Page 1

Operator's Manual
Rad-97™ Pulse CO-Oximeter
®
Page 2

Page 3

These operating instructions provide the necessary information for proper operation of all
models of the Rad-97. There may be information provided in this manual that is not relevant
for your system. General knowledge of pulse oximetry and an understanding of the features
and functions of Rad-97 are prerequisites for its proper use. Do not operate Rad-97 without
completely reading and understanding these instructions.
Note: Cleared Use Only: The device and related accessories are cleared by the Food and Drug
Administration (FDA) and are CE Marked for noninvasive patient monitoring and may not be
used for any processes, procedures, experiments, or any other use for which the device is not
intended or cleared by the applicable regulatory authorities, or in any manner inconsistent
with the directions for use or labeling.
Notice: Purchase or possession of this device does not carry any express or implied license to
use with replacement parts which would, alone or in combination with this device, fall within
the scope of one of the relating patents.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. See
instructions for use for full prescribing information, including indications, contraindications,
warnings and precautions.
For professional use. See instructions for use for full prescribing information, including
indications, contraindications, warnings, and precautions.
Wireless Radio
Contains: FCC ID: VKF-MWM1 | Model: Rad-97 | IC: 7362A- MWM1 | IC Model: MWM1
Masimo Corporation
40 Parker
Irvine, CA 92618, USA
Tel.: 949-297-7000
Fax.: 949-297-7001
www.masimo.com
EU authorized representative for Masimo Corporation:
MDSS GmbH
Schiffgraben 41
D-30175 Hannover, Germany
MEDICAL ELECTRICAL EQUIPMENT
WITH RESPECT TO ELECTRIC SHOCK, FIRE AND MECHANICAL HAZARDS ONLY
IN ACCORDANCE WITH
ANSI/AAMI ES 60601-1:2005,CAN/CSA C22.2 No. 60601-1:2008, and
3149433
applicable Particular (EN/ISO 80601-2-61:2011) and related Collateral (IEC
60601-1-8:2006) Standards for which the product has been found to comply by
Intertek.
Patents: www.masimo.com/patents.htm
®, Adaptive Probe Off Detection®, APOD®, Discrete Saturation Transform®, DST®,
FastSat®, FST®, Masimo®, Pulse CO-Oximeter®, PVi®, rainbow®, rainbow Resposable®,
RRa®, SET®, Signal Extraction Technology®, Signal IQ®, SpCO®, SpHb®, and SpMet® are
federally registered trademarks of Masimo Corporation.
Rad-97™, rainbow Acoustic Monitoring™, rainbow SET™, RAM™, Adaptive Threshold Alarm™, In
Vivo Adjustment™, ORi™, X-Cal™, Kite™, SpOC™, and RRp™ are trademarks of Masimo
Corporation. All other trademarks and registered trademarks are property of their respective
www.masimo.com 1 Masimo
Page 4

owners. The use of the trademark Patient SafetyNet is under license from University
HealthSystem Consortium.
All other registered trademarks and trademarks are property of their respective owners.
© 2017 Masimo Corporation
www.masimo.com 2 Masimo
Page 5

Contents
About This Manual-------------------------------------------------------------------------------------------7
Product Description, Features and Indications for Use ----------------------------------------------- 9
Product Description ------------------------------------------------------------------------------------- 9
Indications for Use ------------------------------------------------------------------------------------- 10
Contraindications -------------------------------------------------------------------------------------- 11
Safety Information, Warnings and Cautions ---------------------------------------------------------- 13
Safety Warnings and Cautions ----------------------------------------------------------------------- 13
Performance Warnings and Cautions --------------------------------------------------------------- 15
Cleaning and Service Warnings and Cautions --------------------------------------------------- 20
Compliance Warnings and Cautions ---------------------------------------------------------------- 21
Chapter 1: Technology Overview ------------------------------------------------------------------------ 23
Signal Extraction Technology® (SET®) ------------------------------------------------------------ 23
rainbow Pulse CO-Oximetry Technology ----------------------------------------------------------- 26
rainbow Acoustic Monitoring™ (RAM™) ------------------------------------------------------------ 31
Chapter 2: Description ----------------------------------------------------------------------------------- 35
General System Description ------------------------------------------------------------------------- 35
Features ------------------------------------------------------------------------------------------------- 36
Chapter 3: Setting Up------------------------------------------------------------------------------------ 39
Unpacking and Inspection -------------------------------------------------------------------------- 39
Preparation for Use ----------------------------------------------------------------------------------- 39
Guidelines for Setting Up ---------------------------------------------------------------------------- 39
Powering the Rad-97 ON and OFF ------------------------------------------------------------------ 40
Initial Battery Charging ------------------------------------------------------------------------------- 41
Nurse Call Connection -------------------------------------------------------------------------------- 41
Attach NIBP Cuff --------------------------------------------------------------------------------------- 42
Masimo Kite --------------------------------------------------------------------------------------------- 42
Third-Party Devices ------------------------------------------------------------------------------------ 42
Chapter 4: Operation -------------------------------------------------------------------------------------- 43
Using the Touchscreen and Home Button --------------------------------------------------------- 43
About the Main Screen --------------------------------------------------------------------------------48
About the System Status Light --------------------------------------------------------------------- 56
www.masimo.com 3 Masimo
Page 6

Rad-97 Contents
Accessing Main Menu Options ---------------------------------------------------------------------- 57
Parameter Settings ----------------------------------------------------------------------------------- 60
Noninvasive Blood Pressure (NIBP) Settings ---------------------------------------------------- 80
Sounds --------------------------------------------------------------------------------------------------- 85
Device Settings ----------------------------------------------------------------------------------------- 86
About ----------------------------------------------------------------------------------------------------- 97
Trends ---------------------------------------------------------------------------------------------------- 97
Rad-97 Screenshot Capture -------------------------------------------------------------------------- 99
Patient Admit/Discharge --------------------------------------------------------------------------- 101
Chapter 5: Profiles --------------------------------------------------------------------------------------- 103
Profiles Overview ------------------------------------------------------------------------------------- 103
Changing Profiles ------------------------------------------------------------------------------------ 103
Profiles Settings -------------------------------------------------------------------------------------- 106
Replacing Factory Default Settings for Adult, Pediatric and Neonatal Profiles ------------107
Chapter 6: Noninvasive Blood Pressure (NIBP) ---------------------------------------------------- 109
Operation - NIBP ------------------------------------------------------------------------------------- 109
Patient Category ------------------------------------------------------------------------------------- 109
Patient Conditions ----------------------------------------------------------------------------------- 109
Cuff Selection and Placement --------------------------------------------------------------------- 110
Blood Pressure Measurement ----------------------------------------------------------------------- 112
Chapter 7: Admit to and Discharge from Patient SafetyNet -------------------------------------- 117
Not Admitted ------------------------------------------------------------------------------------------ 117
Admitting a Patient ---------------------------------------------------------------------------------- 118
Discharging a Patient ------------------------------------------------------------------------------- 120
Chapter 8: Third-Party Devices ------------------------------------------------------------------------- 121
Connect Device to Rad-97 --------------------------------------------------------------------------- 121
Chapter 9: Alarms and Messages --------------------------------------------------------------------- 123
Alarm Interface --------------------------------------------------------------------------------------- 123
About Alarms ------------------------------------------------------------------------------------------ 124
Adaptive Threshold Alarm (ATA) Feature -------------------------------------------------------- 127
3D Alarms --------------------------------------------------------------------------------------------- 128
Messages ---------------------------------------------------------------------------------------------- 130
Noninvasive Blood Pressure (NIBP) Messages ------------------------------------------------- 135
www.masimo.com 4 Masimo
Page 7

Rad-97 Contents
Chapter 10: Troubleshooting -------------------------------------------------------------------------- 137
Troubleshooting Measurements ------------------------------------------------------------------- 137
Troubleshooting Rad-97 ---------------------------------------------------------------------------- 140
Chapter 11: Specifications ------------------------------------------------------------------------------ 145
Pulse CO-Oximetry Specifications ----------------------------------------------------------------- 145
Noninvasive Blood Pressure (NIBP) Specifications -------------------------------------------- 150
Electrical ----------------------------------------------------------------------------------------------- 151
Environmental ---------------------------------------------------------------------------------------- 152
Physical Characteristics ----------------------------------------------------------------------------- 152
Alarms -------------------------------------------------------------------------------------------------- 153
Display Indicators ------------------------------------------------------------------------------------ 153
Compliance -------------------------------------------------------------------------------------------- 154
Connectors -------------------------------------------------------------------------------------------- 155
Wireless Specifications------------------------------------------------------------------------------ 155
Guidance and Manufacturer's Declaration-Electromagnetic Emissions -------------------- 158
Guidance and Manufacturer's Declaration-Electromagnetic Immunity -------------------- 158
Recommended Separation Distances ------------------------------------------------------------ 161
Symbols ------------------------------------------------------------------------------------------------ 162
Citations ----------------------------------------------------------------------------------------------- 164
Chapter 12: Service and Maintenance --------------------------------------------------------------- 167
Cleaning ----------------------------------------------------------------------------------------------- 167
Nurse Call Setting Connections ------------------------------------------------------------------- 167
Performance Verification --------------------------------------------------------------------------- 168
Calibration -------------------------------------------------------------------------------------------- 170
Maintenance ------------------------------------------------------------------------------------------- 172
Repair Policy ------------------------------------------------------------------------------------------ 173
Return Procedure ------------------------------------------------------------------------------------- 173
Contacting Masimo ---------------------------------------------------------------------------------- 173
Appendix: Concepts of Alarm Response Delay ------------------------------------------------------- 177
Concepts of Alarm Response Delay ---------------------------------------------------------------- 177
Index ------------------------------------------------------------------------------------------------------- 179
www.masimo.com 5 Masimo
Page 8

Page 9

About This Manual
This manual explains how to set up and use Rad-97™ Pulse CO-Oximeter®. Important safety
information relating to general use of Rad-97 appears in this manual. Read and follow any
warnings, cautions, and notes presented throughout this manual. The following are
explanations of warnings, cautions, and notes.
A warning is given when actions may result in a serious outcome (for example, injury, serious
adverse effect, death) to the patient or user.
WARNING: This is an example of a warning statement.
A caution is given when any special care is to be exercised by the patient or user to avoid
injury to the patient, damage to this device, or damage to other property.
CAUTION: This is an example of a caution statement.
A note is given when additional general information is applicable.
Note: This is an example of a note.
www.masimo.com 7 Masimo
Page 10

Page 11

Product Description, Features and Indications for Use
Product Description
Rad-97™ Pulse CO-Oximeter® is a non-invasive device intended to monitor functional oxygen
saturation of arterial hemoglobin (SpO
Variability Index (PVi) along with optional non-invasive measurements of total hemoglobin
(SpHb), carboxyhemoglobin (SpCO), total oxygen content (SpOC), methemoglobin (SpMet),
Acoustic Respiration Rate (RRa), Oxygen Reserve Index (ORi)*, and Pleth Respiration Rate
(RRp)*.
The following key features are available for Rad-97:
• Masimo SET and rainbow SET technology performance.
• SpO
• Continuous and non-invasive monitoring of carboxyhemoglobin (SpCO),
• Respiration Rate (RR) is measured using:
• Oxygen Reserve Index (ORi)*, an index to measure changes in oxygen states under
• Wireless radio for transfer of parameter data.
• Sleep Study and optional Home operational modes.
• Optional integrated Noninvasive Blood Pressure (NIBP) technology.
• Designed for third party measurement expansion to allow for additional platform
• Ability to display data on a secondary display.
For all prescribing information and instructions for use of the compatible medical devices
connected to Rad-97, see the Operator's Manual or Instructions for Use for the specific
medical device.
* Currently not available in the U.S.A. and territories relying on FDA market clearance.
and pulse rate monitoring in motion and low perfusion environments.
2
methemoglobin (SpMet), and total hemoglobin (SpHb).
• Acoustic (RRa)
• Plethysmographic waveform (RRp)*
hyperoxic conditions.
measurements.
), pulse rate (PR), perfusion index (Pi), and Pleth
2
Regulatory Notice
The following features are NOT AVAILABLE.
Feature NOT AVAILABLE in U.S.A. and territories relying on FDA market clearance
SpO2
www.masimo.com 9 Masimo
Page 12

Rad-97 Product Description, Features and Indications for Use
Feature NOT AVAILABLE in U.S.A. and territories relying on FDA market clearance
PR
Pi
PVi
SpHb
SpCO
SpOC
SpMet
RRa
RRp X
ORi X
ATA X
In-Vivo X
NIBP
Indications for Use
The Masimo Rad-97 and Accessories are indicated for hospitals, hospital-type facilities,
mobile, and home environments.
The Masimo Rad-97 and Accessories can communicate with network systems for
supplemental remote viewing and alarming (e.g., at a central station).
The Masimo Rad-97 and Accessories are indicated for the continuous non-invasive
monitoring of functional oxygen saturation of arterial hemoglobin (SpO
carboxyhemoglobin saturation (SpCO), methemoglobin saturation (SpMet), total hemoglobin
concentration (SpHb), and/or respiratory rate (RRa). The Masimo Rad-97 and Accessories are
indicated for use with adult, pediatric, and neonatal patients during both no motion and
motion conditions, and for patients who are well or poorly perfused. In addition, the Masimo
Rad-97 and Accessories are indicated to provide the continuous non-invasive monitoring
data obtained from the Masimo Rad-97 and Accessories for functional oxygen saturation of
arterial hemoglobin (SpO
those devices.
) and pulse rate (PR) to multi-parameter devices for the display on
2
The Masimo Rad-97 and Accessories are not intended to be used as the sole basis for making
diagnosis or treatment decisions related to suspected carbon monoxide poisoning; it is
www.masimo.com 10 Masimo
), pulse rate (PR),
2
Page 13
Rad-97 Product Description, Features and Indications for Use
intended to be used in conjunction with additional methods of assessing clinical signs and
symptoms.
The optional Nomoline Capnography product family is intended to be connected to other
medical backboard devices for monitoring of breath rate and CO
Capnography product family is intended to be connected to a patient breathing circuit for
. The Nomoline
2
monitoring of inspired/expired gases during anesthesia, recovery and respiratory care. The
environment is the operating suite, intensive care unit and patient room. The intended
patient population is adult, pediatric and infant patients.
The optional non-invasive blood pressure (NIBP) module is indicated for the noninvasive
measurement of arterial blood pressure. The NIBP module is designed to measure blood
pressure for patient population described in the following table:
Patient Population Approximate Age Range
Newborn (neonate) Birth to 1 month of age
Infant 1 month to 2 years of age
Child 2 to 12 years of age
Adolescent 12-21 years of age
Adult 21 years of age and older
Contraindications
The Rad-97 is not intended for use as an apnea monitor.
www.masimo.com 11 Masimo
Page 14

Page 15

Safety Information, Warnings and Cautions
CAUTION: Rad-97 is to be operated by, or under the supervision of, qualified personnel only.
Read the manual, accessories directions for use, all precautionary information, and
specifications before use. Refer to Operator’s Manuals of Patient SafetyNet and Kite for
additional safety information, warnings, and cautions.
Safety Warnings and Cautions
WARNING: Do not use Rad-97 if it appears or is suspected to be damaged. Damage to the
device can result in exposed electrical circuits that may cause patient harm.
WARNING: Do not adjust, repair, open, disassemble, or modify the Rad-97. Damage to the
device may result in degraded performance and/or patient injury.
WARNING: Do not start or operate the Rad-97 unless the setup was verified to be correct.
Improper set-up of this device may result in degraded performance and/or patient injury.
WARNING: Do not place the Rad-97 or accessories in any position that might cause it to fall
on the patient.
WARNING: Only use Masimo authorized devices with Rad-97. Using unauthorized devices
with Rad-97 may result in damage to the device and/or patient injury.
WARNING: All sensors and cables are designed for use with specific devices. Verify the
compatibility of the device, cable, and sensor before use; otherwise degraded performance
and/or patient injury can result.
WARNING: Do not use the Rad-97 in the presence of flammable anesthetics or other
flammable substance in combination with air, oxygen-enriched environments, or nitrous
oxide to avoid risk of explosion.
WARNING: Do not use the Rad-97 during magnetic resonance imaging (MRI) or in an MRI
environment.
WARNING: Rad-97 may be used during defibrillation. However, to reduce the risk of electric
shock, the operator should not touch the Rad-97 during defibrillation.
WARNING: Electrical Shock Hazard: To protect against injury, follow the directions below:
• Avoid placing the device on surfaces with visible liquid spills.
• Do not soak or immerse the device in liquids.
• Do not attempt to sterilize the device.
• Use cleaning solutions only as instructed in this Operator's Manual.
• Do not attempt to clean the Rad-97 while monitoring patient.
WARNING: To ensure safety, avoid placing anything on the device during operation.
WARNING: As with all medical equipment, carefully route patient cables to reduce the
possibility of patient entanglement or strangulation.
CAUTION: Do not place the Rad-97 where the controls can be changed by the patient.
www.masimo.com 13 Masimo
Page 16

Rad-97 Safety Information, Warnings and Cautions
CAUTION: Do not place Rad-97 where the appliance inlet or the AC power plug cannot be
readily disconnected.
CAUTION: Use a grounded outlet for proper equipment grounding. A hospital-grade outlet is
required.
CAUTION: To avoid risk of electrical shock, this equipment must only be connected to a
supply mains with a protective earth connection. Do not under any circumstances remove the
grounding conductor from the power plug.
CAUTION: Only use the AC power cable provided by Masimo. Using a different AC power cable
could cause damage to Rad-97. Check the power cord and plug to ensure that it is intact and
undamaged.
CAUTION: To ensure patient electrical isolation, all external device connections to the Data
Output/Nurse Call connectors must be IEC 60950-1, IEC 60601-1, or UL1069 compliant.
Note: If there is any doubt about the integrity of the protective earth conductor arrangement,
operate the Rad-97 on internal battery power until the AC power supply protective conductor
is fully functional.
Note: Disconnect the device from AC mains by removing the AC power cord connector from
the appliance inlet.
Note: Do not monitor more than a single patient at a time on Rad-97.
Note: Use and store the Rad-97 in accordance with specifications. See the Specifications
section in this manual.
Noninvasive Blood Pressure
WARNING: Only use Rad-97 in Neonatal mode with a neonatal blood pressure cuff to
measure blood pressure on neonates.
WARNING: Neonatal blood pressure measurements must always use a 3 meter hose in order
to avoid overpressure error caused by lack of air volume within the overall pneumatic system.
WARNING: Frequently check the blood pressure monitoring site to ensure adequate
circulation.
WARNING: Do not apply the cuff to a limb that is on the same side of a mastectomy.
WARNING: Do not use or stop blood pressure measurements if the patient appears to be
affected by the pressurization of the cuff due to a physical condition (i.e. pregnant,
pre-eclamptic, etc.)
WARNING: Too frequent blood pressure measurements can cause injury to the patient due to
blood flow interference.
WARNING: Do not attach the cuff to a limb being used for IV infusions or any other
intravascular access, therapy or an arterio-venous (A-V) shunt. The cuff inflation can
temporarily block blood flow, potentially causing harm to the patient.
CAUTION: Applying the blood pressure cuff over a wound can cause further injury.
CAUTION: A compressed or kinked connection hose may cause continuous cuff pressure
resulting in blood flow interference and potentially harmful injury to the patient.
www.masimo.com 14 Masimo
Page 17

Rad-97 Safety Information, Warnings and Cautions
Kite
WARNING: Do not adjust, repair, open, disassemble, or physically modify the Kite host
device. Injury to personnel or equipment damage could occur. Return the Kite host device for
servicing.
Performance Warnings and Cautions
WARNING: Rad-97 should not be used as the sole basis for medical decisions. It must be used
in conjunction with clinical signs and symptoms.
WARNING: The Rad-97 and Accessories are not intended to be used as the sole basis for
making diagnosis or treatment decisions related to suspected carbon monoxide poisoning; it
is intended to be used in conjunction with additional methods of assessing clinical signs and
symptoms.
WARNING: If any measurement seems questionable, first check the patient’s vital signs by
alternate means and then check Rad-97 for proper functioning.
WARNING: Variation in hemoglobin measurements may be profound and may be affected by
sample type, body positioning, as well as other physiological conditions. As with most
hemoglobin data, Rad-97 trend data should be scrutinized in light of a specific patient
condition. Any results exhibiting inconsistency with the patient's clinical status should be
repeated and/or supplemented with additional data.
WARNING: Rad-97 is not an apnea monitor.
WARNING: Rad-97 should not be used as a replacement or substitute for ECG-based
arrhythmia analysis.
WARNING: Rad-97 may be used during defibrillation. This may affect the accuracy or
availability of the parameters and measurements.
WARNING: Rad-97 may be used during electrocautery. This may affect the accuracy or
availability of the parameters and measurements.
WARNING: Avoid placing Rad-97 against a surface that may cause the alarm to be muffled.
This may result in the inability to detect the audible alarms.
WARNING: Rad-97 may not fully charge in a high ambient temperature environment.
WARNING: Properly apply sensors according to sensor's directions for use. Misapplied sensor
or sensors that become partially dislodged may cause no or incorrect readings.
WARNING: Select a well perfused site for monitoring, very low perfusion at the monitored site
may result in no or incorrect readings.
WARNING: Do not use Rad-97 on patients that have been injected with dyes or any
substance containing dyes, the change usual blood pigmentation may cause no or incorrect
readings.
WARNING: Display parameter may not be accurate when a low SIQ message is provided.
Clinicians should consider additional information to supplement values to completely
understand the patient’s condition.
WARNING: If SpO2 values indicate hypoxemia, a laboratory blood sample should be taken to
confirm the patient’s condition.
www.masimo.com 15 Masimo
Page 18

Rad-97 Safety Information, Warnings and Cautions
WARNING: SpO2 is empirically calibrated in healthy adult volunteers with normal levels of
carboxyhemoglobin (COHb) and methemoglobin (MetHb).
WARNING: Optical, pleth-based measurements (e.g. SpO
and ORi) can be affected by the following:
, SpHb, SpOC, SpMet, SpCO, RRp,
2
• Improper sensor application or use of use of incorrect sensor.
• Blood pressure cuff applied to the same arm as the sensor site.
• Intravascular dyes such as indocyanine green or methylene blue.
• Venous congestion.
• Abnormal venous pulsations (e.g. tricuspid value regurgitation, Trendelenburg
position).
• Abnormal pulse rhythms due to physiological conditions or induced through
external factors (e.g. cardiac arrhythmias, intra-aortic balloon, etc.).
• Externally applied coloring and texture such as nail polish, acrylic nails, glitter, etc.
• Moisture, birthmarks, skin discoloration, nail aberration, deformed fingers, or
foreign objects in the light path.
• Elevated levels of bilirubin.
• Physiological conditions that can significantly shift the oxygen disassociation
curve.
• A physiological condition that may effect vasomotor tone or changes in vasomotor
tone.
WARNING: Inaccurate SpO
readings may be caused by:
2
• Elevated levels of COHb and/or MetHb.
• Severe anemia.
• Extremely low arterial perfusion.
• Excessive induced motion.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (Quantitative defects such as Thalassemias).
WARNING: Inaccurate SpHb and SpOC readings may be caused by:
• Low arterial perfusion.
• Motion induced artifact.
• Low arterial oxygen saturation levels.
• Elevated COHb and/or MetHb levels.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (quantitative defects such as Thalassemias).
• Severe anemia.
WARNING: Inaccurate SpCO readings may be caused by:
• Elevated methemoglobin levels in the range of >15%.
www.masimo.com 16 Masimo
Page 19

Rad-97 Safety Information, Warnings and Cautions
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (quantitative defects such as Thalassemias).
• Extremely elevated hemoglobin levels.
• Low arterial perfusion.
• Low arterial oxygen saturation levels including altitude induced hypoxemia.
• Motion induced artifact.
• Severe anemia.
WARNING: SpCO readings may not be provided if there are Low arterial oxygen saturation
levels or elevated methemoglobin levels.
WARNING: Inaccurate SpMet readings may be caused by:
• Elevated carboxyhemoglobin levels in the range of > 3%.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (quantitative defects such as Thalassemias).
• Extremely elevated hemoglobin levels.
• Low arterial perfusion.
• Low arterial oxygen saturation levels including altitude induced hypoxemia.
• Motion induced artifact.
• Physiological conditions that can significantly shift the oxygen disassociation
curve.
• Severe anemia.
WARNING: Inaccurate RRa measurements may be caused by:
• Improper sensor application or use of use of incorrect sensor.
• Abnormal pulse rhythms due to physiological conditions or induced through
external factors (e.g. Cardiac arrhythmias, intra-aortic balloon, etc.).
• Motion artifact.
• Excessive ambient or environmental noise.
WARNING: Inaccurate RRp readings may be caused by:
• Low arterial perfusion.
• Motion induced artifact.
• Severe anemia.
WARNING: Inaccurate ORi readings may be caused by:
• Low arterial perfusion.
• Motion induced artifact.
• Elevated COHb and/or MetHb levels.
• Hemoglobinopathies (qualitative defects including sickle cell) and Hemoglobin
synthesis disorders (Quantitative defects such as Thalassemias).
www.masimo.com 17 Masimo
Page 20

Rad-97 Safety Information, Warnings and Cautions
• Hypotension, severe vasoconstriction, severe anemia, or hypothermia.
WARNING: ORi is not intended as a replacement for SpO2 monitoring, PaO2 monitoring, or as
a sole indicator of the patient condition.
WARNING: Wireless communication of alarms to a secondary monitoring station should not
be relied upon as a primary alarm.
CAUTION: ORi may not indicate additional changes in oxygen states above 200 mmHg of
.
PaO
2
CAUTION: If using Rad-97 during full body irradiation, keep the sensor out of the radiation
field. If the sensor is exposed to the radiation, the reading might be inaccurate or the device
might read zero for the duration of the active irradiation period.
CAUTION: When patients are undergoing photodynamic therapy they may be sensitive to
light sources. Pulse oximetry may be used only under careful clinical supervision for short
time periods to minimize interference with photodynamic therapy.
CAUTION: High ambient light sources such as surgical lights (especially those with a xenon
light source), bilirubin lamps, fluorescent lights, infrared heating lamps, and direct sunlight
can interfere with the performance of the sensor.
CAUTION: To prevent interference from ambient light, ensure that the sensor is properly
applied, and cover the sensor site with opaque material, if required. Failure to take this
precaution in high ambient light conditions may result in inaccurate measurements.
CAUTION: If the Low Perfusion message is frequently displayed, find a better perfused
monitoring site. In the interim, assess the patient and, if indicated, verify oxygenation status
through other means.
CAUTION: To minimize radio interference, other electrical equipment that emits radio
frequency transmissions should not be in close proximity to Rad-97.
CAUTION: Do not place the Rad-97 near electrical equipment that may affect the device,
preventing it from working properly.
CAUTION: Failure to charge Rad-97 promptly after a Low Battery alarm may result in the
device shutting down.
CAUTION: Do not connect to an electrical outlet controlled by a wall switch or dimmer.
CAUTION: In order to establish and maintain Rad-97’s minimum Quality of Service, the
following network specifications should be met before and after installation:
• Wired Network Connection
During Ping Test, passing result if:
a. At least 98% of packets have latency ≤ 30 milliseconds, and
b. No more than 2 % packets loss.
• Wireless Network Connection
During Ping Test, passing result if:
a. At least 98% of packets have latency ≤ 100 milliseconds,
b. No more than 2 % packets loss, and
c. Primary access point signal strength at least -67 dBm.
CAUTION: The wireless quality of services may be influenced by the presence of other devices
that may create radio frequency interference (RFI). Some RFI devices to consider are as
follows: electrocautery equipment, cellular telephones, wireless PC and tablets, pagers, RFID,
www.masimo.com 18 Masimo
Page 21

Rad-97 Safety Information, Warnings and Cautions
MRI electrically powered wheelchair, etc. When used in the presence of potential RFI devices,
consideration should be taken to maximize separation distances and to observe for any
potential signs of interference such as loss of communication or reduced Wi-Fi signal
strength.
CAUTION: To ensure that alarm limits are appropriate for the patient being monitored, check
the limits each time Rad-97 is used.
CAUTION: Replace the cable or sensor when a replace sensor or when a low SIQ message is
consistently displayed while monitoring consecutive patients after completing the low SIQ
troubleshooting steps listed in the troubleshooting section.
Note: Cables and sensors are provided with X-Cal™ technology to minimize the risk of
inaccurate readings and unanticipated loss of patient monitoring. Refer to the Cable or
Sensor DFU for the specified duration of patient monitoring time.
Note: SpHb readings may be inaccurate for patients with conditions that may cause edema
at the measurement site (eg. kidney disease, pregnancy, etc.).
Note: Physiological conditions that result in loss of pulsatile signal may result in no SpO
SpHb, SpOC, SpCO, SpMet, RRp, and ORi readings.
,
2
Note: Rad-97 is provided with a Wi-Fi signal indicator as an indication of Wi-Fi
communication.
Note: Rad-97’s alarm capabilities have been designed to be independent of the Wi-Fi
communication feature in order to preserve Rad-97's primary alarms.
Note: Always charge Rad-97 when it is not in use to ensure that the Rad-97 Battery Module
remains fully charged.
Note: All batteries lose capacity with age, thus the amount of run time at Low Battery will
vary depending upon the age of the Battery Module.
Note: A functional tester cannot be used to assess the accuracy of Rad-97.
Note: When monitoring acoustic respiration, Masimo recommends minimally monitoring
both oxygenation (SpO
) and respiration (RRa).
2
Note: When using the Maximum Sensitivity setting, performance of the "Sensor Off"
detection may be compromised. If the Rad-97 is in this setting and the sensor becomes
dislodged from the patient, the potential for false readings may occur due to environmental
"noise" such as light, vibration, and excessive air movement.
Noninvasive Blood Pressure
WARNING: Before applying the cuff on the patient, confirm the cuff size is appropriate.
WARNING: When a blood pressure measurement error code occurs, any blood pressure values
reported should be disregarded.
CAUTION: If the blood pressure cuff is on the same limb as monitoring equipment (i.e., pulse
oximeter probe), the pressurization within the cuff can cause temporary loss of function of the
monitoring equipment.
CAUTION: Blood pressure measurements can be affected by the patient's position,
physiological condition, and environmental factors.
www.masimo.com 19 Masimo
Page 22

Rad-97 Safety Information, Warnings and Cautions
Note: Physiological conditions that can affect blood pressure measurements include, but are
not limited to, cardiac arrhythmias, arterial sclerosis, poor perfusion, diabetes, age,
pregnancy, pre-eclampsia, renal diseases, trembling, and shivering.
Kite
WARNING: Kite does not generate or manage alarms. The connected device's alarms, used in
conjunction with clinical signs and symptoms, are the primary sources for determining that
an alarm condition exists.
CAUTION: Kite is not a primary display. All medical decisions should be made using data on
the connected Masimo medical device primary display.
CAUTION: Kite is intended to operate across the facility's network. Unanticipated failure or
alteration of network components (including but not limited to: disconnection or
malfunctioning of a networking device/switch/router/ethernet cable) may result in loss of
connectivity of Kite to other hospital systems. Altering or making changes to the hospital
network should be done with proper knowledge.
Patient SafetyNet
Note: The wireless communication status between Rad-97 and Patient SafetyNet is displayed
by Patient SafetyNet.
Cleaning and Service Warnings and Cautions
WARNING: Do not attempt to remanufacture, recondition or recycle the Rad-97 as these
processes may damage the electrical components, potentially leading to patient harm.
WARNING: To avoid electric shock, always turn off the Rad-97 and physically disconnect the
AC power and all patient connections before cleaning.
WARNING: To avoid electric shock, do not attempt to replace or remove the Battery from the
Rad-97. Service of Rad-97 should be done by qualified personnel only.
WARNING: Do not incinerate the Rad-97 Battery. The battery should be properly disposed
according to local laws and regulations.
CAUTION: Only perform maintenance procedures specifically described in the manual.
Otherwise, return the Rad-97 for servicing.
CAUTION: Do not touch, press, or rub the display panels with abrasive cleaning compounds,
instruments, brushes, rough-surface materials, or bring them into contact with anything that
could scratch the display.
CAUTION: To avoid permanent damage to the Rad-97, do not use undiluted bleach (5% -
5.25% sodium hypochlorite) or any other cleaning solution not recommended.
CAUTION: Do not use petroleum-based or acetone solutions, or other harsh solvents, to clean
the Rad-97. These substances affect the device’s materials and device failure can result.
CAUTION: Do not submerge the Rad-97 in any cleaning solution or attempt to sterilize by
autoclave, irradiation, steam, gas, ethylene oxide or any other method. This will seriously
damage the device.
CAUTION: To prevent damage, do not soak or immerse Rad-97 in any liquid solution.
www.masimo.com 20 Masimo
Page 23

Rad-97 Safety Information, Warnings and Cautions
CAUTION: Electrical Shock Hazard: Carry out periodic tests to verify that leakage currents of
patient-applied circuits and the system are within acceptable limits as specified by the
applicable safety standards. The summation of leakage currents must be checked and in
compliance with IEC 60601-1 and UL60601-1. The system leakage current must be checked
when connecting external equipment to the system. When an event such as a component
drop of approximately 1 meter or greater or a spillage of blood or other liquids occurs, retest
before further use. Injury to personnel could occur.
Compliance Warnings and Cautions
WARNING: Any changes or modifications not expressly approved by Masimo shall void the
warranty for this equipment and could void the user’s authority to operate the equipment.
WARNING: In accordance with international telecommunication requirements, the frequency
band of 2.4 GHz and 5.15 to 5.25 GHz is only for indoor usage to reduce potential for harmful
interference to co-channel mobile satellite systems.
CAUTION: Disposal of Product: Comply with local laws in the disposal of the device and/or its
accessories.
CAUTION: Device contains an internal battery. Dispose of the battery according to required
country or regional requirements.
Note: Use Rad-97 in accordance with the Environmental Specifications section in the
Operator's Manual.
Note: This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and (2) this
device must accept any interference received, including interference that may cause
undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the user
is encouraged to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Note: This equipment has been tested and found to comply with the Class B limits for
medical devices according to the EN 60601-1-2: 2007, Medical Device Directive 93/42/EEC.
These limits are designed to provide reasonable protection against harmful interference in all
establishments, including domestic establishments.
Note: In order to maintain compliance with FCC regulations, shielded cables must be used
with this equipment. Operation with non-approved equipment or unshielded cables is likely
to result in interference to radio and TV reception. The user is cautioned that changes and
www.masimo.com 21 Masimo
Page 24

Rad-97 Safety Information, Warnings and Cautions
modifications made to the equipment without the approval of manufacturer could void the
user's authority to operate this equipment.
Note: To satisfy RF exposure requirements, this device and its antenna must operate with a
separation distance of at least 20 cm from all persons and must not be co-located or
operating in conjunction with any other antenna or transmitter.
Note: This Class B digital apparatus complies with Canadian ICES-003.
Note: This device complies with Industry Canada license-exempt RSS standard(s). Operation
is subject to the following two conditions: (1) this device may not cause interference, and (2)
this device must accept any interference, including interference that may cause undesired
operation of the device.
www.masimo.com 22 Masimo
Page 25
Chapter 1: Technology Overview
The following chapter contains general descriptions about parameters, measurements, and
the technology used by Masimo products.
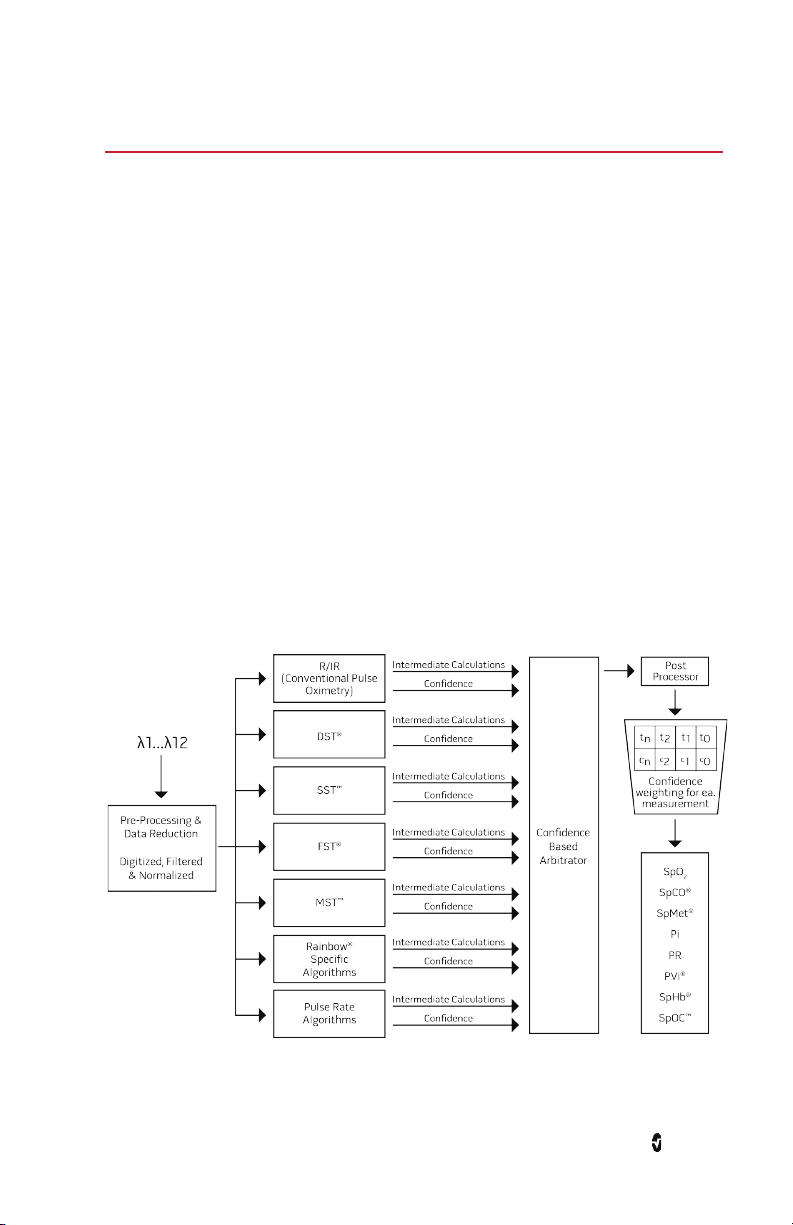
Signal Extraction Technology® (SET®)
Masimo Signal Extraction Technology's signal processing differs from that of conventional
pulse oximeters. Conventional pulse oximeters assume that arterial blood is the only blood
moving (pulsating) in the measurement site. During patient motion, however, the venous
blood also moves, causing conventional pulse oximeters to read low values, because they
cannot distinguish between the arterial and venous blood movement (sometimes referred to
as noise).
Masimo SET
are powerful because they are able to adapt to the varying physiologic signals and/or noise
and separate them by looking at the whole signal and breaking it down to its fundamental
components. The Masimo SET
(DST®), in parallel with Fast Saturation Transform (FST®), reliably identifies the noise, isolates
it and, using adaptive filters, cancels it. It then reports the true arterial oxygen saturation for
display on the monitor.
Masimo rainbow SET® Parallel Engines
This figure is for conceptual purposes only.
®
pulse oximetry utilizes parallel engines and adaptive filtering. Adaptive filters
®
signal processing algorithm, Discrete Saturation Transform
®
www.masimo.com 23 Masimo
Page 26
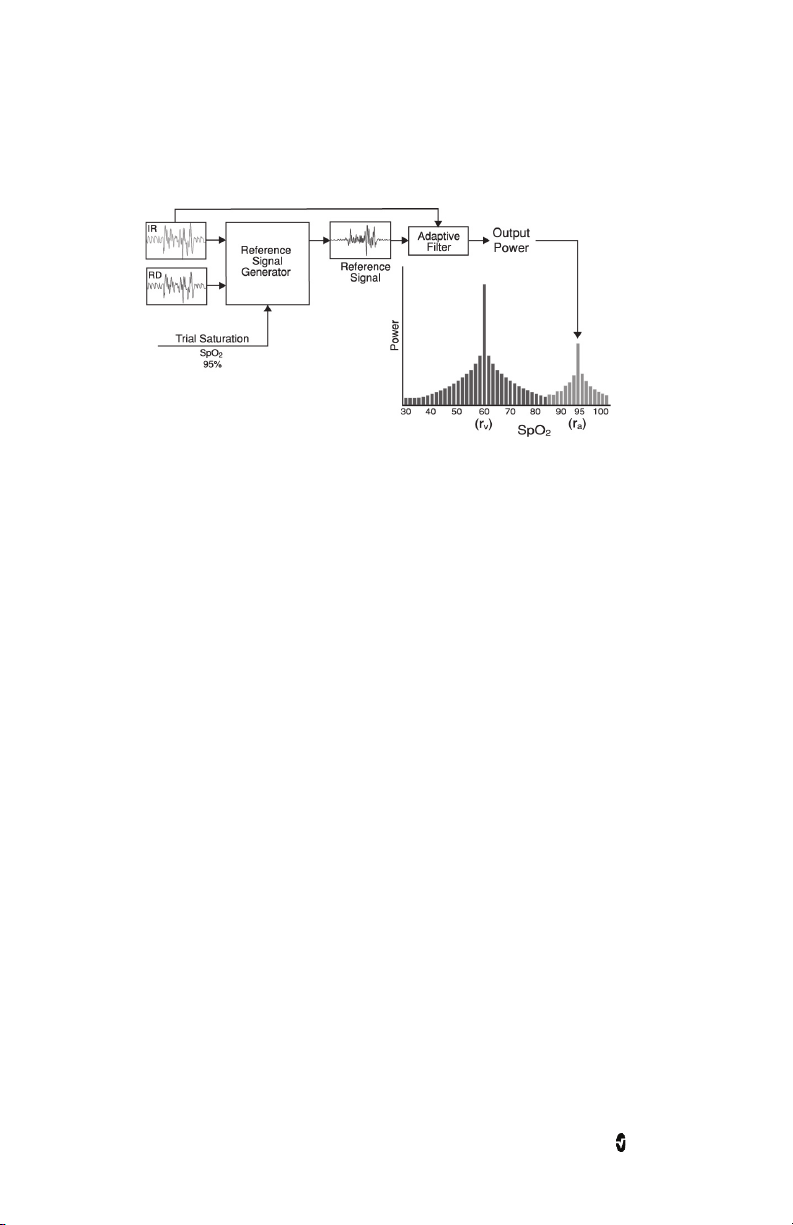
Rad-97 Chapter 1: Technology Overview
Masimo SET® DST
This figure is for conceptual purposes only.
General Description for Oxygen Saturation (SpO2)
Pulse oximetry is governed by the following principles:
1. Oxyhemoglobin (oxygenated blood) and deoxyhemoglobin (non-oxygenated
blood) differ in their absorption of red and infrared light (spectrophotometry).
2. The amount of arterial blood in tissue changes with your pulse
(photoplethysmography). Therefore, the amount of light absorbed by the varying
quantities of arterial blood changes as well.
Successful Monitoring for SpO2, PR and Pi
Stability of the SpO2 readings may be a good indicator of signal validity. Although stability is
a relative term, experience will provide a good feeling for changes that are artifactual or
physiological and the speed, timing, and behavior of each.
The stability of the readings over time is affected by the averaging mode being used. The
longer the averaging time, the more stable the readings tend to become. This is due to a
dampened response as the signal is averaged over a longer period of time than during shorter
averaging times. However, longer averaging times delay the response of the oximeter and
reduce the measured variations of SpO2 and pulse rate.
Functional Oxygen Saturation (SpO2)
The Rad-97 is calibrated to measure and display functional oxygen saturation (SpO2): the
amount of oxyhemoglobin expressed as a percentage of the hemoglobin that is available to
transport oxygen.
Note: Dyshemoglobins are not capable of transporting oxygen, but are recognized as
oxygenated hemoglobins by conventional pulse oximetry.
www.masimo.com 24 Masimo
Page 27

Rad-97 Chapter 1: Technology Overview
General Description for Pulse Rate (PR)
Pulse rate (PR), measured in beats per minute (BPM) is based on the optical detection of
peripheral flow pulse.
General Description for Perfusion Index (Pi)
The Perfusion Index (Pi) is the ratio of the pulsatile blood flow to the non-pulsatile or static
blood in peripheral tissue. Pi thus represents a noninvasive measure of peripheral perfusion
that can be continuously and noninvasively obtained from a pulse oximeter.
General Description for Pleth Variability Index (PVi)
The Pleth Variability Index (PVi) is a measure of the dynamic changes in the Perfusion Index
(Pi) that occur during the respiratory cycle. The calculation is accomplished by measuring
changes in Pi over a time interval where one or more complete respiratory cycles have
occurred. PVi is displayed as a percentage (0-100%).
PVi may show changes that reflect physiological factors such as vascular tone, circulating
blood volume, and intrathoracic pressure excursions.
The utility of PVi has been evaluated in clinical studies [1-11]. Technical and clinical factors
that may affect PVi include probe malposition, probe site, patient motion, skin incision,
spontaneous breathing activity, lung compliance, open pericardium, use of vasopressors or
vasodilators, low perfusion index, subject age, arrhythmias, left or right heart failure, and tidal
volume [12-14].
Citations for Pleth Variability Index (PVi)
1. Cannesson M., Desebbe O., Rosamel P., Delannoy B., Robin J., Bastien O., Lehot J.J.
Pleth Variability Index to Monitor the Respiratory Variations in the Pulse Oximeter
Plethysmographic Waveform Amplitude and Predict Fluid Responsiveness in the
Operating Theatre. Br J Anaesth. 2008 Aug;101(2):200-6.
2. Forget P, Lois F, de Kock M. Goal-Directed Fluid Management Based on the Pulse
Oximeter-Derived Pleth Variability Index Reduces Lactate Levels and Improves Fluid
Management. Anesth Analg. 2010 Oct;111(4):910-4.
3. Zimmermann M., Feibicke T., Keyl C., Prasser C., Moritz S., Graf B.M., Wiesenack C.
Accuracy of Stroke Volume Variation Compared with Pleth Variability Index to
Predict Fluid Responsiveness in Mechanically Ventilated Patients Undergoing Major
Surgery. Eur J Anaesthesiol. 2010 Jun;27(6):555-61.
4. Desebbe O, Boucau C, Farhat F, Bastien O, Lehot JJ, Cannesson M. Anesth Analg. The
Ability of Pleth Variability Index to Predict the Hemodynamic Effects of Positive
End-Expiratory Pressure in Mechanically Ventilated Patients under General
Anesthesia. 2010 Mar 1;110(3):792-8.
5. Tsuchiya M., Yamada T., Asada A. Pleth Variability Index Predicts Hypotension
During Anesthesia Induction. Acta Anesthesiol Scand. 2010 May;54(5):596-602.
6. Loupec T., Nanadoumgar H., Frasca D., Petitpas F., Laksiri L., Baudouin D., Debaene
B., Dahyot-Fizelier C., Mimoz O. Pleth Variability Index Predicts Fluid
Responsiveness in Critically Ill Patients. Crit Care Med. 2011 Feb;39(2):294-9.
www.masimo.com 25 Masimo
Page 28

Rad-97 Chapter 1: Technology Overview
7. Fu Q., Mi W.D., Zhang H. Stroke Volume Variation and Pleth Variability Index to
Predict Fluid Responsiveness during Resection of Primary Retroperitoneal Tumors in
Hans Chinese. Biosci Trends. 2012 Feb;6(1):38-43.
8. Haas S., Trepte C., Hinteregger M., Fahje R., Sill B., Herich L., Reuter D.A. J.
Prediction of Volume Responsiveness using Pleth Variability Index in Patients
Undergoing Cardiac Surgery after Cardiopulmonary Bypass. Anesth. 2012
Oct;26(5):696-701.
9. Byon H.J., Lim C.W., Lee J.H., Park Y. H., Kim H.S., Kim C.S., Kim J.T. Br. J.
Prediction of fluid Responsiveness in Mechanically Ventilated Children Undergoing
Neurosurgery. Anaesth 2013 Apr;110(4):586-91.
10. Feissel M., Kalakhy R., Banwarth P., Badie J., Pavon A., Faller J.P., Quenot JP.
Plethysmographic Variation Index Predicts Fluid Responsiveness in Ventilated
Patients in the Early Phase of Septic Shock in the Emergency Department: A Pilot
Study. J Crit Care. 2013 Oct;28(5):634-9.
11. Yu Y., Dong J., Xu Z., Shen H., Zheng J. Pleth Variability Index-Directed Fluid
Management in Abdominal Surgery under Combined General and Epidural
Anesthesia. J Clin Monit Comput. 2014 Feb 21.
12. Desgranges F.P., Desebbe O., Ghazouani A., Gilbert K., Keller G., Chiari P., Robin
J.,Bastien O., Lehot J.J., Cannesson M. Br. J. Anaesth 2011 Sep;107(3):329-35.
13. Cannesson M. Arterial pressure variation and goal-directed fluid therapy. J
Cardiothorac Vasc Anesth. 2010 Jun;24(3):487-97.
14. Takeyama M, Matsunaga A, Kakihana Y, Masuda M, Kuniyoshi T, Kanmura Y.
Impact of Skin Incision on the Pleth Variability Index. J Clin Monit Comput 2011
Aug;25(4):215-21.
Signal IQ
The Signal IQ provides an indicator of the assessment of the confidence in the displayed SpO2
value. The SpO
SIQ can also be used to identify the occurrence of a patient’s pulse.
2
With motion, the plethysmographic waveform is often distorted and may be obscured by
noise artifact. Shown as a vertical line, the SpO
pulsation. Even with a plethysmographic waveform obscured by artifact, the Signal IQ
SIQ coincides with the peak of an arterial
2
identifies the timing that the algorithms have determined for the arterial pulsation. The
pulse tone (when enabled) coincides with the vertical line of the SpO
SIQ.
2
The height of the vertical line of the SpO2 SIQ provides an assessment of the confidence in
the measurement displayed. A high vertical bar indicates higher confidence in the
measurement. A small vertical bar indicates lower confidence in the displayed measurement.
When the Signal IQ is very low, this suggests that the accuracy of the displayed measurement
may be compromised. See About the Status Bar on page 49.
rainbow Pulse CO-Oximetry Technology
rainbow Pulse CO-Oximetry technology is governed by the following principles:
1. Oxyhemoglobin (oxygenated blood), deoxyhemoglobin (non-oxygenated blood),
carboxyhemoglobin (blood with carbon monoxide content), methemoglobin
(blood with oxidized hemoglobin) and blood plasma constituents differ in their
absorption of visible and infrared light (using spectrophotometry).
www.masimo.com 26 Masimo
Page 29
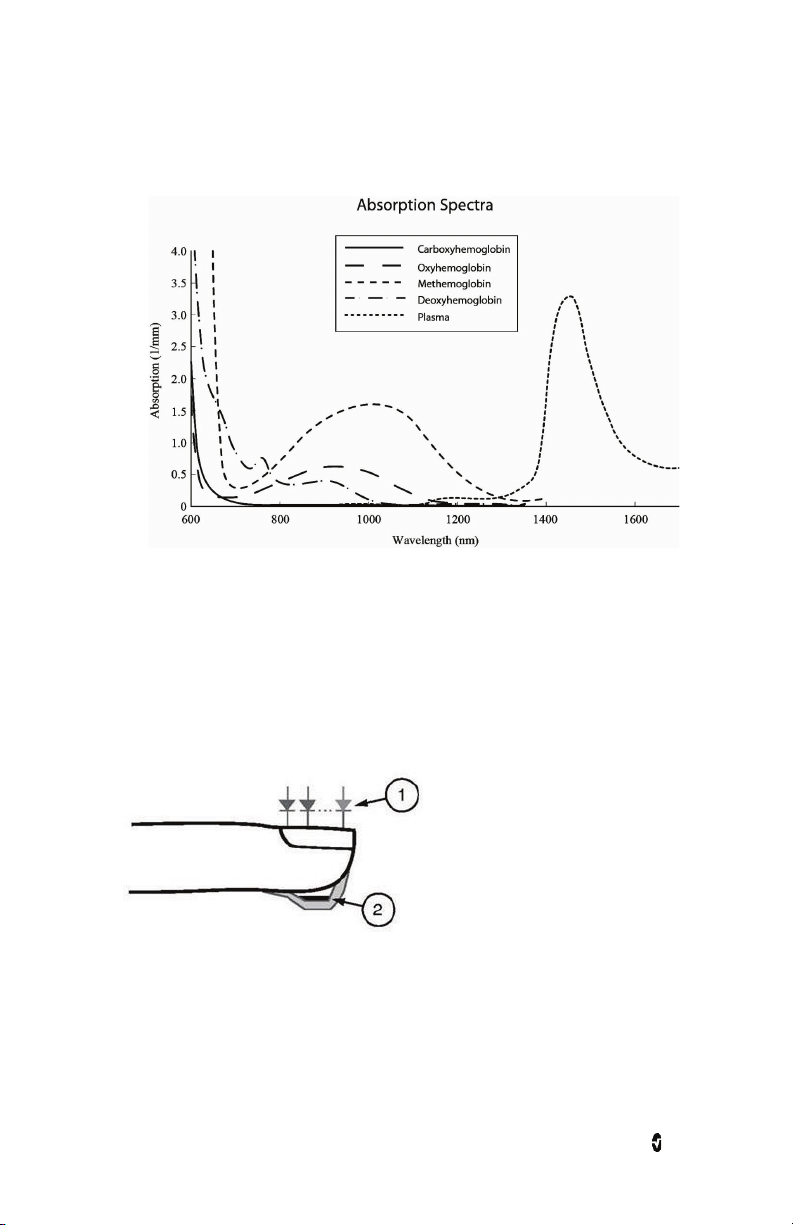
Rad-97 Chapter 1: Technology Overview
2. The amount of arterial blood in tissue changes with pulse
(photoplethysmography). Therefore, the amount of light absorbed by the varying
quantities of arterial blood changes as well.
The Rad-97 uses a multi-wavelength sensor to distinguish between oxygenated blood,
deoxygenated blood, blood with carbon monoxide, oxidized blood and blood plasma.
The Rad-97 utilizes a sensor with various light-emitting diodes (LEDs) that pass light through
the site to a diode (detector). Signal data is obtained by passing various visible and infrared
lights (LEDs, 500 to 1400nm) through a capillary bed (for example, a fingertip, a hand, a foot)
and measuring changes in light absorption during the blood pulsatile cycle. This information
may be useful to clinicians. The maximum radiant power of the strongest light is rated at ≤ 25
mW. The detector receives the light, converts it into an electronic signal and sends it to the
Rad-97 for calculation.
1. Light Emitting Diodes (LEDs)
(7 + wavelengths)
2. Detector
www.masimo.com 27 Masimo
Page 30

Rad-97 Chapter 1: Technology Overview
Once Rad-97 receives the signal from the sensor, it utilizes proprietary algorithms to
calculate the patient’s functional oxygen saturation (SpO
carboxyhemoglobin saturation (SpCO [%]), methemoglobin saturation (SpMet [%]), total
[%]), blood levels of
2
hemoglobin concentration (SpHb [g/dL]) and pulse rate (PR). The SpCO, SpMet and SpHb
measurements rely on a multi-wavelength calibration equation to quantify the percentage of
carbon monoxide and methemoglobin and the concentration of total hemoglobin in arterial
blood. Maximum skin-sensor interface temperature was tested to be less than 41º C (106º F)
in a minimum ambient temperature of 35º C (95º F). The tests were conducted with sensors
operating at reasonable worst case power.
Pulse CO-Oximetry vs. Drawn Whole Blood Measurements
When SpO2, SpCO, SpMet, and SpHb measurements obtained from the Rad-97 (noninvasive)
are compared to drawn whole blood (invasive) measurements by blood gas and/or laboratory
CO-Oximetry methods, caution should be taken when evaluating and interpreting the results.
The blood gas and/or laboratory CO-Oximetry measurements may differ from the SpO
SpMet, SpHb, and SpOC measurements of the Rad-97. Any comparisons should be
simultaneous, meaning the measurement on the device should be noted at the exact time
that blood is drawn.
In the case of SpO
the calculated measurement is not appropriately corrected for the effects of variables that
, different results are usually obtained from the arterial blood gas sample if
2
shift the relationship between the partial pressure of oxygen (pO2) and saturation, such as:
pH,temperature, the partial pressure of carbon dioxide (pCO
), 2,3-DPG, and fetal hemoglobin.
2
In the case of SpCO, different results are also expected if the level of methemoglobin (MetHb)
in the blood gas sample is abnormal (greater than 2% for MetHb).
In the case of SpHb, variation in hemoglobin measurements may be profound and may be
affected by sampling technique as well as the patient's physiological conditions. Any results
exhibiting inconsistency with the patient's clinical status should be repeated and/or
supplemented with additional test data. As with most hemoglobin tests, a laboratory blood
sample should be analyzed prior to clinical decision making.
High levels of bilirubin may cause erroneous SpO
samples are usually taken over a period of 20 seconds (the time it takes to draw the blood) a
, SpMet, SpCO, and SpHb readings. As blood
2
meaningful comparison can only be achieved if the oxygen saturation (SaO2), levels of
carboxyhemoglobin (COHb), and MetHb of the patient are stable and not changing over the
period of time that the blood gas sample is taken. Subsequently, blood gas and laboratory
CO-Oximetry measurements of SpO
administration of fluids and in procedures such as dialysis. Additionally, drawn whole blood
, SpCO, SpMet, SpHb, and SpOC may vary with the rapid
2
testing can be affected by sample handling methods and time elapsed between blood draw
and sample testing.
Measurements with Low Signal IQ should not be compared to laboratory measurements.
, SpCO,
2
General Description for Total Hemoglobin (SpHb)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of total
hemoglobin (SpHb) in arterial blood. It relies on the same principles of pulse oximetry to
make its SpHb measurement.
www.masimo.com 28 Masimo
Page 31

Rad-97 Chapter 1: Technology Overview
Successful Monitoring for SpHb
A stable SpHb reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion at the
measurement site. Physiological changes at the measurement site are mainly caused by
fluctuations in the oxygen saturation, blood concentration and perfusion. See Safety
Information, Warnings and Cautions on page 13 and Troubleshooting Measurements on
page 137.
General Description for Total Arterial Oxygen Content (CaO2)
Oxygen (O2) is carried in the blood in two forms, either dissolved in plasma or combined with
hemoglobin. The amount of oxygen in the arterial blood is termed the oxygen content (CaO
and is measured in units of ml O
oxygen, whereas 100 ml of blood plasma may carry approximately 0.3 ml of oxygen*. The
oxygen content is determined mathematically as:
CaO2 = 1.34 (ml O2/g) x Hb (g/dL) x HbO2 + PaO2 (mmHg) x 0.003 (ml O2/dL/mmHg)
Where HbO
arterial oxygen.
For typical PaO
based on PaO
and methemoglobin levels, the functional saturation (SpO
is the fractional arterial oxygen saturation and PaO2 is the partial pressure of
2
values, the second part of the above equation is approximately 0.3 ml O2/dL
2
being approximately 100 mmHg. Furthermore, for typical carboxyhemoglobin
2
is given by:
*Martin, Laurence. All You Really Need to Know to Interpret Arterial Blood Gases, Second
Edition. New York: Lippincott Williams & Wilkins, 1999.
/dL blood. One gram of hemoglobin (Hb) can carry 1.34 ml of
2
) as measured by a pulse oximeter
2
SpO
= 1.02 x HbO2
2
)
2
General Description for SpOC
The above approximations result in the following reduced equation for oxygen content via the
Pulse CO-Oximeter:
SpOC (ml/dL*) = 1.31 (ml O
*When ml O
cancels the gram unit in the numerator of g/dL resulting in ml/dL (ml of oxygen in one dL of
/g Hb is multiplied by g/dL of SpHb, the gram unit in the denominator of ml/g
2
/g) x SpHb (g/dL) x SpO2 + 0.3 (ml O2/dL)
2
blood) as the unit of measure for SpOC. See Safety Information, Warnings and Cautions on
page 13.
General Description for Carboxyhemoglobin (SpCO)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of
carboxyhemoglobin saturation (SpCO) in arterial blood. It relies on the same basic principles
of pulse oximetry (spectrophotometry) to make its SpCO measurement.
The measurement is obtained by placing a sensor on a patient, usually on the fingertip for
adults and the hand or foot for infants. The sensor connects either directly to the Pulse
CO-Oximetry device or through a device patient cable.
www.masimo.com 29 Masimo
Page 32

Rad-97 Chapter 1: Technology Overview
The sensor collects signal data from the patient and sends it to the device. The device
displays the calculated data as percentage value for the SpCO, which reflect blood levels of
carbon monoxide bound to hemoglobin.
Successful Monitoring for SpCO
A stable SpCO reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion in the patient's
fingertip (measurement site). Physiological changes at the measurement site are mainly
caused by fluctuations in the oxygen saturation, blood concentration and perfusion.
General Description for Methemoglobin (SpMet)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of
methemoglobin saturation (SpMet) in arterial blood. It relies on the same basic principles of
pulse oximetry (spectrophotometry) to make its SpMet measurement.
The measurement is obtained by placing a sensor on a patient, usually on the fingertip for
adults and the hand or foot for infants. The sensor connects either directly to the Pulse
CO-Oximetry device or through a patient cable.
The sensor collects signal data from the patient and sends it to the device. The device
displays the calculated data as percentage value for the SpMet.
Successful Monitoring for SpMet
A stable SpMet reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion in the patient’s
fingertip (measurement site).
Physiological changes at the measurement site are mainly caused by fluctuations in the
oxygen saturation, blood concentration and perfusion. See Safety Information, Warnings and
Cautions on page 13.
General Description for Respiration Rate (RRp)
RRp is currently not available in the U.S.A. and territories relying on FDA market
clearance.
Respiration rate can be determined by the plethysmographic waveform (RRp). This method
measures respirations per minute (rpm) based on plethysmographic amplitude changes that
correspond to the respiratory cycle.
www.masimo.com 30 Masimo
Page 33

Rad-97 Chapter 1: Technology Overview
General Description for Oxygen Reserve Index (ORi)
ORi is currently not available in the U.S.A. and territories relying on FDA market clearance.
Pulse CO-Oximetry is a continuous and noninvasive method of measuring changes in oxygen
states in hyperoxic conditions. It relies on the same principles of pulse oximetry to make its
ORi measurement.
The measurement is taken by a sensor capable of measuring ORi, usually on the fingertip for
adult or pediatric patients. The sensor connects directly to the Pulse CO-Oximeter or with a
patient cable. The sensor collects signal data from the patient and sends it to the device. The
device displays the processed data as an indicator of changes in oxygen states in hyperoxic
conditions.
Successful Monitoring for ORi
A stable ORi reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion at the
measurement site. Physiological changes at the measurement site are mainly caused by
fluctuations in the oxygen saturation, blood concentration and perfusion. See Safety
Information, Warnings and Cautions on page 13 and Troubleshooting Measurements on
page 137.
SpCO, SpMet, and SpHb Measurements During Patient Motion
The Rad-97 displays measurements of SpCO, SpMet, and SpHb during patient motion.
However, because of the changes in the physiological parameters such as blood volume,
arterial-venous coupling, etc. that occur during patient motion, the accuracy of such
measurements may not be reliable during excessive motion. In this case, the measurement
value for SpCO, SpMet, or SpHb displays as dashes (---) and a message (Low SpCO SIQ, Low
SpMet SIQ, or Low SpHb SIQ) displays to alert the clinician that the device does not have
confidence in the value due to poor signal quality caused by excessive motion or other signal
interference.
rainbow Acoustic Monitoring™ (RAM™)
Note: This feature is currently available on Rad-97 devices only.
rainbow Acoustic Monitoring (RAM) continuously measures a patient’s respiration rate based
on airflow sounds generated in the upper airway. The Acoustic Sensor, which is applied on the
patient's neck, translates airflow sounds generated in the upper airway to an electrical signal
that can be processed to produce a respiration rate, measured as breaths per minute.
Respiratory sounds include sounds related to respiration such as breath sounds (during
inspiration and expiration), adventitious sounds, cough sounds, snoring sounds, sneezing
sounds, and sounds from the respiratory muscles [1].
These respiratory sounds often have different characteristics depending on the location of
recording [2] and they originate in the large airways where air velocity and air turbulence
induce vibration in the airway wall. These vibrations are transmitted, for example, through
www.masimo.com 31 Masimo
Page 34
Rad-97 Chapter 1: Technology Overview
the lung tissue, thoracic wall and trachea to the surface where they may be heard with the aid
of a stethoscope, a microphone or more sophisticated devices.
rainbow Acoustic Monitoring Architecture
The following figure illustrates how a respiratory sound produced by a patient can be turned
into a numerical measurement that corresponds to a respiratory parameter.
Patient
Respiratory airflow to sound
Signal
Processing
Digital signal to respiratory
measurement
Sensor
Sound to
electrical signal
Envelope
Detection
Acquisition
System
Electrical signal to
digital signal
RRa Estimation
Patient
The generation of respiratory sounds is primarily related to turbulent respiratory airflow in
upper airways. Sound pressure waves within the airway gas and airway wall motion contribute
to the vibrations that reach the body surface and are recorded as respiratory sounds.
Although the spectral shape of respiratory sounds varies widely from person to person, it is
often reproducible within the same person, likely reflecting the strong influence of individual
airway anatomy [2-6].
Sensor
The sensor captures respiratory sounds (and other biological sounds) much like a microphone
does. When subjected to a mechanical strain, (e.g., surface vibrations generated during
breathing), the sensor becomes electrically polarized.
The degree of polarization is proportional to the applied strain. The output of the sensor is an
electric signal that includes a sound signal that is modulated by inspiratory and expiratory
phases of the respiratory cycle.
www.masimo.com 32 Masimo
Page 35

Rad-97 Chapter 1: Technology Overview
Acquisition System
The acquisition system converts the electric signal provided by the sensor into a digital
signal. This format allows the signal to be processed by a computing device.
Signal Processing
The digital signal produced by the acquisition system is converted into a measurement that
corresponds to the respiratory parameter of interest. As shown in the previous figure, this can
be performed by, for example, determining the digital signal envelope or outline which in turn
may be utilized to determine the respiratory rate. In this way, a real-time, continuous breath
rate parameter can be obtained and displayed on a monitor which, in many cases, may be
real-time and continuous.
The respiratory cycle envelope signal processing principle is similar to methods that sample
airway gasses and subsequently determine a respiratory rate.
Citations
[1] A.R.A. Sovijärvi, F. Dalmasso, J. Vanderschool, L.P. Malmberg, G. Righini, S.A.T. Stoneman.
Definition of terms for applications of respiratory sounds. Eur Respir Rev 2000; 10:77,
597-610.
[2] Z. Moussavi. Fundamentals of respiratory sounds analysis. Synthesis lectures on biomedical
engineering #8. Morgan & Claypool Publishers, 2006.
[3] Olsen, et al. Mechanisms of lung sound generation. Semin Respir Med 1985; 6: 171-179.
[4] Pastercamp H, Kraman SS, Wodicka GR. Respiratory sounds – Advances beyond the
stethoscope. Am J Respir Crit Care Med 1977; 156: 974-987.
[5] Gavriely N, Cugell DW. Airflow effects on amplitude and spectral content of normal breath
sounds. J Appl Physiol 1996; 80: 5-13.
[6] Gavrieli N, Palti Y, Alroy G. Spectral characteristics of normal breath sounds. J Appl Physiol
1981; 50: 307-314.
www.masimo.com 33 Masimo
Page 36

Page 37

Chapter 2: Description
This chapter contains the description of the Rad-97 physical features.
General System Description
The Rad-97 system includes the following:
• Rad-97 Device
• AC Power Cord
• Patient Cable
• Sensor
For a complete list of compatible sensors and cables, visit http://www.masimo.com.
www.masimo.com 35 Masimo
Page 38

Rad-97 Chapter 2: Description
Features
Front View
1. Display and Touchscreen
Provides a user interface to view and change
settings.
2. Home Button
Provides a multipurpose user interface that
allows for navigation to the home screen as
well as turning the device on and off.
3. Patient Cable Connector
Provides a connection to a patient cable or
sensor.
4. NIBP Nib*
Allows connection to a cuff for blood pressure
measurements.
*Optional feature.
www.masimo.com 36 Masimo
Page 39

Rad-97 Chapter 2: Description
Back View
1. Nurse Call Connector
Allows connection to a Nurse Call system.
Caution: To ensure patient electrical isolation, all
external device connections to the Analog
Output/Nurse Call connectors must be IEC 60950-1,
IEC 60601-1, or UL 1069 compliant.
See Nurse Call Connection on page 41.
2. Ethernet
Allows network connection to Rad-97 using an RJ-45
cable.
3. USB
Provides USB 2.0 connectivity.
4. Equipotential Ground Connector
Provides optional functional earthing for Rad-97 to
eliminate potential differences between the earth
connections for Rad-97 and another medical device.
The use of the Equipotential Ground Connector
should be in accordance with IEC 60601-1.
5. Power Entry Module
Provides connection to an AC power cord.
Note: Always connect the Rad-97 to the mains power
for continuous operation and/ or battery recharging.
Note: Use the power cord as the means to disconnect
the device from AC power. To disconnect the device
from AC power, first disconnect the power cord from
the power outlet, rather than from the device.
www.masimo.com 37 Masimo
Page 40

Rad-97 Chapter 2: Description
Side and Top Views
1. Speaker
The speaker provides audio alarms.
Care should be taken not to cover
the speaker.
2. Swivel Foot
Provides stability when placing
Rad-97 on a surface in a vertical
position.
3. Foot Pads
Provides physical support to
Rad-97 when placed on a surface in
a horizontal position.
4. System Status Light
Provides an indication of alarm
status. See About the System
Status Light on page 56.
www.masimo.com 38 Masimo
Page 41

Chapter 3: Setting Up
This chapter contains information about setting up Rad-97 before use.
Unpacking and Inspection
To unpack and inspect the Rad-97:
1. Remove the Rad-97 from the shipping carton and examine it for signs of shipping
damage.
2. Check all materials against the packing list. Save all packing materials, invoice
and bill of lading. These may be required to process a claim with the carrier.
3. If anything is missing or damaged, contact the Masimo Technical Service
Preparation for Use
Prior to setting up the Rad-97 for monitoring, perform the following steps:
Department. See Return Procedure on page 173.
1. Confirm that you have all system components:
• Rad-97 Device
• AC Power Cord
• Patient Cable
• Sensor
2. Read the Safety Information, Warnings and Cautions on page 13.
3. Setup the Rad-97 according to the directions provided in this Operator's Manual.
Guidelines for Setting Up
When setting up Rad-97, follow these guidelines:
1. Place on a stable, hard, flat, dry surface near the patient.
Caution: Do not place the Rad-97 where the controls can be changed by the
patient.
Note: If placed in a vertical position, rotate the swivel foot at the base of the
device as shown in Side and Top Views on page 38 for stability.
2. Maintain a minimum of three (3) centimeters (approximately one [1] inch) of free
space around Rad-97.
3. Ensure that the Speaker is not covered to avoid a muffled alarm sound.
4. Charge Rad-97's battery fully before use. See Initial Battery Charging on page 41.
5. Rad-97 should not be operated outside the environmental conditions listed in the
www.masimo.com 39 Masimo
specifications section. See Environmental on page 152.
Page 42

Rad-97 Chapter 3: Setting Up
Powering the Rad-97 ON and OFF
To Power ON Rad-97
1. Press and hold the Home Button for more than two (2) seconds, until one (1)
audible tone sounds.
2. The Home Button will illuminate Green and the Rad-97 will power on.
To Power OFF Rad-97
When powering off the Rad-97, the device remembers the last settings if the Power on Profile
is set to Previous Profile. See Access Control on page 94. The device also remembers the
Device Mode when turned off, and will turn back on in that mode.
1. Press and hold the Home Button for more than 8 seconds, until two (2) audible
tones sound.
2. The Home Button will flash Orange.
3. The Rad-97 will power down and turn off.
www.masimo.com 40 Masimo
Page 43

Rad-97 Chapter 3: Setting Up
Initial Battery Charging
Before use, the Rad-97 battery must be charged completely.
To charge Rad-97
1. Plug the AC power cord into the power entry module. Make sure it is securely
plugged in.
2. Plug the AC power cord into an AC power source.
3. Verify that the battery is charging:
• When Rad-97 is ON and charging, the AC Power Indicator lightning bolt icon
will appear on the screen.
• When Rad-97 is OFF and charging, the Home button will illuminate Orange.
4. When the battery is fully charged:
• When Rad-97 is ON and fully charged, the AC Power Indicator will change to
a plug icon.
Touch the AC Power Indicator icon to view battery charge details. See Rad-97 Battery on page
93. For additional information, see Battery Operation and Maintenance on page 172.
Nurse Call Connection
To connect to a Nurse Call System
1. Identify the Nurse Call connection end (1/4 inch round male connector) of the
cable.
2. Insert the Nurse Call cable connector securely into the compatible port (1/4 inch
round female connector) on the rear of the Rad-97. See Back View on page 37.
3. Depending on the connection type of the Nurse Call System, it may be necessary to
orient the other end of the Nurse Call connection cable to fit correctly into the
system connection.
4. It may be necessary to configure the settings of the Nurse Call output. See Device
Output on page 96 and Nurse Call Setting Connections on page 167 for additional
information.
www.masimo.com 41 Masimo
Page 44

Rad-97 Chapter 3: Setting Up
Attach NIBP Cuff
1. Connect the cuff to the patient hose.
2. Connect the opposite end of the patient hose to the NIBP Nib located on the front
of Rad-97. See Chapter 6: Noninvasive Blood Pressure (NIBP) on page 109.
Masimo Kite
Masimo Kite Software Application is a passive monitoring interface to Point-of-Care (POC)
Masimo medical devices (Rad-97 for example) that co-exist under the same Wi-Fi network.
Kite remotely displays system and parameter status reported by the POC device on a separate
monitor.
Rad-97 must be on the same network as Kite.
Note: If the device is not on the same network, it can be added, but Kite will not be able to
connect it to view the parameters monitored by that device until both Kite and the device are
connected to the same network.
To add Rad-97 to Kite to view parameter status, refer to the Masimo Kite Software Application
Operator's Manual.
Third-Party Devices
Third-party devices can be connected to Rad-97 through Bluetooth communication. For more
information, including how to connect a device to Rad-97, see Chapter 8: Third-Party Devices
on page 121.
www.masimo.com 42 Masimo
Page 45

Chapter 4: Operation
The information in this chapter assumes that Rad-97 is set up and ready for use. This chapter
provides necessary information for proper operation of the device. Do not operate Rad-97
without completely reading and understanding these instructions.
Using the Touchscreen and Home Button
1. Main Screen
To access settings and other screens, touch a
value or icon on the Display View. See About the
Main Screen on page 48.
2. Home / Power button
To return to the Main Screen, press the Home
button.
The Home button is also used to power the
device ON and OFF. See Powering the Rad-97
ON and OFF on page 40.
The Home Button changes color depending on
the selected profile. See Profiles Overview on
page 103.
www.masimo.com 43 Masimo
Page 46

Rad-97 Chapter 4: Operation
Using the Touchscreen Interface
Using the gestures described below, the user is able to customize the viewing experience,
including displaying the highest priority parameters and measurements. Feature navigation
availability is dependent on which medical devices are connected to Rad-97.
Action Illustration Example Description
Touch
Touch and
Hold
Swipe
(Touch
and
Move)
Flick
Pinch
Drag and
Drop
See Understanding Windows
on page 53.
Touch and release. Action
performed once finger is released.
Touch and hold. Action performed
once hold duration is reached. A
notification is displayed.
Touch, move (left, right, up or
down), and release. Moves an
object across the display.
Touch and quickly swipe (left,
right, up or down), and release.
Touch, move, and release via two
touch points. Moving touch points
apart zooms in, and moving them
together zooms out.
Touch, hold, drag an object to
desired position, and drop it by
releasing.
www.masimo.com 44 Masimo
Page 47

Rad-97 Chapter 4: Operation
Below is a list of all the different types of controls available on Rad-97 and the various ways to
interact with each type of control.
Control Applicable Actions Description
Toggle Touch and slide knob
Touch and slide left or
right of toggle
Labeled Toggle Touch and slide knob
Touch and slide left or
right of toggle
Touch label
Spinner Touch center (focused)
tile
Swipe up or down
Touch unfocused tile
Touch anywhere
outside spinner
Slider Touch and slide knob
Press anywhere along
slider path
Slider Spinner Touch and slide knob
• Switches between toggle states
• Quickly moves knob left or right
• Switches between toggle states
• Quickly moves knob left or right
• Quickly moves knob left or right
• When closed, expands spinner
• When open, collapses spinner
• When open, scrolls through spinner tiles
• When open, scrolls tile into center (focused)
position
• When open, collapses spinner
• Moves knob
• Quickly moves knob to tap position
• Moves knob
Touch anywhere along
• Quickly moves knob to tap position
slider path
Touch center (focused)
tile
Swipe up/down
Touch unfocused tile
• When closed, expands spinner
• When open, collapses spinner
• When open, scrolls through spinner tiles
• When open, scrolls tile into center (focused)
position
www.masimo.com 45 Masimo
Page 48

Rad-97 Chapter 4: Operation
Control Applicable Actions Description
Touch anywhere
outside spinner
Button Touch
Icon Menu Touch tile
Swipe left or right
(anywhere)
Touch bottom
indicator icon
Window Touch parameter or
measurement
Touch and hold
Well Touch parameter or
measurement
• When open, collapses spinner
• Performs action (as defined by the button
description)
• Opens menu specified by tile
• Scrolls icons left or right
• Quickly centers tile corresponding to
indicator icon
• When no parameter or measurement alarm
is present, opens parameter or measurement
menu
• When parameter or measurement alarm is
present, silences parameter or measurement
alarm
• Enables parameter and measurement drag
and drop
• When no parameter or measurement alarm
is present, opens parameter or measurement
menu
• When parameter or measurement alarm is
present, silences parameter or measurement
alarm
Touch and hold
Live Waveform Swipe down
Swipe up
Trend Line Pinch in
Pinch out
Pan
www.masimo.com 46 Masimo
• Enables parameter and measurement drag
and drop
• Separates pleth and acoustic waveforms
• Combines pleth and acoustic waveforms
• Zooms out
• Zooms in
• Changes time range
Page 49

Rad-97 Chapter 4: Operation
Control Applicable Actions Description
Touch y-axis
Trend Zoom Touch '+'
Touch '-'
Touch time label
Alarm Silence
Touch
icon
Audio Pause
Touch
icon
Other Status
Touch
Bar icons
Back Arrow Touch
• Opens parameter or measurement trend
menu
• Increases time range
• Decreases time range
• Resets time range to default
• Silences all alarms
• Enables Audio Pause
• Opens relevant menu
• Exits menu, abandons any changes
www.masimo.com 47 Masimo
Page 50

Rad-97 Chapter 4: Operation
About the Main Screen
The Main Screen consists of different areas.
Ref. Feature Information
1 Status Bar See About the Status Bar on page 49.
2 Action Menu See About the Action Menu on page 51.
3 Waveform View See Waveform Mode on page 79.
4 Parameter Display See Understanding Windows on page 53.
5 Well See Understanding Windows on page 53.
6 Alarm Silence See About Alarms on page 124.
7 Main Menu See Accessing Main Menu Options on page 57.
8 Patient Admit/Discharge See Patient Admit/Discharge on page 101.
www.masimo.com 48 Masimo
Page 51

Rad-97 Chapter 4: Operation
About the Status Bar
The Status Bar is visible at the top of the Main Screen.
Ref. Feature Description
Suspends all audible alarms and displays remaining Audio
1 Audio Pause
Pause Duration time when activated during an alarm event.
Visual alarms are not impacted and will still display.
See Audio Pause on page 126.
2 Alarm Silence
3 Profiles
4 Device Output
5 Bluetooth
6 Wi-Fi
7 Ethernet
www.masimo.com 49 Masimo
Displays alarm status and mutes all active audible alarms.
See Silencing Alarms on page 125.
Provides access to the Profiles screen. The example shown
illustrates the current Profile is Adult, for an adult patient.
See Chapter 5: Profiles on page 103.
Provides access to the Device Output screen.
See Device Output on page 96.
Provides access to the Bluetooth screen. If this icon is visible,
then Bluetooth connectivity has been enabled.
See Bluetooth on page 92.
Provides access to the Wi-Fi screen. If this icon is visible, then
Wi-Fi connectivity has been enabled. The icon itself also
indicates the strength of the wireless signal.
See Wi-Fi on page 92.
Provides access to the Ethernet screen. If this icon is visible,
then Ethernet connectivity has been enabled.
See Ethernet on page 91.
Page 52

Rad-97 Chapter 4: Operation
Ref. Feature Description
Displays charging status. Provides access to the Battery screen.
Rad-97 Battery
8
Charge/AC Power
Indicator
The example shows that AC power is connected and the battery
is currently charging.
See AC Power Indicator on page 50and Battery Charge Status
Indicator on page 51.
Provides access to the Sounds screen to adjust alarm and pulse
9 Sounds
tone volume. This icon does not indicate the actual volume level
of the alarm and pulse tone.
See Sounds on page 85.
Displays the current time and provides access to the
10 Current Time
Localization screen, which contains settings related to local
time, language, and geography.
See Localization on page 87.
AC Power Indicator
Whenever Rad-97 is connected to an AC power source and ON, the AC Power Indicator icon
will appear on the display as follows:
Icon Status
Battery is currently charging
Battery is fully charged
Touch the AC Power Indicator icon to view battery charge details. See Rad-97 Battery on page
93.
www.masimo.com 50 Masimo
Page 53

Rad-97 Chapter 4: Operation
Battery Charge Status Indicator
When unplugged from AC power, the Battery Charge Status Indicator icon provides a visual
indication of the current battery charge condition.
When the battery charge reaches a low level:
• The Battery Charge Status Indicator icon will change color (Red).
• A “Low Battery” message appears and a medium priority alarm tone will sound
with a Red border on the display. The system status light will flash Yellow.
Connect the battery to AC power to prevent the device from powering off and to charge the
battery. When connected to power, the AC Power Indicator icon will be displayed.
Touch the Battery Charge Status Indicator icon to view battery details. See Rad-97 Battery on
page 93.
About the Action Menu
To expand the Action Menu, select the arrow in the upper right corner of the window.
Note: After approximately 10 seconds without interaction, the Action Menu will retract.
The Action Menu allows quick access to the following settings directly from the Main Screen:
• Sensitivity - Selecting this option cycles through the available sensitivity modes:
APOD, NORM and MAX. See Sensitivity Modes Overview on page 52.
• Trend View - Displays values in Trend View. See Customizing Trend View on page
54.
• Numeric View - Displays values in a standard grid view.
On NIBP models, the Action Menu provides access to the following settings:
• Intervals - Opens the intervals setting screen. See Stat Interval NIBP
Measurement on page 114.
www.masimo.com 51 Masimo
Page 54

Rad-97 Chapter 4: Operation
• Auto - Starts the automatic blood pressure check operation. See Automatic
Interval Measurement on page 113.
• Trend View - Displays values in Trend View. See Trends for NIBP on page 82.
• Numeric View - Displays values in a standard grid view.
Sensitivity Modes Overview
Three sensitivity levels enable a clinician to tailor the response of Rad-97 to the needs of the
particular patient situation. Sensitivity Modes are accessed through the Action Menu. See
About the Action Menu on page 51.
The sensitivity levels are as follows:
• NORM (Normal Sensitivity)
NORM is the recommended sensitivity mode for patients who are experiencing
some compromise in blood flow or perfusion. It is advisable for care areas where
patients are observed frequently, such as an intensive care unit (ICU).
• APOD® (Adaptive Probe Off Detection® Sensitivity)
APOD is the recommended sensitivity mode for situations which there is a high
probability of the sensor becoming detached. It is also the suggested mode for
care areas where patients are not visually monitored continuously. This mode
delivers enhanced protection against erroneous pulse rate and arterial oxygen
saturation readings when a sensor becomes inadvertently detached from a patient
due to excessive movement.
• MAX (Maximum Sensitivity)
MAX is the recommended sensitivity mode for patients with low perfusion or when
a low perfusion message displays in APOD or NORM mode. MAX mode is not
recommended for care areas where patients are not monitored visually, such as
medical-surgical floors. It is designed to display data at the measuring site when
the signal may be weak due to decreased perfusion. When a sensor becomes
detached from a patient, it will have compromised protection against erroneous
pulse rate and arterial saturation readings.
www.masimo.com 52 Masimo
Page 55

Rad-97 Chapter 4: Operation
Understanding Windows
The following information describes how to customize the information viewed on the Main
Screen.
Customizing Windows
Windows can be customized by expanding and minimizing parameters and measurements in
both Trend View and Numeric View. When a parameter is minimized, it is only displayed in
the Well with its Numeric Value and Parameter Label. When a parameter is expanded, it will
be shown in the Parameter display with or without its trend, depending on Trend View setup.
See Customizing Trend View on page 54.
To expand a parameter or measurement
Order Instruction
Step 1 Touch and hold the Numeric Value until it dims.
Step 2 Drag the Numeric Value over any Trend Display.
Step 3 Release the Numeric Value.
www.masimo.com 53 Masimo
Page 56

Rad-97 Chapter 4: Operation
To minimize a parameter or measurement
Order Instruction
Step 1 Touch and hold the Numeric Value until it shrinks.
Step 2 Drag the Numeric Value to the Well.
Step 3 Release the Numeric Value.
Customizing Trend View
There are different ways to view trend information. The following is an example of trend
information for SpO
horizontal position:
www.masimo.com 54 Masimo
, PR, and Pi as they appear within the Main Screen with the device in the
2
Page 57

Rad-97 Chapter 4: Operation
In Trend View, a parameter or measurement is displayed as a graph of its values over time.
The following diagram and table describe key features of a parameter's trend display in
Trend View.
Ref. Feature Description
1 Value Range
measurement. Press to access the Trend Menu from which the
minimum and maximum values in the range can be modified.
Indicates current range of the displayed parameter or
2 Trend Graph
Displays parameter measurement over a period of time. Zoom in
and out of a Trend Graph by pinching out and in.
3 Numeric Value Indicates current reading of the parameter or measurement.
4 Alarm Limits
Parameter or
5
Measurement Label
Indicate high and low alarm limits for the parameter or
measurement, if applicable.
Indicates the name of the parameter or measurement.
Data can be added to or removed from Trend View in the same manner as described in
Customizing Windows on page 53. Data can be manipulated using the touchscreen as
follows:
1. Swipe the trend view display left or right to scroll the Trend View data forward or
backward in time.
2. Tap the Trend View in a specific spot to view the values at that time.
3. Touch the box in the lower right corner of the screen to change the time range of
Trend View data displayed on the screen. Select from 0:10h (10 minutes) to
24:00h (24 hours).
4. Using a pinch gesture with two fingers, zoom in and out of the Trend View data
displayed on the screen from 0:10h (10 minutes) to 24:00h (24 hours) in
increments of 0:01h.
www.masimo.com 55 Masimo
Page 58

Rad-97 Chapter 4: Operation
About the System Status Light
The System Status Light provides visual indications of alarms and system messages. The
light will illuminate in different colors depending on the state of the device.
To locate the System Status Light, see Side and Top Views on page 38.
Light Status Alarm
Indication
Priority
None None System is off
Green None System is monitoring patient; no alarms.
There is an active, low priority alarm.
Examples of low priority alarms:
Yellow Low
• No cable is connected.
• Cable connected but there is no sensor connected to
cable.
• Sensor is off patient and has been acknowledged.
There is an active medium priority alarm.
Flashing
Yellow
Medium
Example of a medium priority alarm:
• Device battery is low.
There is an active high priority alarm.
Flashing Red High
Example of a high priority alarm:
• The sensor has come off of the patient.
www.masimo.com 56 Masimo
Page 59

Rad-97 Chapter 4: Operation
Accessing Main Menu Options
To access Main Menu options, press the Main Menu icon in the bottom left corner of the
touchscreen:
The Main Menu options are:
Parameter Settings
Displayed on devices without NIBP.
See Parameter Settings on page 60.
rainbow Parameter Settings
Displayed on devices with NIBP.
See rainbow Parameter Settings on page 59.
Noninvasive Blood Pressure Settings
See Noninvasive Blood Pressure (NIBP) Settings on page 80.
Additional Settings*
Displayed on devices without NIBP. Devices with NIBP display this icon under the
rainbow Parameters Settings menu.
See Additional Settings on page 78.
Profiles*
See Chapter 5: Profiles on page 103.
Sounds
See Sounds on page 85.
Device Settings
See Device Settings on page 86.
About
See About on page 97.
www.masimo.com 57 Masimo
Page 60

Rad-97 Chapter 4: Operation
3D Alarms*
Displayed on devices without NIBP. Devices with NIBP display this icon under the
rainbow Parameters Settings menu.
See 3D Alarms on page 128.
Trends*
See Trends on page 97.
Rad-97
This icon is displayed when the Rad-97 is in optional Home mode (when
available), allowing device settings to be changed.
See Home on page 89.
* This icon is neither available nor displayed in the Main Menu when the device is in optional
Home mode (when available). See Home on page 89.
Navigating the Main Menu
Once the Main Menu screen is displayed, users can access additional screens, information
and settings. Swipe the screen left or right to pan through the menu icons. Touch the arrow
icon to return to the Main Screen.
Note: Non-NIBP device used in the examples below.
Icons at the bottom edge of the displayed menu screen correspond to the settings. Touch an
icon to jump to the setting on the displayed menu screen.
www.masimo.com 58 Masimo
Page 61
Rad-97 Chapter 4: Operation
Display Timeout
When viewing any of the menu screens, and no user interaction occurs within one (1) minute,
the display times out and returns to the Main Screen.
Navigating Through Menus
When configuring settings, all changes must be confirmed by selecting OK. To cancel the
changes, select Cancel.
Any screen requiring selection of option(s) will time out after one (1) minute of inactivity and
return to the Display View.
To navigate to the previous screen, press the arrow in the top left corner of the
touchscreen.
To return to the Main Screen, press the Home Button at any time.
rainbow Parameter Settings
On Rad-97, the rainbow menu allows the user to view and customize settings for rainbow
parameters:
Parameter Settings
See Parameter Settings on page 60.
3D Alarms
See 3D Alarms on page 128.
Additional Settings
See Additional Settings on page 78.
www.masimo.com 59 Masimo
Page 62

Rad-97 Chapter 4: Operation
Parameter Settings
The following is an example of the Rad-97 Parameter Settings screen.
To access any of the available parameter setting screens:
1. From the Parameter Settings screen, to access the desired parameter, swipe the
on-screen icons left or right.
2. Touch the icon of the desired parameter.
Parameter Settings include:
• See SpO2 Settings on page 62
• See PR Settings on page 64
• See Pi Settings on page 65
• See PVi Settings on page 66
• See Respiration Rate (RR) Settings on page 67
• See SpHb Settings on page 71
• See SpOC Settings on page 74
• See SpMet Settings on page 74
• See SpCO Settings on page 76
• See ORi Settings on page 77
www.masimo.com 60 Masimo
Page 63

Rad-97 Chapter 4: Operation
In Vivo Adjustment Overview
In Vivo is currently not available in the U.S.A. and territories relying on FDA market
clearance.
The In Vivo Adjustment feature lets clinicians manually adjust the displayed value(s) of one
or more clinical parameters to match a corresponding laboratory reference during continuous
trending. To remind clinicians that the feature is active, an offset value displays alongside
the adjusted parameter value.
The In Vivo Adjustment feature for a parameter can be turned on by accessing the In Vivo
screen in the Settings menu for that parameter. After enabling the feature, set an offset
value. Once the feature is enabled, a positive or a negative offset value appears, as shown in
the below illustration.
The In Vivo offset amount is set to zero when any of the following conditions occur:
• Cable or sensor is disconnected from device.
• Sensor goes off patient causing a sensor initialization to occur.
• Eight hours has elapsed since the In Vivo offset was activated.
• Restore of factory defaults.
• User turns off In Vivo.
Offset Value
When In Vivo Adjustment is activated for a specific parameter, the offset value appears
beneath that specific parameter. A positive value means that the displayed parameter value
has been increased (according to a laboratory reference value, as entered by a clinician) and a
negative value means the displayed parameter value has been decreased (according to a
laboratory reference value, as entered by a clinician).
In the example below, the displayed SpO
the displayed SpHb value of 16.0 takes into account an offset of +0.4.
value of 96 takes into account an offset of -1.0, and
2
The In Vivo Adjustment feature can be set to On or Off. The factory default setting is Off. If set
to On, the parameter value is adjusted and an offset value appears. The offset value is set by
the user.
The feature applies to any of the following parameters:
• See In Vivo for SpO2 on page 64
• See In Vivo for SpHb on page 73
• See In Vivo for SpCO on page 76
• See In Vivo for SpMet on page 75
www.masimo.com 61 Masimo
Page 64
Rad-97 Chapter 4: Operation
High Limit is the upper threshold that
SpO2 Settings
Allows access to any of the following options:
SpO2 Alarms on page 62
Additional Settings for SpO2 on page 63
In Vivo for SpO2 on page 64
Trends on page 97
About Parameter Information on page 78
About Desat Index on page 128
SpO2 Alarms
From the Alarms screen, change any of the following options:
Options Description
High Limit
Low Limit
triggers an alarm.
Low Limit is the lower threshold that
triggers an alarm.
Sets the Rapid Desat limit threshold
to the selected amount below the Low
Alarm Limit. When an SpO2 value falls
Rapid Desat
below the Rapid Desat limit the audio
and visual alarms are immediately
triggered without respect to alarm
delay.
When an alarm condition is met, this
Alarm Delay
feature delays the audible part of an
alarm.
Silence
Duration
Sets the amount of time that the
alarm is silenced.
Alarm
Priority
Factory
Default
Settings
Medium Off
High 88%
NA -10%
NA
15
seconds
NA 2 minutes
User
Configurable
Settings
2% to 99% in
steps of 1%, or
Off
When set to
Off, alarm is
disabled
1% to 98% in
steps of 1%
Off, -5%, or
-10%
0, 5, 10, or 15
seconds
30 sec, 1 or 2
minutes
www.masimo.com 62 Masimo
Page 65

Rad-97 Chapter 4: Operation
Options Description
Alarm
Priority
Factory
Default
Settings
User
Configurable
Settings
ATA establishes patient-specific limit
Adaptive
Threshold
Alarm (ATA)
thresholds based upon the baseline
value of the parameter.
See Adaptive Threshold Alarm (ATA)
NA Off Off or On
Feature on page 127.
Additional Settings for SpO2
From the Additional Settings screen, change any of the following options:
Options Description Factory
Default
Settings
Averaging
Time*
The length of time over which the
system calculates the average of all
8 seconds** 2-4, 4-6, 8, 10, 12, 14,
data points.
FastSat See FastSat Overview on page 63. Off Off or On
* With FastSat the averaging time is dependent on the input signal.
** Defaults to 2-4 seconds when in Sleep Study mode. See Sleep Study on page 90.
*** For the 2 and 4 second settings the averaging time may range from 2-4 and 4-6 seconds,
respectively.
User Configurable
Settings
or 16 seconds***
FastSat Overview
FastSat enables rapid tracking of arterial oxygen saturation changes. Arterial oxygen
saturation data is averaged using pulse oximeter averaging algorithms to smooth the trend.
When Rad-97 is set to FastSat On, the averaging algorithm evaluates all saturation values,
providing an averaged saturation value that is a better representation of the patient’s current
oxygenation status. With FastSat set to On, the averaging time is dependent on the input
signal.
www.masimo.com 63 Masimo
Page 66

Rad-97 Chapter 4: Operation
In Vivo for SpO2
In Vivo is currently not available in the U.S.A. and territories relying on FDA market
clearance.
From the In Vivo screen, change any of the following options:
Options Description Factory Default
Settings
Enabled See In Vivo Adjustment
Off On or Off
Overview on page 61.
Offset
Amount
See In Vivo Adjustment
Overview on page 61.
0.0 when turned
on
PR Settings
From the PR Settings screen, change any of the following options:
PR Alarms on page 64
Trends on page 97
About Parameter Information on page 78
PR Alarms
From the PR Alarms screen, change any of the following options:
Options Description Alarm
Priority
Factory
Default
Settings
User Configurable
Settings
-6.0% to +6.0%, in steps
of 0.1%
User Configurable
Settings
High Limit High Limit is the upper
threshold that triggers an
alarm.
Low Limit Low Limit is the lower
threshold that triggers an
High 140 bpm
35 bpm to 235 bpm,
in steps of 5 bpm
High 50 bpm 30 bpm to 230 bpm,
in steps of 5 bpm
alarm.
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 sec, 1, 2 or 5
minutes
www.masimo.com 64 Masimo
Page 67

Rad-97 Chapter 4: Operation
Pi Settings
From the Pi Settings screen, access any of the following screens:
Pi Alarms on page 65
Additional Settings for Pi on page 66
Trends on page 97
About Parameter Information on page 78
Pi Delta on page 130
Pi Alarms
From the Alarms screen, change any of the following options:
Options Description Alarm
Priority
High Limit High Limit is the upper
Medium Off 0.04 to 0.09 in
threshold that triggers an
alarm.
Low Limit Low Limit is the lower
Medium 0.3 Off, or 0.03 to 0.09
threshold that triggers an
alarm.
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 seconds or 1, 2, or
Factory
Default
Settings
User Configurable
Settings
steps of 0.01
0.10 to 0.90 in steps
of 0.10
1 to 19 in steps of 1,
or Off
in steps of 0.01
0.10 to 0.90 in steps
of 0.10
1 to 18 in steps of 1
5 minutes
www.masimo.com 65 Masimo
Page 68

Rad-97 Chapter 4: Operation
system calculates the average of all data
Additional Settings for Pi
From the Additional Settings screen, change the following option:
Options Description Factory
Default
Settings
Averaging
The length of time over which the
Long Short or Long
Time
points.
PVi Settings
From the PVi Settings screen, access any of the following options:
PVi Alarms on page 66
Additional Settings for PVi on page 67
Trends on page 97
About Parameter Information on page 78
PVi Alarms
From the Additional Settings screen, change any of the following options:
Options Description Alarm
Priority
High Limit High Limit is the upper
Medium 40 2 to 99, in steps of 1,
threshold that triggers an
alarm.
Factory
Default
Settings
User Configurable
Settings
User Configurable
Settings
or Off
When set to Off,
alarms are disabled.
Low Limit Low Limit is the lower
threshold that triggers an
alarm.
Silence
Duration
Sets the amount of time
that the alarm is silenced.
www.masimo.com 66 Masimo
Medium 5 Off or 1 to 98 in steps
of 1
When set to Off,
alarms are disabled.
NA 2 minutes 30 seconds or 1, 2, 5,
or 10 minutes
Page 69

Rad-97 Chapter 4: Operation
system calculates the average of all data
Additional Settings for PVi
From the Additional Settings screen, change the following option:
Options Description Factory
Default
User Configurable
Settings
Settings
Averaging
The length of time over which the
Long Short or Long
Time
points.
Respiration Rate (RR) Settings
RRp is currently not available in the U.S.A. and territories relying on FDA market
clearance.
Rad-97 can determine Respiration Rate (RR) either by the acoustic signal (RRa) or the
plethysmographic waveform (RRp). For more information, see:
RRa Settings on page 68
RRp Settings on page 69
From the RR Settings screen, change any of the following options:
RRa Alarms on page 68
RRp Alarms on page 70
Additional Settings for RRa on page 69
Additional Settings for RRp on page 71
Trends on page 97
About Parameter Information on page 78
www.masimo.com 67 Masimo
Page 70

Rad-97 Chapter 4: Operation
RRa Settings
RRa is active when the following conditions are all met:
• RRa is installed on the Rad-97.
• A dual rainbow cable is connected.
• An acoustic sensor is connected.
Note: See the Directions for Use provided with the acoustic sensor.
When using an acoustic sensor, Respiration Rate (RR) is determined by the acoustic (RRa)
signal. See rainbow Acoustic Monitoring™ (RAM™) on page 31. When the respiratory rate is
determined by the acoustic signal, the Main Screen labels respiratory rate as RRa, as shown
below.
From the RR Settings screen, access any of the following screens:
RRa Alarms on page 68
Additional Settings for RRa on page 69
About Parameter Information on page 78
RRa Alarms
From the Alarms screen, change any of the following options:
Options Description Alarm
Priority
High Limit High Limit is the upper
High 30 breaths
threshold that triggers an
alarm.
Low Limit Low Limit is the lower
High 6 breaths
threshold that triggers an
alarm.
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 seconds or 1, 2 or 5
www.masimo.com 68 Masimo
Factory
Default
Settings
per minute
per minute
User Configurable
Settings
6 to 69 breaths per
minute in steps of 1
breath per minute, or
Off
Off, or 5 to 68 breaths
per minute in steps of 1
breath per minute
minutes
Page 71

Rad-97 Chapter 4: Operation
Options Description Alarm
Priority
Factory
Default
User Configurable
Settings
Settings
Respiratory
Pause
The duration of time that
triggers an alarm if no
NA 30 seconds 15, 20, 25, 30, 35, or 40
seconds
breaths are detected.
Alarm Delay When a High or Low alarm
condition occurs, this
NA 30 seconds 0, 10, 15, 30, or 60
seconds
feature delays the audible
part of an alarm.
Additional Settings for RRa
From the Additional Settings screen, change any of the following options:
Options Description Factory
Default
Settings
Averaging
Time
The length of time over which the
system calculates the average of all
Slow Trending, No
data points.
Freshness The duration of time that, during
5 minutes 0, 1, 5, 10, or 15
interference, the system displays the
last valid reading.
User Configurable
Settings
Averaging, Fast,
Medium, or Slow
minutes
RRp Settings
RRp is currently not available in the U.S.A. and territories relying on FDA market
clearance.
When using a pulse oximetry or pulse CO-Oximetry sensor with Rad-97, respiration rate can
be determined by the plethysmographic waveform (RRp). This method measures a patient's
respiratory rate based on plethysmographic amplitude changes corresponding to the
respiratory cycle. When using a pulse oximetry or pulse CO-Oximetry sensor, RRp alarms and
RRp settings are active and the Main Screen labels respiratory rate as RRp, as shown below.
www.masimo.com 69 Masimo
Page 72

Rad-97 Chapter 4: Operation
Note that Rad-97 can monitor RRa or RRp but not both simultaneously.
RRp is active when the following conditions have all been met:
• RRp is installed on the Rad-97.
• No dual rainbow cable is connected.
• A pulse oximetry or pulse CO-Oximetry sensor is connected.
• The optical sensor must support RRp.
From the RR Settings screen, access any of the following screens:
RRp Alarms on page 70
Additional Settings for RRp on page 71
RRp Alarms
From the Alarms screen, change any of the following options:
Options Description Alarm
Priority
High
Limit
High Limit is the upper
threshold that triggers an
High 30 breaths
alarm.
Low Limit Low Limit is the lower
High 6 breaths
threshold that triggers an
alarm.
Silence
Duration
Alarm
Delay
Sets the amount of time
that the alarm is silenced.
When an alarm condition is
met, this feature delays the
NA 2 minutes 30 seconds or 1, 2, or 5
NA 30 seconds 0, 10, 15, 30, or 60
audible part of an alarm.
Factory
Default
Settings
per minute
per minute
User Configurable
Settings
6 to 69 breaths per
minute in steps of 1
breath per minute, or Off
Off, or 5 to 68 breaths
per minute in steps of 1
breath per minute
minutes
seconds
www.masimo.com 70 Masimo
Page 73

Rad-97 Chapter 4: Operation
Additional Settings for RRp
From the Additional Settings screen, change any of the following options:
Options Description Factory
Default
Settings
Averaging
Time
The length of time over which the
system calculates the average of all
Slow No Averaging, Fast,
data points.
Freshness The duration of time that, during
5 minutes 0, 1, 5, 10, or 15
interference, the system displays the
last valid reading.
SpHb Settings
From the SpHb Settings screen, access any of the following screens:
SpHb Alarms on page 72
Additional Settings for SpHb on page 73
In Vivo for SpHb on page 73
Trends on page 97
About Parameter Information on page 78
User Configurable
Settings
Medium, Slow,
Trending
minutes
www.masimo.com 71 Masimo
Page 74

Rad-97 Chapter 4: Operation
SpHb Alarms
From the Alarms screen, change any of the following options:
Options Description Alarm
Priority
High Limit High Limit is the upper
High 17.0 g/dL
threshold that triggers
an alarm.
Low Limit Low Limit is the lower
High 7.0 g/dL
threshold that triggers
an alarm.
Factory
Default
Settings
(11.0
mmol/L)
(170 g/L)
(4.0
mmol/L)
(70 g/L)
User Configurable
Settings
2.0 g/dL to 24.5 g/dL in
steps of 0.1 g/dL, or Off
(2.0 mmol/L to 15.0
mmol/L in steps of 0.1
mmol/L, or Off)
(20 g/L to 245 g/L in steps
of 1 g/L, or Off)
When SpHb Precision is
set to 1.0, values are
rounded down.
When set to Off, alarm is
disabled.
Off, or 1.0 g/dL to 23.5
g/dL in steps of 0.1 g/dL
(Off, or 1.0 mmol/L to 14.5
mmol/L, in steps of 0.1
mmol/L)
(Off, or 10 g/L to 235 g/L
in steps of 1 g/L)
When SpHb Precision is
set to 1.0, values are
rounded down.
When set to Off, alarm is
disabled.
Silence
Duration
Sets the amount of time
that the alarm is
NA 2 minutes 30 seconds, 1, 2 or 5
minutes
silenced.
www.masimo.com 72 Masimo
Page 75

Rad-97 Chapter 4: Operation
Additional Settings for SpHb
From the Additional Settings screen, change any of the following options:
Options Description Factory
Default
Settings
Averaging
Time
Calibration Provides an arterial or venous value that
The length of time over which the system
calculates the average of all data points.
Medium Short, Medium,
Arterial Arterial or
displays on the main screen.
Precision
(units of g/dL
and mmol/L)
Allows the user to set the precision of the
displayed SpHb value.
Note: When unit is g/L, Precision is always 1
0.1 0.1, 0.5, or 1.0
User
Configurable
Settings
or Long
Venous
(whole numbers)
Unit of
Measure*
Displays total hemoglobin (SpHb) as g/dL
(grams per deciliter), g/L (grams per liter), or
g/dL g/dL, g/L, or
mmol/L
mmol/L (millimoles per liter). Unit of
Measure cannot be changed during active
monitoring.
*Changing Unit of Measure will delete all prior trend data for all parameters.
In Vivo for SpHb
In Vivo is currently not available in the U.S.A. and territories relying on FDA market
clearance.
From the In Vivo screen, change any of the following options:
Options Description Factory Default
User Configurable Settings
Settings
Enabled See In Vivo Adjustment
Off On or Off
Overview on page 61.
Offset
Amount
See In Vivo Adjustment
Overview on page 61.
0.0 when turned
on
-3.0 g/dL to +3.0 g/dL in
steps of ± 0.1 g/dL
www.masimo.com 73 Masimo
Page 76

Rad-97 Chapter 4: Operation
SpOC Settings
From the SpOC Settings screen, access the following screens:
SpOC Alarms on page 74
Trends on page 97
About Parameter Information on page 78
SpOC Alarms
From the SpOC Alarms screen, access the following screens:
Options Description Alarm
Priority
Factory
Default
Settings
High Limit High Limit is the upper
Medium 25 2 ml/dl to 34 ml/dl in
threshold that triggers an
alarm.
Low Limit Low Limit is the lower
High 10 Off, or 1 ml/dl to 33
threshold that triggers an
alarm.
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 seconds or 1, 2, or 5
SpMet Settings
From the SpMet Settings screen, access the following screens:
SpMet Alarms on page 75
In Vivo for SpMet on page 75
Trends on page 97
About Parameter Information on page 78
User Configurable
Settings
steps of 1 ml/dl, or Off
ml/dl in steps of 1
ml/dl
minutes
www.masimo.com 74 Masimo
Page 77

Rad-97 Chapter 4: Operation
SpMet Alarms
From the Alarms screen, change any of the following options:
Options Description Alarm
Priority
Factory
Default
User Configurable
Settings
Settings
High Limit High Alarm Limit is the
upper threshold that
triggers an alarm.
High 3.0 1.0% to 2.0% in
steps of 0.1%
2.5% to 99.5% in
steps of 0.5%, or Off
Low Limit Low Alarm Limit is the lower
threshold that triggers an
alarm.
Medium Off Off, or 0.1% to 2.0%
in steps of 0.1%
2.5% to 99% in steps
of 0.5%
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 seconds, or 1, 2,
or 5 minutes
In Vivo for SpMet
In Vivo is currently not available in the U.S.A. and territories relying on FDA market
clearance.
From the In Vivo screen, access the following screens:
Options Description Factory Default
Settings
User Configurable
Settings
Enabled See In Vivo Adjustment
Off On or Off
Overview on page 61
Offset
Amount
See In Vivo Adjustment
Overview on page 61.
0.0 when turned
on
-3.0% to +3.0% in steps
of 0.1%
www.masimo.com 75 Masimo
Page 78

Rad-97 Chapter 4: Operation
SpCO Settings
From the SpCO Settings screen, access the following screens:
SpCO Alarms on page 76
In Vivo for SpCO on page 76
Trends on page 97
About Parameter Information on page 78
SpCO Alarms
From the SpCO Settings screen, access the following screens:
Options Description Alarm
Priority
Factory
Default
User Configurable
Settings
Settings
High Limit High Limit is the upper
threshold that triggers an
alarm.
High 10 2% to 98%, in steps
of 1%, or Off
When set to Off,
alarm is disabled
Low Limit Low Limit is the lower
threshold that triggers an
alarm.
Medium Off Off or 1% to 97%, in
steps of 1%
When set to Off,
alarm is disabled
Silence
Duration
Sets the amount of time
that the alarm is silenced.
NA 2 minutes 30 seconds, 1, 2, or 5
minutes
In Vivo for SpCO
In Vivo is currently not available in the U.S.A. and territories relying on FDA market
clearance.
From the In Vivo screen, access the following screens:
Options Description Factory Default
Settings
User Configurable
Settings
Enabled See In Vivo Adjustment
Off On or Off
Overview on page 61.
www.masimo.com 76 Masimo
Page 79

Rad-97 Chapter 4: Operation
Options Description Factory Default
Settings
Offset
Amount
See In Vivo Adjustment
Overview on page 61.
0.0 when turned
on
User Configurable
Settings
-9.0% to +9.0% in steps
of 0.1%
ORi Settings
ORi is currently not available in the U.S.A. and territories relying on FDA market clearance.
From the ORi Settings screen, access the following screens:
ORi Alarms on page 77
Trends on page 97
About Parameter Information on page 78.
ORi Alarms
From the ORi Alarms screen, access the following screens:
Options Description Alarm
Priority
Low Limit Low Limit is the lower
Medium Off Off, or 0.01 to 0.98
threshold that triggers an
alarm.
Factory
Default
Settings
User Configurable
Settings
in steps of 0.01
Trending
Down Alarm
Trending Down Alarm is
displayed when a rapid
N/A Off On or Off
decrease in ORi is measured.
Silence
Duration
Sets the amount of time that
the alarm is silenced.
N/A 2 minutes 30 seconds or 1, 2,
or 5 minutes
www.masimo.com 77 Masimo
Page 80

Rad-97 Chapter 4: Operation
About Parameter Information
Additional information about each parameter is available.
To access additional information about parameters:
1. From the Parameter Settings screen, touch the About icon. The following is an
example for SpO
.
2
2. An About screen appears for the selected parameter and displays information
about the parameter.
Additional Settings
Use the Additional Settings screen to configure the following:
Option Description Factory
Default
Settings
Sensitivity
Mode
Change Sensitivity
Mode.
See Sensitivity
Modes Overview on
APOD MAX, APOD, NORM
page 52.
Waveform
Mode
Change the
Waveform View.
Pleth + Sig IQ
+ Acoustic
See Waveform Mode
on page 79.
SmartTone Enable or disable the
Off On, Off
SmartTone.
See Sounds on page
85.
User Configurable Settings
Acoustic, Pleth + Sig IQ, Pleth + Sig IQ
+ Acoustic, PVi Pleth + Sig IQ, or PVi
Pleth + Sig IQ + Acoustic
www.masimo.com 78 Masimo
Page 81

Rad-97 Chapter 4: Operation
Option Description Factory
User Configurable Settings
Default
Settings
SpO2 low %
limit
Set the SpO2 low
limit alarm.
See SpO2 Settings on
page 62.
Off Off, 1% to 98%
Waveform Mode
The following section contains examples of some of the waveforms viewable on the Main
Screen.
Signal IQ Indicators
Signal IQ (SIQ) indicators are displayed as vertical bars for each individual pulsation. The
height of the bar provides an assessment of the confidence in the SpO
displayed.
measurement
2
Acoustic Waveform View
The RRa waveform is located above the parameter values. Acoustic Respiratory Rate (RRa)
must be available for this feature to be shown. This view contains RRa waveform only.
www.masimo.com 79 Masimo
Page 82

Rad-97 Chapter 4: Operation
Pleth + Sig IQ + Acoustic View
The waveform is located above the parameter values. This view contains the Pleth waveform,
signal quality indicators, and acoustic waveform (if RRa is available).
Noninvasive Blood Pressure (NIBP) Settings
Note: This feature is optional on Rad-97 devices.
The NIBP menu allows the user to view and customize settings for the NIBP module by
changing any of the following options:
Parameter Settings
See Parameter Settings for Noninvasive Blood Pressure (NIBP) on page 81.
Intervals
See Intervals for NIBP on page 84.
Additional Settings
See Additional Settings for NIBP on page 84.
Calibration
See Calibration for NIBP on page 85.
www.masimo.com 80 Masimo
Page 83

Rad-97 Chapter 4: Operation
Parameter Settings for Noninvasive Blood Pressure (NIBP)
From the NIBP screen, touch Parameter Settings, and then change individual parameter
settings/alarms by selecting one of the following parameters:
Systolic/Diastolic
See SYS/DIA Settings on page 81.
Mean Arterial Pressure
See MAP Settings on page 82.
Pulse Rate
See Pulse Rate (PR) on page 83.
SYS/DIA Settings
From the Systolic/Diastolic Settings screen, access the following screens:
SYS/DIA Alarms on page 81
Trends for NIBP on page 82
About Parameter Information on page 78
SYS/DIA Alarms
From the Systolic/Diastolic Settings screen, touch Alarms, and then change any of the
following options:
Options Description
Systolic
High Limit
High Limit is the upper
threshold that triggers an
alarm.
Alarm
Priority
Medium 220
www.masimo.com 81 Masimo
Factory
Default
Settings
User Configurable
Settings
42-259 in steps of 1,
or Off
When set to Off,
alarm is disabled
Page 84

Rad-97 Chapter 4: Operation
Options Description
Systolic Low
Limit
Diastolic
High Limit
Diastolic
Low Limit
Low Limit is the lower
threshold that triggers an
alarm.
The High Limit is upper
threshold that triggers an
alarm.
Low Limit is the lower
threshold that triggers an
alarm.
Alarm
Priority
Medium 75
Medium 110
Medium 35
Factory
Default
Settings
User Configurable
Settings
Off, or 41-258 in
steps of 1
When set to Off,
alarm is disabled
22-199 in steps of 1,
or Off
When set to Off,
alarm is disabled
Off, or 21-198 in
steps of 1
When set to Off,
alarm is disabled
Trends for NIBP
From the Systolic/Diastolic Settings screen, touch Trends, and then change any of the
following options:
Options Description
Factory Default
Settings
Configurable
Options
Y-Axis
Max
Y-Axis
Min
The NIBP Trend Max, indicating the
highest value that will be shown.
The NIBP Trend Min, indicating the lowest
value that will be shown.
260
20
21-260 in steps
of 1
20-259 in steps
of 1
MAP Settings
From the Mean Arterial Pressure Settings screen, access the following screens:
MAP Alarms on page 83
About Parameter Information on page 78
www.masimo.com 82 Masimo
Page 85

Rad-97 Chapter 4: Operation
MAP Alarms
From the Mean Arterial Pressure screen, touch Alarms, and then change any of the following
options:
Options Description
High
Limit
Low
Limit
High Limit is the upper
threshold that triggers an
alarm.
Low Limit is the lower
threshold that triggers an
alarm.
Alarm
Priority
Medium 120
Medium 50
Factory
Default
Settings
User Configurable
Settings
28-219 in steps of 1,
or Off
When set to Off,
alarm is disabled
Off, or 27-218 in
steps of 1
When set to Off,
alarm is disabled
Pulse Rate (PR)
From the Pulse Rate Settings screen, access the following screens:
Pulse Rate Alarms on page 83
About Parameter Information on page 78
Pulse Rate Alarms
From the Pulse Rate Settings screen, touch Alarms, and then change any of the following
options:
Options Description
High
Limit
Low
Limit
High Limit is the upper
threshold that triggers an
alarm.
Low Limit is the lower
threshold that triggers an
alarm.
Alarm
Priority
Medium 120
Medium 50
Factory
Default
Settings
User Configurable
Settings
40-210 in steps of 5,
or Off
When set to Off,
alarm is disabled
Off, or 35-215 in
steps of 5
When set to Off,
alarm is disabled
www.masimo.com 83 Masimo
Page 86

Rad-97 Chapter 4: Operation
mode
Intervals for NIBP
From the NIBP screen, touch Intervals, and then change any of the following options:
Options Description Factory
Default
User Configurable
Settings
Settings
Set Mode The mode of measurement for NIBP. Automatic Automatic or Stat
Note: Option available when
Interval
Automatic mode is selected.
Automatic interval measurement mode
will take blood pressure measurements
15 minutes
2, 3, 4, 5, 10, 15, 30,
60, or 90 minutes
once every desired interval.
Note: Option available when Stat
Stat
Duration
is selected.
Stat interval measurement mode will
take blood pressure measurements
10 minutes 5 or 10 minutes
continually for the desired duration.
Start
Auto/Start
Starts NIBP measurement NA NA
Stat
Additional Settings for NIBP
Use the Additional Settings screen to configure the following option:
Options Description Factory Default
Settings
Measurement
Timeout
Set the measurement
timeout value.
15 minutes
User Configurable
Settings
5, 10, 15, 30, 60, or 90
minutes
www.masimo.com 84 Masimo
Page 87

Rad-97 Chapter 4: Operation
Calibration for NIBP
The Calibration option on the NIBP menu allows a qualified service professional to access
calibration settings and tools for the NIBP module. For more information, see Chapter 12:
Service and Maintenance on page 167.
Note: This section is provided as a reference and intended for qualified service professionals
only.
Sounds
Use the Sounds screen to control the volume of sounds and duration of audio pause on
Rad-97. Users can also access the Sounds screen by pressing the Sounds icon on the Status
Bar. See About the Status Bar on page 49.
Option Description Factory
Default
User Configurable
Settings
Settings
Alarm
Volume
Pulse Tone
Volume
Sets the alarm volume level.
Sets the pulse tone volume level. 3
Sets the length of time that the
Audio Pause
Duration
audible alarm remains silenced,
when Audio Pause is enabled. See
Audio Pause on page 126.
SmartTone Allows the audible pulse to continue
4 (Highest
volume)
2 minutes
Off On or Off
1 to 4 - Slide towards the
left to decrease volume
and to silence.
0 to 4 - Slide towards the
left to decrease volume
and to silence.
1, 2, or 3 minutes,
Permanent *,**, or
Permanent with
Reminder *,***.
to beep when the pleth graph shows
signs of motion.
* Requires user to have All Mute Enabled turned on in the Access Control menu. See Access
Control on page 94.
** If Permanent is selected, there will be no audible alarms, but visual alarms will still display.
www.masimo.com 85 Masimo
Page 88

Rad-97 Chapter 4: Operation
*** If Permanent with Reminder is selected, a tone will sound every three (3) minutes as a
reminder that Permanent is active.
Device Settings
The Device Settings menu allows the user to view and customize settings for Rad-97.
Note: When in Home mode, items below marked with an * are displayed in the Main Menu;
no other device settings are available. See Home on page 89.
The Device Settings options are:
Localization
See Localization on page 87.
Device Mode
See Device Mode on page 88.
Screen Orientation*
See Screen Orientation on page 91.
Ethernet
See Ethernet on page 91.
Wi-Fi
See Wi-Fi on page 92.
Bluetooth
See Bluetooth on page 92.
Rad-97 Battery*
See Rad-97 Battery on page 93.
Brightness*
See Brightness on page 94.
Access Control
See Access Control on page 94.
www.masimo.com 86 Masimo
Page 89

Rad-97 Chapter 4: Operation
Device Output
See Device Output on page 96.
Localization
Use the Localization screen to view the current date and time and configure settings related
to local time, language and geography. The user can also access the Localization screen by
pressing the current time on the Status Bar. See About the Status Bar on page 49.
Option Description Factory Default
Settings
Language
Date Format
Time Format
Line
Frequency
Select the language display
for Rad-97.
Set the display format for
current date.
Set the display format for
current time.
Set to match regional power
line frequency.
English
mm/dd/yy mm/dd/yy or dd/mm/yy
12 hour 12 or 24 hour
60 Hz 50 Hz or 60 Hz
User Configurable
Settings
Choose from available
languages.
Date Set the current date. N/A month, date, and year
Time Set the current time. N/A
hour and minutes
AM or PM
www.masimo.com 87 Masimo
Page 90

Rad-97 Chapter 4: Operation
Device Mode
The Device Mode screen allows the user to select the device operating mode. Continuous
Monitoring is the default device mode. When the Rad-97 is turned off, the device mode is
stored. Rad-97 will start in the same mode when turned on again.
Note: Home mode is an optional feature.
Access to the Device Mode screen is password protected.
1. When the screen displays, press the key.
2. Enter the following: 6 2 7 4
To undo an entry, press the Backspace key.
3. Press the Enter key to access the Device Mode screen.
4. Select the desired option and select OK to set the device mode.
Continuous Monitoring
Continuous Monitoring mode is the standard mode of operation of the Rad-97 and includes
all functionality outlined in this Operator's Manual.
www.masimo.com 88 Masimo
Page 91

Rad-97 Chapter 4: Operation
Home
When optional Home mode is available, Rad-97 operates using the Continuous Monitoring
settings in effect at the time Home mode is enabled (Profiles, Alarms, Trends, etc.). Rad-97
operation changes as follows when in Home mode:
• Alarm tone volume is set to the highest level and cannot be changed.
• Pulse tone volume can be changed; however, all other sound settings are disabled.
• The Alarm Silence button is not included on the Main Screen. See About Alarms on
page 124.
• Profile settings are not available (device operates in the profile set during
Continuous Monitoring mode).
• When parameters in the Main Menu are selected, only parameter information is
displayed. Settings are not displayed or available. See About Parameter
Information on page 78.
• Profiles, Device Settings, and Trend Settings are not displayed in the Main Menu.
Change Settings/Exit Home Mode
To change settings or switch to a different device mode, select the Rad-97 menu from the
Main Menu.
1. When the screen displays, press the key.
2. Enter the following: 6 2 7 4
To undo an entry, press the Backspace key.
3. Press the Enter key. The Main Menu screen will display.
• From the Main Menu, make changes to Rad-97 settings as necessary and
select the back button to return to Home mode operation.
• From the Main Menu, select Device Settings > Device Mode to change
operating modes.
www.masimo.com 89 Masimo
Page 92

Rad-97 Chapter 4: Operation
Sleep Study
When in Sleep Study mode, Rad-97 operates using the Continuous Monitoring settings in
effect at the time Sleep Study mode is enabled (Profiles, Alarms, etc.). Rad-97 operation
changes as follows when in Sleep Study mode:
• Audible alarms are disabled.
• Visual alarms display. If the display is off when an alarm triggers, the display
wakes and displays the visual alarm until the alarm event is resolved.
• Sounds are disabled and cannot be changed.
• Profile settings are not available (device operates in the profile set during
Continuous Monitoring mode).
• Home button illumination turns off.
• SpO2 averaging time defaults to 2-4 seconds and cannot be changed. See
Additional Settings for SpO2 on page 63.
• The display times out and turns off after approximately 10 seconds. Touch the
display to wake.
Change Settings or Device Mode
To make changes to unavailable settings listed above or switch to a different device mode,
select Device Settings > Device Mode.
1. When the screen displays, press the key.
2. Enter the following: 6 2 7 4
To undo an entry, press the Backspace key.
3. Select Continuous Monitoring to change operating modes, allowing changes to
previously unavailable settings.
4. After making changes, go back to Device Mode and select Sleep Study to resume.
www.masimo.com 90 Masimo
Page 93

Rad-97 Chapter 4: Operation
Screen Orientation
Use Screen Orientation to set screen preferences.
From the Screen Orientation screen, change any of the following options:
Options Description Factory Default
Settings
Auto
Orientation
Allows the device to
automatically adjust screen
On Off or On
User Configurable
Settings
content depending on device
orientation.
Orientation When Auto Orientation is Off,
allows the user to manually set
screen orientation.
Portrait (with
device in vertical
position)
Portrait, Inverted
Portrait, Landscape, or
Inverted Landscape
Landscape (with
device in
horizontal
position)
Ethernet
Use the Ethernet screen to enable or disable Ethernet connectivity. When Ethernet
connectivity is enabled, the Ethernet icon will appear in the Status Bar. The user can also
access the Ethernet screen by pressing the Ethernet icon on the Status Bar. See About the
Status Bar on page 49.
Option Description Factory Default
Settings
User Configurable
Settings
Ethernet
Enables or disables Ethernet
connectivity.
On On or Off
Additional fields in the Ethernet screen display read-only settings about the Ethernet
connectivity that cannot be configured by the user.
www.masimo.com 91 Masimo
Page 94

Rad-97 Chapter 4: Operation
Wi-Fi
The Wi-Fi radio allows for networked communication of data and alarm signals between
Rad-97 and a secondary patient monitoring station, Masimo Patient SafetyNet, over an IEEE
802.11 a/b/g wireless network.
Rad-97 uses only configured MAC addresses to establish wireless communications to prevent
unauthorized connections to other wireless devices. As risk mitigation, in the event of the
loss of wireless communication, Rad-97 alarm capabilities are designed to be independent of
Wi-Fi communication in order to ensure alarms are received.
Use the Wi-Fi screen to enable or disable Wi-Fi connectivity. When Rad-97 is connected to a
Wi-Fi network, the Wi-Fi icon on the Status Bar indicates the strength of the connection. The
user can also access the Wi-Fi screen by pressing the Wi-Fi icon on the Status Bar. See About
the Status Bar on page 49.
Option Description Factory Default
Settings
Wi-Fi
Enables or disables Wi-Fi
connectivity.
Off On or Off
User Configurable
Settings
Additional fields in the Wi-Fi screen display read-only settings about the Wi-Fi connection
that cannot be configured by the user.
Your Masimo sales representative can provide necessary information regarding an initial
Wi-Fi connection.
Bluetooth
Use the Bluetooth screen to enable or disable Bluetooth connectivity. When Bluetooth
connectivity is enabled, the Bluetooth icon will appear in the Status Bar. The user can also
access the Bluetooth screen by pressing the Bluetooth icon on the Status Bar. See About the
Status Bar on page 49.
Option Description Factory
Default
Settings
Bluetooth
Enables or disables Bluetooth
connectivity.
Off On or Off
User
Configurable
Settings
www.masimo.com 92 Masimo
Page 95

Rad-97 Chapter 4: Operation
Option Description Factory
Default
Settings
User
Configurable
Settings
Used in conjunction with MyView on
Presence
Monitoring
Masimo Patient SafetyNet (see the
Masimo Patient SafetyNet Operator's
Off On or Off
Manual).
Additional fields in the Bluetooth screen display read-only settings about the Bluetooth
connection that cannot be configured by the user.
Your Masimo sales representative can provide necessary information regarding an initial
Bluetooth connection.
Note: Presence Monitoring must be disabled in order for Rad-97 to function. For more
information on how to configure the Masimo MyView Presence Tag, see the Masimo Patient
SafetyNet Operator's Manual.
Rad-97 Battery
Use the Battery screen to view the specific percentage of charge remaining in Rad-97's
battery. The user can also access the Battery screen by pressing the Battery icon on the
Status Bar. See About the Status Bar on page 49.
www.masimo.com 93 Masimo
Page 96

Rad-97 Chapter 4: Operation
Option Description
State of Charge Provides a read-only display of battery level remaining.
Battery Diagnostics Allows trained personnel to access battery diagnostic information.
Brightness
Use the Brightness screen to adjust the brightness of Rad-97's display.
Option Description Factory
Default
User Configurable
Settings
Settings
Auto
Brightness
Brightness
Allows automatic adjustment of display
brightness based on the ambient light
level.
Adjust the brightness level of the
display manually.
Off On or Off
4
1 (dimmest), 2, 3, 4
(brightest)
Access Control
The Access Control screen contains configurable options and settings that require a password
to view or change.
To enter Access Control
1. Press the key.
2. When the screen displays, enter the following: 6 2 7 4
Asterisks (****) will be displayed.
www.masimo.com 94 Masimo
Page 97

Rad-97 Chapter 4: Operation
To undo an entry, press Backspace.
3. Press Enter to access the password-protected screen.
Note: The password will have to be entered every time this screen is accessed.
Option Description Factory
Default
Settings
Power on
Profile
All Mute
Enabled
Lock Alarm
Volume
Sets the profile used when the
device is powered on. See
Chapter 5: Profiles on page 103.
Enables parameter Alarm Silence
menu option. See Sounds on
page 85.
Sets the lowest alarm volume
level.
Previous
Profile
Off On or Off
Off 3, 4, or Off
Allows the user to lock the
Screen Lock
touchscreen to prevent
Off On or Off
accidental changes.
USB Port
Baudrate
Data
Collection
Enabled
Save as
Adult*
Sets the USB port
communication speed.
Enables or disables physical data
collection mode.
Saves current profile parameter
as the Adult Profile.
921600
Off On or Off
N/A
User Configurable Settings
Previous Profile, Adult,
Pediatric, Neonatal, or User
defined profile (up to 8)
9600, 19200, 38400,
57600, 115200, 230400,
or 921600
Press Save to update the
profile.
Save as
Pediatric*
Save as Neo*
Factory
Defaults
Saves current profile parameter
as the Pediatric Profile.
Saves current profile parameter
as the Neonatal Profile.
Options are restored to factory
values.
N/A
N/A
Press Save to update the
profile.
Press Save to update the
profile.
N/A Press Restore.
* See Replacing Factory Default Settings for Adult, Pediatric and Neonatal Profiles on page
107.
www.masimo.com 95 Masimo
Page 98
Rad-97 Chapter 4: Operation
Device Output
The Device Output screen allows the user to configure additional data output options. A Nurse
Call can be triggered based on alarm, low Signal IQ events, or both. In addition, Nurse Call
Polarity can be inverted to accommodate local Nurse Call station requirements.
The Device Output screen can also be accessed by selecting the Device Output icon on the
Status Bar. See About the Status Bar on page 49.
Option Description Factory
Default
Configurable
Settings
Setting
Nurse Call
Trigger
Controls the source of monitoring which
sets off the trigger.
Alarms
Alarms,
Alarms+SIQ, or
Low SIQ
Controls the mechanism of action for
Nurse Call
Polarity
triggering to occur. Should be changed to
accommodate institutional Nurse Call
Normal
Normal or
Inverted
settings.
USB Port*
Controls the communication protocol used
to transmit parameter data to a 3rd party
device or an EMR system.
IAP
None, IAP, ASCII
1, or IntelliBridge
Identifies the type of IntelliBridge Module
IntelliBridge
Module
connected to the USB Port.
Note: USB Port selection must be
EC-10/B EC-10/B or A
Intellibridge for option to be available.
* When IAP, ASCII 1, or IntelliBridge is selected, the Device Output icon displays in the Status
Bar. If None is selected, no Device Output icons display in the Status Bar. See About the
Status Bar on page 49.
Note: The Nurse Call feature is disabled when Audio Pause is enabled and Nurse Call Trigger
is set to Alarms. For more information about Audio Pause, see Audio Pause on page 126.
www.masimo.com 96 Masimo
Page 99

Rad-97 Chapter 4: Operation
About
For information about individual parameters, see About Parameter Information on page 78.
Use the About screen to view the serial number as well as Rad-97 software and hardware
version information. These details may be helpful during troubleshooting.
Option * Description
Serial Number Displays the serial number for the device.
MCU Displays the version number of the device board software.
Processor Displays the version number of the system level software.
MX Board Displays the version number of the technology level software.
* These fields are read-only and cannot be configured by the user.
Trends
Trend settings allow the user to configure the Y-axis maximum and Y-axis minimum for each
parameter. The maximum and minimum possible values differ depending on the selected
parameter. See Customizing Trend View on page 54 for additional information.
Trend Settings
Use the Trend Settings screen to configure Trend Views on the Main Screen and trend data
storage on Rad-97.
Option Description Factory Default
Default
Duration
Clear Trends Deletes all stored trend data. N/A
www.masimo.com 97 Masimo
Sets the time duration
displayed in trend lines.
Settings
2 hours
User Configurable Settings
15, 30, or 45 minutes
1, 2, 4, 8, 12, or 24 hours
Press Clear to delete all
stored trend data.
Page 100

Rad-97 Chapter 4: Operation
Option Description Factory Default
Settings
Y-axis Min 50 0 to 95 in steps of 5
SpO2
Y-axis Max 100 5 to 100 in steps of 5
Y-axis Min 25 25 to 235 in steps of 5
PR
Y-axis Max 200 30 to 240 in steps of 5
Y-axis Min 5.0 g/dL
SpHb g/dL
Y-axis Max 20.0 g/dL
Y-axis Min 3.1 mmol/L
SpHb
mmol/L
Y-axis Max 12.4 mmol/L
Y-axis Min 50 g/L 0 to 249 g/L in steps of 1
SpHb g/L
Y-axis Max 200 g/L 1 to 250 g/L in steps of 1
Y-axis Min 0 0 to 69 in steps of 1
RRa
Y-axis Max 35 1 to 70 in steps of 1
User Configurable Settings
0.0 to 24.9 g/dL in
increments of 0.1
0.1 to 25.0 g/dL in
increments of 0.1
0.0 to 15.4 mmol/L in
increments of 0.1
0.1 to 15.5 mmol/L in
increments of 0.1
Y-axis Min 0 0 to 69 in steps of 1
RRp*
Y-axis Max 35 1 to 70 in steps of 1
Y-axis Min 0 0 to 99 in steps of 1
SpCO
Y-axis Max 40 1 to 100 in steps of 1
Y-axis Min 0.0
SpMet
Y-axis Max 15.0
www.masimo.com 98 Masimo
0.0 to 99.5 in increments
of 0.5
1.0 to 100.0 in increments
of 0.5
 Loading...
Loading...