealthSystem
Consortium.
© 2017 Masimo Corporation
www.masimo.com 2 Masimo
Page 5

Contents
About this Manual -------------------------------------------------------------------------------------------7
Product Description, Features and Indications for Use ----------------------------------------------- 9
Product Description ------------------------------------------------------------------------------------- 9
Indications for Use -------------------------------------------------------------------------------------- 9
Contraindications --------------------------------------------------------------------------------------- 9
Safety Information, Warnings and Cautions ---------------------------------------------------------- 11
Safety Warnings and Cautions ----------------------------------------------------------------------- 11
Performance Warnings and Cautions --------------------------------------------------------------- 12
Cleaning and Service Warnings and Cautions ---------------------------------------------------- 17
Compliance Warnings and Cautions ---------------------------------------------------------------- 17
Chapter 1: Technology Overview ------------------------------------------------------------------------ 19
Signal Extraction Technology® (SET®) ------------------------------------------------------------ 19
rainbow Pulse CO-Oximetry Technology ----------------------------------------------------------- 22
rainbow Acoustic Monitoring™ (RAM™) ------------------------------------------------------------ 26
Chapter 2: System Components ------------------------------------------------------------------------- 29
General System Description -------------------------------------------------------------------------- 29
Radius-7 Instrument Module ------------------------------------------------------------------------ 30
Radius-7 Battery Module ------------------------------------------------------------------------------ 31
Radius-7 Armband ------------------------------------------------------------------------------------- 32
Radius-7 Battery Charging Adapter ---------------------------------------------------------------- 33
Chapter 3: Setting Up------------------------------------------------------------------------------------ 35
Unpacking and Inspection -------------------------------------------------------------------------- 35
Preparation for Use ----------------------------------------------------------------------------------- 35
Charging the Radius-7 Battery Module ------------------------------------------------------------ 36
Connecting Radius-7 to Root via Bluetooth ------------------------------------------------------ 36
Connecting Radius-7 to Patient SafetyNet -------------------------------------------------------- 37
Securing Radius-7 to Patient ------------------------------------------------------------------------- 37
Removing Radius-7 from Patient ------------------------------------------------------------------- 40
Chapter 4: Operation -------------------------------------------------------------------------------------- 41
Using the Touchpad ----------------------------------------------------------------------------------- 41
About the Main Screen -------------------------------------------------------------------------------- 42
www.masimo.com 3 Masimo
Page 6

Radius-7 Contents
Navigating Radius-7 Main Menu -------------------------------------------------------------------- 42
Navigating Radius-7 Settings on Root ------------------------------------------------------------- 43
Parameter Settings ------------------------------------------------------------------------------------ 46
Trends ---------------------------------------------------------------------------------------------------- 61
Chapter 5: Alarms and Messages ----------------------------------------------------------------------- 63
About Alarms ------------------------------------------------------------------------------------------- 63
3D Alarms ----------------------------------------------------------------------------------------------- 64
Messages ------------------------------------------------------------------------------------------------ 66
Chapter 6: Troubleshooting ----------------------------------------------------------------------------- 71
Troubleshooting Measurements--------------------------------------------------------------------- 71
Troubleshooting Radius-7 ---------------------------------------------------------------------------- 73
Radius-7 Error Codes ---------------------------------------------------------------------------------- 74
Chapter 7: Specifications --------------------------------------------------------------------------------- 75
Measurement Range ---------------------------------------------------------------------------------- 75
Accuracy (ARMS*) ------------------------------------------------------------------------------------- 75
ARMS Performance Specifications ------------------------------------------------------------------ 77
Resolution ---------------------------------------------------------------------------------------------- 80
Electrical------------------------------------------------------------------------------------------------- 81
Environmental ------------------------------------------------------------------------------------------ 81
Physical Characteristics ------------------------------------------------------------------------------ 82
Alarms --------------------------------------------------------------------------------------------------- 82
Display Indicators -------------------------------------------------------------------------------------- 83
EMC Compliance --------------------------------------------------------------------------------------- 83
Safety Standards Compliance ----------------------------------------------------------------------- 83
Wireless Specifications ------------------------------------------------------------------------------- 84
Guidance and Manufacturer's Declaration- Electromagnetic Emissions --------------------- 86
Guidance and Manufacturer's Declaration- Electromagnetic Immunity --------------------- 87
Recommended Separation Distances -------------------------------------------------------------- 89
Symbols------------------------------------------------------------------------------------------------- 90
Citations ------------------------------------------------------------------------------------------------- 92
Chapter 8: Service and Maintenance ------------------------------------------------------------------ 95
Cleaning ------------------------------------------------------------------------------------------------- 95
Battery Operation and Maintenance --------------------------------------------------------------- 95
www.masimo.com 4 Masimo
Page 7

Radius-7 Contents
Safety Checks ------------------------------------------------------------------------------------------ 96
Repair Policy -------------------------------------------------------------------------------------------- 97
Return Procedure --------------------------------------------------------------------------------------- 97
Contacting Masimo ----------------------------------------------------------------------------------- 98
Appendix: Concepts of Alarm Response Delay ------------------------------------------------------ 101
Concepts of Alarm Response Delay --------------------------------------------------------------- 101
Index ------------------------------------------------------------------------------------------------------- 103
www.masimo.com 5 Masimo
Page 8

Page 9

About this Manual
This manual explains how to set up and use the Radius-7® Wearable Pulse CO-Oximeter®.
Important safety information relating to general use of the Radius-7
appears in this manual.
Read and follow any warnings, cautions, and notes presented throughout this manual. The
following are explanations of warnings, cautions, and notes.
A warning is given when actions may result in a serious outcome (for example, injury, serious
adverse effect, death) to the patient or user.
WARNING: This is an example of a warning statement.
A caution is given when any special care is to be exercised by the patient or user to avoid
injury to the patient, damage to this device or damage to other property.
CAUTION: This is an example of a caution statement.
A note is given when additional general information is applicable.
Note: This is an example of a note.
www.masimo.com 7 Masimo
Page 10

Page 11

Product Description, Features and Indications for Use
Product Description
The Radius-7® Wearable Pulse CO-Oximeter® is a noninvasive device that measures arterial
oxygen saturation (SpO
(PVi®) along with optional measurements of hemoglobin (SpHb®), carboxyhemoglobin
, pulse rate (PR), perfusion index (Pi), and Pleth Variability Index
2)
(SpCO®), total oxygen content (SpOC™), methemoglobin (SpMet®), and Acoustic Respiration
Rate (RRa®).
The following key features are available for the Radius-7:
• Patient wearable device for continuous monitoring when the patient is
ambulatory.
• Bluetooth radio for transfer of parameter data to the Root patient monitoring and
connectivity platform.
• Optional Wi-Fi for direct communication throughout the hospital to the Patient
SafetyNet™ remote monitoring system.
• Masimo SET® and rainbow® SET technology performance.
• SpO
and pulse rate monitoring in motion and low perfusion environments.
2
• Continuous and noninvasive monitoring of carboxyhemoglobin (SpCO),
methemoglobin (SpMet), and total hemoglobin (SpHb).
• Respiration Rate (RR) is measured by acoustic signal (RRa).
Indications for Use
The Masimo Radius-7® Wearable Pulse CO-Oximeter® and Accessories are indicated for the
continuous noninvasive monitoring of functional oxygen saturation of arterial hemoglobin
(SpO2), pulse rate, carboxyhemoglobin saturation (SpCO), methemoglobin saturation
(SpMet), total hemoglobin concentration (SpHb), and/or respiratory rate (RRa). The Masimo
Radius-7® Wearable Pulse CO-Oximeter® and accessories are indicated for use with adult
and pediatric patients during both no motion and motion conditions, and for patients who
are well or poorly perfused in hospitals and hospital-type facilities.
Contraindications
There are no contraindications.
www.masimo.com 9 Masimo
Page 12

Page 13

Safety Information, Warnings and Cautions
CAUTION: Radius-7 is to be operated by, or under the supervision of, qualified personnel only.
Read the manual, accessories, directions for use, all precautionary information, and
specifications should be read before use. Refer to the Operator’s Manual for Root for
additional safety information, warnings, and cautions.
Safety Warnings and Cautions
WARNING: Do not use Radius-7 if it appears or is suspected to be damaged.
WARNING: Always use Radius-7 in conjunction with Root. Do not use parts from other
systems. Injury to personnel or equipment damage could occur.
WARNING: Do not adjust, repair, open, disassemble, or modify the Radius-7. Damage to the
device may result in degraded performance and/or patient injury.
WARNING: Do not start or operate the Radius-7 unless the setup was verified to be correct.
Improper set-up of this device may result in degraded performance and/or patient injury.
WARNING: Only use Masimo authorized devices with Radius-7. Using unauthorized devices
with Radius-7 may result in damage to the device and/or patient injury.
WARNING: All sensors and cables are designed for use with specific devices. Verify the
compatibility of the device, cable, and sensor before use; otherwise degraded performance
and/or patient injury can result.
WARNING: Do not use the Radius-7 in the presence of flammable anesthetics or other
flammable substance in combination with air, oxygen-enriched environments, or nitrous
oxide to avoid risk of explosion.
WARNING: Do not use the Radius-7 during magnetic resonance imaging (MRI) or in an MRI
environment.
WARNING: Radius-7 may be used during defibrillation. However, to reduce the risk of electric
shock, the operator should not touch the Radius-7 during defibrillation.
WARNING: Electrical Shock Hazard: To protect against injury, follow the directions below:
• Avoid placing the device on surfaces with visible liquid spills.
• Do not soak or immerse the device in liquids.
• Do not attempt to sterilize the device.
• Use cleaning solutions only as instructed in this Operator's Manual.
• Do not attempt to clean the Radius-7 while monitoring patient.
WARNING: To ensure safety, avoid placing anything on the device during operation.
WARNING: As with all medical equipment, carefully route patient cables to reduce the
possibility of patient entanglement or strangulation.
WARNING: The Armband site must be checked frequently or per clinical protocol to ensure
adequate securement, circulation and skin integrity.
www.masimo.com 11 Masimo
Page 14

Radius-7 Safety Information, Warnings and Cautions
WARNING: Armbands applied too tightly or that become tight due to edema will cause
inaccurate readings and can cause pressure injury.
WARNING: Discontinue and dispose of Armband if it appears to be stained or becomes
excessively moist to minimize risk of skin irritation.
CAUTION: Electrical Shock Hazard: Do not place the Battery Charging Adapter of Radius-7 on
or near the patient. Injury to patient could occur.
Note: Use and store the Radius-7 in accordance with specifications. See the Specifications
section in this manual.
Performance Warnings and Cautions
General
WARNING: Radius-7 should not be used as the sole basis for medical decisions. It must be
used in conjunction with clinical signs and symptoms.
WARNING: The Radius-7 and Accessories are not intended to be used as the sole basis for
making diagnosis or treatment decisions related to suspected carbon monoxide poisoning; it
is intended to be used in conjunction with additional methods of assessing clinical signs and
symptoms.
WARNING: If any measurement seems questionable, first check the patient’s vital signs by
alternate means and then check Radius-7 for proper functioning.
WARNING: Variation in hemoglobin measurements may be profound and may be affected by
sample type, body positioning, as well as other physiological conditions. As with most
hemoglobin data, Radius-7 trend data should be scrutinized in light of a specific patient
condition. Any results exhibiting inconsistency with the patient's clinical status should be
repeated and/or supplemented with additional data.
WARNING: Radius-7 is not an apnea monitor.
WARNING: Radius-7 should not be used as a replacement or substitute for ECG-based
arrhythmia analysis.
WARNING: Radius-7 may be used during defibrillation, but this may affect the accuracy or
availability of the parameters and measurements.
WARNING: Do not use during electrocautery. This may affect the accuracy or availability of
the parameters and measurements.
WARNING: When the Radius-7 is connected to Root, all audible alarms will be provided on
the Root.
WARNING: Always pair Radius-7 with Root.
WARNING: Avoid placing Radius-7 against a surface that may cause the alarm to be muffled.
This may result in the inability to detect the audible alarms.
WARNING: Properly apply sensors according to the sensor’s directions for use. Misapplied
sensor or sensors that become partially dislodged may cause no or incorrect readings.
WARNING: Display parameter may not be accurate when a low SIQ message is provided.
Clinicians should consider additional information to supplement values to completely
understand the patient’s condition.
www.masimo.com 12 Masimo
Page 15

Radius-7 Safety Information, Warnings and Cautions
WARNING: With very low perfusion at the monitored site, the reading may read lower than
core arterial oxygen saturation.
WARNING: If SpO
confirm the patient’s condition.
WARNING: SpO
carboxyhemoglobin (COHb) and methemoglobin (MetHb).
values indicate hypoxemia, a laboratory blood sample should be taken to
2
is empirically calibrated in healthy adult volunteers with normal levels of
2
WARNING: Variation in hemoglobin measurements may be profound and may be affected by
sample type, body positioning, as well as other physiological conditions. As with most
hemoglobin data, Radius-7 trend data should be scrutinized in light of a specific patient
condition. Any results exhibiting inconsistency with the patient's clinical status should be
repeated and/or supplemented with additional data.
WARNING: The Radius-7 should be considered an early warning instrument. Blood samples
should be analyzed by laboratory instruments to completely understand the patient's
condition prior to making clinical decision.
WARNING: SpHb measurements in the ranges of 0 to 8g/dL and 17 to 25 g/dL are provided
for reference information only. The monitor shall display Low SpHb SIQ message along with
the SpHb measurement whenever the measurement is displayed in these ranges.
Furthermore, the display window also changes color providing a visual alarm to alert the user
that the SpHb values are either in the 0 to 8g/dL or 17 to 25 g/dL ranges. Clinicians should
consider additional information to supplement SpHb values, including laboratory diagnostic
tests using blood samples, to completely understand the patient’s condition.
WARNING: Optical, pleth-based measurements (e.g. SpO
can be affected by the following:
, SpHb, SpOC, SpMet, and SpCO)
2
• Improper sensor application or use of use of incorrect sensor.
• Blood pressure cuff applied to the same arm as the sensor site.
• Intravascular dyes such as indocyanine green or methylene blue.
• Venous congestion.
• Abnormal venous pulsations (e.g. tricuspid value regurgitation, Trendelenburg
position).
• Abnormal pulse rhythms due to physiological conditions or induced through
external factors (e.g. cardiac arrhythmias, intra-aortic balloon, etc.).
• Externally applied coloring and texture such as nail polish, acrylic nails, glitter, etc.
• Moisture, birthmarks, skin discoloration, nail aberration, deformed fingers, or
foreign objects in the light path.
• Elevated levels of bilirubin.
• Physiological conditions that can significantly shift the oxygen disassociation
curve.
• A physiological condition that may effect vasomotor tone or changes in vasomotor
tone.
WARNING: Inaccurate SpO
readings may be caused by:
2
• Elevated levels of COHb and/or MetHb.
• Severe anemia.
www.masimo.com 13 Masimo
Page 16

Radius-7 Safety Information, Warnings and Cautions
• Extremely low arterial perfusion.
• Excessive induced motion.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (Quantitative defects such as Thalassemias).
WARNING: Inaccurate SpHb and SpOC readings may be caused by:
• Low arterial perfusion.
• Motion induced artifact.
• Low arterial oxygen saturation levels.
• Elevated COHb and/or MetHb levels.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (quantitative defects such as Thalassemias).
• Severe anemia.
WARNING: Inaccurate SpCO readings may be caused by:
• Elevated methemoglobin levels in the range of >15%.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (quantitative defects such as Thalassemias).
• Extremely elevated hemoglobin levels.
• Low arterial perfusion.
• Low arterial oxygen saturation levels including altitude induced hypoxemia.
• Motion induced artifact.
• Severe anemia.
WARNING: SpCO readings may not be provided if there are Low arterial oxygen saturation
levels or elevated methemoglobin levels.
WARNING: Inaccurate SpMet readings may be caused by:
• Elevated carboxyhemoglobin levels in the range of >3%.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (quantitative defects such as Thalassemias).
• Extremely elevated hemoglobin levels.
• Low arterial perfusion.
• Low arterial oxygen saturation levels including altitude induced hypoxemia.
• Motion induced artifact.
• Physiological conditions that can significantly shift the oxygen disassociation
curve.
• Severe anemia.
WARNING: Inaccurate RRa measurements may be caused by:
• Improper sensor application or use of use of incorrect sensor.
www.masimo.com 14 Masimo
Page 17

Radius-7 Safety Information, Warnings and Cautions
• Abnormal pulse rhythms due to physiological conditions or induced through
external factors (e.g. Cardiac arrhythmias, intra-aortic balloon, etc.).
• Motion artifact.
• Excessive ambient or environmental noise.
CAUTION: Do not place the Radius-7 near electrical equipment that may affect the device,
preventing it from working properly.
CAUTION: Failure to charge Radius-7 promptly after a Low Battery alarm may result in the
device shutting down.
CAUTION: If using Radius-7 during full body irradiation, keep the sensor out of the radiation
field. If the sensor is exposed to the radiation, the reading might be inaccurate or the device
might read zero for the duration of the active irradiation period.
CAUTION: When patients are undergoing photodynamic therapy they may be sensitive to
light sources. Pulse oximetry may be used only under careful clinical supervision for short
time periods to minimize interference with photodynamic therapy.
CAUTION: High ambient light sources such as surgical lights (especially those with a xenon
light source), bilirubin lamps, fluorescent lights, infrared heating lamps, and direct sunlight
can interfere with the performance of the sensor.
CAUTION: To prevent interference from ambient light, ensure that the sensor is properly
applied, and cover the sensor site with opaque material, if required. Failure to take this
precaution in high ambient light conditions may result in inaccurate measurements.
CAUTION: If the Low Perfusion message is frequently displayed, find a better perfused
monitoring site. In the interim, assess the patient and, if indicated, verify oxygenation status
through other means.
CAUTION: To minimize radio interference, other electrical equipment that emits radio
frequency transmissions should not be in close proximity to Radius-7.
CAUTION: In order to maintain Bluetooth connectivity with Root, ensure that the Radius-7 is
within approximately 7 m radius and line of sight of Root.
CAUTION: When using Radius-7 in Wi-Fi mode, be aware of the patient's location. Alarms
relayed to Patient SafetyNet will not provide patient location.
CAUTION: When using multiple Radius-7 and Root systems, re-dock the Battery Module to
Root to ensure proper pairing before connecting the Radius-7 to the patient.
CAUTION: If the Radius-7 and Root become unable to communicate, parameters and
measurements will not show on the Root; however, this will not affect Radius-7's ability to
monitor the patient.
CAUTION: In order to establish and maintain Radius-7’s minimum Quality of Service, the
following network specifications should be met before and after installation:
• Wireless Network Connection
During Ping Test, passing result if:
a. At least 98% of packets have latency ≤ 100 milliseconds,
b. No more than 2 % packets loss, and
c. Primary access point signal strength at least -67 dBm.
CAUTION: The wireless quality of services may be influenced by the presence of other devices
that may create radio frequency interference (RFI). Some RFI devices to consider are as
www.masimo.com 15 Masimo
Page 18

Radius-7 Safety Information, Warnings and Cautions
follows: electrocautery equipment, cellular telephones, wireless PC and tablets, pagers, RFID,
MRI electrically powered wheelchair, etc. When used in the presence of potential RFI devices,
consideration should be taken to maximize separation distances and to observe for any
potential signs of interference such as loss of communication or reduced Wi-Fi signal
strength.
CAUTION: To ensure that alarm limits are appropriate for the patient being monitored, check
the limits each time Radius-7 is used.
CAUTION: Replace the cable or sensor when a replace sensor or when a low SIQ message is
consistently displayed while monitoring consecutive patients after completing the low SIQ
troubleshooting steps listed in the troubleshooting section.
Note: Cables and sensors are provided with X-Cal™ technology to minimize the risk of
inaccurate readings and unanticipated loss of patient monitoring. Refer to the Cable or
Sensor DFU for the specified duration of patient monitoring time.
Note: SpHb readings may be inaccurate for patients with conditions that may cause edema
at the measurement site (eg. kidney disease, pregnancy, etc.).
Note: Physiological conditions that result in loss of pulsatile signal may result in no SpO
SpHb, SpOC, SpCO, and SpMet readings.
,
2
Note: Radius-7 is provided with a Wi-Fi signal indicator as an indication of Wi-Fi
communication.
Note: Radius-7’s alarm capabilities have been designed to be independent of the Wi-Fi
communication feature in order to preserve Radius-7’s primary alarms.
Note: When the Radius-7 is connected directly via Wi-Fi to Patient SafetyNet, the Radius-7
will provide audible alarms.
Note: Before securing Radius-7 onto the patient, make sure the Battery Module is sufficiently
charged.
Note: Always charge Radius-7 when it is not in use to ensure that the Radius-7 Battery
Module remains fully charged.
Note: All batteries lose capacity with age, thus the amount of run time at Low Battery will
vary depending upon the age of the Battery Module.
Note: The Radius-7 display enters standby mode after 30s of inactivity. The Radius-7 display
entering standby mode does not affect the monitoring of the patient.
Note: A functional tester cannot be used to assess the accuracy of Radius-7.
Note: When monitoring acoustic respiration, Masimo recommends minimally monitoring
both oxygenation (SpO
) and respiration (RRa).
2
Note: When using Radius-7 in the Maximum Sensitivity setting, performance of the "Sensor
Off" detection may be compromised. If the sensor becomes dislodged from the patient in this
setting, false readings may occur due to environmental "noise" such as light, vibration, and
excessive air movement.
Patient SafetyNet
Note: The wireless communication status between Radius-7 and Patient SafetyNet is
displayed by Patient SafetyNet.
www.masimo.com 16 Masimo
Page 19

Radius-7 Safety Information, Warnings and Cautions
Cleaning and Service Warnings and Cautions
WARNING: Do not attempt to remanufacture, recondition or recycle the Radius-7 as these
processes may damage the electrical components, potentially leading to patient harm.
WARNING: To avoid electric shock, do not attempt to service the Radius-7 or the Battery
Module. Servicing of the Radius-7 should be done by qualified personnel only.
CAUTION: Only perform maintenance procedures specifically described in the manual.
Otherwise, return the Radius-7 for servicing.
CAUTION: Do not touch, press, or rub the display panels with abrasive cleaning compounds,
instruments, brushes, rough-surface materials, or bring them into contact with anything that
could scratch the display.
CAUTION: To avoid electric shock, always turn off the Radius-7 and physically disconnect it
from Root before cleaning Radius-7.
CAUTION: Do not use petroleum-based or acetone solutions, or other harsh solvents, to clean
the Radius-7. These substances affect the device’s materials and device failure can result.
CAUTION: Do not submerge the Radius-7 in any cleaning solution or attempt to sterilize by
autoclave, irradiation, steam, gas, ethylene oxide or any other method. This will seriously
damage the device.
CAUTION: To prevent damage, do not soak or immerse Radius-7 in any liquid solution.
Compliance Warnings and Cautions
WARNING: Any changes or modifications not expressly approved by Masimo shall void the
warranty for this equipment and could void the user’s authority to operate the equipment.
WARNING: In accordance with international telecommunication requirements, the frequency
band of 2.4 GHz and 5.15 to 5.25 GHz is only for indoor usage to reduce potential for harmful
interference to co-channel mobile satellite systems.
CAUTION: Disposal of Product: Comply with local laws in the disposal of the device and/or its
accessories.
CAUTION: Dispose of used batteries according to required country or regional requirements.
Note: Use Radius-7 in accordance with the Environmental Specifications section in the
Operator's Manual.
Note: This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and (2) this
device must accept any interference received, including interference that may cause
undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the user
is encouraged to try to correct the interference by one or more of the following measures:
www.masimo.com 17 Masimo
Page 20

Radius-7 Safety Information, Warnings and Cautions
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Note: This equipment has been tested and found to comply with the Class B limits for
medical devices according to the EN 60601-1-2: 2007, Medical Device Directive 93/42/EEC.
These limits are designed to provide reasonable protection against harmful interference in all
establishments, including domestic establishments.
Note: This Class B digital apparatus complies with Canadian ICES-003.
Note: This device complies with Industry Canada license-exempt RSS standard(s). Operation
is subject to the following two conditions: (1) this device may not cause interference, and (2)
this device must accept any interference, including interference that may cause undesired
operation of the device.
www.masimo.com 18 Masimo
Page 21
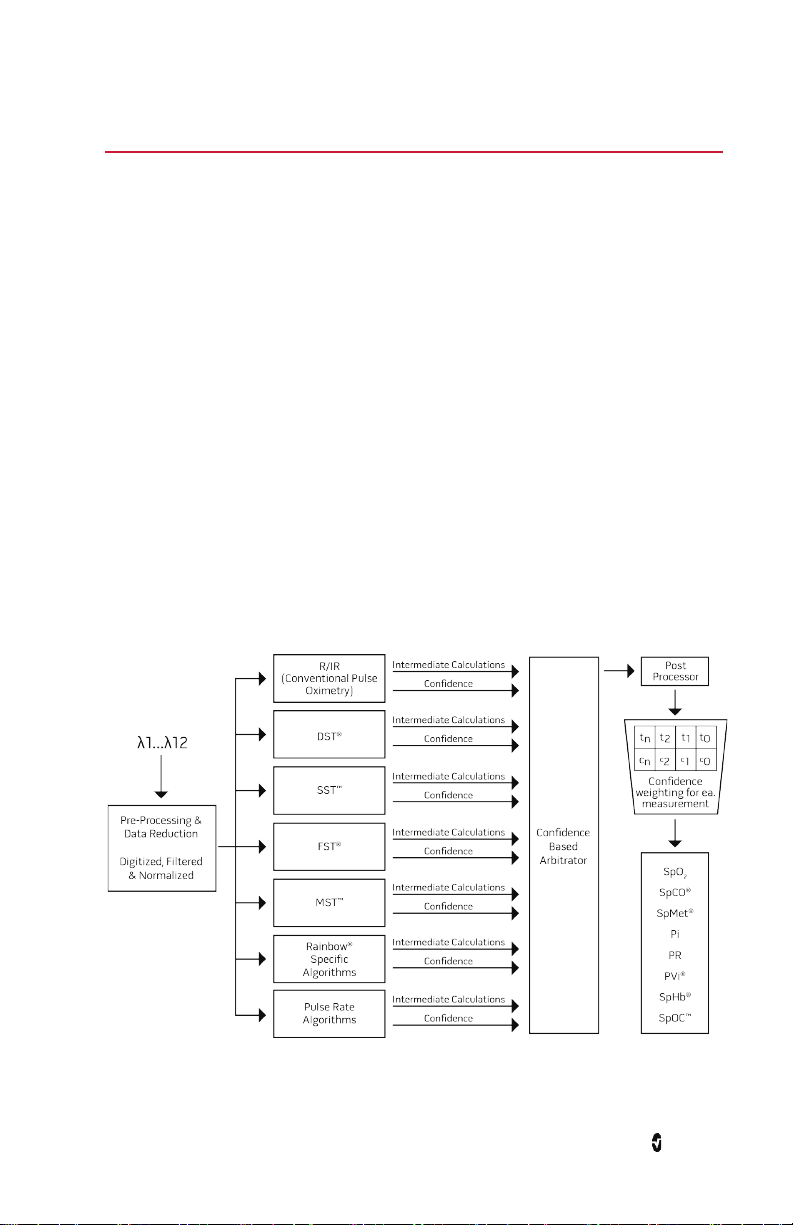
Chapter 1: Technology Overview
The following chapter contains general descriptions about parameters, measurements, and
the technology used by Masimo products.
Signal Extraction Technology® (SET®)
Masimo Signal Extraction Technology's signal processing differs from that of conventional
pulse oximeters. Conventional pulse oximeters assume that arterial blood is the only blood
moving (pulsating) in the measurement site. During patient motion, however, the venous
blood also moves, causing conventional pulse oximeters to read low values, because they
cannot distinguish between the arterial and venous blood movement (sometimes referred to
as noise).
Masimo SET pulse oximetry utilizes parallel engines and adaptive filtering. Adaptive filters
are powerful because they are able to adapt to the varying physiologic signals and/or noise
and separate them by looking at the whole signal and breaking it down to its fundamental
components. The Masimo SET signal processing algorithm, Discrete Saturation Transform®
(DST®), in parallel with Fast Saturation Transform (FST®), reliably identifies the noise,
isolates it and, using adaptive filters, cancels it. It then reports the true arterial oxygen
saturation for display on the monitor.
Masimo rainbow SET® Parallel Engines
This figure is for conceptual purposes only.
www.masimo.com 19 Masimo
Page 22
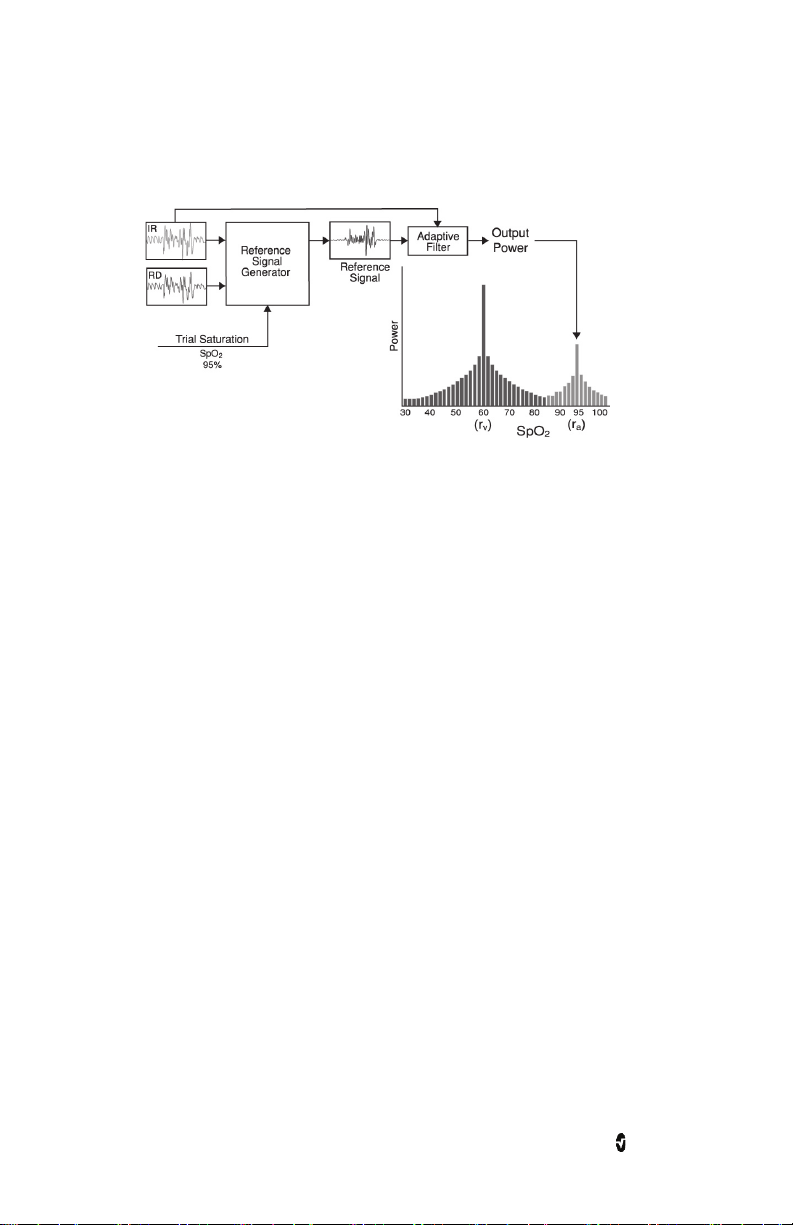
Radius-7 Chapter 1: Technology Overview
Masimo SET® DST
This figure is for conceptual purposes only.
General Description for Oxygen Saturation (SpO2)
Pulse oximetry is governed by the following principles:
• Oxyhemoglobin (oxygenated blood) and deoxyhemoglobin (non-oxygenated
blood) differ in their absorption of red and infrared light (spectrophotometry).
• The amount of arterial blood in tissue changes with your pulse
(photoplethysmography). Therefore, the amount of light absorbed by the varying
quantities of arterial blood changes as well.
Successful Monitoring for SpO2, PR and Pi
Stability of the SpO2 readings may be a good indicator of signal validity. Although stability is
a relative term, experience will provide a good feeling for changes that are artifactual or
physiological and the speed, timing, and behavior of each.
The stability of the readings over time is affected by the averaging time being used. The
longer the averaging time, the more stable the readings tend to become. This is due to a
dampened response as the signal is averaged over a longer period of time than during shorter
averaging times. However, longer averaging times delay the response of the oximeter and
reduce the measured variations of SpO2 and pulse rate.
Functional Oxygen Saturation (SpO2)
The Radius-7 is calibrated to measure and display functional oxygen saturation (SpO2): the
amount of oxyhemoglobin expressed as a percentage of the hemoglobin that is available to
transport oxygen.
Note: Dyshemoglobins are not capable of transporting oxygen, but are recognized as
oxygenated hemoglobins by conventional pulse oximetry.
www.masimo.com 20 Masimo
Page 23

Radius-7 Chapter 1: Technology Overview
General Description for Pulse Rate (PR)
Pulse rate (PR), measured in beats per minute (BPM) is based on the optical detection of
peripheral flow pulse.
General Description for Perfusion Index (Pi)
The Perfusion Index (Pi) is the ratio of the pulsatile blood flow to the non-pulsatile or static
blood in peripheral tissue. Pi thus represents a noninvasive measure of peripheral perfusion
that can be continuously and noninvasively obtained from a pulse oximeter.
General Description for Pleth Variability Index (PVi)
The Pleth Variability Index (PVi) is a measure of the dynamic changes in the Perfusion Index
(Pi) that occur during the respiratory cycle. The calculation is accomplished by measuring
changes in Pi over a time interval where one or more complete respiratory cycles have
occurred. PVi is displayed as a percentage (0-100%).
PVi may show changes that reflect physiological factors such as vascular tone, circulating
blood volume, and intrathoracic pressure excursions.
The utility of PVi has been evaluated in clinical studies [1-11]. Technical and clinical factors
that may affect PVi include probe malposition, probe site, patient motion, skin incision,
spontaneous breathing activity, lung compliance, open pericardium, use of vasopressors or
vasodilators, low perfusion index, subject age, arrhythmias, left or right heart failure, and tidal
volume [12-14].
Citations for Pleth Variability Index (PVi)
1. Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot J.J.
Pleth Variability Index to Monitor the Respiratory Variations in the Pulse Oximeter
Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the
Operating Theatre. Br J Anaesth. 2008 Aug;101(2):200-6.
2. Forget P, Lois F, de Kock M. Goal-Directed Fluid Management Based on the Pulse
Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid
Management. Anesth Analg. 2010 Oct;111(4):910-4.
3. Zimmermann M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B.M., Wiesenack C.
Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to
Predict Fluid Responsiveness in Mechanically Ventilated Patients Undergoing Major
Surgery. Eur J Anaesthesiol. 2010 Jun;27(6):555-61.
4. Desebbe O, Boucau C, Farhat F, Bastien O, Lehot JJ, Cannesson M. Anesth Analg. The
Ability of Pleth Variability Index to Predict the Hemodynamic Effects of Positive
End-Expiratory Pressure in Mechanically Ventilated Patients under General
Anesthesia. 2010 Mar 1;110(3):792-8.
5. Tsuchiya M., Yamada T., Asada A. Pleth Variability Index Predicts Hypotension
During Anesthesia Induction. Acta Anesthesiol Scand. 2010 May;54(5):596-602.
6. Loupec T., Nanadoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene
B., Dahyot-Fizelier C., Mimoz O. Pleth Variability Index Predicts Fluid
Responsiveness in Critically Ill Patients. Crit Care Med. 2011 Feb;39(2):294-9.
www.masimo.com 21 Masimo
Page 24

Radius-7 Chapter 1: Technology Overview
7. Fu Q., Mi W.D., Zhang H. Stroke Volume Variation and Pleth Variability Index to
Predict Fluid Responsiveness during Resection of Primary Retroperitoneal Tumors in
Hans Chinese. Biosci Trends. 2012 Feb;6(1):38-43.
8. Haas S., Trepte C., Hinteregger M., Fahje R., Sill B., Herich L., Reuter D.A. J.
Prediction of Volume Responsiveness using Pleth Variability Index in Patients
Undergoing Cardiac Surgery after Cardiopulmonary Bypass. Anesth. 2012
Oct;26(5):696-701.
9. Byon H.J., Lim C.W., Lee J.H., Park Y. H., Kim H.S., Kim C.S., Kim J.T. Br. J.
Prediction of fluid Responsiveness in Mechanically Ventilated Children Undergoing
Neurosurgery. Anaesth 2013 Apr;110(4):586-91.
10. Feissel M., Kalakhy R., Banwarth P., Badie J., Pavon A., Faller J.P., Quenot JP.
Plethysmographic Variation Index Predicts Fluid Responsiveness in Ventilated
Patients in the Early Phase of Septic Shock in the Emergency Department: A Pilot
Study. J Crit Care. 2013 Oct;28(5):634-9.
11. Yu Y., Dong J., Xu Z., Shen H., Zheng J. Pleth Variability Index-Directed Fluid
Management in Abdominal Surgery under Combined General and Epidural
Anesthesia. J Clin Monit Comput. 2014 Feb 21.
12. Desgranges F.P., Desebbe O., Ghazouani A., Gilbert K., Keller G., Chiari P., Robin
J.,Bastien O., Lehot J.J., Cannesson M. Br. J. Anaesth 2011 Sep;107(3):329-35.
13. Cannesson M. Arterial pressure variation and goal-directed fluid therapy. J
Cardiothorac Vasc Anesth. 2010 Jun;24(3):487-97.
14. Takeyama M, Matsunaga A, Kakihana Y, Masuda M, Kuniyoshi T, Kanmura Y.
Impact of Skin Incision on the Pleth Variability Index. J Clin Monit Comput 2011
Aug;25(4):215-21.
Signal IQ
The Signal IQ provides an indicator of the assessment of the confidence in the displayed SpO2
value. The SpO
SIQ can also be used to identify the occurrence of a patient’s pulse.
2
With motion, the plethysmographic waveform is often distorted and may be obscured by
noise artifact. Shown as a vertical line, the SpO
pulsation. Even with a plethysmographic waveform obscured by artifact, the Signal IQ
SIQ coincides with the peak of an arterial
2
identifies the timing that the algorithms have determined for the arterial pulsation. The
pulse tone (when enabled) coincides with the vertical line of the SpO
SIQ.
2
The height of the vertical line of the SpO2 SIQ provides an assessment of the confidence in
the measurement displayed. A high vertical bar indicates higher confidence in the
measurement. A small vertical bar indicates lower confidence in the displayed measurement.
When the Signal IQ is very low, this suggests that the accuracy of the displayed measurement
may be compromised. See About the Main Screen on page 42.
rainbow Pulse CO-Oximetry Technology
rainbow Pulse CO-Oximetry technology is governed by the following principles:
1. Oxyhemoglobin (oxygenated blood), deoxyhemoglobin (non-oxygenated blood),
carboxyhemoglobin (blood with carbon monoxide content), methemoglobin
(blood with oxidized hemoglobin) and blood plasma constituents differ in their
absorption of visible and infrared light (using spectrophotometry).
www.masimo.com 22 Masimo
Page 25
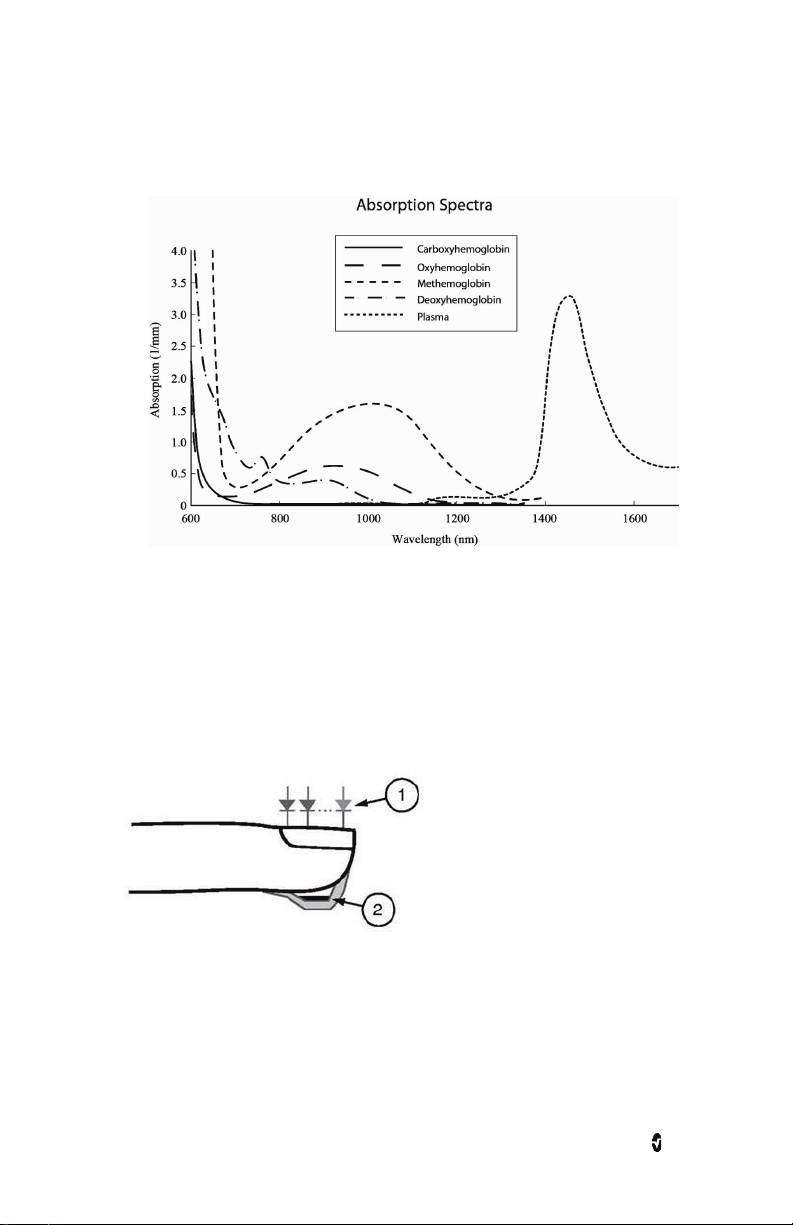
Radius-7 Chapter 1: Technology Overview
2. The amount of arterial blood in tissue changes with pulse
(photoplethysmography). Therefore, the amount of light absorbed by the varying
quantities of arterial blood changes as well.
The Radius-7 uses a multi-wavelength sensor to distinguish between oxygenated blood,
deoxygenated blood, blood with carbon monoxide, oxidized blood and blood plasma.
The Radius-7 utilizes a sensor with various light-emitting diodes (LEDs) that pass light
through the site to a diode (detector). Signal data is obtained by passing various visible and
infrared lights (LEDs, 500 to 1400nm) through a capillary bed (for example, a fingertip, a
hand, a foot) and measuring changes in light absorption during the blood pulsatile cycle. This
information may be useful to clinicians. The maximum radiant power of the strongest light is
rated at ≤ 25 mW. The detector receives the light, converts it into an electronic signal and
sends it to the Radius-7 for calculation.
1. Light Emitting Diodes (LEDs)
(7 + wavelengths)
2. Detector
www.masimo.com 23 Masimo
Page 26

Radius-7 Chapter 1: Technology Overview
Once Radius-7 receives the signal from the sensor, it utilizes proprietary algorithms to
calculate the patient’s functional oxygen saturation (SpO
carboxyhemoglobin saturation (SpCO [%]), methemoglobin saturation (SpMet [%]), total
[%]), blood levels of
2
hemoglobin concentration (SpHb [g/dL]) and pulse rate (PR). The SpCO, SpMet and SpHb
measurements rely on a multi-wavelength calibration equation to quantify the percentage of
carbon monoxide and methemoglobin and the concentration of total hemoglobin in arterial
blood. Maximum skin-sensor interface temperature was tested to be less than 41º C (106º F)
in a minimum ambient temperature of 35º C (95º F). The tests were conducted with sensors
operating at reasonable worst case power.
Pulse CO-Oximetry vs. Drawn Whole Blood Measurements
When SpO2, SpCO, SpMet, and SpHb measurements obtained from the Radius-7
(noninvasive) are compared to drawn whole blood (invasive) measurements by blood gas
and/or laboratory CO-Oximetry methods, caution should be taken when evaluating and
interpreting the results.
The blood gas and/or laboratory CO-Oximetry measurements may differ from the SpO
SpMet, SpHb, and SpOC measurements of the Radius-7. Any comparisons should be
simultaneous, meaning the measurement on the device should be noted at the exact time
that blood is drawn.
In the case of SpO
the calculated measurement is not appropriately corrected for the effects of variables that
, different results are usually obtained from the arterial blood gas sample if
2
shift the relationship between the partial pressure of oxygen (pO2) and saturation, such as:
pH, temperature, the partial pressure of carbon dioxide (pCO
hemoglobin.
), 2,3-DPG, and fetal
2
In the case of SpCO, different results are also expected if the level of methemoglobin (MetHb)
in the blood gas sample is abnormal (greater than 2% for MetHb).
In the case of SpHb, variation in hemoglobin measurements may be profound and may be
affected by sampling technique as well as the patient's physiological conditions. Any results
exhibiting inconsistency with the patient's clinical status should be repeated and/or
supplemented with additional test data. As with most hemoglobin tests, a laboratory blood
sample should be analyzed prior to clinical decision making.
High levels of bilirubin may cause erroneous SpO
samples are usually taken over a period of 20 seconds (the time it takes to draw the blood) a
, SpMet, SpCO, and SpHb readings. As blood
2
meaningful comparison can only be achieved if the oxygen saturation (SaO2), levels of
carboxyhemoglobin (COHb), and MetHb of the patient are stable and not changing over the
period of time that the blood gas sample is taken. Subsequently, blood gas and laboratory
CO-Oximetry measurements of SpO
administration of fluids and in procedures such as dialysis. Additionally, drawn whole blood
, SpCO, SpMet, SpHb, and SpOC may vary with the rapid
2
testing can be affected by sample handling methods and time elapsed between blood draw
and sample testing.
Measurements with Low Signal IQ should not be compared to laboratory measurements.
, SpCO,
2
General Description for Total Hemoglobin (SpHb)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of total
hemoglobin (SpHb) in arterial blood. It relies on the same principles of pulse oximetry to
make its SpHb measurement.
www.masimo.com 24 Masimo
Page 27

Radius-7 Chapter 1: Technology Overview
Successful Monitoring for SpHb
A stable SpHb reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion at the
measurement site. Physiological changes at the measurement site are mainly caused by
fluctuations in the oxygen saturation, blood concentration and perfusion. See Safety
Information, Warnings and Cautions on page 11 and Troubleshooting Measurements on
page 71.
General Description for SpOC
The above approximations result in the following reduced equation for oxygen content via the
Pulse CO-Oximeter:
SpOC (ml/dL*) = 1.31 (ml O
*When ml O2/g Hb is multiplied by g/dL of SpHb, the gram unit in the denominator of ml/g
cancels the gram unit in the numerator of g/dL resulting in ml/dL (ml of oxygen in one dL of
blood) as the unit of measure for SpOC. See Safety Information, Warnings and Cautions on
page 11.
/g) x SpHb (g/dL) x SpO2 + 0.3 (ml O2/dL)
2
General Description for Carboxyhemoglobin (SpCO)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of
carboxyhemoglobin saturation (SpCO) in arterial blood. It relies on the same basic principles
of pulse oximetry (spectrophotometry) to make its SpCO measurement.
The measurement is obtained by placing a sensor on a patient, usually on the fingertip for
adults and the hand or foot for infants. The sensor connects either directly to the Pulse
CO-Oximetry device or through a device patient cable.
The sensor collects signal data from the patient and sends it to the device. The device
displays the calculated data as percentage value for the SpCO, which reflect blood levels of
carbon monoxide bound to hemoglobin.
Successful Monitoring for SpCO
A stable SpCO reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion in the patient's
fingertip (measurement site). Physiological changes at the measurement site are mainly
caused by fluctuations in the oxygen saturation, blood concentration and perfusion.
General Description for Methemoglobin (SpMet)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of
methemoglobin saturation (SpMet) in arterial blood. It relies on the same basic principles of
pulse oximetry (spectrophotometry) to make its SpMet measurement.
The measurement is obtained by placing a sensor on a patient, usually on the fingertip for
adults and the hand or foot for infants. The sensor connects either directly to the Pulse
CO-Oximetry device or through a patient cable.
www.masimo.com 25 Masimo
Page 28

Radius-7 Chapter 1: Technology Overview
The sensor collects signal data from the patient and sends it to the device. The device
displays the calculated data as percentage value for the SpMet.
Successful Monitoring for SpMet
A stable SpMet reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion in the patient’s
fingertip (measurement site).
Physiological changes at the measurement site are mainly caused by fluctuations in the
oxygen saturation, blood concentration and perfusion. See Safety Information, Warnings and
Cautions on page 11.
SpCO, SpMet, and SpHb Measurements During Patient Motion
The Radius-7 displays measurements of SpCO, SpMet, and SpHb during patient motion.
However, because of the changes in the physiological parameters such as blood volume,
arterial-venous coupling, etc. that occur during patient motion, the accuracy of such
measurements may not be reliable during excessive motion. In this case, the measurement
value for SpCO, SpMet, or SpHb displays as dashes (---) and a message (Low SpCO SIQ, Low
SpMet SIQ, or Low SpHb SIQ) displays to alert the clinician that the device does not have
confidence in the value due to poor signal quality caused by excessive motion or other signal
interference.
rainbow Acoustic Monitoring™ (RAM™)
rainbow Acoustic Monitoring (RAM) continuously measures a patient’s respiration rate based
on airflow sounds generated in the upper airway. The Acoustic Sensor, which is applied on the
patient's neck, translates airflow sounds generated in the upper airway to an electrical signal
that can be processed to produce a respiration rate, measured as breaths per minute.
Respiratory sounds include sounds related to respiration such as breath sounds (during
inspiration and expiration), adventitious sounds, cough sounds, snoring sounds, sneezing
sounds, and sounds from the respiratory muscles [1].
These respiratory sounds often have different characteristics depending on the location of
recording [2] and they originate in the large airways where air velocity and air turbulence
induce vibration in the airway wall. These vibrations are transmitted, for example, through
the lung tissue, thoracic wall and trachea to the surface where they may be heard with the aid
of a stethoscope, a microphone or more sophisticated devices.
www.masimo.com 26 Masimo
Page 29
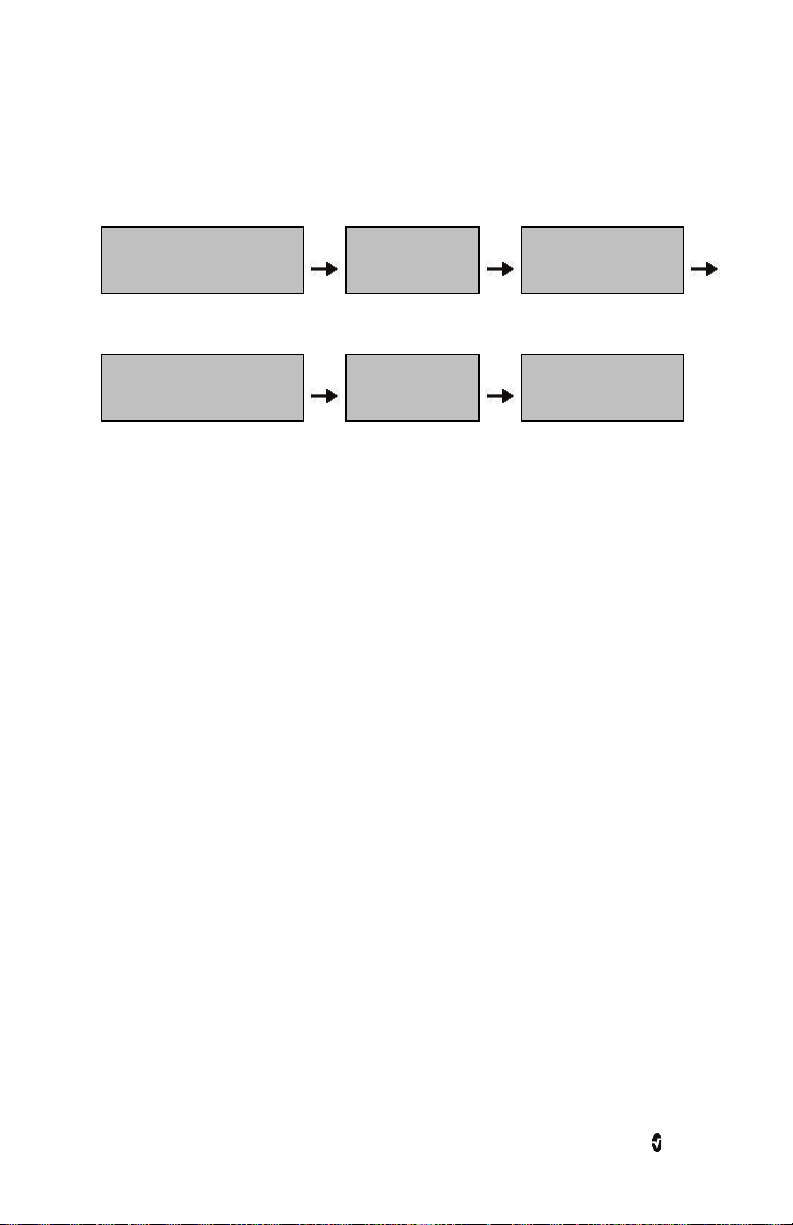
Radius-7 Chapter 1: Technology Overview
rainbow Acoustic Monitoring Architecture
The following figure illustrates how a respiratory sound produced by a patient can be turned
into a numerical measurement that corresponds to a respiratory parameter.
Patient
Respiratory airflow to sound
Signal
Processing
Digital signal to respiratory
measurement
Sensor
Sound to
electrical signal
Envelope
Detection
Acquisition System
Electrical signal to
digital signal
RRa Estimation
Patient
The generation of respiratory sounds is primarily related to turbulent respiratory airflow in
upper airways. Sound pressure waves within the airway gas and airway wall motion contribute
to the vibrations that reach the body surface and are recorded as respiratory sounds.
Although the spectral shape of respiratory sounds varies widely from person to person, it is
often reproducible within the same person, likely reflecting the strong influence of individual
airway anatomy [2-6].
Sensor
The sensor captures respiratory sounds (and other biological sounds) much like a microphone
does. When subjected to a mechanical strain, (e.g., surface vibrations generated during
breathing), the sensor becomes electrically polarized.
The degree of polarization is proportional to the applied strain. The output of the sensor is an
electric signal that includes a sound signal that is modulated by inspiratory and expiratory
phases of the respiratory cycle.
Acquisition System
The acquisition system converts the electric signal provided by the sensor into a digital
signal. This format allows the signal to be processed by a computing device.
www.masimo.com 27 Masimo
Page 30

Radius-7 Chapter 1: Technology Overview
Signal Processing
The digital signal produced by the acquisition system is converted into a measurement that
corresponds to the respiratory parameter of interest. As shown in the previous figure, this can
be performed by, for example, determining the digital signal envelope or outline which in turn
may be utilized to determine the respiratory rate. In this way, a real-time, continuous breath
rate parameter can be obtained and displayed on a monitor which, in many cases, may be
real-time and continuous.
The respiratory cycle envelope signal processing principle is similar to methods that sample
airway gasses and subsequently determine a respiratory rate.
Citations
[1] A.R.A. Sovijärvi, F. Dalmasso, J. Vanderschool, L.P. Malmberg, G. Righini, S.A.T. Stoneman.
Definition of terms for applications of respiratory sounds. Eur Respir Rev 2000; 10:77,
597-610.
[2] Z. Moussavi. Fundamentals of respiratory sounds analysis. Synthesis lectures on biomedical
engineering #8. Morgan & Claypool Publishers, 2006.
[3] Olsen, et al. Mechanisms of lung sound generation. Semin Respir Med 1985; 6: 171-179.
[4] Pastercamp H, Kraman SS, Wodicka GR. Respiratory sounds – Advances beyond the
stethoscope. Am J Respir Crit Care Med 1977; 156: 974-987.
[5] Gavriely N, Cugell DW. Airflow effects on amplitude and spectral content of normal breath
sounds. J Appl Physiol 1996; 80: 5-13.
[6] Gavrieli N, Palti Y, Alroy G. Spectral characteristics of normal breath sounds. J Appl Physiol
1981; 50: 307-314.
www.masimo.com 28 Masimo
Page 31

Chapter 2: System Components
This chapter contains the description of the Radius-7 physical features.
General System Description
The Radius-7® Wearable Pulse CO-Oximeter® system consists of the following components:
• Instrument Module
• Battery Module
• Armband
• Battery Charging Adapter
The Battery Charging Adapter docks onto the Root to function as both a charger and holder
for the Radius-7. The Battery Module snaps onto the Instrument Module and together they
can be strapped onto a patient's arm using the Armband.
For a complete list of compatible sensors and cables, visit http://www.masimo.com.
www.masimo.com 29 Masimo
Page 32

Radius-7 Chapter 2: System Components
Radius-7 Instrument Module
The Instrument Module connects both optical and acoustic rainbow sensors and has a
Bluetooth, or optional Wi-Fi radio to connect with Root.
The following table describes the features of the Instrument Module:
Ref. Feature Description
An acoustic sensor can be connected to Radius-7 via this
Acoustic Sensor
1
Connector
connector.
CAUTION: Refer to the Directions for Use for the sensor before
applying it on patients.
2 Contact Pins The pins provide a data and power connection to the Battery
Module.
3 Key for Armband The key allows for proper positioning of the Armband used to
secure Radius-7 to the patient.
4 rainbow SET Sensor
Connector
A rainbow SET sensors can be connected to Radius-7 via this
connector.
CAUTION: Refer to the Directions for Use for the sensor before
applying it on patients.
5 Wi-Fi Antenna Antenna for wireless communication.
www.masimo.com 30 Masimo
Page 33

Radius-7 Chapter 2: System Components
Radius-7 Battery Module
The Battery Module features a Display panel, Touchpad, Speaker and rechargeable
lithium-ion battery. The Battery Module is designed to snap onto the Instrument Module.
Front View Back View
The following table describes the features of the Battery Module:
Ref. Feature Description
1 Speaker Radius-7 is provided with a speaker to provide alarms in the event the
communication to secondary display is lost.
2 Release
Buttons
3 Display
Panel
These buttons are used to release the Battery Module from the
Instrument Module and Battery Charging Adapter.
This display area shows parameter values and visual alarms. If the
device is connected to a secondary display, parameter data is displayed
continuously on the secondary display.
4 Touchpad This feature is used to navigate the menu screens and acknowledge
alarms.
5 Connection
Pins
The pins enable the Battery Module to dock onto the Battery Charging
Adapter and provide power and communication to the Battery Module.
www.masimo.com 31 Masimo
Page 34

Radius-7 Chapter 2: System Components
Radius-7 Armband
The Armband is used to secure Radius-7 to the patient. The Armband comes in three different
sizes; small (11.9”), medium (16.4”) and large (25.4”). The Instrument Module and Armband
are keyed so that they can only be connected properly in the right orientation. See Radius-7
Armband on page 32.
www.masimo.com 32 Masimo
Page 35

Radius-7 Chapter 2: System Components
Radius-7 Battery Charging Adapter
The Battery Charging Adapter fits into the docking station on Root and allows the Battery
Module to be docked for charging or storage. Once the Battery Charging Adapter is installed
on the Root docking station during initial setup, the adapter should not be removed during
patient monitoring.
The following table describes the features of the Battery Charging Adapter:
Ref. Feature Description
1 Battery Pocket The Battery Pocket can be used to store the entire Radius-7 or
Instrument Module separately.
2 Battery Module
Connector
The Battery Module Connector allows for docking and charging of
the Battery Module. See Charging the Radius-7 Battery Module on
page 36.
www.masimo.com 33 Masimo
Page 36

Page 37

Chapter 3: Setting Up
This chapter contains information about setting up Radius-7 before use.
Unpacking and Inspection
To unpack and inspect the device perform the following steps:
1. Remove the device from the shipping carton and examine it for signs of shipping
damage.
2. Check all materials against the packing list. Save all packing materials, invoice
and bill of lading. These may be required to process a claim with the carrier.
3. If anything is missing or damaged, contact the Technical Service Department. See
Preparation for Use
Prior to setting up the Radius-7 for monitoring, perform the following steps:
Chapter 8: Service and Maintenance on page 95.
1. Confirm that you have all system components:
• Battery Module (2)
• Instrument Module
• Armband
• Battery Charging Adapter
• Root
• Sensors
2. Read the Safety Information, Warnings and Cautions on page 11.
3. Setup the Root system according to the directions provided in the Operator's
Manual for Root.
4. Power on the Root and ensure it is connected to AC power supply. See Operator's
Manual for Root.
5. Ensure the Battery Module is fully charged. See Charging the Radius-7 Battery
Module on page 36.
www.masimo.com 35 Masimo
Page 38

Radius-7 Chapter 3: Setting Up
Charging the Radius-7 Battery Module
Before use, the Radius-7 Battery Module needs to be fully charged. To charge the Battery
Module for the first time perform the following steps:
1. Attach the Battery Charging Adapter to the Root by aligning the bottom of the
adapter with the two groves at the bottom of the docking interface on the Root
and snap it in place.
2. Ensure that the Root is powered on and connected to an AC power supply.
3. Dock the Battery Module onto the Battery Charging Adapter.
Note: Charge the Battery Module on the Root System you intend to pair with the
Radius-7. Docking the Battery Module onto Root automatically pairs the device
with Root. See Connecting Radius-7 to Root via Bluetooth on page 36.
4. Verify that the Battery Module is charging. A battery icon will be displayed on the
Radius-7 screen to indicate that the Battery Module is charging. See About the
Main Screen on page 42.
5. Once sufficiently charged you may un-dock the Battery Module by pressing the
Release Buttons on the Battery Module.
6. Enable Bluetooth Connectivity on Root. See Operator's Manual for Root.
For additional battery information, See Battery Operation and Maintenance on page 95.
Connecting Radius-7 to Root via Bluetooth
In order connect the Radius-7 to Root via Bluetooth connection perform the following steps:
1. Enable Bluetooth Connectivity on Root. See Operator's Manual for Root.
2. Dock the Battery Module of the Radius-7 to the Root that you intend to make the
Bluetooth connection.
3. Allow enough time for the Root to acknowledge the Radius-7 is docked. The user
will hear a beep tone to indicate that the Bluetooth connection between Root and
Radius-7 has been established.
4. Verify that the Bluetooth Mac address on Radius-7 matches the Mac Address listed
on Root. See Navigating Radius-7 Main Menu on page 42.
5. Un-dock the Battery Module from Root and connect it to the Instrument Module to
complete Bluetooth connection.
6. You can verify the Bluetooth connection is successful when the Root screen begins
to display the Radius-7’s measurement data.
WARNING: When the Radius-7 is connected via Bluetooth to Root all audible alarms will be
provided on the Root.
CAUTION: In order to maintain Bluetooth connectivity with Root, ensure that the Radius-7 is
within approximately a 7m radius and line of sight of Root.
CAUTION: When using multiple Radius-7 and Root systems, re-dock the Battery Module to
Root to ensure proper pairing before connecting the Radius-7 to the patient.
www.masimo.com 36 Masimo
Page 39

Radius-7 Chapter 3: Setting Up
Connecting Radius-7 to Patient SafetyNet
Radius-7 can be configured to connect to Patient SafetyNet wirelessly by authorized and
trained personnel only.
The wireless icon in the Status Bar on Radius-7 displays the current connection status. See
About the Main Screen on page 42.
Icon Description
A gray icon indicates Radius-7 wireless radio is on, but it is not connected to a
wireless network.
A blue icon indicates Radius-7 is connected to a wireless network, but not
communicating with Patient SafetyNet.
A green icon indicates Radius-7 is connected to a wireless network and
communicating directly with Patient SafetyNet.
Securing Radius-7 to Patient
Before securing Radius-7 onto the patient, make sure the Battery Module is sufficiently
charged.
Note: Safety Information, Warnings and Cautions should be read before use. See Safety
Information, Warnings and Cautions on page 11.
See Chapter 2: System Components on page 29 for information on the different components.
www.masimo.com 37 Masimo
Page 40

Radius-7 Chapter 3: Setting Up
To secure the Radius-7 to a patient, follow the instructions below with the help of the visual
aid:
www.masimo.com 38 Masimo
Page 41

Radius-7 Chapter 3: Setting Up
1. Remove the Armband from the packaging.
2. Slide the Instrument Module between the Armband fabric and the Armband
plastic as shown in the figure above.
3. The shaped hole in the Armband plastic should fit over the matching key
on the front side of the Instrument Module.
4. Connect the Battery Module securing the Armband Adapter between the Battery
Module and the Instrument Module.
5. Select a site on the patient's arm to secure Radius-7. Place the Radius-7 on the
arm with the Masimo logo on the top and making sure the Armband fabric is
between the Radius-7 and the arm.
CAUTION: If the device is being applied directly to the patient's skin, select a site
that is free from skin irritation or signs of chaffing.
CAUTION: Only the smooth side of the Armband fabric should make contact with
the patient when properly applied.
Note: Do not to dispose of the Radius-7 instrument module when changing the
replaceable armband.
Note: The Radius-7 should be oriented so that the Acoustic Sensor connector is
the closest connector to the patient's neck.
6. Loop the Armband strap around the patient's arm and thread the strap through
the remaining open slot of the Armband plastic from the rear and secure the end
of the Armband strap by pressing the tab on the end onto the Armband fabric.
7. Check to ensure the strap fits comfortably around the patient's arm.
WARNING: Armbands applied too tightly or that become tight due to edema will
cause inaccurate readings and can cause pressure injury.
WARNING: The Armband site must be checked frequently or per clinical protocol
to ensure adequate securement, circulation and skin integrity.
WARNING: Discontinue and dispose of Armband if it appears to be stained or
becomes excessively moist to minimize risk of skin irritation.
CAUTION: Ensure that the Armband does not slide off the arm.
8. Connect sensor(s) to the Instrument Module.
9. See Directions for Use for each sensor for proper application of the sensor to the
patient.
WARNING: As with all medical equipment, carefully route patient cabling to
reduce the possibility of patient entanglement or strangulation.
www.masimo.com 39 Masimo
Page 42

Radius-7 Chapter 3: Setting Up
Removing Radius-7 from Patient
To remove the Radius-7 from a patient, perform the following steps:
1. Disconnect sensor(s) from the Instrument Module.
2. Detach the end of the armband strap from the Armband fabric.
3. Un-thread armband strap from Instrument Module slot and remove the Radius-7
from the patient’s arm.
4. Press the Release Buttons on the Battery Module, and slide the Battery Module off
of the Instrument Module.
5. Undo the key of the armband plastic and slide the Instrument Module away from
the Armband.
Note: Do not to dispose of the Radius-7 instrument module when changing the
replaceable armband.
6. Dispose of the armband according to local laws and regulations.
WARNING: Do not reuse the strap to avoid possible cross contamination.
7. Disinfect and clean the Battery Module and Instrument Module. See Cleaning on
page 95.
8. Return the Battery Module to the battery charging adapter for charging. See
Charging the Radius-7 Battery Module on page 36.
9. Store the Instrument Module in the Battery Pocket of the Battery Charging
Adapter.
www.masimo.com 40 Masimo
Page 43

Chapter 4: Operation
Using the Touchpad
The Touchpad on the Radius-7 is located below the display panel on the Battery Module.
Note: The display panel is not a touch screen.
Using the gestures described below, the user is able to view all parameters and
measurements, navigate through menu options, and silence/acknowledge alarms on
Radius-7.
Action Description Function
Touch Touch and release. Action
Touch
and Hold
Swipe Touch, move (left, right, up or
Flick Touch, quickly swipe across (left,
After 30 seconds of inactivity on the Touchpad, the Display Panel turns off automatically and
switches to Standby mode to conserve power. To turn the Display Panel back on, tap
anywhere on the Touchpad.
Note: The Radius-7 display entering Standby mode does not affect the monitoring of the
patient.
www.masimo.com 41 Masimo
performed once finger is released.
Touch and stay for a prescribed
amount of time. Release finger
once action had been performed.
down) and release.
right, up or down) and release.
Select a menu item or action
Enter and Exit the Main Screen
Silence/acknowledge alarms.
View all selectable menu options.
View all selectable menu options. Similar to
the Swipe gesture. It allows user to scroll
through menu options faster.
Page 44

Radius-7 Chapter 4: Operation
About the Main Screen
The Main Screen is composed of the following:
Ref Feature Description
1 Status Bar Visible at the top of the Main Screen and displays Exception Messages,
Wi-Fi connectivity status, Bluetooth connectivity status and battery life.
2 Parameter
Display
3 Waveform
Field
Majority portion of Main Screen. Displays up to four parameters
simultaneously.
Displays SIQ and the pleth waveform with the respiration waveform
(blue) in the background.
Navigating Radius-7 Main Menu
From the Main Screen, touch and hold the Touchpad on the Battery Module screen to access
the Main Menu.
Use the Touchpad Swipe gesture to scroll through the Main Menu Options. Use the Touch
gesture on the Touchpad to select the Main Menu Option that is centered in th window. Use
the same gestures to adjust settings.
The Main Menu options are:
Main Menu
Options
Back
www.masimo.com 42 Masimo
Description Default Options
Returns you to the main screen or previous
menu.
N/A N/A
Page 45

Radius-7 Chapter 4: Operation
Main Menu
Description Default Options
Options
Waveform
Brightness
Display
Timeout
About
Allows the user to choose if the waveform will
be displayed on the screen.
Allows the user to change the brightness of the
Display Panel.
Allows the user to choose the amount of time
before the display turns off.
Hardware and software information about the
device including Wi-Fi Bluetooth Mac Address.
On On or Off
4
30
seconds
1 through 4 in
steps of 1
10, 30, 60, or
120 seconds
N/A N/A
Power Restarts the Radius-7 N/A N/A
Navigating Radius-7 Settings on Root
The following settings on Radius-7 can be configured with Root:
• Parameter settings including alarms and trends. See Configuring Parameters on
page 44.
• Additional settings including Averaging time and FastSat. See Additional
Settings on page 45.
The Radius-7 settings may be configured with Root when connected via Bluetooth. See
Connecting Radius-7 to Root via Bluetooth on page 36 for information on how to pair
Radius-7 with Root. For general information on Root, see Operator’s Manual for Root.
About the Action Menu
To expand the Action Menu, select the arrow in the upper right corner of the window.
The Action Menu allows quick access to the following settings directly from the Main Screen:
• Sensitivity - Selecting this option cycles through the available sensitivity modes:
APOD, NORM and MAX. See Sensitivity Modes Overview on page 44.
• Trend View - Displays values in Trend View. See Trends on page 61.
• Analog View - Displays values in an analog gauge style view.
www.masimo.com 43 Masimo
Page 46

Radius-7 Chapter 4: Operation
Note: After approximately 10 seconds without interaction, the Action Menu will retract.
Sensitivity Modes Overview
Three sensitivity levels enable a clinician to tailor the response of Radius-7 to the needs of
the particular patient situation. Sensitivity Modes are accessed through the Action Menu or
through Additional Settings. See About the Action Menu on page 43 or Additional Settings
on page 45.
The sensitivity levels are as follows:
• NORM (Normal Sensitivity)
NORM is the recommended sensitivity mode for patients who are experiencing
some compromise in blood flow or perfusion. It is advisable for care areas where
patients are observed frequently, such as an intensive care unit (ICU).
• APOD® (Adaptive Probe Off Detection® Sensitivity)
APOD is the recommended sensitivity mode for situations which there is a high
probability of the sensor becoming detached. It is also the suggested mode for
care areas where patients are not visually monitored continuously. This mode
delivers enhanced protection against erroneous pulse rate and arterial oxygen
saturation readings when a sensor becomes inadvertently detached from a patient
due to excessive movement.
• MAX (Maximum Sensitivity)
MAX is the recommended sensitivity mode for patients with low perfusion or when
a low perfusion message displays in APOD or NORM mode. MAX mode is not
recommended for care areas where patients are not monitored visually, such as
medical-surgical floors. It is designed to display data at the measuring site when
the signal may be weak due to decreased perfusion. When a sensor becomes
detached from a patient, it will have compromised protection against erroneous
pulse rate and arterial saturation readings.
Configuring Parameters
Each parameter displayed on Root and Radius-7 can be configured in its respective menu on
Root. Configurable options include Alarm Settings and Averaging Time.
There are two ways to access any parameter’s settings menu on Root:
1. From the Main Screen on Root, press on any of the parameters displayed in the
rainbow window to access its respective settings menu.
Or
2. Press the gear icon on the bottom right-hand corner of the Main Screen on
Root to access the Main Menu. Then press the rainbow tile to access the
rainbow menu. See rainbow Parameter Settings on page 45.
www.masimo.com 44 Masimo
Page 47

Radius-7 Chapter 4: Operation
rainbow Parameter Settings
The rainbow menu allows the user to view and customize settings for rainbow parameters:
Parameter Settings
See Parameter Settings on page 46.
3D Alarms
See 3D Alarms on page 64.
Additional Settings
See Additional Settings on page 45.
Additional Settings
Use the Additional Settings screen to configure the following:
Option Description Default
Setting
Sensitivity
Mode
Change Sensitivity Mode.
See Sensitivity Modes Overview on
APOD MAX, APOD, NORM
Configurable
Settings
page 44.
SmartTone Enable or disable the SmartTone. Off On, Off
SpO2 low %
limit
Set the SpO2 low limit alarm.
See SpO2 Settings on page 46.
Off Off, 1% to 98%
www.masimo.com 45 Masimo
Page 48

Radius-7 Chapter 4: Operation
Parameter Settings
Only parameters that have been loaded onto the system will be visible.
To access any of the available parameter setting screens:
1. From the Parameter Settings screen, to access the desired parameter, flick the
on-screen icons left or right.
2. Touch the icon of the desired parameter. For details, see any of the following
sections:
• See SpO2 Settings on page 46.
• See PR Settings on page 48.
• See Pi Settings on page 49.
• See PVi Settings on page 51.
• See Respiration Rate (RR) Settings on page 53.
• See SpHb Settings on page 55.
• See SpOC Settings on page 58.
• See SpMet Settings on page 59.
• See SpCO Settings on page 60.
SpO2 Settings
From the SpO2 Settings screen, change any of the following options:
SpO2 Alarms on page 47
Additional Settings for SpO2 on page 48
About Parameter Information on page 60
Desat Index on page 65
About Desat Index on page 64
Trends on page 61
www.masimo.com 46 Masimo
Page 49

Radius-7 Chapter 4: Operation
SpO2 Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
Priority
High
Limit
Low Limit Low Limit is the lower threshold that
High Limit is the upper threshold that
triggers an alarm.
Medium Off 2% to 99% in
High 88% 1% to 98% in
triggers an alarm.
High
Caution
Range
Low
Caution
Range
Silence
Duration
Rapid
Desat
Provides an early indicator for a
parameter approaching the high limit
threshold value that triggers an alarm.
Provides an early indicator for a
parameter approaching the low limit
threshold value that triggers an alarm.
Sets the amount of time that the alarm
is silenced.
Sets the Rapid Desat limit threshold to
the selected amount below the Low
NA Off Off or 1 to 10 in
NA 4 Off or 1 to 10 in
NA 2 minutes 30 seconds, 1,
NA -10% -5%, or -10%, or
Alarm Limit. When SpO2 value falls
below rapid desat limit the audio and
visual alarm are immediately triggered
without respect to the alarm delay.
Factory
Default
Settings
User
Configurable
Options
steps of 1%, or
Off
When set to Off,
alarm is
disabled
steps of 1%
steps of 1
steps of 1
or 2 minutes
Off
Alarm
Delay
When an alarm condition is met, this
feature delays the audible part of an
NA 15
seconds
0, 5, 10, or 15
seconds
alarm
www.masimo.com 47 Masimo
Page 50

Radius-7 Chapter 4: Operation
Additional Settings for SpO2
From the Additional Settings screen, change any of the following options:
Options Description Factory
Default
User Configurable
Settings
Settings
Averaging
Time*
The length of time over which the
system calculates the average of all
8 seconds 2-4, 4-6, 8, 10, 12, 14,
or 16 seconds
data points.
FastSat See FastSat Overview on page 48. Off Off or On
* With FastSat the averaging time is dependent on the input signal.
FastSat Overview
FastSat enables rapid tracking of arterial oxygen saturation changes. Arterial oxygen
saturation data is averaged using pulse oximeter averaging algorithms to smooth the trend.
When Radius-7 is set to FastSat On, the averaging algorithm evaluates all saturation values,
providing an averaged saturation value that is a better representation of the patient’s current
oxygenation status. With FastSat set to On, the averaging time is dependent on the input
signal.
PR Settings
From the PR Settings screen, change any of the following options:
PR Alarms on page 49
About Parameter Information on page 60
Trends on page 61
www.masimo.com 48 Masimo
Page 51

Radius-7 Chapter 4: Operation
PR Alarms
From the PR Alarms screen, change any of the following options:
Option Description Alarm
Priority
High Limit High Limit is the upper threshold
High 140 bpm 35 bpm to 235
that triggers an alarm.
Low Limit Low Limit is the lower threshold
High 50 bpm 30 bpm to 230
that triggers an alarm.
High
Caution
Range
Provides an early indicator for a
parameter approaching the high
limit threshold value that triggers
NA 20 Off or 5 to 25 in
an alarm.
Low
Caution
Range
Provides an early indicator for a
parameter approaching the low
limit threshold value that triggers
NA 10 Off or 5 to 25 in
an alarm.
Silence
Duration
Sets the amount of time that the
alarm is silenced.
NA 2 minutes 30 seconds, or 1, 2,
Pi Settings
Factory
Default
Settings
User Configurable
Options
bpm, in steps of 5
bpm
bpm, steps of 5
bpm
steps of 5
steps of 5
or 5 minutes
From the Pi Settings screen, access any of the following screens:
Pi Alarms on page 50
Additional Settings for Pi on page 51
About Parameter Information on page 60
Pi Delta on page 66
About Pi Delta on page 65
Trends on page 61
www.masimo.com 49 Masimo
Page 52

Radius-7 Chapter 4: Operation
Pi Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
Priority
High Limit High Limit is the upper threshold
Medium Off Step size:
that triggers an alarm.
Low Limit Low Limit is the lower threshold
Medium 0.30 Step size:
that triggers an alarm.
High
Caution
Range
Provides an early indicator for a
parameter approaching the high
limit threshold value that
NA Off Off or 0.01 to 0.09
triggers an alarm.
Low
Caution
Range
Provides an early indicator for a
parameter approaching the low
limit threshold value that
NA 0.30 Off or 0.01 to 0.09
triggers an alarm.
Factory
Default
Settings
User Configurable
Options
0.04 to 0.09 in
steps of 0.01
0.10 to 0.90 in
steps of 0.10
1 to 19 in steps of
1, or Off
Off or 0.03 to 0.09
in steps of 0.01
0.10 to 0.90 in
steps of 0.10
1 to 18 in steps of 1
in increments of
0.01
0.1 to 1.0 in
increments of 0.1
in increments of
0.01
0.1 to 1.0 in
increments of 0.1
Silence
Duration
Sets the amount of time that the
alarm is silenced.
NA 2 minutes 30 seconds, or 1, 2,
or 5 minutes
www.masimo.com 50 Masimo
Page 53
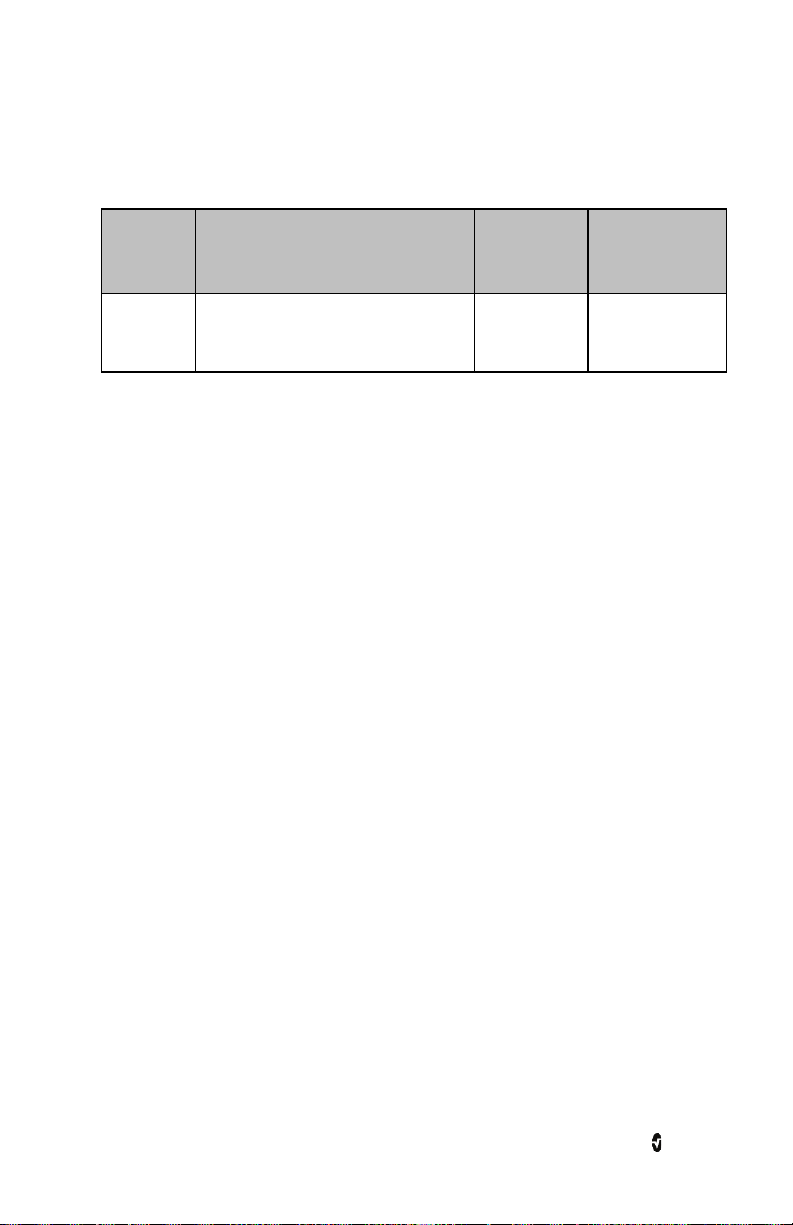
Radius-7 Chapter 4: Operation
Additional Settings for Pi
From the Additional Settings screen, change the following option:
Options Description Factory
Default
Settings
Averaging
Time
The length of time over which the
system calculates the average of all data
Long Short or Long
points.
PVi Settings
From the PVi Settings screen, access any of the following options:
PVi Alarms on page 52
Additional Settings for PVi on page 53
About Parameter Information on page 60
Trends on page 61
User Configurable
Settings
www.masimo.com 51 Masimo
Page 54

Radius-7 Chapter 4: Operation
PVi Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
Priority
High Limit High Limit is the upper threshold
Medium 40 2 to 99 in steps of
that triggers an alarm.
Low Limit Low Limit is the lower threshold
Medium 5 Off or 1 to 98 in
that triggers an alarm.
High
Caution
Range
Provides an early indicator for a
parameter approaching the high
limit threshold value that triggers
NA 5 Off or 1 to 10 in
an alarm.
Low
Caution
Range
Provides an early indicator for a
parameter approaching the low
limit threshold value that triggers
NA 3 Off or 1 to 10 in
an alarm.
Silence
Duration
Sets the amount of time that the
alarm is silenced.
NA 2 minutes 30 seconds, or 1,
Factory
Default
Settings
User
Configurable
Options
1, or Off
When set to Off,
alarms are
disabled.
steps of 1
When set to Off,
alarms are
disabled.
steps of 1
steps of 1
2, 5, or 10
minutes
www.masimo.com 52 Masimo
Page 55

Radius-7 Chapter 4: Operation
system calculates the average of all data
Additional Settings for PVi
From the Additional Settings screen, change the following option:
Options Description Factory
Default
User Configurable
Settings
Settings
Averaging
The length of time over which the
Long Short or Long
Time
points.
Respiration Rate (RR) Settings
Radius-7 can determine Respiration Rate (RR) either by the acoustic signal (RRa). For more
information, see:
RRa Settings on page 53
From the RR Settings screen, change any of the following options:
RRa Alarms on page 54
Additional Settings for RRa on page 55
About Parameter Information on page 60
Trends on page 61
RRa Settings
RRa is active when the following conditions are all met:
• RRa is installed on the Radius-7.
• An acoustic sensor is connected.
When using an acoustic sensor, Respiration Rate (RR) is determined by the acoustic (RRa)
signal. See rainbow Acoustic Monitoring™ (RAM™) on page 26. When the respiratory rate is
determined by the acoustic signal, the Main Screen labels respiratory rate as RRa, as shown
below.
www.masimo.com 53 Masimo
Page 56

Radius-7 Chapter 4: Operation
From the RR Settings screen, access any of the following screens:
RRa Alarms on page 54
Additional Settings for RRa on page 55
About Parameter Information on page 60
RRa Alarms
From the Alarms screen, change any of the following options:
Options Description Alarm
Priority
High Limit High Limit is the upper
High 30 breaths
threshold that triggers an
alarm.
Low Limit Low Limit is the lower
High 6 breaths
threshold that triggers an
alarm.
High
Caution
Range
Provides an early indicator for
a parameter approaching the
high limit threshold value
NA 5 Off or 1 to 7 in steps of
that triggers an alarm.
Low Caution
Range
Provides an early indicator for
a parameter approaching the
NA 2 Off or 1 to 7 in steps of
low limit threshold value that
triggers an alarm.
Silence
Duration
Respiratory
Pause
Sets the amount of time that
the alarm is silenced.
The duration of time that
triggers an alarm if no
NA 2 minutes 30 seconds or 1, 2 or 5
NA 30 seconds 15, 20, 25, 30, 35, or
breaths are detected.
Alarm Delay When a High or Low alarm
NA 30 seconds 0, 10, 15, 30, or 60
condition occurs, this feature
delays the audible part of an
alarm.
Factory
Default
Settings
per minute
per minute
Configurable Options
6 to 69 breaths per
minute in steps of 1
breath per minute, or
Off
Off, or 5 to 68 breaths
per minute in steps of
1 breath per minute
1
1
minutes
40 seconds
seconds
www.masimo.com 54 Masimo
Page 57

Radius-7 Chapter 4: Operation
Additional Settings for RRa
From the Additional Settings screen, change any of the following options:
Options Description Factory
Default
Settings
Averaging
Time
The length of time over which the
system calculates the average of all
Slow Trending, No
data points.
Freshness The duration of time that, during
5 minutes 0, 1, 5, 10, or 15
interference, the system displays the
last valid reading.
SpHb Settings
From the SpHb Settings screen, access any of the following screens:
SpHb Alarms on page 56
Additional Settings for SpHb on page 57
About Parameter Information on page 60
Trends on page 61
User Configurable
Settings
Averaging, Fast,
Medium, or Slow
minutes
www.masimo.com 55 Masimo
Page 58

Radius-7 Chapter 4: Operation
SpHb Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
High
Limit
Low Limit Low Limit is the lower
High Limit is the upper
threshold that triggers an
alarm.
threshold that triggers an
alarm.
Priority
High 17.0 g/dL
High 7.0 g/dL
Factory
Default
Settings
(11.0
mmol/L)
(170 g/L)
(4.0
mmol/L)
(70 g/L)
User Configurable
Options
2.0 g/dL to 24.5 g/dL in
steps of 0.1 g/dL, or Off
(2.0 mmol/L to 15.0
mmol/L in steps of 0.1
mmol/L, or Off)
(20 g/L to 245 g/L in
steps of 1 g/L, or Off)
When SpHb Precision is
set to 1.0, the values are
rounded to the nearest
whole number.
When set to Off, alarm is
disabled.
1.0 g/dL to 23.5 g/dL in
steps of 0.1 g/dL. or Off
(1.0 mmol/L to 14.5
mmol/L, in steps of 0.1
mmol/L, or Off)
(10 g/L to 235 g/L in
steps of 1 g/L, or Off)
When SpHb Precision is
set to 1.0, values are
rounded to the nearest
whole number.
When set to Off, alarm is
disabled.
High
Caution
Range
www.masimo.com 56 Masimo
Provides an early indicator
for a parameter approaching
the high limit threshold
value that triggers an alarm.
NA 1.0 g/dL
Off
(mmol/L)
(10 g/L)
Off or 0.1 g/dL to 2.5
g/dL in increments of 0.1
Off or 0.1 mmol/L to 1.5
mmol/L in increments of
0.1
Off or 1 g/L to 25 g/L in
increments of 1
Page 59

Radius-7 Chapter 4: Operation
Option Description Alarm
Priority
Factory
Default
User Configurable
Options
Settings
Low
Caution
Range
Provides an early indicator
for a parameter approaching
the low limit threshold
value that triggers an alarm.
NA 2.0 g/dL
Off
(mmol/L)
(20 g/L)
Off or 0.1 g/dL to 2.5
g/dL in increments of 0.1
Off or 0.1 mmol/L to 1.5
mmol/L in increments of
0.1
Off or 1 g/L to 25 g/L in
increments of 1
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 seconds, or 1, 2, or 5
minutes
Additional Settings for SpHb
Option Description Factory
Averaging Time The length of time over which the system
calculates the average of all data points.
Default
Settings
Medium Short, Medium,
User
Configurable
Options
or Long
Calibration Provides an arterial or venous value that
displays on the main screen.
Unit of
Measure*
Displays total hemoglobin (SpHb) as g/dL
(grams per deciliter), mmol/L (millimoles
Arterial Arterial or
Venous
g/dL g/dL, mmol/L,
or g/L
per liter), or g/L (grams per liter). Unit of
Measure cannot be changed during active
monitoring.
Precision
(units of g/dL
and mmol/L)
Allows the user to set the decimal for SpHb.
Note: When unit is g/L, Precision is always
1 (whole numbers).
0.1 0.1, 0.5, or 1.0
*Changing Unit of Measure will delete all prior trend data for all parameters.
www.masimo.com 57 Masimo
Page 60

Radius-7 Chapter 4: Operation
SpOC Settings
From the SpOC Settings screen, access any of the following screens:
SpOC Alarms on page 58
About Parameter Information on page 60
Trends on page 61
SpOC Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
Priority
High Limit High Limit is the upper threshold
Medium 25
that triggers an alarm.
Low Limit Low Limit is the lower threshold
High 10 Off or 1% to 33%,
that triggers an alarm.
High
Caution
Range
Provides an early indicator for a
parameter approaching the high
limit threshold value that triggers
NA Off Off or 1 to 4 in
an alarm.
Low
Caution
Range
Provides an early indicator for a
parameter approaching the low
limit threshold value that triggers
NA 2 Off or 1 to 4 in
an alarm.
Silence
Duration
Sets the amount of time that the
alarm is silenced.
NA 2 minutes 30 seconds, or 1,
Factory
Default
Settings
User
Configurable
Options
2% to 34%, in
steps of 1%, or Off
When set to Off,
alarm is disabled
in steps of 1%
When set to Off,
alarm is disabled.
steps of 1
steps of 1
2, or 5 minutes
www.masimo.com 58 Masimo
Page 61

Radius-7 Chapter 4: Operation
SpMet Settings
From the SpMet Settings screen, access any of the following screens:
SpMet Alarms on page 59
About Parameter Information on page 60
Trends on page 61
SpMet Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
High Limit High Limit is the upper threshold
Low Limit Low Limit is the lower threshold
High
Caution
Range
Low
Caution
Range
that triggers an alarm.
that triggers an alarm.
Provides an early indicator for a
parameter approaching the high
limit threshold value that triggers
an alarm.
Provides an early indicator for a
parameter approaching the low
limit threshold value that triggers
an alarm.
Priority
High 3.0
Medium Off Off or 0.1% to 2%,
NA 1.0 Off or 0.1 to 2.0 in
NA Off Off or 0.1 to 2.0 in
Factory
Default
Settings
User Configurable
Options
1.0% to 2.0%, in
steps of 0.1%,
2.5% to 99.5% in
steps of 0.5%, or
Off
When set to Off,
alarm is disabled
in steps of 0.1%
2.5% to 99%, in
steps 0.5%
When set to Off,
alarm is disabled.
increments of 0.1
2.5 to 10.0 in
increments of 0.5
increments of 0.1
2.5 to 10.0 in
increments of 0.5
Silence
Duration
www.masimo.com 59 Masimo
Sets the amount of time that the
alarm is silenced.
NA 2 minutes 30 seconds, or 1,
2, or 5 minutes
Page 62

Radius-7 Chapter 4: Operation
SpCO Settings
From the SpCO Settings screen, access any of the following screens:
SpCO Alarms on page 60
About Parameter Information on page 60
Trends on page 61
SpCO Alarms
From the Alarms screen, change any of the following options:
Option Description Alarm
Priority
High Limit High Limit is the upper threshold
High 10
that triggers an alarm.
Low Limit Low Limit is the lower threshold
Medium Off Off or 1% to 97%,
that triggers an alarm.
High
Caution
Range
Provides an early indicator for a
parameter approaching the high
limit threshold value that triggers
NA 3 Off or 1 to 10 in
an alarm.
Low
Caution
Range
Provides an early indicator for a
parameter approaching the low
limit threshold value that triggers
NA Off Off or 1 to 10 in
an alarm.
Silence
Duration
Sets the amount of time that the
alarm is silenced.
NA 2 minutes 30 seconds, or 1,
About Parameter Information
Factory
Default
Settings
User
Configurable
Options
2% to 98%, in
steps of 1%, or Off
When set to Off,
alarm is disabled
in steps of 1%
When set to Off,
alarm is disabled.
steps of 1
steps of 1
2, or 5 minutes
Additional information about each parameter is available.
www.masimo.com 60 Masimo
Page 63

Radius-7 Chapter 4: Operation
To access additional information about parameters:
1. From the Parameter Settings screen, touch the About icon. The following is an
example for SpO
.
2
2. An About screen appears for the selected parameter and displays information
about the parameter.
Trends
Trend settings allow the user to configure the Y-axis maximum and Y-axis minimum for each
parameter. The maximum and minimum possible values differ depending on the selected
parameter.
Trend Settings
Use the Trend Settings screen under each Parameter Setting screen to configure Trend Views
on Root.
Option Description Factory Default
Setting
Default
Duration*
Sets the time duration
displayed in trend lines.
2 hours
Clear Trends* Deletes all stored trend data. N/A
Y-axis Min 50 0 to 95 in steps of 5
SpO2
Y-axis Max 100 5 to 100 in steps of 5
User Configurable Settings
15, 30, or 45 minutes
1, 2, 4, 8, 12, or 24 hours
Press Clear to delete all
stored trend data.
Y-axis Min 25 25 to 235 in steps of 5
PR
Y-axis Max 200 30 to 240 in steps of 5
SpHb g/dL Y-axis Min 5.0 g/dL
0.0 to 24.9 g/dL in
increments of 0.1
www.masimo.com 61 Masimo
Page 64

Radius-7 Chapter 4: Operation
Option Description Factory Default
Setting
Y-axis Max 20.0 g/dL
Y-axis Min 3.1 mmol/L
SpHb
mmol/L
Y-axis Max 12.4 mmol/L
Y-axis Min 50 g/L 0 to 249 g/L in steps of 1
SpHb g/L
Y-axis Max 200 g/L 1 to 250 g/L in steps of 1
Y-axis Min 0 0 to 69 in steps of 1
RRa
Y-axis Max 35 1 to 70 in steps of 1
Y-axis Min 0 0 to 99 in steps of 1
SpCO
Y-axis Max 40 1 to 100 in steps of 1
Y-axis Min 0.0
SpMet
Y-axis Max 15.0
User Configurable Settings
0.1 to 25.0 g/dL in
increments of 0.1
0.0 to 15.4 mmol/L in
increments of 0.1
0.1 to 15.5 mmol/L in
increments of 0.1
0.0 to 99.5 in increments
of 0.5
1.0 to 100.0 in increments
of 0.5
Y-axis Min 0.0
0.0 to 19.0 in increments
of 1.0
Pi
Y-axis Max 20.0
1.0 to 20.0 in increments
of 1.0
Y-axis Min 0.0 0 to 99 in steps of 1
PVi
Y-axis Max 30 1 to 100 in steps of 1
Y-axis Min 0 0 to 34 in steps of 1
SpOC
Y-axis Max 20 1 to 35 in steps of 1
*Available under the Root Main Menu Settings screen. For information about viewing and
manipulating Trend Settings on Root, refer to the Root Operator's Manual.
www.masimo.com 62 Masimo
Page 65
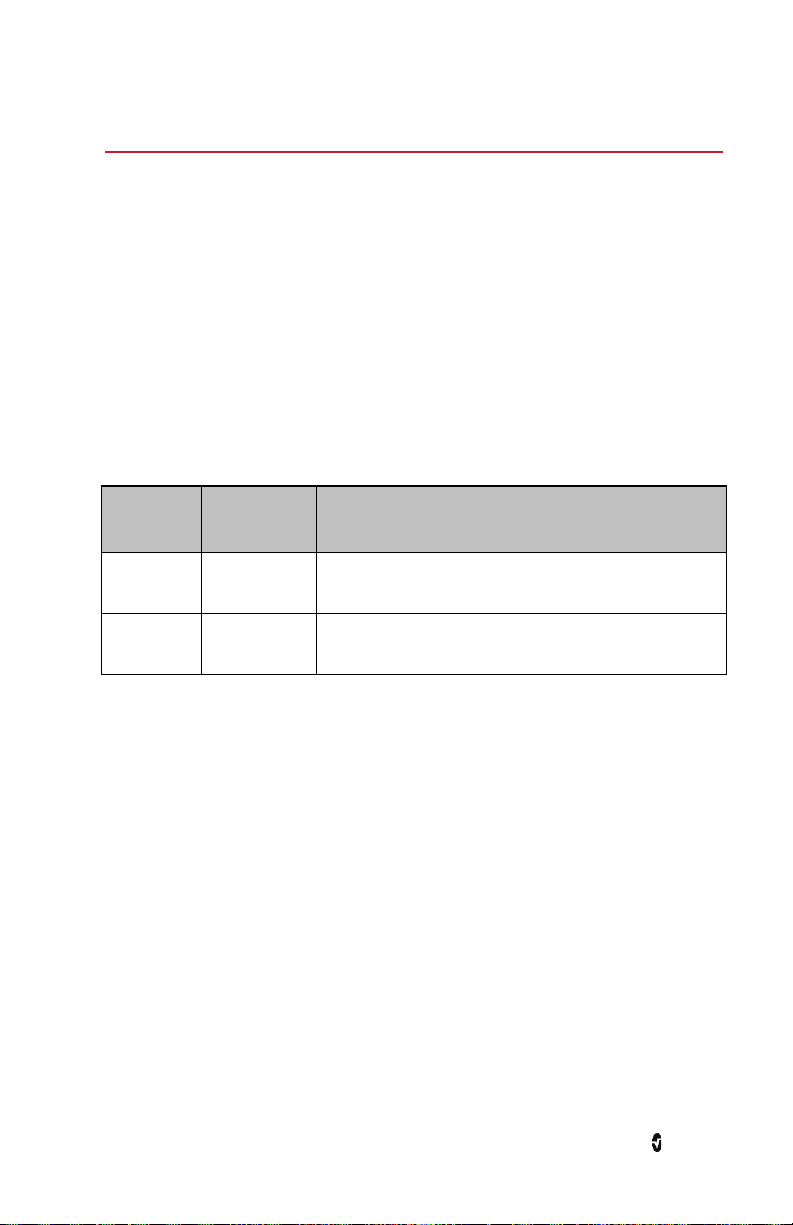
Chapter 5: Alarms and Messages
About Alarms
The Radius-7 visually and audibly indicates alarm conditions that the system detects. Audible
alarms may be silenced, without affecting the operation of visual alarms. See Safety
Information, Warnings and Cautions on page 11.
Alarm Priorities
There are two priorities for alarms:
• High
• Medium
The following are the audible and visual characteristics for different alarm priorities:
Alarm
Priority
High Priority
Medium
Priority
Alarm Status
Color
Flashing red 571 Hz tone, 10-pulse burst, pulse spacing: 0.25s, 0.25s,
Flashing yellow 550 Hz tone, 3-pulse burst, pulse spacing: 0.375s, 0.375s,
Audio Alarm Description
0.50s, 0.25s, repeat time:10s
repeat time: 7s
Alarm Management
In order to minimize accidental changes to Radius-7's critical settings, alarm management is
restricted to Root.
When Radius-7 is connected to Root, audible alarms will sound on Root but not Radius-7. In
this case, audible alarms can be temporarily silenced on Root. Visual alarms will display on
both Radius-7 and Root until the alarm condition has been addressed. For alarm
management on Root, see Operator’s Manual for Root.
When Radius-7 is not connected to Root, audible alarms will sound on Radius-7. Audible
alarms can be temporarily silenced by touching and holding the Touchpad for 2 seconds.
Visual alarms will continue to display on Radius-7 until the alarm condition has been
addressed.
When Radius-7 is connected to Patient SafetyNet (and not communicating to Root through
Bluetooth), audible alarms will sound on Radius-7 and Patient SafetyNet. Audible alarms can
be temporarily silenced by touching and holding the Touchpad on Radius-7 for 2 seconds.
Visual alarms will continue to display on Radius-7 until the alarm condition has been
addressed.
Note: In the event of temporary loss of power to Radius-7, the Root will restore alarm setting
to Radius-7 through the re-established Bluetooth connection. If the Radius-7 is used without
www.masimo.com 63 Masimo
Page 66

Radius-7 Chapter 5: Alarms and Messages
a Bluetooth connection to Root, then the alarm settings will be restored to the factory
default.
3D Alarms
3D Alarms, accessible from the Main Menu, include the following:
Desat Index on page 65
About Desat Index on page 64
Pi Delta on page 66
About Pi Delta on page 65
About Desat Index
The 3D Desat Index Alarm allows a clinician to request audible and visual alarms if a patient
experiences a specified number of desaturations beyond a defined level from the patient's
baseline saturation over a specific period of time.
Traditional high and low SpO
user-selected thresholds. These thresholds are typically established to detect significant
changes from patients’ baseline saturation levels. However, in select patient populations,
substantial desaturation events that remain above a typical low alarm limit threshold may be
preceded by a cycle of smaller transient desaturations over a limited period of time. The
ability to alert clinicians when a cycle of smaller transient desaturations occur may provide
an earlier indication of a potential significant decline in patient status, allowing for more
focused monitoring and/or a change in treatment.
To address the select patient populations in which detecting a cycle of transient
desaturations may be helpful, set a 3D Desat Index Alarm.
To set a 3D Desat Index Alarm see Desat Index on page 65.
www.masimo.com 64 Masimo
alarm limits alert clinicians to saturation levels that exceed
2
Page 67

Radius-7 Chapter 5: Alarms and Messages
Desat Index
From the Desat Index menu screen, change any of the following options:
Options Description Factory
Default
User Configurable
Settings
Settings
Delta The change in saturation from the
patient's baseline measurement.
Time The period of time in which saturation
events that exceed the delta will be
4% 2% to 10%, in steps of
1%.
1 hour 1 to 4 hours, in steps
of 1 hour.
monitored.
Number of
Events
The number of desaturations exceeding
the delta which will activate audible and
visual alarms.
Off Off, 1 to 24
desaturations in
steps of 1.
About Pi Delta
The Perfusion Index (Pi) Delta Alarm allows a clinician to request audible and visual alarms if
perfusion at the monitored site decreases by a specified level (delta) over a specific period of
time.
Perfusion Index gives an indication of the level of perfusion at the monitored site. Radius-7
measures perfusion at the monitored SpO
non-pulsatile signal, and expressing that ratio as a percentage. Pi has been clinically proven
to be useful as a predictor of the level of illness in neonates and adults. It has also been
shown that Pi may change dramatically in response to sympathetic changes caused by
inhalational agents and pain stimulation.* If Pi decreases over time, there may be underlying
physiological reasons that need to be addressed.
Pi Delta audibly and visually alerts the user to important changes in a patient's perfusion, as
compared to the patient’s baseline Pi rate. The baseline is set by Radius-7 once the user has
enabled the alarm and represents 30 seconds of currently averaged Pi. To set a Pi Delta
alarm, see Pi Delta on page 66. The feature includes a user-selectable Pi Delta Alarm. This
allows the clinician to request an audible and visual alarm if perfusion at the monitored site
decreases by a specified level (delta) over a specified window of time. Three of the variables
are selectable by the user within established ranges as noted in Pi Delta Alarms.
*De Felice C, Latini G, Vacca P, Kopotic RJ. The pulse oximeter perfusion index as a predictor for
high illness severity in neonates. Eur J Pediatr. 2002;161:561-562.
site by comparing the pulsatile signal to the
2
www.masimo.com 65 Masimo
Page 68
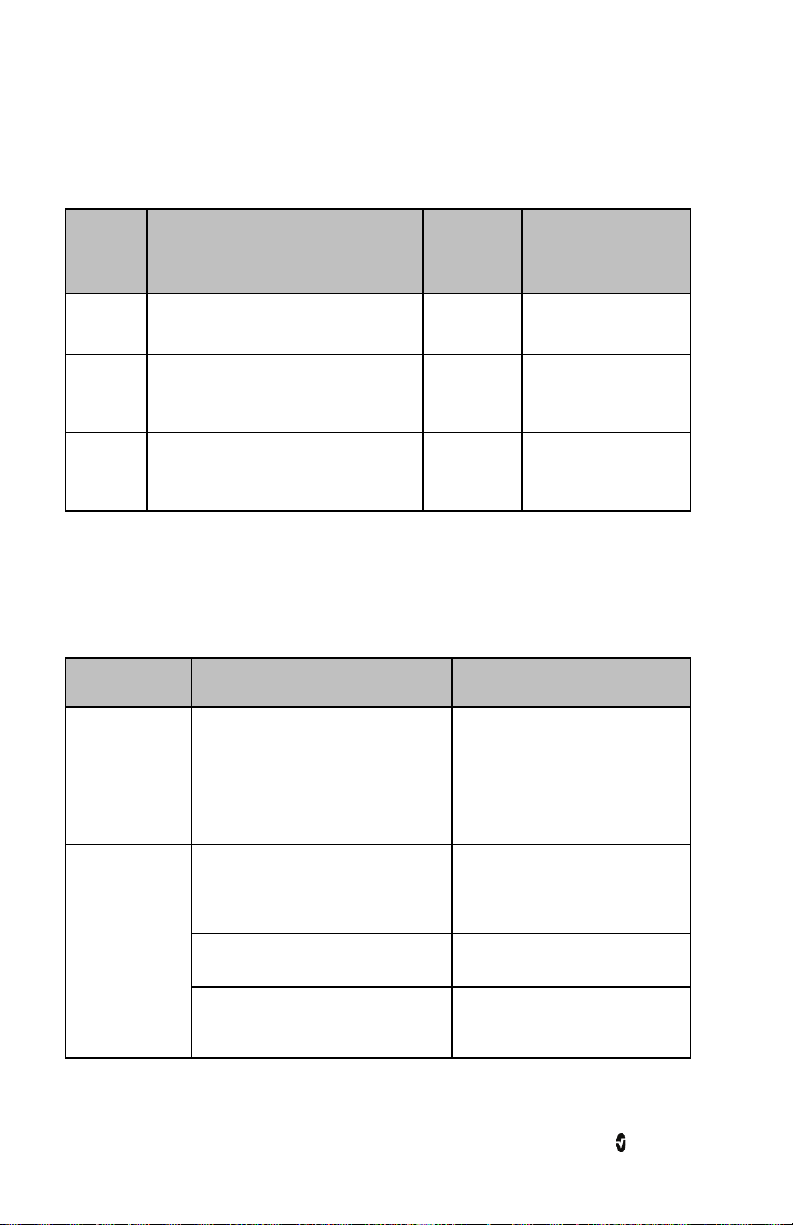
Radius-7 Chapter 5: Alarms and Messages
Instrument
Pi Delta
From the Pi Delta menu screen, change any of the following options:
Options Description Factory
Default
User Configurable
Settings
Settings
Set
Baseline
Percent
Change
Sets the Perfusion Index (Pi) value to be
used as the baseline.
The change in Pi from the baseline that,
if maintained for the Timeout length,
Off On or Off
50% 10% to 99%, in steps of
1%
will trigger audible and visual alarms.
Timeout The length of time over which the
percent change in Pi is monitored.
None None or 1, 5, 30
minutes, 1, 4, 8, 12, 24,
36, 48 hours
Messages
The following section lists common messages, their potential causes, and next steps.
Alarm Message Description Next Step
(Pulse CO-Ox)
Replace Cable
or
(RAM) Replace
Cable
• Defects in the Instrument
Module
• Return the device for
servicing.
(Pulse CO-Ox) No
Sensor
Connected
or
(RAM) No Sensor
Connected
• Sensor not fully inserted into
the connector.
• Incompatible or defective
sensor.
• Device is searching for patient’s
pulse.
• Disconnect and reconnect
sensor. See the instructions
for use provided with your
sensor.
• Replace with a compatible
Masimo sensor.
• Disconnect and reconnect
the sensor to the
Module.
www.masimo.com 66 Masimo
Page 69

Radius-7 Chapter 5: Alarms and Messages
Alarm Message Description Next Step
(Pulse CO-Ox)
Replace Sensor
or
(RAM) Replace
Sensor
(Pulse CO-Ox)
Incompatible
Sensor
or
(RAM)
Incompatible
Sensor
(Pulse CO-Ox)
Sensor Near
Expiration
or
(RAM) Sensor
Near Expiration
(Pulse CO-Ox) No
Adhesive Sensor
Connected
or
(RAM) No
Adhesive Sensor
Connected
• Sensor has used all of its
available monitoring time
• Sensor is non-functional.
• Defective sensor.
• Not a compatible Masimo
sensor.
• Sensor is attached to a device
without an appropriate
parameter installed.
• Sensor has less than 10%
patient monitoring time
remaining.
• The adhesive portion of the
sensor is not connected.
• Replace sensor.
• Replace with a compatible
Masimo sensor.
• Use a compatible sensor.
Contact your local Masimo
Representative to learn
more about optional
parameter upgrades.
• Replace sensor.
• Ensure the adhesive portion
is firmly connected to the
sensor.
(Pulse CO-Ox)
Replace
Adhesive Sensor
or
(RAM) Replace
Adhesive Sensor
(Pulse CO-Ox)
Incompatible
www.masimo.com 67 Masimo
• When a disposable or
resposable sensor is used, the
adhesive portion of the sensor is
non-functional, or the life of the
adhesive portion of the sensor
has expired.
• Not a compatible Masimo
disposable or resposable sensor.
• Replace the adhesive
portion of the sensor.
• Replace with a compatible
Masimo disposable or
resposable sensor.
Page 70

Radius-7 Chapter 5: Alarms and Messages
Adhesive Sensor
Replace with new disposable
Alarm Message Description Next Step
or
(RAM)
Incompatible
Adhesive Sensor
(Pulse CO-Ox)
Adhesive Near
Expiration
or
(RAM) Adhesive
Near Expiration
(Pulse CO-Ox)
Sensor Off
Patient
or
(RAM) Sensor Off
Patient
(Pulse CO-Ox)
Pulse Search
(Pulse CO-Ox)
Sensor
Initializing
• Sensor is attached to a device
without an appropriate
parameter installed.
• Disposable or resposable sensor
has less than 10% patient
monitoring time remaining.
• Sensor off patient.
• Sensor not connected to patient
properly. Sensor is damaged.
• Device is searching for pulse. • If device fails to display
• Device is checking the sensor for
proper functioning and
performance.
• Use a compatible sensor.
Contact your local Masimo
Representative to learn
more about optional
parameter upgrades.
•
or resposable sensor.
• Disconnect and reconnect
sensor. Reattach sensor.
• Properly reapply the sensor
on the patient and
reconnect the sensor to the
Instrument Module. If the
sensor is damaged, replace
the sensor.
within 30 seconds,
disconnect and reconnect. If
pulse search continues,
move sensor to better
perfused site.
• If values are not displayed
within 30 seconds,
disconnect and reconnect
sensor. If values are still not
displayed, replace with a
new sensor.
(RAM) RAM
Check Sensor
(RAM) Sensor
Initializing
www.masimo.com 68 Masimo
• RAM unable to collect data
through RAM Sensor.
• Device is checking the sensor for
proper function and
performance.
• Ensure proper sensor
application. Check that no
object is pulling on the
sensor cable, which may
cause the sensor to peel off.
• If values are not displayed
within 30 seconds,
disconnect and reconnect
sensor. If values are still not
displayed, replace with a
new sensor.
Page 71

Radius-7 Chapter 5: Alarms and Messages
Alarm Message Description Next Step
(Pulse CO-Ox)
Interference
Detected
or
(RAM)
Interference
Detected
(Pulse CO-Ox)
SpO
Only Mode
2
(Pulse CO-Ox)
Low Perfusion
Index
(Pulse CO-Ox)
Low Signal IQ
Low SpCO SIQ
Low SpMet SIQ
• High intensity light such as
pulsating strobe lights,
• Place a Masimo Optical
Light Shield over the sensor.
excessive ambient light sources
such as surgical lights or direct
sunlight, or other monitor
displays.
• Occurs during an unsuccessful
sensor initialization/pulse
search routine or during
monitoring.
• See the directions for use
provided with your sensor.
Use a Masimo light shield to
cover the sensor and adjust
the sensor.
• Signal strength is too weak. • Move sensor to better
perfused site.
• Indicates low signal confidence
in the value displayed due to
poor signal strength.
• Ensure proper sensor
application. Move sensor to
a better perfused site. See
Signal IQ on page 22.
• Indicates low signal confidence
in the SpCO measurement
displayed.
• Ensure proper sensor
application. Check sensor to
see if it is working properly.
If not, replace the sensor.
See Successful Monitoring
for SpHb on page 25.
• Indicates low signal quality of
SpMet measurement.
• Ensure proper sensor
application. Check sensor to
see if it is working properly.
If not, replace the sensor.
See Successful Monitoring
for SpMet.
Low SpHb SIQ
• Indicates low signal quality of
SpHb measurement.
• Ensure proper sensor
application. Check sensor to
see if it is working properly.
If not, replace the sensor.
See Successful Monitoring
for SpHb on page 25.
"- -" (Dashes
shown as
• Unable to provide a parameter
value.
• Check patient's vital
condition.
parameter value)
www.masimo.com 69 Masimo
Page 72

Radius-7 Chapter 5: Alarms and Messages
Alarm Message Description Next Step
Low battery
Device
disconnected
Speaker Failure
• Battery charge is low. • Charge Battery Module by
docking into Battery
Charging Adapter on Root
and powering Root with AC
line power. Replace Battery
Module if necessary.
• Device has lost Bluetooth
connectivity with Root.
• Check if Bluetooth is enabled
on Root. See the Operator's
Manual for Root.
• Check connection between
the Instrument Module and
Battery Module.
• Re-dock the Battery Module
on Root to re-establish
Bluetooth connectivity.
• Device requires service. • Contact Masimo Tech
Support. See Contacting
Masimo on page 98.
www.masimo.com 70 Masimo
Page 73

Chapter 6: Troubleshooting
Troubleshooting Measurements
Symptom Potential Causes Next Steps
Low signal quality.
Difficulty obtaining a
reading.
• Sensor is damaged or not
functioning.
• Improper sensor type or
application.
• Excessive motion.
• Low perfusion.
• Interference from line
frequency induced noise.
• Misaligned sensor
• Inappropriate sensor or sensor
size.
• Excessive ambient or strobing
light.
• Excessive motion.
• Verify Sensor type and
size and re-apply sensor.
See Directions for Use for
Sensor.
• Check and see if blood
flow to the site is
restricted.
• Check the placement of
the sensor. Re-apply
sensor or move to a
different site.
• Replace Sensor.
• Minimize or eliminate
motion at the monitoring
site.
• Set to Maximum
Sensitivity. See
Sensitivity Modes
Overview on page 44.
• Verify/set 50/60hz menu
setting. See Operator's
Manual for Root.
• Verify Sensor type and
size and re-apply sensor.
See Directions for Use for
Sensor.
• Check and see if blood
flow to the site is
restricted.
• Check the placement of
the sensor. Re-apply
sensor or move to a
different site.
• Minimize or eliminate
motion at the monitoring
site.
• Shield the sensor from
excessive or strobing
light.
www.masimo.com 71 Masimo
Page 74

Radius-7 Chapter 6: Troubleshooting
Symptom Potential Causes Next Steps
SpCO reading
displays as dashes.
SpHb reading is dim
and "Low SpHb SIQ"
message displays.
• SpCO parameter may have not
stabilized.
• The SpHb reading is outside of
the accuracy range. See
Chapter 7: Specifications on
page 75.
• Improper sensor type or
application.
• Excessive motion.
• Low perfusion.
• Allow time for parameter
reading to stabilize.
• Verify Sensor type and
size and re-apply sensor.
See Directions for Use for
Sensor.
• Check and see if blood
flow to the site is
restricted.
• Check the placement of
the sensor. Re-apply
sensor or move to a
different site.
• Replace Sensor.
• Submit blood sample for
laboratory CO-Oximetry
test for comparison.
• Check patient conditions
indicated to affect SpCO
accuracy.
• Verify Sensor type and
size and re-apply sensor.
See Directions for Use for
Sensor.
• Check and see if blood
flow to the site is
restricted.
• Check the placement of
the sensor. Re-apply
sensor or move to a
different site.
• Replace Sensor.
• Minimize or eliminate
motion at the monitoring
site.
• Submit blood sample for
laboratory CO-Oximetry
test for comparison.
• Check patient conditions
indicated to affect SpHb
accuracy.
www.masimo.com 72 Masimo
Page 75

Radius-7 Chapter 6: Troubleshooting
Troubleshooting Radius-7
Symptom Potential Cause Next Step
Device turns on
but Display Panel
is blank.
Touchpad does
not respond to
gestures.
Speaker makes
no sound when
device is not
connected to
Root.
Unable to pair
with Root
Intermittent
Connection with
Root
The viewing contrast
is not correct
Internal failure
Internal failure
• Bluetooth not
enabled on Root
• Internal failure
• Presence
Monitoring is
enabled on Root
• Environmental
interference
• Adjust the brightness setting. See
Navigating Radius-7 Main Menu on page
42.
• If the condition persists, issue requires
service. See Contacting Masimo on page
98.
• Requires service. See Contacting Masimo
on page 98.
• Requires service. See Contacting Masimo
on page 98.
1. Check if Bluetooth is enabled on Root.
See the Operator's Manual for Root.
2. Check if Bluetooth is enabled on
Radius-7 by accessing the About
panel on the Main Menu. See
Connecting Radius-7 to Root via
Bluetooth on page 36.
3. Verify the Mac address on Radius-7
matches the one on Root. The Mac
address on Radius-7 can be found by
accessing the About panel on the
Main Menu of Radius-7. For
information on accessing the Mac
address listed on Root refer to
Operator's Manual for Root.
4. Re-dock the Battery Module on Root
to pair the device with Root.
5. Call Service.
1. Check to see if Presence Monitoring
feature is enabled. Refer to the
Operator's Manual for Root.
2. Ensure Presence Monitoring feature is
disabled when using Radius-7 with
Root.
3. Check for sources of potential
environmental interference. See
Compliance Warnings and Cautions
on page 17.
www.masimo.com 73 Masimo
Page 76

Radius-7 Chapter 6: Troubleshooting
Radius-7 Error Codes
The following section lists possible Radius-7 error codes and potential causes. Contact
Masimo Service if any of these error codes appear. See Contacting Masimo on page 98.
Code Name
1 Instrument Module (IB) Crystal fault
2 Instrument Module (IB) Watchdog fault
3 Instrument Module (IB) RAM fault
4 Instrument Module (IB) ROM fault
5 Instrument Module (IB) Processor fault
6 Instrument Module (IB) Temperature out of range
7 Voltage out of range
8 MX Technology Board fault
11 Bluetooth fault
51 Battery Module (BB) Crystal fault
52 Battery Module (BB) Watchdog fault
53 Battery Module (BB) RAM fault
54 Battery Module (BB) ROM fault
55 Battery Module (BB) Processor fault
56 Battery Module (BB) Temperature out of range
57 Voltage out of range
58 Battery Gauge fault
59 Battery Charger fault
www.masimo.com 74 Masimo
Page 77

Chapter 7: Specifications
Measurement Range
Measurement Display Range
SpO2 (Oxygen Saturation) 0% to 100%
PR (Pulse Rate) 0 bpm to 240 bpm
Pi (Perfusion Index) 0.00 to 20
PVi (Pleth Variability Index) 0 to 100
RRa (Respiration Rate) 0 bpm to 70 bpm
SpHb (Hemoglobin) 0.0 g/dL to 25.0 g/dL
0 g/L to 250 g/L
(0.0 mmol/L to 15.5 mmol/L)
SpCO (Carboxyhemoglobin) 0% to 99%
SpMet (Methemoglobin) 0.0% to 99.9%
SpOC (Oxygen Content) 0 ml of O2/dL to 35 ml of O2/dL of blood
Accuracy (ARMS*)
Oxygen Saturation (SpO2) [1]
No Motion [1]
(SpO2 from 60% to 80%)
No Motion [2]
(SpO2 from 70% to 100%)
www.masimo.com 75 Masimo
Adults, Pediatrics 3%
Adults, Pediatrics 2%
Page 78

Radius-7 Chapter 7: Specifications
Motion [3]
from 70% to 100%)
(SpO
2
Low perfusion [4]
from 70% to 100%)
(SpO
2
Adults, Pediatrics 3%
Adults, Pediatrics 2%
Pulse Rate (PR)
Range 25 to 240 bpm
No motion Adults, Pediatrics 3 bpm
Motion [5] Adults, Pediatrics 5 bpm
Low Perfusion Adults, Pediatrics 3 bpm
Carboxyhemoglobin Level (SpCO) [1]
Range of 1% to 40% Adults, Pediatrics 3%
Methemoglobin Level (SpMet) [1]
Range 1% to 15% Adults, Pediatrics 1%
Total Hemoglobin SpHb [6]
Range of 8 g/dL to 17 g/dL Adults, Pediatrics 1 g/dL
Respiratory Rate (RRa) [7]
Range of 4 to 70 bpm Adults, Pediatrics 1 bpm
* A
accuracy is a statistical calculation of the difference between device measurements and
RMS
reference measurements. Approximately two-thirds of the device measurements fell within
+/- A
of the reference measurements in a controlled study.
RMS
www.masimo.com 76 Masimo
Page 79

Radius-7 Chapter 7: Specifications
ARMS Performance Specifications
The tables below provides A
Masimo rainbow SET Technology, which is included in the Radius-7, with Masimo rainbow
(Accuracy Root Mean Square) values measured using the
rms
sensors in clinical studies under no motion conditions. This SpO2 data is representative of
data obtained from Masimo rainbow SET Technology and compatible Masimo sensors.
The below Bland-Altman plots represent the correlation of the (SpO
SaO
) under no motion with an upper 95% and lower 95% limits of agreement.
2
+ SaO2)/2 versus (SpO2 -
2
Measurement Arms Values for rainbow Reuse (DCI) Sensors
SpO2 Accuracy Range (%) Arms (%)
60-70 2.00
70-80 1.61
60-80 1.83
Figure 1: rainbow Reuse Sensors (Arms 60-80%)
www.masimo.com 77 Masimo
Page 80

Radius-7 Chapter 7: Specifications
Measurement Arms Values for rainbow Reuse (DCI) Sensors
SpO2 Accuracy Range (%) Arms (%)
70-80 1.88
80-90 1.72
90-100 1.21
70-100 1.63
Figure 2: rainbow Reuse Sensors (Arms 70-100%)
www.masimo.com 78 Masimo
Page 81

Radius-7 Chapter 7: Specifications
Measurement Arms Values for rainbow Adhesive (R1 Series) Sensors
SpO2 Accuracy Range (%) Arms (%)
60-70 3.42
70-80 2.49
60-80 2.99
Figure 3: rainbow R1 Series (Arms 60-80%)
www.masimo.com 79 Masimo
Page 82

Radius-7 Chapter 7: Specifications
Measurement Arms Values for rainbow Adhesive (R1 Series) Sensors
SpO2 Accuracy Range (%) Arms (%)
70-80 2.47
80-90 1.80
90-100 1.57
70-100 1.98
Figure 4: rainbow R1 Series (Arms 70-100%)
Resolution
Parameter Resolution
SpO2 1%
PR 1 bpm
RRa 1 bpm
SpHb 0.1 g/dL
www.masimo.com 80 Masimo
Page 83

Radius-7 Chapter 7: Specifications
Parameter Resolution
1 g/L
0.1 mmol/L
SpCO 1%
SpMet 0.1%
SpOC 1.0 ml/dl
Electrical
Radius-7 Battery Module
Type Lithium ion
Run Time ≥ 12 hours (continuous Masimo SET monitoring, Display off, Bluetooth on,
Wi-Fi off)
Charging
≤ 6 hours
Time
Note: For information on Root Battery see Specifications in the Operator's Manual for Root
Environmental
Radius-7 Environmental Conditions
Operating Temperature 41°F to 95°F
Storage Temperature -40°F to 158°F
Operating Humidity 10% to 95%, non-condensing
Storage Humidity 10% to 95%, non-condensing
Atmospheric Pressure 540 mbar to 1060 mbar
www.masimo.com 81 Masimo
(5°C to 35°C)
(-40°C to 70°C)
Page 84

Radius-7 Chapter 7: Specifications
For Environmental Specifications for Root with Battery Charging Adapter see Operator's
Manual for Root.
Physical Characteristics
Item Description
Dimensions
4.7” x 2.5” x 1.1”
(119.5 mm x 63.2 mm x 27.2 mm)
Weight
Battery Module 0.21 lbs. (93g)
Instrument Module 0.15 lbs. (67g)
Alarms
Alarm
Priority
High Priority
Medium
Priority
Alarm Characteristic
Alarm Volume*
Alarm Status
Color
Flashing red 571 Hz tone, 10-pulse burst, pulse spacing: 0.25s, 0.25s,
Flashing yellow 550 Hz tone, 3-pulse burst, pulse spacing: 0.375s, 0.375s,
Description
High Priority: 75 dB (min)
Medium Priority: 70 dB (min)
Audio Alarm Description
0.50s, 0.25s, repeat time:10s
repeat time: 7s
Sensitivity NORM, MAX, APOD [8]
* When volume is set to the highest level.
www.masimo.com 82 Masimo
Page 85

Radius-7 Chapter 7: Specifications
Display Indicators
Item Description
Data Update Rate 1 second
Type OLED
Pixels 160 X 128
Dot Pitch 0.073 (W) mm X 0.219 (H) mm
EMC Compliance
EMC Compliance
IEC 60601-1-2:2007, Class B
Safety Standards Compliance
Safety Standards Compliance
ANSI/AAMI ES 60601-1:2005
CAN/CSA C22.2 No. 60601-1:2008
IEC 60601-1:2005
EN 60601-1:2006
ANSI/AAMI/IEC 60601-1-8:2006
IEC 60601-2-49:2011
EN/ISO 80601-2-61:2011
www.masimo.com 83 Masimo
Page 86

Radius-7 Chapter 7: Specifications
Equipment Classification per IEC 60601-1
Type of Protection Internally powered (battery powered)
Degree of Protection against
Defibrillation Proof Type BF-Applied Part
Electrical Shock
Protection against harm from
Water and Particulate Matter
IP24 (Protection from solid foreign objects ≥12.5 mm
diameter and against ingress from splashing water)
Mode of Operation Continuous
Environment Not suitable for use in the presence of flammable
anesthetics
Wireless Specifications
Communication (Wi-Fi)
Type WLAN Radio: IEEE 802.11 a/b/g
Frequency
Max Peak Output Power WLAN 17 dBm
Classification of Output Power Rating Conducted
Output Power Type Fixed at the Factory
802.11a: 5180-5240 MHz, 5745-5825 MHz
802.11b/g: 2412-2462 MHz
Modulation Types OFDM, BPSK, CCK
Modulation Signals Analog and Digital
802.11a - 6, 9, 12, 18, 24, 36, 48, 54 Mbps.
Available Data Rates
802.11b - 1, 2, 5.5, 11 Mbps.
802.11g - 6, 9, 12, 18, 24, 36, 48, 54 Mbps.
www.masimo.com 84 Masimo
Page 87
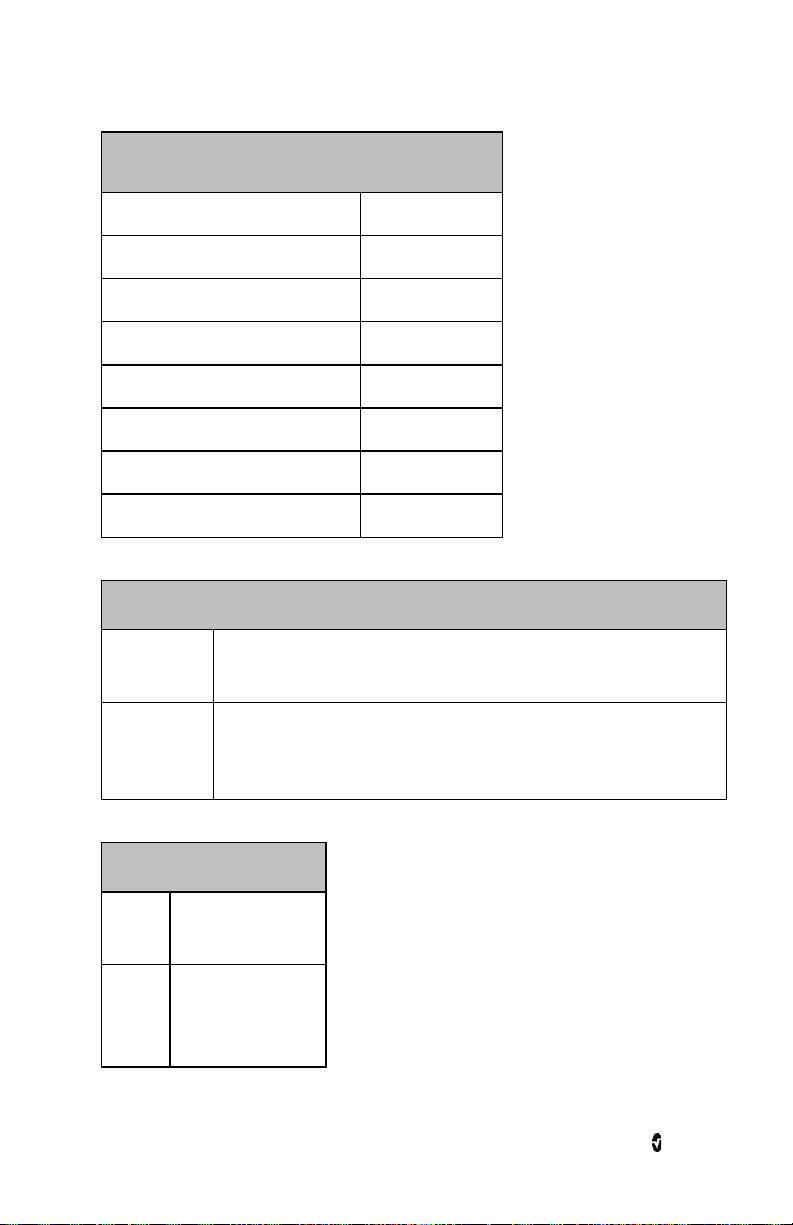
Radius-7 Chapter 7: Specifications
Communication (Bluetooth)
Type Bluetooth
Frequency 2402-2480 MHz
Max Peak Output Power Bluetooth 1.3 dBm
Classification of Output Power Rating Conducted
Output Power Type Fixed at the Factory
Modulation Types DH5
Modulation Signals Analog and Digital
Available Data Rates Bluetooth 1 Mbps
Security and Authentication
Encryption 64/128-bit WEP, Dynamic WEP, WPA-TKIP, WPA2-AES
Authentication
Radio Compliance
USA FCC ID: VKF-MWM1
Canada* IC: 7362A-MWM1
www.masimo.com 85 Masimo
Open System, Shared Key, Pre-Shared Key (PSK), 802.1X: LEAP, PEAP< TTLS,
TLS, EAP-FAST
Model: Radius-7
IC Model: MWM1
RSS-247
Page 88
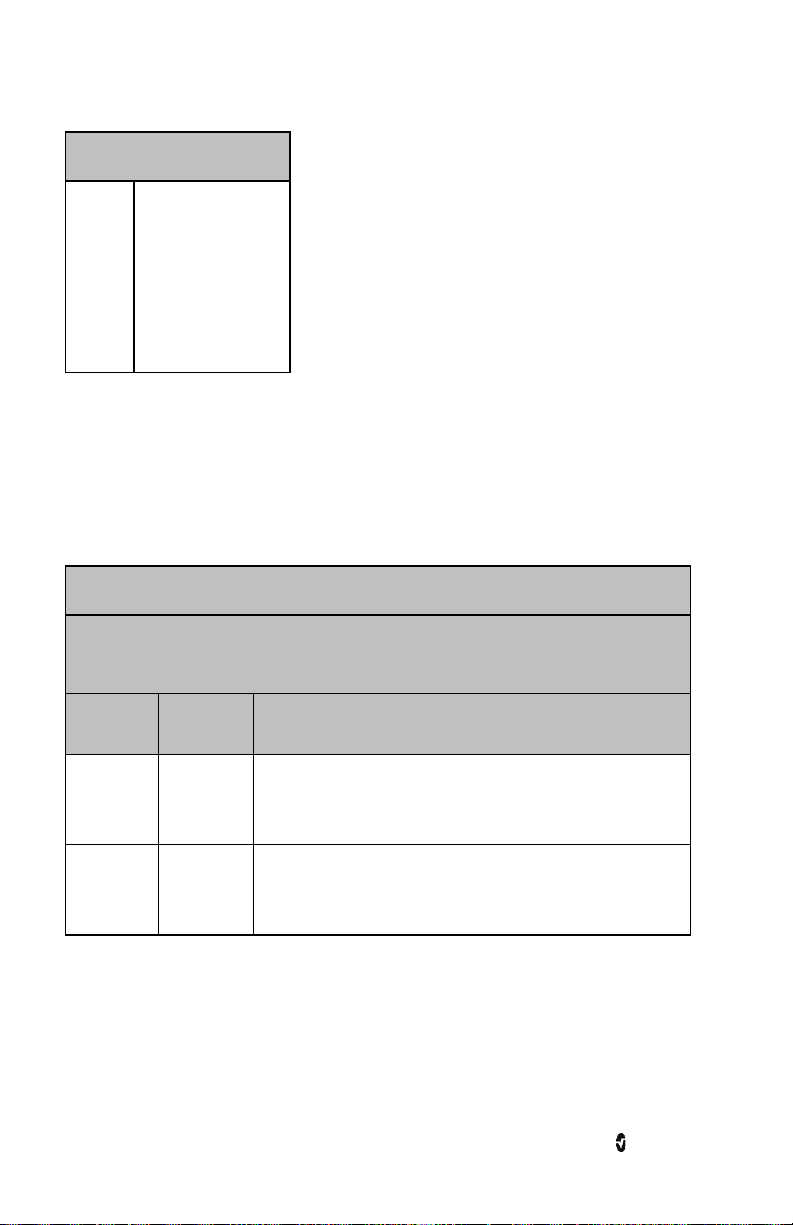
Radius-7 Chapter 7: Specifications
Radio Compliance
Europe EN 301 489-17:
EN 300 328 V2.2.0
EN 301 489-1 V2.1.1
EN 301 489-17 V3.1.1
EN 301 893 V2.0.7
R & TTE Directive
* The Per RSS-Gen, Section 8.4 This device complies with Industry Canada license-exempt
RSS standard(s). Operation is subject to the following two conditions: (1) this device may not
cause interference, and (2) this device must accept any interference, including interference
that may cause undesired operation of the device.
Guidance and Manufacturer's Declaration- Electromagnetic Emissions
Guidance and Manufacturer's Declarations - Electromagnetic Emissions
The ME Equipment is intended for use in the electromagnetic environment specified below.
The customer or the user of the ME Equipment should assure that it is used in such an
environment.
Emission
Test
RF
Emissions
CISPR 11
RF
Emissions
Compliance Electromagnetic Environment - Guidance
The ME Equipment must emit electromagnetic energy in order to
Group 2
perform its intended function. Nearby electronic equipment may
be affected.
Suitable for use in all establishments, including domestic
Class B
environments.
CISPR 11
www.masimo.com 86 Masimo
Page 89

Radius-7 Chapter 7: Specifications
recommended separation distance in meters
Guidance and Manufacturer's Declaration- Electromagnetic Immunity
Guidance and Manufacturer's Declaration - Electromagnetic Immunity
The ME Equipment is intended for use in the electromagnetic environment specified below.
The customer or the user of the ME Equipment should assure that it is used in such an
environment.
Immunity Test IEC
Electrostatic
discharge (ESD)
IEC 61000-4-2
Power frequency
(50 / 60 Hz)
magnetic field.
IEC 61000-4-8
Radiated RF
IEC 61000-4-3
60601
Test
Level
+6 kV
contact
+8 kV air
3 A/m 3 A/m Power frequency magnetic fields should be at
3 V/m
80 MHz
to 2.5
GHz
Compliance
Level
+6 kV
contact
+8 kV air
3 V/m
Electromagnetic Environment - Guidance
Floors should be wood, concrete or ceramic
tile. If floors are covered with synthetic
material, the relative humidity should be at
least 30%.
levels characteristic of typical location in a
typical hospital environment.
Portable and mobile RF communications
equipment should be used no closer to any
part of the ME Equipment, including cables,
than the recommended separation distance
calculated from the equation applicable to
the frequency of the transmitter.
Recommended separation distance
80 MHz to 800 MHz
www.masimo.com 87 Masimo
800 MHz to 2,5 GHz
where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
Page 90

Radius-7 Chapter 7: Specifications
(m).
Guidance and Manufacturer's Declaration - Electromagnetic Immunity
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey,
should be less than the compliance level in
each frequency range
b
.
Interference may occur in the vicinity of
equipment marked with the following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the ME Equipment is
used exceeds the applicable RF compliance level above, the ME Equipment should be
observed to verify normal operation. If abnormal performance is observed, additional
measures may be necessary, such as re-orienting or relocating the ME Equipment.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1]
V/m.
www.masimo.com 88 Masimo
Page 91

Radius-7 Chapter 7: Specifications
Recommended Separation Distances
Recommended Separation Distance Between Portable and Mobile RF Communication
Equipment and the ME Equipment
The ME Equipment is intended for use in an electromagnetic environment in which radiated
RF disturbances are controlled. The customer or the user of the ME Equipment can help
prevent electromagnetic interference by maintaining a minimum distance between portable
and mobile RF communications equipment (transmitters) and the ME Equipment as
recommended below, according to the maximum output power of the communication
equipment.
Rated maximum output power of
transmitter (W)
Separation Distance According to Frequency of
Transmitter (m)
150 K Hz to 80
MHz
d = 1.17*Sqrt (P)
80 MHz to 800
MHz
d = 1.17*Sqrt (P)
800 MHz to
2.5GHz
d = 2.33*Sqrt (P)
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.17 1.17 2.33
10 3.7 3.7 7.37
100 11.7 11.7 23.3
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter
in watts (W) according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
www.masimo.com 89 Masimo
Page 92

Radius-7 Chapter 7: Specifications
Symbols
The following symbols may appear on the product or product labeling:
Symbol Description Symbol Description
Follow instructions for use
Mark of conformity to
European medical device
directive 93/42/EEC
Non-Sterile
Defibrillation-proof. Type BF
applied part
Authorized representative in
the European community
Caution: Federal (USA) law
restricts this device to sale
by or on the order of a
physician
Non-ionizing
electromagnetic radiation
Warning, electricity
Consult instructions for use
UL LLC certification
Protection from ingress and
particulates and water spray
from any direction
Separate collection for
electrical and electronic
equipment (WEEE)
Recyclable
Federal Communications
Commission (FCC) Licensing
Identifies unit has been
registered as a radio device
Industry Canada Identification
Electrostatic
No parameter alarms
Biohazardous Waste
Not for continuous monitoring
(No alarm for SpO
)
2
www.masimo.com 90 Masimo
Page 93

Radius-7 Chapter 7: Specifications
Symbol Description Symbol Description
Caution
Manufacturer
Date of manufacture
YYYY-MM-DD
Storage temperature range
Keep dry
Storage humidity limitation
Atmospheric pressure
limitation
Nurse Call Interface
Product contains no PVC
(polyvinyl chloride) material
Not made with natural rubber
latex
Catalog number (model
number)
Masimo reference number
Serial number
Fragile, handle with care
Do not use if package is
damaged
Equipotential Ground Terminal
AC current
Fuse
Stand-By
RS-232 Interface
www.masimo.com 91 Masimo
SatShare Interface
Wireless Symbol level
Wireless features can be used in
member states with the
restriction of indoor use in
France-Class 2 wireless device
Iris Connection
Page 94

Radius-7 Chapter 7: Specifications
Symbol Description Symbol Description
Analog Out Interface
Greater than
Less than
Ethernet
USB port
China Restriction of Hazardous
Substances
The names and content of the
toxic and hazardous substances
--- ---
or elements shall be provided
in the product instruction
manual
Instructions/Directions for Use/Manuals are available in electronic format
@http://www.Masimo.com/TechDocs
Note: eIFU is not available for CE mark countries.
Citations
[1] SpO2, SpCO, and SpMet accuracy was determined by testing on healthy adult male and
female volunteers in the range 60% to 100% SpO
against a laboratory CO-Oximeter. SpO
NICU patients ranging in age from 7 days to 135 days old and weighing between 0.5 kg and
and SpMet accuracy was determined on 16 neonatal
2
4.25 kg. Seventy-nine data samples were collected over a range of 70% to 100% SaO2 and 0.5%
to 2.5% HbMet with a resultant accuracy of 2.9% SpO
testing specifications.
[2] The Masimo rainbow SET technology with Masimo sensors has been validated for no motion
accuracy in human blood studies on healthy adult male and female volunteers with light to
dark skin pigmentation in induced hypoxia studies in the range of 70%-100% SpO
laboratory CO-Oximeter and ECG monitor. This variation equals ±1 standard deviation which
encompasses 68% of the population weight.
[3] The Masimo rainbow SET technology with Masimo sensors has been validated for motion
accuracy in human blood studies on healthy adult male and female volunteers with light to
dark skin pigmentation in induced hypoxia studies while performing rubbing and touching
motions, at 2 to 4 Hz at an amplitude of 1 to 2 cm and a non-repetitive motion between 1 to 5
Hz at an amplitude of 2 to 3 cm in induced hypoxia studies in the range of 70%-100% SpO2
against a laboratory CO-Oximeter and ECG monitor. This variation equals ±1 standard
deviation. Plus or minus one standard deviation encompasses 68% of the population.
, 0% to 40% SpCO, and 0% to 15% SpMet
2
and 0.9% SpMet. Contact Masimo for
2
against a
2
www.masimo.com 92 Masimo
Page 95

Radius-7 Chapter 7: Specifications
[4] The Radius-7 has been validated for low perfusion accuracy in bench-top testing against a
Biotek Index 2TM* simulator and Masimo's simulator with signal strengths of greater than
0.02% and transmission of greater than 5% for saturations ranging from 70%-100%. This
variation equals ±1 standard deviation. Plus or minus one standard deviation encompasses
68% of the population.
[5] Masimo rainbow SET technology with Masimo sensors has been validated for pulse rate
accuracy for the range of 25-240 bpm in bench top testing against a Biotek Index 2 simulator.
This variation equals ±1 standard deviation which encompasses 68% of the population.
[6] SpHb accuracy has been validated on healthy adult male and female volunteers and on
surgical patients with light to dark skin pigmentation in the range of 8 g/dL to 17 g/dL SpHb
against a laboratory CO-Oximeter. The variation equals ±1 standard deviation which
encompasses 68% of the population. The SpHb accuracy has not been validated with motion or
low perfusion.
[7] Respiration rate accuracy for the Masimo Acoustic Respiration Sensor and device has been
validated for the range of 4 to 70 breaths per minute in bench top testing. Clinical validation
for up to 30 breaths per minute was also performed with the Masimo Acoustic Respiration
Sensor and Instrument.
[8] Maximum sensitivity mode fixes perfusion limit to 0.02%.
*Registered trademark of Fluke Biomedical Corporation, Everett, Washington.
www.masimo.com 93 Masimo
Page 96

Page 97

Chapter 8: Service and Maintenance
Cleaning
The Radius-7 is a reusable device. The device is supplied and used non-sterile.
The Radius-7 should be cleaned before and after it has been applied to a patient and/or in
accordance with local and governmental regulations to minimize the risk of
cross-contamination.
The Battery Charging Adapter should also be cleaned periodically or according to local and
governmental regulations to minimize the risk of cross-contamination.
CAUTION: Check the enclosure for possible cracks or opening before cleaning.
CAUTION: Do not allow liquids to enter the interior of the device.
The outer surfaces can be cleaned either with a soft cloth dampened with a mild detergent
and warm water solution or they can be wiped down with the following cleaning solutions:
• Cidex Plus (3.4% glutaraldehyde)
• 10% bleach solution
• 70% isopropyl alcohol solution
Using the recommended cleaning solutions on the display panel will not affect the
performance of the Radius-7.
WARNING: Do not attempt to clean or re-use the Armband on multiple patients.
WARNING: Discontinue and dispose of Armband if it appears to be stained or becomes
excessively moist to minimize risk of skin irritation.
Battery Operation and Maintenance
The Radius-7 includes a Battery Module containing a lithium ion rechargeable battery.
Before using the Radius-7, the Battery Module should be fully charged. See Charging the
Radius-7 Battery Module on page 36.
The Battery Module requires approximately 6 hours for charging.
Memory effects of the battery may shorten run-time. When Battery Module run time is
significantly reduced, it is advisable to completely discharge and fully recharge the Battery
Module.
Note: Always store the Battery Module on the Root. Do not place it on a conductive surface
where the connection pins may be shorted.
www.masimo.com 95 Masimo
Page 98

Radius-7 Chapter 8: Service and Maintenance
Safety Checks
Under normal operation, no internal adjustment or recalibration is required. Safety tests and
internal adjustments should be done by qualified personnel only. Safety checks should be
performed by trained personnel at regular intervals or in accordance with local and
governmental regulations.
Before conducting Safety Checks examine the device. Look for cracks or possible openings in
the enclosure. If the device appears or is suspected to be damaged, return for Servicing.
To conduct Safety Checks follow the procedure outlined in this chapter. If Radius-7 fails any of
the described tests, discontinue its use and refer to the Troubleshooting section.
Before performing the following tests, do the following:
• Disconnect any sensors or patient cables.
• Disconnect the Battery Module from the Instrument Module. See Chapter 3:
Setting Up on page 35.
• Ensure that the Battery Module is charged.
Speaker, Display and Touchpad Function Test
To Conduct a Speaker, Display and Touchpad Function Test
1. Snap the Battery Module onto the front side of the Instrument Module.
2. Upon connection, verify the Radius-7 emits a tone and the Masimo logo is
displayed on the screen.
3. Follow instructions for using the Touchpad. See Using the Touchpad on page 41.
Alarm Limit Test
To Conduct an Alarm Limit Test
1. Pair the Radius-7 device to Root. See Connecting Radius-7 to Root via Bluetooth
on page 36.
2. Use Root to change the High SpO
the currently selected value. See SpO
3. Verify that the newly set parameter is shown on the Display screen.
4. Return the parameter to its original setting.
5. Repeat steps 1 to 3 for all active parameters.
6. Reset the alarm limits again to the original settings.
www.masimo.com 96 Masimo
Alarm parameter to a value two points below
2
Settings on page 46.
2
Page 99

Radius-7 Chapter 8: Service and Maintenance
Battery Test
To Conduct a Battery test
1. Dock the Battery Module on the Battery Charging Adapter on Root. Make sure the
connection pins of the Battery Module are in contact with the adapter.
2. Verify that the Battery Module is charging. A battery icon will be displayed on the
Radius-7 screen to indicate that the Battery is charging. See Battery Operation
and Maintenance on page 95.
3. Un-dock the Battery Module from Root and connect to the Instrument Module.
4. Upon connection, verify the device emits a tone and the device turns on.
Repair Policy
Masimo or an authorized service department must perform warranty repair and service. Do
not use malfunctioning equipment. Have the device repaired.
Clean contaminated and/or dirty equipment before returning, following the cleaning
procedure described in Cleaning. Make sure the equipment is fully dry before packing.
To return the device for service, refer to Return Procedure on page 97.
Return Procedure
Clean contaminated/dirty equipment before returning, following instructions in Cleaning.
Make sure the equipment is fully dry before packing. Call Masimo at 800-326-4890 and ask
for Technical Support. Ask for an RMA number. Package the equipment securely, in the
original shipping container if possible, and enclose or include the following information and
items:
• A letter describing in detail any difficulties experienced with the Radius-7. Include
the RMA number in the letter.
• Warranty information, a copy of the invoice or other applicable documentation
must be included.
• Purchase order number to cover repair if the Radius-7 is not under warranty, or for
tracking purposes if it is.
• Ship-to and bill-to information.
• Person (name, telephone/Telex/fax number, and country) to contact for any
questions about the repairs.
• A certificate stating the Radius-7 has been decontaminated for bloodborne
pathogens.
• Return the Radius-7 to the shipping address listed in Contacting Masimo on page
98 below.
www.masimo.com 97 Masimo
Page 100

Radius-7 Chapter 8: Service and Maintenance
Contacting Masimo
Masimo Corporation
52 Discovery
Irvine, California 92618
Tel:+1 949 297 7000
Fax:+1 949 297 7001
Limited Warranty
Masimo warrants to the original end-user purchaser the Masimo-branded hardware product
(Radius-7® Wearable Pulse CO-Oximeter®) and any software media contained in the original
packaging against defects in material and workmanship when used in accordance with
Masimo’s user manuals, technical specifications, and other Masimo published guidelines for a
period of 12 months and any batteries for six (6) months from the original date the Product
was obtained by the end-user purchaser.
Masimo’s sole obligation under this warranty is the repair or replacement, at its option, of any
defective Product or software media that is covered under the warranty.
To request a replacement under warranty, Purchaser must contact Masimo and obtain a
returned goods authorization number so that Masimo can track the Product. If Masimo
determines that a Product must be replaced under warranty, it will be replaced and the cost of
shipment covered. All other shipping costs must be paid by purchaser.
Exclusions
The warranty does not apply to any non-Masimo branded product or any software, even if
packaged with the Product, or any Product that was: (a) not new or in its original packaging
when supplied to purchaser; (b) modified without Masimo’s written permission; (c) supplies,
devices, or systems external to the Product; (d) disassembled, reassembled, or repaired by
anyone other than a person authorized by Masimo; (e) used with other products, like new
sensors, reprocessed sensors, or other accessories, not intended by Masimo to be used with
the Product; (f) not used or maintained as provided in the operator’s manual or as otherwise
provided in its labeling; (g) reprocessed, reconditioned, or recycled; and (h) damaged by
accident, abuse, misuse, liquid contact, fire, earthquake or other external cause.
No warranty applies to any Product provided to Purchaser for which Masimo, or its authorized
distributor, is not paid; and these Products are provided AS-IS without warranty.
Limitation of Warranty
Except as otherwise required by law or altered by the purchase agreement, the above warranty
is the exclusive warranty that applies to the Product and software media, and Masimo does
not make any other promises, conditions, or warranties regarding the Product. No other
warranty applies, express or implied, including without limitation, any implied warranty of
merchantability, fitness for a particular purpose, satisfactory quality, or as to the use of
reasonable skill and care. See the licensing terms for the terms and conditions that apply to
and Software accompanying the Product. Additionally, Masimo will not be liable for any
incidental, indirect, special, or consequential loss, damage, or expense arising from the use or
www.masimo.com 98 Masimo
 Loading...
Loading...