Page 1

Operator's Manual
Pronto® Pulse CO-Oximeter®
Page 2

Page 3
These operating instructions provide the necessary information for proper operation of all
models of the Pronto. There may be information provided in this manual that is not relevant
for your system. General knowledge of pulse oximetry and an understanding of the features
and functions of Pronto are prerequisites for its proper use. Do not operate Pronto without
completely reading and understanding these instructions.
Notice: Purchase or possession of this device does not carry any express or implied license to
use with replacement parts which would, alone or in combination with this device, fall within
the scope of one of the relating patents.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Note: Cleared Use Only: The device and related accessories are cleared by the Food and Drug
Administration (FDA) and are CE Marked for noninvasive patient monitoring and may not be
used for any processes, procedures, experiments, or any other use for which the device is not
intended or cleared by the applicable regulatory authorities, or in any manner inconsistent
with the directions for use or labeling.
For professional use. See instructions for use for full prescribing information, including
indications, contraindications, warnings, and precautions.
Masimo Corporation
40 Parker
Irvine, CA 92618, USA
Tel.: 949-297-7000
Fax.: 949-297-7001
www.masimo.com
EU authorized representative for Masimo Corporation:
MDSS GmbH
Schiffgraben 41
D-30175 Hannover, Germany
Medical electrical equipment with respect to electric shock, fire, and mechanical
hazards only in accordance with UL 60601-1/CAN/CSA C22.2 No. 601.1
80fk
Patents: www.masimo.com/patents.htm
© 2016 Masimo Corporation
, Discrete Saturation Transform, DST, FST, Masimo, Pronto, Pulse CO-Oximeter, rainbow,
rainbow ReSposable, SET, Signal Extraction Technology, Signal I.Q., and SpHb are federally
registered trademarks of Masimo Corporation.
All other trademarks and registered trademarks are property of their respective owners.
www.masimo.com 1 Masimo
Page 4

Page 5

Contents
About This Manual------------------------------------------------------------------------------------------ 5
Product Description, Features, and Indications for Use -----------------------------------------------7
Product Description and Features ---------------------------------------------------------------------7
Indications for Use ---------------------------------------------------------------------------------------7
Contraindications ----------------------------------------------------------------------------------------7
Safety Information, Warnings, and Cautions ----------------------------------------------------------- 9
Safety Warnings and Cautions ------------------------------------------------------------------------ 9
Performance Warnings and Cautions --------------------------------------------------------------- 10
Cleaning and Service Warnings and Cautions ---------------------------------------------------- 13
Compliance Warnings and Cautions ---------------------------------------------------------------- 14
Chapter 1: Technology Overview ------------------------------------------------------------------------ 15
Masimo Signal Extraction Technology (Masimo SET®) ----------------------------------------- 15
rainbow Pulse CO-Oximetry Technology ----------------------------------------------------------- 17
Chapter 2: System Description -------------------------------------------------------------------------- 21
Front Panel ---------------------------------------------------------------------------------------------- 21
Rear Panel ----------------------------------------------------------------------------------------------- 23
Chapter 3: Setup ------------------------------------------------------------------------------------------- 25
Unpacking and Inspection --------------------------------------------------------------------------- 25
Power Requirements ----------------------------------------------------------------------------------- 25
Initial Setup --------------------------------------------------------------------------------------------- 25
Chapter 4: Operation -------------------------------------------------------------------------------------- 27
Basic Operation ---------------------------------------------------------------------------------------- 27
Default Settings ---------------------------------------------------------------------------------------- 29
Audible Alerts ------------------------------------------------------------------------------------------ 30
Battery Level Indicator ------------------------------------------------------------------------------- 30
Navigating the Menu ---------------------------------------------------------------------------------- 31
Trend Setup and Use --------------------------------------------------------------------------------- 33
Chapter 5: Messages ------------------------------------------------------------------------------------- 35
Messages ----------------------------------------------------------------------------------------------- 35
Chapter 6: Troubleshooting ----------------------------------------------------------------------------- 39
Troubleshooting --------------------------------------------------------------------------------------- 39
www.masimo.com 3 Masimo
Page 6

Pronto Contents
Low Perfusion ------------------------------------------------------------------------------------------- 40
Low Signal I.Q. (Low SIQ) ---------------------------------------------------------------------------- 40
Low Battery Audible Alert ---------------------------------------------------------------------------- 41
Chapter 7: Specifications --------------------------------------------------------------------------------- 43
Performance Specifications -------------------------------------------------------------------------- 43
Display Ranges ----------------------------------------------------------------------------------------- 44
Resolution ----------------------------------------------------------------------------------------------- 44
Electrical------------------------------------------------------------------------------------------------- 44
Environmental ------------------------------------------------------------------------------------------ 45
Physical Characteristics ------------------------------------------------------------------------------ 45
Trend Memory ------------------------------------------------------------------------------------------ 45
Alerts ----------------------------------------------------------------------------------------------------- 45
Display Indicators -------------------------------------------------------------------------------------- 46
Compliance --------------------------------------------------------------------------------------------- 46
Citations ------------------------------------------------------------------------------------------------- 48
Symbols-------------------------------------------------------------------------------------------------- 49
Guidance and Manufacturer's Declaration - Electromagnetic Emissions -------------------- 51
Guidance and Manufacturer's Declaration - Electromagnetic Immunity -------------------- 52
Recommended Separation Distances -------------------------------------------------------------- 55
Chapter 8: Service and Maintenance ------------------------------------------------------------------ 57
Cleaning ------------------------------------------------------------------------------------------------- 57
Battery Replacement ---------------------------------------------------------------------------------- 57
Performance Verification ----------------------------------------------------------------------------- 58
Service and Repair ------------------------------------------------------------------------------------- 58
Limited Warranty ------------------------------------------------------------------------------------- 60
Exclusions ---------------------------------------------------------------------------------------------- 60
Limitation of Warranty ------------------------------------------------------------------------------- 60
Sales & End-User License Agreement -------------------------------------------------------------- 61
Restrictions --------------------------------------------------------------------------------------------- 61
Index --------------------------------------------------------------------------------------------------------- 63
www.masimo.com 4 Masimo
Page 7

About This Manual
This manual explains how to set up and use Pronto® Pulse CO-Oximeter®. Important safety
information relating to general use of Pronto appears in this manual. Read and follow any
warnings, cautions, and notes presented throughout this manual. The following are
explanations of warnings, cautions, and notes.
A warning is given when actions may result in a serious outcome (for example, injury, serious
adverse effect, death) to the patient or user.
WARNING: This is an example of a warning statement.
A caution is given when any special care is to be exercised by the patient or user to avoid
injury to the patient, damage to this device, or damage to other property.
CAUTION: This is an example of a caution statement.
A note is given when additional general information is applicable.
Note: This is an example of a note.
www.masimo.com 5 Masimo
Page 8

Page 9

Product Description, Features, and Indications for Use
Product Description and Features
Pronto® Pulse CO-Oximeter® is a noninvasive device intended to monitor functional oxygen
saturation of arterial hemoglobin (SpO2), pulse rate (PR), perfusion index (PI), and total
hemoglobin (SpHb®).
The following key features are available for Pronto:
• Masimo SET® and rainbow® SET technology performance.
• SpO2 and pulse rate monitoring in motion and low perfusion environments.
• Noninvasive spot-checking of total arterial hemoglobin concentration (SpHb).
• Download capabilities to transfer data from the device to a computer.
Indications for Use
The Masimo Rainbow SET® Pronto Pulse CO-Oximeter and Accessories are indicated for
noninvasive spot checking of functional saturation of arterial oxygen hemoglobin (SpO2),
pulse rate, and total hemoglobin concentration (SpHb). The Masimo Rainbow SET® Pronto
Pulse CO-Oximeter and Accessories are indicated for use, by trained personnel, with adult and
pediatric individuals during both no motion and motion conditions, and for individuals who
are well or poorly perfused in clinical and nonclinical settings (e.g., hospitals, hospital-type
facilities, mobile environments, homes, clinics, physician offices, blood donation facilities,
and ambulatory surgery centers).
Contraindications
Pronto is contraindicated for use as an apnea monitor. Pronto is also contraindicated for use
as a continuous monitor.
www.masimo.com 7 Masimo
Page 10

Page 11

Safety Information, Warnings, and Cautions
Caution: Pronto is to be operated by, or under the supervision of, qualified personnel only.
The manual, accessories, directions for use, all precautionary information, and specifications
should be read before use.
Safety Warnings and Cautions
WARNING: Do not use the Pronto if it appears or is suspected to be damaged.
WARNING: Do not start or operate Pronto unless the setup was verified to be correct.
WARNING: Do not use Pronto during magnetic resonance imaging (MRI) or in an MRI
environment.
WARNING: Explosion hazard: Do not use the Pronto in the presence of flammable
anesthetics or other flammable substance in combination with air, oxygen-enriched
environments, or nitrous oxide.
WARNING: Do not place the Pronto or accessories in any position that might cause it to fall
on the patient.
WARNING: To ensure safety, avoid stacking multiple devices or placing anything on the
device during operation.
WARNING: To protect against injury, follow the directions below:
• Avoid placing the device on surfaces with visible liquid spills.
• Do not soak or immerse the device in liquids.
• Do not attempt to sterilize the device.
• Use cleaning solutions only as instructed in this operator's manual.
• Do not attempt to clean Pronto while monitoring patient.
WARNING: To protect from electric shock, always remove the sensor and completely
disconnect Pronto before bathing the patient.
WARNING: As with all medical equipment, carefully route patient cabling to reduce the
possibility of patient entanglement or strangulation.
CAUTION: Do not place the Pronto where the controls can be changed by the patient.
www.masimo.com 9 Masimo
Page 12

Pronto Safety Information, Warnings, and Cautions
Performance Warnings and Cautions
WARNING: Pronto should not be used as the sole basis for medical decisions. It must be used
in conjunction with clinical signs and symptoms.
WARNING: Variation in hemoglobin measurements may be profound and may be affected by
sampling technique as well as the patient's physiological conditions. Any results exhibiting
inconsistency with the patient’s clinical status should be repeated and/or supplemented with
additional test data. Blood samples should be analyzed by laboratory instruments prior to
clinical decision making to completely understand the patient’s condition.
WARNING: If any measurement seems questionable, first check the patient’s vital signs by
alternate means and then check Pronto for proper functioning.
WARNING: Do not use Pronto for continuous monitoring.
WARNING: Pronto is not an apnea monitor.
WARNING: Pronto should not be used for arrhythmia analysis.
WARNING: Do not use Pronto during defibrillation.
WARNING: Do not use Pronto during electrocautery.
WARNING: Do not place containers with liquids on or near Pronto. Liquids spilled on Pronto
may cause it to perform inaccurately or fail.
WARNING: Interfering Substances: Dyes, or any substance containing dyes, that change
usual blood pigmentation may cause erroneous readings.
WARNING: SpO2 and SpHb are empirically calibrated in healthy adult volunteers with
normal levels of carboxyhemoglobin (COHb) and methemoglobin (MetHb).
WARNING: Inaccurate SpO2 readings may be caused by:
• Improper sensor application and placement
• Elevated levels of COHb or MetHb: High levels of COHb or MetHb may occur with a
seemingly normal SpO2. When elevated levels of COHb or MetHb are suspected,
laboratory analysis (CO-Oximetry) of a blood sample should be performed.
• Elevated levels of bilirubin
• Elevated levels of dyshemoglobin
• Vasospastic disease, such as Raynaud’s, and peripheral vascular disease
• Hemoglobinopathies and synthesis disorders such as thalassemias, Hb s, Hb c,
sickle cell, etc.
• Hypocapnic or hypercapnic conditions
• Severe anemia
• Very low arterial perfusion
• Extreme motion artifact
• Abnormal venous pulsation or venous constriction
• Severe vasoconstriction or hypothermia
• Arterial catheters and intra-aortic balloon
www.masimo.com 10 Masimo
Page 13

Pronto Safety Information, Warnings, and Cautions
• Intravascular dyes, such as indocyanine green or methylene blue
• Externally applied coloring and texture, such as nail polish, acrylic nails, glitter,
etc.
• Birthmark(s), tattoos, skin discolorations, moisture on skin, deformed or abnormal
fingers. etc.
• Skin color disorders
Warning: Inaccurate SpHb readings may be caused by:
• Improper sensor application and placement
• Low arterial oxygen saturation levels
• Elevated carboxyhemoglobin levels
• Elevated methemoglobin levels
• Elevated levels of bilirubin
• Elevated levels of dyshemoglobin
• Elevated PaO2 levels
• Vasospastic disease, such as Raynaud’s, and peripheral vascular disease
• Hemoglobinopathies and synthesis disorders such as thalassemias, Hb s, Hb c,
sickle cell, etc.
• Hypocapnic or hypercapnic conditions
• Peripheral vascular disease
• Liver disease
• Severe anemia
• Low arterial perfusion
• Motion artifact
• Abnormal venous pulsation or venous constriction
• Severe vasoconstriction or hypothermia
• Arterial catheters and intra-aortic balloon
• Intravascular dyes, such as indocyanine green or methylene blue
• Externally applied coloring and texture, such as nail polish, acrylic nails, glitter,
etc.
• Birthmark(s), tattoos, skin discolorations, moisture on skin, deformed or abnormal
fingers. etc.
• Skin color disorders
• Elevated altitude
• EMI radiation interference
CAUTION: Do not place the Pronto on electrical equipment that may affect the device,
preventing it from working properly.
www.masimo.com 11 Masimo
Page 14

Pronto Safety Information, Warnings, and Cautions
CAUTION: The device must be configured to match your local power line frequency to allow
for the cancelation of noise introduced by fluorescent lights and other sources.
CAUTION: If the Low SIQ Indicator illuminates frequently, find a better perfused monitoring
site. In the interim, assess the patient and, if indicated, verify oxygenation status through
other means.
CAUTION: If using pulse oximetry during full body irradiation, keep the sensor out of the
radiation field. If the sensor is exposed to the radiation, the reading might be inaccurate or
the device might read zero for the duration of the active irradiation period.
CAUTION: When patients are undergoing photodynamic therapy they may be sensitive to
light sources. Pulse oximetry may be used only under careful clinical supervision for short
time periods to minimize interference with photodynamic therapy.
CAUTION: If SpO2 values indicate hypoxemia, a laboratory blood sample should be taken to
confirm the patient’s condition.
Note: A functional tester cannot be used to assess the accuracy of the Pronto.
Note: It is recommended that Pronto battery is fully charged prior to use.
Note: All batteries lose capacity with age, thus the amount of run time at Low Battery will
vary depending upon the age of the battery.
Note: Do not loop the patient cabling into a tight coil or wrap around the device, as this can
damage the patient cabling.
Note: Additional information specific to the Masimo sensors compatible with Pronto,
including information about parameter/measurement performance during motion and low
perfusion, may be found in the sensor's directions for use (DFU).
Note: High-intensity extreme lights (such as pulsating strobe lights) directed on the sensor,
may not allow the Pronto to obtain vital sign readings.
www.masimo.com 12 Masimo
Page 15

Pronto Safety Information, Warnings, and Cautions
Cleaning and Service Warnings and Cautions
WARNING: Do not adjust, repair, open, disassemble, or modify Pronto. Injury to personnel or
equipment damage could occur. Return Pronto for servicing.
WARNING: Electrical Shock Hazard: The battery should be installed and/or removed from the
Pronto by qualified personnel only.
WARNING: Do not incinerate battery.
WARNING: Do not use petroleum-based or acetone solutions, or other harsh solvents, to
clean the Pronto. These substances affect the device’s materials and device failure can result.
WARNING: Do not touch, press, or rub the display panels with abrasive cleaning compounds,
instruments, brushes, rough-surface materials, or bring them into contact with anything that
could scratch the display.
CAUTION: An operator may only perform maintenance procedures specifically described in
the manual. Refer servicing to qualified service personnel trained in the repair of this
equipment.
CAUTION: Do not submerge Pronto in any cleaning solution or attempt to sterilize by
autoclave, irradiation, steam, gas, ethylene oxide or any other method. This will seriously
damage Pronto.
CAUTION: Electric shock hazard: Do not open the Pronto cover except to replace the battery
or batteries.
CAUTION: Electrical shock and flammability hazard: Before cleaning, always turn off the
device and remove batteries.
CAUTION: Electrical Shock Hazard: Carry out periodic tests to verify that leakage currents of
patient-applied circuits and the system are within acceptable limits as specified by the
applicable safety standards. The summation of leakage currents must be checked and in
compliance with IEC 60601-1 and UL60601-1. The system leakage current must be checked
when connecting external equipment to the system. When an event such as a component
drop of approximately 1 meter or greater or a spillage of blood or other liquids occurs, retest
before further use. Injury to personnel could occur.
Note: Excessive cleaning solution can flow into the monitor and cause damage to internal
components.
www.masimo.com 13 Masimo
Page 16

Pronto Safety Information, Warnings, and Cautions
Compliance Warnings and Cautions
WARNING: Changes or modifications not expressly approved by the party responsible for
compliance could void the user's authority to operate the equipment.
CAUTION: Disposal of product - Comply with local laws in the disposal of the device and/or
its accessories.
CAUTION: Dispose of used batteries according to country or regional regulations.
CAUTION: To minimize radio interference, other electrical equipment that emits radio
frequency transmissions should not be in close proximity to Pronto.
Note: Use the Pronto in accordance with Environmental Specifications section in this
manual.
Note: This device complies with part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and (2) this
device must accept any interference received, including interference that may cause
undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the user
is encouraged to try to correct the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Note: This equipment has been tested and found to comply with the Class B limits for
medical devices according to the EN 60601-1-2: 2007, Medical Device Directive 93/42/EEC.
These limits are designed to provide reasonable protection against harmful interference in a
typical medical installation.
Note: In order to maintain compliance with FCC regulations, shielded cables must be used
with this equipment. Operation with non-approved equipment or unshielded cables is likely
to result in interference to radio and TV reception. The user is cautioned that changes and
modifications made to the equipment without the approval of manufacturer could void the
user's authority to operate this equipment.
Note: To satisfy RF exposure requirements, this device and its antenna must operate with a
separation distance of at least 20 cm from all persons and must not be co-located or
operating in conjunction with any other antenna or transmitter.
Note: This Class B digital apparatus complies with Canadian ICES-003.
www.masimo.com 14 Masimo
Page 17
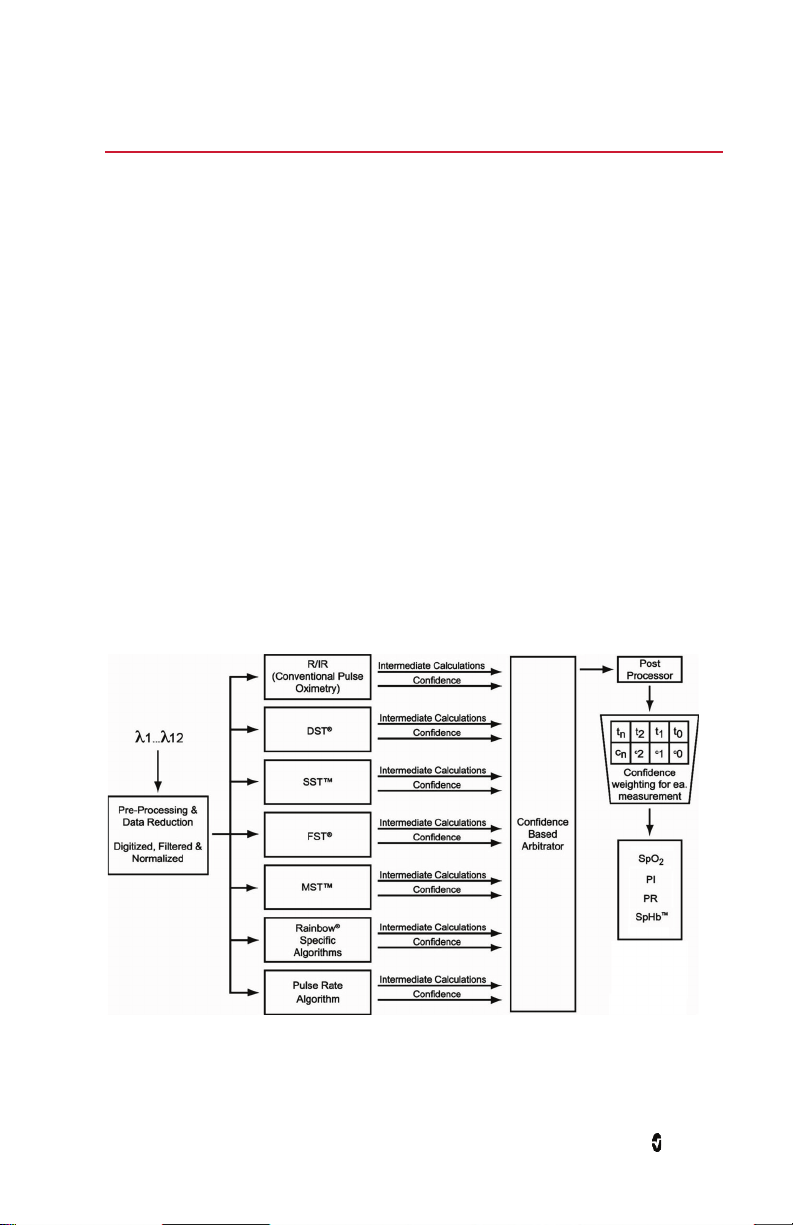
Chapter 1: Technology Overview
The following chapter contains general descriptions for the parameters, measurements, and
technology used within Masimo products.
Masimo Signal Extraction Technology (Masimo SET®)
The signal processing in Masimo Signal Extraction Technology (Masimo SET®) differs from
that of conventional pulse oximeters. Conventional pulse oximeters assume that arterial
blood is the only blood moving (pulsating) in the measurement site. During patient motion,
however, the venous blood also moves, causing conventional pulse oximeters to read low
values, because they cannot distinguish between the arterial and venous blood movement
(sometimes referred to as noise).
Masimo SET® pulse oximetry utilizes parallel engines and adaptive filtering. Adaptive filters
are powerful because they are able to adapt to the varying physiologic signals and/or noise
and separate them by looking at the whole signal and breaking it down to its fundamental
components. The Masimo SET® signal processing algorithm, Discrete Saturation Transform®
(DST®), in parallel with Fast Saturation Transform (FST®), reliably identifies the noise,
isolates it and, using adaptive filters, cancels it. It then reports the true arterial oxygen
saturation for display on the monitor.
Masimo SET® Parallel Engines
This figure is for conceptual purposes only.
www.masimo.com 15 Masimo
Page 18
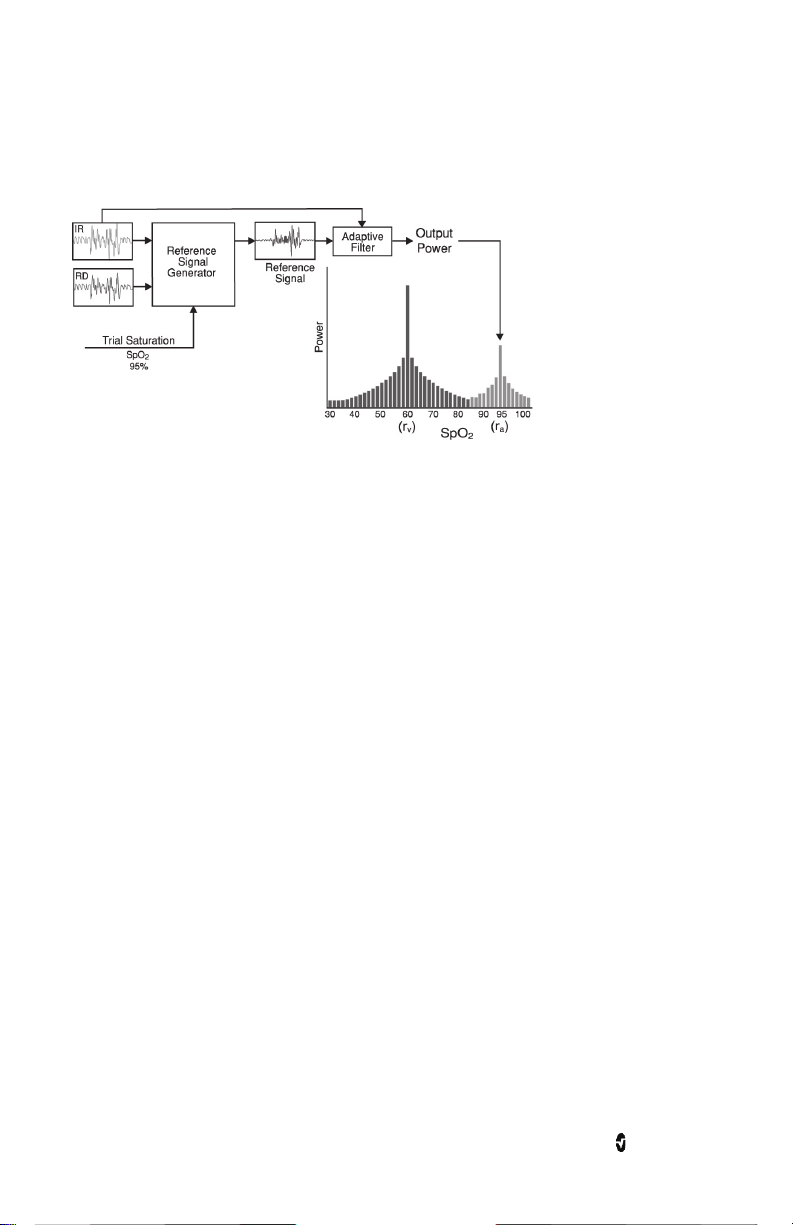
Pronto Chapter 1: Technology Overview
Masimo SET® DST
This figure is for conceptual purposes only.
General Description for Oxygen Saturation (SpO2)
Pulse oximetry is governed by the following principles:
1. Oxyhemoglobin (oxygenated blood) and deoxyhemoglobin (non-oxygenated
blood) differ in their absorption of red and infrared light (spectrophotometry).
2. The amount of arterial blood in tissue changes with your pulse
(photoplethysmography). Therefore, the amount of light absorbed by the varying
quantities of arterial blood changes as well.
Functional Oxygen Saturation (SpO2)
The Pronto is calibrated to measure and display functional oxygen saturation (SpO2): the
amount of oxyhemoglobin expressed as a percentage of the hemoglobin that is available to
transport oxygen.
Note: Dyshemoglobins are not capable of transporting oxygen, but are recognized as
oxygenated hemoglobins by conventional pulse oximetry.
General Description for Pulse Rate (PR)
Pulse rate (PR), measured in beats per minute (bpm), is based on the optical detection of
peripheral flow pulse.
General Description for Perfusion Index (PI)
The Perfusion Index (PI) is the ratio of the pulsatile blood flow to the non-pulsatile or static
blood in peripheral tissue. PI thus represents a noninvasive measure of peripheral perfusion
that can be continuously and noninvasively obtained from a pulse oximeter.
www.masimo.com 16 Masimo
Page 19
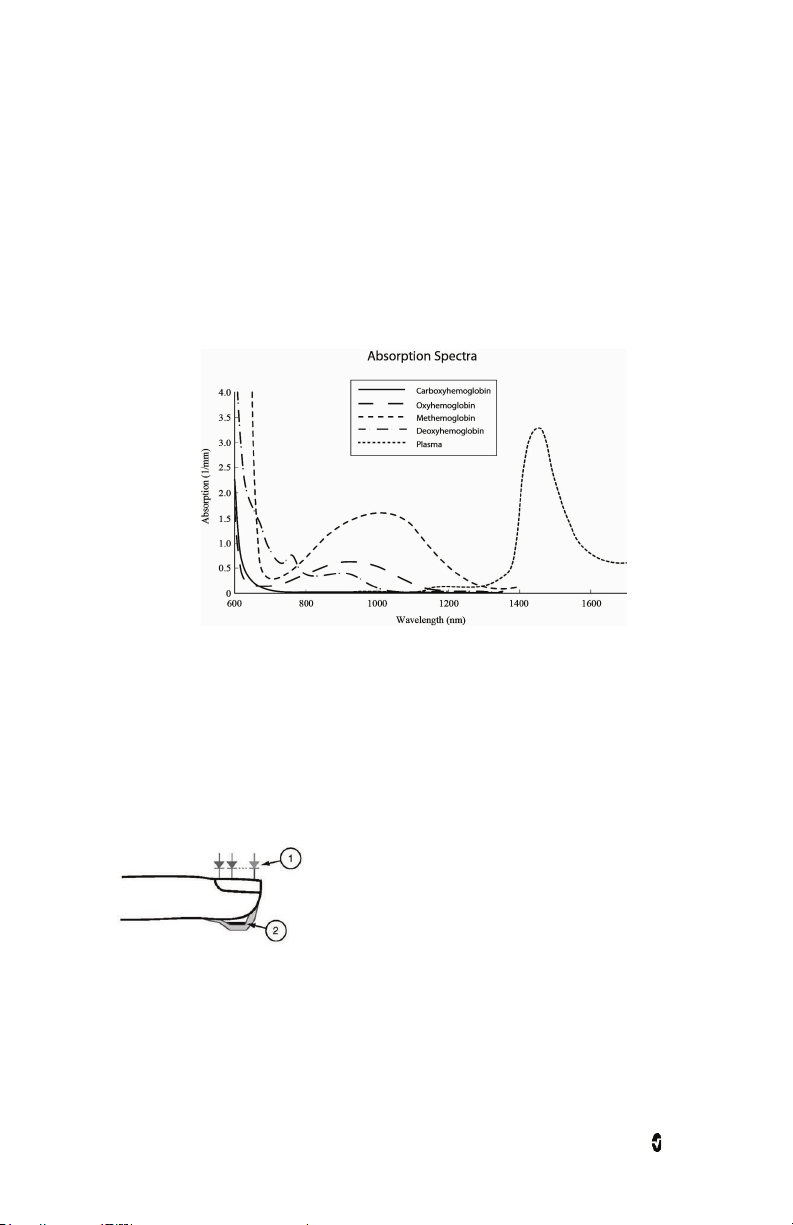
Pronto Chapter 1: Technology Overview
rainbow Pulse CO-Oximetry Technology
rainbow Pulse CO-Oximetry technology is governed by the following principles:
1. Oxyhemoglobin (oxygenated blood), deoxyhemoglobin (non-oxygenated blood),
carboxyhemoglobin (blood with carbon monoxide content), methemoglobin
(blood with oxidized hemoglobin) and blood plasma constituents differ in their
absorption of visible and infrared light (using spectrophotometry).
2. The amount of arterial blood in tissue changes with pulse
(photoplethysmography). Therefore, the amount of light absorbed by the varying
quantities of arterial blood changes as well.
Pronto uses a multi-wavelength sensor to distinguish between oxygenated blood,
deoxygenated blood, blood with carbon monoxide, oxidized blood, and blood plasma.
Pronto utilizes a sensor with various light-emitting diodes (LEDs) that pass light through the
site to a diode (detector). Signal data is obtained by passing various visible and infrared
lights (LEDs, 500 to 1400nm) through a capillary bed (for example, a fingertip, a hand, a foot)
and measuring changes in light absorption during the blood pulsatile cycle. This information
may be useful to clinicians. The maximum radiant power of the strongest light is rated at ≤ 25
mW. The detector receives the light, converts it into an electronic signal, and sends it to
Pronto for calculation.
1. Light Emitting Diodes (LEDs)
(7 + wavelengths)
2. Detector
Once the Pronto receives the signal from the sensor, it utilizes proprietary algorithms to
calculate the patient’s functional oxygen saturation (SpO2 [%]), total hemoglobin
concentration (SpHb [g/dL]), and pulse rate (PR). The SpHb measurement relies on a
multi-wavelength calibration equation to quantify the percentage of carbon monoxide and
methemoglobin and the concentration of total hemoglobin in arterial blood. Maximum
skin-sensor interface temperature was tested to be less than 41º C (106º F) in a minimum
ambient temperature of 35º C (95º F). The tests were conducted with sensors operating at
reasonable worst case power.
www.masimo.com 17 Masimo
Page 20

Pronto Chapter 1: Technology Overview
Pulse CO-Oximetry vs. Drawn Whole Blood Measurements
When SpO2 and SpHb measurements obtained from Pronto (noninvasive) are compared to
drawn whole blood (invasive) measurements by blood gas and/or laboratory CO-Oximetry
methods, caution should be taken when evaluating and interpreting the results.
The blood gas and/or laboratory CO-Oximetry measurements may differ from the SpO2 and
SpHb measurements of Pronto. Any comparisons should be simultaneous, meaning the
measurement on the device should be noted at the exact time that blood is drawn.
In the case of SpO2, different results are usually obtained from the arterial blood gas sample
if the calculated measurement is not appropriately corrected for the effects of variables that
shift the relationship between the partial pressure of oxygen (pO2) and saturation, such as:
pH,temperature, the partial pressure of carbon dioxide (pCO2), 2,3-DPG, and fetal
hemoglobin.
In the case of SpHb, variation in hemoglobin measurements may be profound and may be
affected by sampling technique as well as the patient's physiological conditions. Any results
exhibiting inconsistency with the patient's clinical status should be repeated and/or
supplemented with additional test data. As with most hemoglobin tests, a laboratory blood
sample should be analyzed prior to clinical decision making.
High levels of bilirubin may cause erroneous SpO2 and SpHb readings. As blood samples are
usually taken over a period of 20 seconds (the time it takes to draw the blood), a meaningful
comparison can only be achieved if the oxygen saturation, carboxyhemoglobin, and
methemoglobin concentration of the patient are stable and not changing over the period of
time that the blood gas sample is taken. Subsequently, blood gas and laboratory CO-Oximetry
measurements of SpO2 and SpHb may vary with the rapid administration of fluids and in
procedures such as dialysis. Additionally, drawn whole blood testing can be affected by
sample handling methods and time elapsed between blood draw and sample testing.
Measurements with Low Signal IQ should not be compared to laboratory measurements.
General Description for Total Hemoglobin (SpHb)
Pulse CO-Oximetry is a continuous and noninvasive method of measuring the levels of total
hemoglobin (SpHb) in arterial blood. It relies on the same principles of pulse oximetry to
make its SpHb measurement. The measurement is taken by a sensor capable of measuring
SpHb, usually on the fingertip for adults and pediatric patients.
The sensor connects directly to the Pulse CO-Oximeter or with a patient cable. The sensor
collects signal data from the patient and sends it to the device. The device displays the
calculated data as measurement of total hemoglobin concentration.
Successful Monitoring for SpHb
A stable SpHb reading is associated with correct sensor placement, small physiological
changes during the measurement and acceptable levels of arterial perfusion at the
measurement site. Physiological changes at the measurement site are mainly caused by
fluctuations in the oxygen saturation, blood concentration, and perfusion. See Safety
Information, Warnings, and Cautions on page 9.
www.masimo.com 18 Masimo
Page 21

Pronto Chapter 1: Technology Overview
SpO2 and SpHb Measurements During Patient Motion
Pronto displays measurements of SpO2 and may display SpHb during patient motion.
However, because of the changes in the physiological parameters such as blood volume,
arterial-venous coupling, etc. that occur during patient motion, the accuracy of such
measurements may not be reliable during excessive motion. In this case, the Low SIQ
indicator illuminates to alert the clinician that the device does not have confidence in the
value displayed due to poor signal strength caused by excessive motion or other signal
interference. For more information, see Low Signal I.Q. (Low SIQ) on page 40.
www.masimo.com 19 Masimo
Page 22

Page 23

Chapter 2: System Description
Pronto is a Spot Check Pulse CO-Oximeter that includes noninvasive total hemoglobin
(SpHb) measurement. All hemoglobin pulse oximetry measurement information, as well as
device status data, is displayed on the front panel of the device. All user input is handled by
control buttons on the front panel, and the sensor cable connection is located at the top edge
of the device.
Pronto is powered by four (4) AA alkaline batteries to provide up to eight (8) hours of
continuous use when used with new, fresh batteries. Continuous use is defined as consecutive
spot check tests with each consecutive spot check test initiated immediately upon the
conclusion of the previous spot check test.
A spot check sensor or patient cable attaches to the connector on the top of Pronto.
Front Panel
www.masimo.com 21 Masimo
Page 24

Pronto Chapter 2: System Description
Ref. Feature Description
Parameter/
1
Measurement
Numeric Display
Displays parameter/measurement numeric values once a spot check test is
complete.
2 Pulse Indicator Flashes with patient's pulse reading (PR) during spot check test period.
Spot Check
3
Progress Indicator
Parameter/
4
Measurement
Label Display
5 Power Button
Battery Level
6
Indicator
Patient Cable /
7
Sensor Connector
8 Low SIQ Indicator
Sensor Use
9
Indicator
Incrementally illuminates upward after a SpHb spot check has been initiated.
This indicates progress towards completion of a SpHb spot check. A fully
illuminated spot check progress indicator indicates a competed spot check.
Displays parameter/measurement label once a spot check test is complete.
Powers the device on or off. Press the button once to power on. Press and hold
the button for two (2) seconds to power off.
Battery charge level is indicated by four LED indicators. All four indicators will
be lit when the batteries are full, with fewer indicators being lit as the
batteries lose their charge. For more information, see Battery Level Indicator
on page 30.
The Patient Cable/Sensor connector is where a compatible sensor is
connected to the device.
This illuminates to indicate low confidence in the measurement displayed.
For more information, see Low Signal I.Q. (Low SIQ) on page 40.
This illuminates to display the approximate number of uses remaining for the
attached sensor. The bottom LED will turn red when the remaining uses for
the connected sensor are low. The approximate number of sensor uses
remaining is displayed upon power up (if a sensor is attached) and when a
sensor is connected.
Press to initiate total hemoglobin (SpHb) spot check information on display
10 SpHb Button
Up/Down Arrow
11
Buttons
12 Speaker
or to display a total hemoglobin (SpHb) spot check test. When navigating the
device menu, pressing this button will move to the next menu option and will
confirm a setting change. For more information, see Navigating the Menu on
page 31.
Use the Up or Down arrow buttons to scroll between parameter or
measurement spot check results. When in the device menu, use the Up or
Down arrow buttons to scroll through menu setting options. For more
information, see Navigating the Menu on page 31.
Provides audible indication of alert conditions, pulse tone, and feedback for
control button presses.
www.masimo.com 22 Masimo
Page 25

Pronto Chapter 2: System Description
Rear Panel
Ref. Feature
1 Serial Number Label
2 Certification Label
3 Battery Cover
4 Battery Cover Release
www.masimo.com 23 Masimo
Page 26

Page 27

Chapter 3: Setup
This chapter contains information about setting up Pronto before use.
Unpacking and Inspection
To unpack and inspect the device
1. Remove the device from the shipping carton and examine it for signs of shipping
damage.
2. Check all materials against the packing list. Save all packing materials, invoice,
and bill of lading. These may be required to process a claim with the carrier.
3. If anything is missing or damaged, contact the Technical Service Department. See
Power Requirements
Pronto uses four (4) AA alkaline batteries. Use of non-alkaline batteries may affect the
accuracy of the battery level indicator on the device. Use of batteries with cell voltage of more
than 1.5V could damage the device.
See Battery Level Indicator on page 30.
See Battery Replacement on page 57.
Return Procedure on page 59.
Initial Setup
1. Inspect Pronto case for damage.
2. If your device is equipped with a protective boot, remove it. To remove the boot,
gently bend down on the boot at the bottom end of the device next to the speaker.
Push up on the device and remove the boot.
3. Install four (4) new AA alkaline batteries. Fasten the boot onto the device, if
required.
4. Turn on Pronto. All LEDs will briefly illuminate and an audible tone will sound.
5. If necessary, configure the device for your regional power line frequency (LF) (50
www.masimo.com 25 Masimo
hz or 60 hz). See Default Settings on page 29.
Page 28

Page 29

Chapter 4: Operation
The information in this chapter assumes that Pronto is set up and ready for use. This chapter
provides necessary information for proper operation of the device. Do not operate Pronto
without completely reading and understanding these instructions.
Basic Operation
1. Select a compatible sensor. Remove any substances that may interfere with the
transmission of light between the sensor’s light source and detector.
2. Connect the sensor, or the patient cable and sensor, to the connector of Pronto.
Make sure it is a secure connection and the cable is not twisted, sliced, or frayed.
See Chapter 5: Messages on page 35 to view messages that may be displayed
pertaining to sensors and cables.
3. Press the Power button to turn on Pronto.
• All front-panel indicators will illuminate momentarily, and an audible tone
will sound.
• If applicable, the device will display the number of sensor uses remaining.
• The device displays
4. Attach the sensor to the patient. For more information, see the Directions for Use
for the sensor.
5. Press the SpHb button to start a spot check. Values for PI, PR, and SpO2 will
display while SpHb is being calculated. The Spot Check Progress Indicator begins
to illuminate.
6. Verify that the Pulse Indicator light is illuminated. The light flashes when the
pulse rate (PR) is acquired.
Note: It will take about 30 seconds to three (3) minutes for Pronto to acquire an
accurate spot check. During this period, the sensor is initializing and adjusting to
the patient. PI, PR, and SpO2 parameters/measurements (depending on user
configuration) will appear on the main display. No other quality control activities
(such as calibration) are required.
RDY, indicating that it is ready to use.
www.masimo.com 27 Masimo
Page 30

Pronto Chapter 4: Operation
7. The Spot Check Progress Indicator incrementally illuminates from bottom to top.
When the Spot Check Progress Indicator is fully illuminated, the SpHb
parameter/measurement value is displayed, and an audible tone sounds.
8. Use the Up or Down arrow buttons to navigate through the parameter and
measurement values that have been spot checked. While the sensor remains on
the finger of the patient, the parameter values will continue to update and be
displayed for five (5) minutes from the time the SpHb parameter value was first
displayed.
9. After removing the finger of the patient from the sensor, verify at each
parameter/measurement display that the Low SIQ Indicator is not illuminated. If
the Low SIQ Indicator is illuminated, the value may be checked again.
10. After the spot check is complete, remove the sensor from the patient and store or
dispose of the sensor according to the governing rules. For more information, see
the Directions for Use for the sensor.
11. Pronto will power off automatically after five (5) minutes of inactivity to save
battery life, except when downloading trend data. The user can also press and hold
the Power button for two (2) seconds to turn off Pronto.
Spot Check Results
1. SpHb data displays for five (5) minutes. After five (5) minutes, the data can only
be obtained by downloading the data through the trend monitor or when another
test is performed.
2. To view older readings after a reading has been performed, press the Up or Down
arrow buttons to view different parameters. The parameter label blinks indicating
the value obtained might be older or not correlate with the patient. Parameters
display in this order: SpHb, PI, PR, and SpO2.
www.masimo.com 28 Masimo
Page 31

Pronto Chapter 4: Operation
YR NN
Default Settings
Option Display Factory
Date and Time
Default
Setting
N/A Year/Month/Day (YY/MM/DD)
D
H N
Clear Trend
No No, Yes
CLR
TND
Oxygen Saturation
(SpO2)
Pulse Rate (PR)
02
On On, Off
On On, Off
PR
Perfusion Index (PI)
On On, Off
PI
Line Frequency (LF)
60 Hz 50, 60 Hz
LF
Software Version
HH HH, MX (read only)
UER
SpHb Calibration
HB
Venous
(SpHbv)
Configurable Settings
Hour/Minute(hh:mm)
Venous, Arterial
Pulse Tone
Off On, Off
TON
SpHb Units of
Measurement
Display Measurements
During Low SIQ
www.masimo.com 29 Masimo
HBU
DPL
Grams per
Deciliter
On On, Off
Grams per Deciliter, Millimoles per
Liter
Page 32

Pronto Chapter 4: Operation
Audible Alerts
Pronto visually and audibly indicates the following conditions, using a three (3) beep audible
tone with visual indicator:
• Low battery
• System failure
Battery Level Indicator
Four LED indicators provide information on the remaining battery capacity. The operator
should monitor these indicators periodically to determine remaining spot check uses and if
the batteries should be replaced.
Battery capacity is indicated in the following chart:
LED Indicators Battery Capacity
4 LEDs 100% to 75%
3 LEDs 74% to 50%
2 LEDs 49% to 25%
1 LED 24% to 10%
1 Flashing LED with audible alert 9% to 0%
www.masimo.com 30 Masimo
Page 33

Pronto Chapter 4: Operation
Navigating the Menu
Pronto settings are accessed through the menu system.
1. To access the menu, press and hold both Up and Down arrow buttons for
five (5) seconds
2. To scroll through the menu options (see table below), press the SpHb button
repeatedly.
3. To change a setting (see table below) for a menu option selected in the previous
step, press Up and Down arrow buttons .
4. Press the SpHb button to confirm the change.
Menu Option Settings
Year (Current Year)
YR
NN
Sets Year (00-99)
Month (current month)
Sets Month (01-12)
Day (current day)
D
H
NN
CLR TND
www.masimo.com 31 Masimo
Sets Day (00-31)
Hour (current hour)
Sets Hour (00-23)
Minute (current minute)
Set Minute (00-59)
Clear Trend (Yes)
Clear Trend (No) (Default)
Page 34

Pronto Chapter 4: Operation
Menu Option Settings
SpO2 On (Default)
O2
SpO2 Off
PR On (Default)
PR
PI
LF
UER
HB*
TON
HBU
PR Off
PI On (Default)
PI Off
LF 60(Default)
LF 50
Software Version
HH Software (Default)
Software Version
MX Software
SpHbv Calibration
Venous(Default)
SpHb Calibration Arterial
Pulse Tone Off (Default)
Pulse Tone On
Grams per Deciliter (g/dL) (Default)
Milimoles per Liter (mmol/L)
On (Default)
DPL**
www.masimo.com 32 Masimo
Off
Page 35

Pronto Chapter 4: Operation
*The hemorheologic profile of arterial and venous blood samples can vary. To accommodate
this difference, Pronto provides the option of displaying a SpHb parameter that is based on
either Arterial SpHb or Venous SpHbv laboratory blood sample data. Changing the
calibration setting from SpHbv to SpHb (and vice versa) will clear the trend memory.
**Allows user to choose whether to display values under Low SIQ conditions. For more
information, see Low Signal I.Q. (Low SIQ) on page 40.
Exit the Menu and Power Off the Device
To exit the menu, allow 10 minutes of inactivity, or press the Up and Down arrow buttons
simultaneously. Press the Power button for two (2) seconds to turn off the device.
Trend Setup and Use
Pronto can store at least 10,000 spot checks. The trend data can then be transferred to a PC
for evaluation. The data is not intended to be used for trending purposes.
A Data Transfer Cable is required to connect Pronto to a PC. Patient measurement is not
possible while trend memory is being transferred to a PC.
Trend data is stored in non-volatile memory, so it is not erased when the device is shut off. A
trend data download is initiated using the TrendCom utility (not included) which downloads
the spot check trend data and saves it to an ASCII text (.out) file with an output delimiter
option.
Note: Before collecting trend data, it is recommended to set (or reset) the date and time on
the device.
TrendCom Utility Installation and Operation
Copy the TrendCom utility from the TrendCom CD onto a PC running MS-Windows. For
detailed download and operation instructions, see the TrendCom Directions for Use (DFU).
Note: During download of spot check trend information, all normal Pronto functions are
unavailable and the keypad is locked, except for the Power button.
Erasing Trend Memory
Pronto automatically captures all parameters/measurements. When performing a new study
and gathering data on a new patient, it is highly recommended the Clear Trend function be
utilized prior to data collection in order for the results to be separate. Turning Pronto off will
not erase the trend data. For detailed instructions on erasing trend memory, see the
TrendCom Directions for Use (DFU).
Note: Do not turn off the device for at least one minute after clearing the trend.
www.masimo.com 33 Masimo
Page 36

Pronto Chapter 4: Operation
digit, ASCII encoded, hexadecimal
Trend Data Format
After a successful download of the trend data, a .out file will be created containing the
trend-dump information in ASCII delimited format. The format is defined in the following
table:
Parameter Specification
Date MM\DD\YY
Time HH:MM:SS
Installed Parameter/
Measurement
Numeric value (see the display ranges in the Default Settings on page
29)
The exceptions are displayed as a 3value. The binary bits of the hexadecimal value are encoded as follows:
000 = Normal operation; no exceptions
Exception Messages
004 = Low Perfusion
400 = Low Signal IQ
800 = Masimo SET. This flag means the algorithm is running in full
Masimo SET® mode. It requires a Masimo SET® sensor and needs to
acquire some clean data for this flag to be set.
Sample Trend Output
11/30/11 00:09:36 SpO2=100 PR=070 PI=01.32 SpCO=00.00 Met=00.00 SpHb=15.1 PVI=000
EXC=00000000
12/01/11 00:12:45 SpO2=096 PR=068 PI=03.52 SpCO=00.00 Met=00.00 SpHb=13.8 PVI=000
EXC=00000000
12/01/11 00:13:14 SpO2=100 PR=069 PI=02.20 SpCO=00.00 Met=00.00 SpHb=14.3 PVI=000
EXC=00000000
12/02/11 00:09:27 SpO2=100 PR=068 PI=01.52 SpCO=00.00 Met=00.00 SpHb=14.2 PVI=000
EXC=00000000
12/02/11 00:10:58 SpO2=099 PR=071 PI=03.64 SpCO=00.00 Met=00.00 SpHb=15.8 PVI=000
EXC=00000000
12/02/11 00:15:04 SpO2=097 PR=068 PI=01.52 SpCO=00.00 Met=00.00 SpHb=10.8 PVI=000
EXC=00000000
12/03/11 00:11:31 SpO2=100 PR=072 PI=04.69 SpCO=00.00 Met=00.00 SpHb=12.5 PVI=000
EXC=00000000
Note: Trend output data appears for the parameters/measurements noted above. Pronto only
stores output data for SpO2, PR, PI, and SpHb parameters/measurements. SpCO, SpMet, and
PVI measurements are not available with Pronto.
Note: Pronto does not store continuous output data for SpO2, PR, PI, and SpHb. Pronto only
stores data related to SpHb, SpO2, PR, and PI during spot check tests results.
www.masimo.com 34 Masimo
Page 37

Chapter 5: Messages
Messages
The following messages are specific to Pronto:
Message Explanation Next Steps
1. Connect sensor to cable.
2. Check sensor connection to
Assemble the ReSposable sensor system
and then connect to the device.
1. Reattach sensor to patient.
2. Verify proper sensor
Wait for pulse detection. (This search
should occur whenever a spot check is
performed). If necessary, shield the
sensor from excessive ambient or
strobing light.
NO SEN
3.
SEN OFF
Circulating LEDs
No Sensor Connected
The Masimo rainbow
ReSposable® sensor
system is not connected to
the device
Sensor off patient
Sensor is initializing/
determining measurement
cable.
placement.
1. Rule out occlusion of blood
flow.
Low SIQ Indicator
illuminates
Single Battery Level
Indicator flashes
(with audible alert)
ERR
##
RPL SEN
www.masimo.com 35 Masimo
Low Signal IQ
Battery level too low
System Fault
Defective sensor Replace sensor.
The Masimo ReSposable
sensor system is
non-functional.
2. Verify placement of sensor.
3. Move sensor to a better
perfused site.
4. See Low Signal I.Q. (Low SIQ)
on page 40.
Replace batteries immediately. See
Battery Replacement on page 57.
Return for service. There are several error
codes. All error codes require return of
the device to an authorized service
center for repair. See Return Procedure
on page 59.
Replace the Masimo ReSposable sensor
system.
Page 38

Pronto Chapter 5: Messages
Message Explanation Next Steps
INC DET
(Blinking)
INC SEN
INC CBL
NO CBL
RPL CBL
Temporarily blinking
message:
CBL
Temporarily blinking
message: RPL
SEN
Temporarily blinking
message:
ADH
RPL
RPL
Interference detected Ensure that the sensor is properly
Incompatible sensor Attach appropriate sensor.
Incompatible cable Attach appropriate cable.
No cable Attach appropriate cable.
Cable life expired
Cable life expired
Sensor life expired
Adhesive sensor life
expired
applied, and cover the sensor site with
opaque material, if required.
Replace cable.
Replace cable as soon as possible.
Replace sensor as soon as possible.
Replace adhesive sensor as soon as
possible.
CHC SEN
SEN 000
RE TST
www.masimo.com 36 Masimo
Check sensor connection
Zero sensor uses remaining
Spot check incomplete
Reattach sensor.
Attach new sensor. Dispose of the old
sensor per local governing ordinances.
See Compliance Warnings and Cautions
on page 14.
Confirm sensor placement and press
SpHb button again.
Page 39

Pronto Chapter 5: Messages
Message Explanation Next Steps
NO ADH
INC ADH
RPL ADH
The reusable part of the
Masimo ReSposable sensor
system is connected to the
device, but the adhesive
part is not connected.
The adhesive part of the
Masimo ReSposable sensor
system is incompatible or
unrecognized.
The adhesive part of the
Masimo ReSposable sensor
system is non-functional.
Disconnect the reusable part of the
ReSposable sensor system. Assemble
the ReSposable sensor system and then
connect to the device.
Replace the adhesive part of the
ReSposable sensor system.
Replace the adhesive part of the
ReSposable sensor system.
www.masimo.com 37 Masimo
Page 40

Page 41

Chapter 6: Troubleshooting
Troubleshooting
The following chart describes what to do if Pronto system does not operate properly or fails.
Symptom Possible Cause Recommendation
Minimize or eliminate interference from
surgical or fluorescent lighting.
Verify/set 50/60hz menu setting. See
Initial Setup.
Verify use of a SpHb capable sensor.
Minimize or eliminate motion at the
measurement site.
Difficulty or no SpHb
reading
Low battery
Interference from line
frequency induced noise
Inappropriate sensor or
sensor size
Excessive motion
Unit does not power
on
Continuous speaker
tone
Buttons don’t work
when pressed
Low battery alert
sounds. Battery Level
Indicator shows low
battery capacity less
than expected
capacity.
Excessive ambient or
strobing light
See Safety Information, Warnings, and Cautions on page 9 and the
sensor Directions for Use (DFU).
Low battery Check/replace batteries. See Battery
Internal failure Unit requires service. If audible tone
Internal failure Return for service. See Return Procedure
Effective spot check uses
will be reduced when
operating the device
below 5ºF (-15ºC) due to
alkaline battery
technology
Shield the sensor from excessive light.
Replacement on page 57.
continues to sound, power down unit
and/or remove batteries. See Return
Procedure on page 59
on page 59.
Remove the batteries and allow them to
warm up to room temperature, re-install
them, and then check the battery
indicator level. If the battery capacity
remains low, replace batteries. See
Battery Replacement on page 57.
www.masimo.com 39 Masimo
Page 42

Pronto Chapter 6: Troubleshooting
Low Perfusion
It has been suggested that at extremely low perfusion levels, Pulse CO-Oximeters can
measure peripheral saturation which may differ from central arterial saturation*. This
“localized hypoxemia” may result from the metabolic demands of other tissues extracting
oxygen proximal to the measurement site under conditions of sustained peripheral
hypoperfusion. (This may occur even with a pulse rate that correlates with the ECG heart
rate.)
Low Signal I.Q. (Low SIQ)
Note: The Display Measurements During Low SIQ option (DPL) must be turned on to
display measurements (SpO2, PR, PI, and SpHb) under Low SIQ conditions. For more
information about changing this setting, see Default Settings on page 29 and Navigating the
Menu on page 31.
Pronto provides a visual indicator (LED), the Low SIQ Indicator, which provides an
assessment of the confidence of the measurement displayed.
When the Low SIQ Indicator illuminates, confidence in the measurement displayed is low.
Proceed with caution and do the following:
• Assess the patient.
• Check the sensor and ensure proper sensor application. The sensor must be well
secured to the site to obtain accurate readings. Also, misalignment of the sensor’s
emitter and detector can result in low SIQ.
• Determine if an extreme change in the patient’s physiology and blood flow at the
measurement site occurred, (e.g. an inflated blood pressure cuff, a squeezing
motion, sampling of an arterial blood specimen from the hand containing the
pulse oximetry sensor, severe hypotension, peripheral vasoconstriction in
response to hypothermia, medications, or an episode of Raynaud’s syndrome.)
• Read Safety Information, Warnings, and Cautions on page 9 and the sensor
Directions for Use (DFU).
After performing the above, perform another spot check. An arterial blood specimen for
laboratory CO-Oximetry analysis may be considered to verify the oxygen saturation and
hemoglobin values.
www.masimo.com 40 Masimo
Page 43

Pronto Chapter 6: Troubleshooting
Low Battery Audible Alert
If a low battery condition occurs while a measurement is being taken, an audible alert will
sound. If a low battery condition occurs, immediately replace the batteries.
WARNING: Failure to replace batteries promptly after a low battery alert may result in the
device shutting down and leaving the patient in an unmonitored condition.
WARNING: Use only alkaline batteries. Use of non-alkaline batteries may affect the accuracy
of the Battery Level Indicator.
WARNING: Use of batteries with a cell voltage of more than 1.5V could cause damage to the
device.
WARNING: Effective battery life will be reduced when operating the device below 5ºF (-15ºC)
due to alkaline battery technology.
Note: Remove batteries when storing device for prolonged periods to maintain battery life.
www.masimo.com 41 Masimo
Page 44

Page 45

Chapter 7: Specifications
Performance Specifications
Functional Oxygen Saturation (SpO2)
Condition Range A
*
rms
No Motion [1] 60% to 80% 3%
No Motion [2] 70% to 100% 2%
Motion [3] 70% to 100% 3%
Low Perfusion [4] 70% to 100% 2%
Pulse Rate (PR) [5]
Condition Range A
*
rms
No Motion 25 - 240 bpm 3 bpm
Motion [3] 25 - 240 bpm 5 bpm
Low Perfusion [4 ] 25 - 240 bpm 3 bpm
Total Hemoglobin (SpHb) [6]
Condition Range A
*
rms
No Motion 8 g/dL - 17 g/dL 1 g/dL
*The A
Accuracy is calculated based upon measurement values that are statistically
rms
distributed; approximately 68% of the measured values fell within +/- the A
compared to the reference device under a controlled study.
value when
rms
www.masimo.com 43 Masimo
Page 46

Pronto Chapter 7: Specifications
Display Ranges
Measurement Display Range
SpO2 (Functional Oxygen Saturation) 0% to 100%
SpHb (Hemoglobin) 0 g/dL to 25 g/dL
PR (Pulse Rate) 25 bpm to 240 bpm
PI (Perfusion Index) 0.02% to 20%
Resolution
Parameter Resolution
SpO2 1%
SpHb 0.1 g/dL
Pulse Rate 1 bpm
Electrical
Battery
Type Four (4) AA alkaline
Capacity Operates continuously for up to eight (8) hours without changing batteries [7]
Isolation No external power or ground connection, internally powered only, DC current
www.masimo.com 44 Masimo
Page 47

Pronto Chapter 7: Specifications
Environmental
Operating Temperature 41°F to +104°F (5°C to +40°C)
Storage Temperature -40°F to +158°F (-40°C to +70°C)
Storage Humidity 5% to 95%, non-condensing
Operating Altitude
500 mbar to 1060 mbar
-1000 ft to 18,000 ft (-304 m to 5,486 m),
Physical Characteristics
Dimensions 6.2” x 3.0” x 1.4” (15.8 cm x 7.6cm x 3.6 cm)
Weight 13oz. (0.37 kg)
Trend Memory
Trending
Memory
Stores a minimum of 10,000 time-stamped spot check result data in trend
memory
Alerts
Audible Alerts Low Battery, System Failure
Audible Tone 500 Hz tone, three (3) pulse burst, repeat time: 5s
www.masimo.com 45 Masimo
Page 48

Pronto Chapter 7: Specifications
Display Indicators
Data
Display
Indicators
%SpO2, SpHb g/dl, Pulse Rate bpm, PI
Low Signal IQ Indicator, Battery Level Indicator, Spot Check Progress Indicator,
Pulse Indicator, and Sensor Use Indicator
Type LED
Compliance
Safety Compliance
UL 60601-1
CSA C22.2 No. 601.1
IEC/EN 60601-1, 2nd Ed.
IEC/EN 60601-1, 3rd Ed.
IEC 60601-1-11
IEC 62366
ISO 80601-2-61
www.masimo.com 46 Masimo
Page 49

Pronto Chapter 7: Specifications
EMC Compliance
EN 60601-1-2, Class B
Equipment Classification per IEC 60601-1
Type of Protection Internally powered (battery powered)
Degree of Protection Against Electric
Shock
Environment
Type BF-Applied Part
Not for use in the presence of flammable
anesthetics
Mode of Operation Continuous Operation
www.masimo.com 47 Masimo
Page 50

Pronto Chapter 7: Specifications
Citations
1. SpO2 accuracy was determined by testing on healthy adult volunteers in the range
60% - 100% SpO2, against a laboratory CO-Oximeter. Contact Masimo for testing
specifications.
2. The Masimo sensors have been validated for no motion accuracy in human blood
studies on healthy adult male and female volunteers with light to dark skin
pigmentation in induced hypoxia studies in the range of 70-100% SpO2 against a
laboratory CO-Oximeter and ECG monitor.
3. The Masimo sensors have been validated for motion accuracy in human blood
studies on healthy adult male and female volunteers with light to dark skin
pigmentation in induced hypoxia studies while performing rubbing and tapping
motions, at 2 to 4 Hz at an amplitude of 1 to 2 cm and a non-repetitive motion
between 1 to 5 Hz at an amplitude of 2 to 3 cm in induced hypoxia studies in the
range of 70-100% SpO2 against a laboratory CO-Oximeter and ECG monitor.
4. The Pronto has been validated for low perfusion accuracy in bench-top testing
against a Fluke Biotek Index 2
strengths of greater than 0.02% and transmission of greater than 5% for
saturations ranging from 70-100%.
5. Masimo sensors have been validated for pulse rate accuracy for the range of
25-240 bpm in bench top testing against a Fluke Biotek Index 2 simulator.
6. SpHb accuracy has been validated on healthy adult male and female volunteers
and on surgical patients with light to dark skin pigmentation in the range of 8
g/dL to 17 g/dL SpHb against a laboratory CO-Oximeter. The SpHb accuracy has
not been validated with motion or low perfusion.
7. Continuous use is defined as consecutive spot check tests with each consecutive
spot check test initiated immediately upon the conclusion of the previous spot
check test.
*Registered trademark of Fluke Biomedical Corporation, Everett, Washington.
TM
* simulator and Masimo's simulator with signal
www.masimo.com 48 Masimo
Page 51

Pronto Chapter 7: Specifications
Symbols
The following symbols may be found on Pronto or its packaging and are defined below:
Symbol Definition
Follow Instructions for Use
Consult Instructions for Use
Type BF Applied Part
Not for Continuous Monitoring
Separate Collection for Electronic Waste (WEEE)
Mark of Conformity to European Medical Device Directive 93/42/EEC
Federal law (USA) restricts this device to sale by or on the order of a
physician
Manufacturer
Date of Manufacture
Product contains no natural rubber latex
Product contains no PVC (polyvinyl chloride) material
www.masimo.com 49 Masimo
Page 52

Pronto Chapter 7: Specifications
Symbol Definition
Non-Sterile
Atmospheric Pressure Limitation
Storage Temperature Range
Storage/Transport Relative Humidity Range
Keep Dry
Fragile/Breakable, Handle with Care
Authorized representative in the European community
UL, LLC. Certification
Instructions/Directions for Use/Manuals are available in electronic format
@http://www.Masimo.com/TechDocs
Note: eIFU is not available for CE mark countries.
www.masimo.com 50 Masimo
Page 53

Pronto Chapter 7: Specifications
Guidance and Manufacturer's Declaration - Electromagnetic Emissions
Guidance and Manufacturer's Declarations - Electromagnetic Emissions
The ME Equipment is intended for use in the electromagnetic environment specified below.
The customer or the user of the ME Equipment should assure that it is used in such an
environment.
Emission Test Compliance Electromagnetic Environment - Guidance
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Group 1
Class B
ME Equipment uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
Suitable for use in all establishments, including domestic
environments.
Harmonic
emissions
N/A
IEC 61000-3-2
Voltage
fluctuations/
flicker
N/A
emissions
IEC 61000-3-3
www.masimo.com 51 Masimo
Page 54

Pronto Chapter 7: Specifications
Guidance and Manufacturer's Declaration - Electromagnetic Immunity
Guidance and Manufacturer's Declaration - Electromagnetic Immunity
The ME Equipment is intended for use in the electromagnetic environment specified below.
The customer or the user of the ME Equipment should assure that it is used in such an
environment.
Immunity Test IEC 60601
Test Level
Electrostatic
discharge (ESD)
+6 kV contact
+8 kV air
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
± 2 kV for
power supply
lines
± 1 kV for
input/output
lines
Surge
IEC 61000-4-5
± 1 kV line(s)
to line(s)
± 2 kV line(s)
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
to earth
<5 % UT (>95
% dip in UT)
for 0,5 cycle
40 % UT (60 %
dip in UT) for
5 cycles
70 % UT (30 %
dip in UT) for
25 cycles
<5 % UT (>95
% dip in UT)
for 5 s
Compliance
Level
+6 kV
contact
+8 kV air
Electromagnetic Environment Guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative
humidity should be at least 30%.
N/A Mains power quality should be that of a
typical commercial or hospital
environment.
N/A Mains power quality should be that of a
typical commercial or hospital
environment.
N/A Mains power quality should be that of a
typical commercial or hospital
environment. If the user of the [ME
EQUIPMENT or ME SYSTEM] requires
continued operation during power
mains interruptions, it is recommended
that the [ME EQUIPMENT or ME
SYSTEM] be powered from an
uninterruptible power supply or a
battery.
www.masimo.com 52 Masimo
Page 55

Pronto Chapter 7: Specifications
Guidance and Manufacturer's Declaration - Electromagnetic Immunity
Power frequency
3 A/m 3 A/m Power frequency magnetic fields should
(50/ 60 Hz)
magnetic field
IEC 61000-4-3
Conducted RF IEC
61000-4-6
Radiated RF IEC
61000-4-3
ISO 80601-2-61,
Clause 202
3 Vrms 150
kHz to 80 MHz
3 V/m 150 kHz
to 80MHz
20 V/m 80
MHz to 2.5
GHz
3 Vrms
3 V/m
20 V/m
be at levels characteristic of typical
location in a typical hospital
environment.
Portable and mobile RF
communications equipment should be
used no closer to any part of the ME
Equipment, including cables, than the
recommended separation distance
calculated from the equation applicable
to the frequency of the transmitter.
Recommended separation distance
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2.5 GHz
where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter
manufacturer and d is the
recommended separation distance in
meters (m).
Field strengths from fixed RF
transmitters, as determined by an
electromagnetic site survey
a
, should be
less than the compliance level in each
frequency rangeb.
Interference may occur in the vicinity of
equipment marked with the following
symbol:
www.masimo.com 53 Masimo
Page 56

Pronto Chapter 7: Specifications
If the measured field strength in the location in which the ME Equipment is used
Guidance and Manufacturer's Declaration - Electromagnetic Immunity
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be
considered.
exceeds the applicable RF compliance level above, the ME Equipment should be observed to
verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as re-orienting or relocating the ME Equipment.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m.
www.masimo.com 54 Masimo
Page 57

Pronto Chapter 7: Specifications
Recommended Separation Distances
Recommended Separation Distance Between Portable and Mobile RF Communication
Equipment and the ME Equipment
The ME Equipment is intended for use in an electromagnetic environment in which radiated
RF disturbances are controlled. The customer or the user of the ME Equipment can help
prevent electromagnetic interference by maintaining a minimum distance between portable
and mobile RF communications equipment (transmitters) and the ME Equipment as
recommended below, according to the maximum output power of the communication
equipment.
Rated maximum
Separation Distance According to Frequency of Transmitter (m)
output power of
transmitter (W)
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5GHz
0.01 0.12 0.018 0.035
0.1 0.37 0.057 0.11
1 1.17 0.18 0.35
10 3.7 0.57 1.1
100 11.7 1.8 3.5
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter
in watts (W) according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
www.masimo.com 55 Masimo
Page 58

Page 59

Chapter 8: Service and Maintenance
The following chapter contains information about cleaning, battery operation, performance
verification, service, repair, and warranty.
Cleaning
Pronto is a non-sterile and reusable device. The surface of the Pronto should be cleaned when
the device is visibly dirty, before and after each procedure, and/or according to hospital
practice.
To surface clean, wipe down the outer surface of Pronto using any of the following:
• A soft cloth dampened with a mild detergent and warm water solution
• Cidex Plus (3.4% glutaraldehyde)
• 10% bleach solution
• 70% isopropyl alcohol solution
Note: Do not allow liquids to enter the interior of the device.
Note: The performance of a device with a touchscreen will not be affected when using the
recommended cleaning solutions.
Battery Replacement
Pronto is powered by four (4) AA alkaline batteries. Do not use any other type of batteries or
power source to run the device.
Replacing the batteries
1. Locate the battery compartment on the back of the device.
2. Remove the battery cover by depressing the small rectangular button at the
bottom of the cover, and sliding the cover down off the bottom of the device.
3. Remove the batteries and install new batteries in the directions indicated by the
battery orientation icons (+ and -) inside the battery compartment.
4. Replace the battery cover by sliding it back up from the bottom of the device until
the rectangular locking button snaps back into position.
For more information about battery disposal, see Compliance Warnings and Cautions on page
14.
www.masimo.com 57 Masimo
Page 60

Pronto Chapter 8: Service and Maintenance
Performance Verification
To test the performance of the Pronto following repairs or during routine maintenance, follow
the procedure outlined in this section. If the Pronto fails any of the described tests,
discontinue its use and correct the problem before returning the device back to the user.
Before performing the following tests, verify or install new batteries into the Pronto. See
Battery Replacement on page 57. Also disconnect any patient cables, serial cables, or sensors
from the device.
Power-On Self-Test
1. Turn on the device by pressing the Power button. For about 5 seconds, all
available LEDs are illuminated and a brief beep tone sounds.
2. Verify that the sensor uses remaining displays.
3. Pronto is ready for use (the
RDY message displays).
Service and Repair
Repair Policy
Masimo or an authorized service department must perform warranty repair and service. Do
not use malfunctioning equipment. Have the device repaired.
Clean contaminated and/or dirty equipment before returning, following the cleaning
procedure described in Cleaning on page 57. Make sure the equipment is fully dry before
packing.
To return the device for service, refer to Return Procedure on page 59.
www.masimo.com 58 Masimo
Page 61

Pronto Chapter 8: Service and Maintenance
Return Procedure
Clean contaminated/dirty equipment before returning, following instructions in Cleaning on
page 57. Make sure the equipment is fully dry before packing. Call Masimo at 800-326-4890
and ask for Technical Support. Ask for an RMA number. Package the equipment securely, in
the original shipping container if possible, and enclose or include the following information
and items:
• A letter describing in detail any difficulties experienced with the Pronto. Include
the RMA number in the letter.
• Warranty information, a copy of the invoice or other applicable documentation
must be included.
• Purchase order number to cover repair if the Pronto is not under warranty, or for
tracking purposes if it is.
• Ship-to and bill-to information.
• Person (name, telephone/Telex/fax number, and country) to contact for any
questions about the repairs.
• A certificate stating the Pronto has been decontaminated for bloodborne
pathogens.
• Return the Pronto to the shipping address listed in Contacting Masimo on page
59 below.
Contacting Masimo
Masimo Corporation
40 Parker
Irvine, California 92618
Tel:+1 949 297 7000
Fax:+1 949 297 7001
www.masimo.com 59 Masimo
Page 62

Pronto Chapter 8: Service and Maintenance
Limited Warranty
Masimo warrants to the original end-user purchaser the Masimo-branded hardware product
(Pronto® Pulse CO-Oximeter®) and any software media contained in the original packaging
against defects in material and workmanship when used in accordance with Masimo’s user
manuals, technical specifications, and other Masimo published guidelines for a period of 12
months from the original date the Product was obtained by the end-user purchaser.
Masimo’s sole obligation under this warranty is the repair or replacement, at its option, of any
defective Product or software media that is covered under the warranty.
To request a replacement under warranty, Purchaser must contact Masimo and obtain a
returned goods authorization number so that Masimo can track the Product. If Masimo
determines that a Product must be replaced under warranty, it will be replaced and the cost of
shipment covered. All other shipping costs must be paid by purchaser.
Exclusions
The warranty does not apply to any non-Masimo branded product or any software, even if
packaged with the Product, or any Product that was: (a) not new or in its original packaging
when supplied to purchaser; (b) modified without Masimo’s written permission; (c) supplies,
devices, or systems external to the Product; (d) disassembled, reassembled, or repaired by
anyone other than a person authorized by Masimo; (e) used with other products, like new
sensors, reprocessed sensors, or other accessories, not intended by Masimo to be used with
the Product; (f) not used or maintained as provided in the operator’s manual or as otherwise
provided in its labeling; (g) reprocessed, reconditioned, or recycled; and (h) damaged by
accident, abuse, misuse, liquid contact, fire, earthquake or other external cause.
No warranty applies to any Product provided to Purchaser for which Masimo, or its authorized
distributor, is not paid; and these Products are provided AS-IS without warranty.
Limitation of Warranty
Except as otherwise required by law or altered by the purchase agreement, the above warranty
is the exclusive warranty that applies to the Product and software media, and Masimo does
not make any other promises, conditions, or warranties regarding the Product. No other
warranty applies, express or implied, including without limitation, any implied warranty of
merchantability, fitness for a particular purpose, satisfactory quality, or as to the use of
reasonable skill and care. See the licensing terms for the terms and conditions that apply to
and Software accompanying the Product. Additionally, Masimo will not be liable for any
incidental, indirect, special, or consequential loss, damage, or expense arising from the use or
loss of use of any Products or Software. In no event shall Masimo’s liability arising from any
Product or Software (under contract, warranty, tort, strict liability, or otherwise) exceed the
amount paid by purchaser for the Product or Software. The above limitations do not preclude
any liability that cannot legally be disclaimed by contract.
www.masimo.com 60 Masimo
Page 63

Pronto Chapter 8: Service and Maintenance
Sales & End-User License Agreement
This document is a legal agreement between you (“purchaser”) and Masimo Corporation
(“Masimo”) for the purchase of this Product (“Product”) and a license in the included or
embedded Software (“Software”) except as otherwise expressly agreed in a separate contract
for the acquisition of this Product, the following terms are the entire agreement between the
parties regarding your purchase of this Product. If you do not agree to the terms of this
agreement, promptly return the entire Product, including all accessories, in their original
packages, with your sales receipt to Masimo for a full refund.
Restrictions
1. Copyright Restrictions: The Software and the accompanying written materials are
copyrighted. Unauthorized copying of the Software, including Software that has
been modified, merged, or included with other software, or the written materials is
expressly forbidden. Purchaser may be held legally responsible for any copyright
infringement that is caused or incurred by Purchaser’s failure to abide by the terms
of this Agreement. Nothing in this License provides any rights beyond those
provided by 17 U.S.C. §117.
2. Use Restrictions: Purchaser may physically transfer the Product from one location
to another provided that the Software is not copied. Purchaser may not
electronically transfer the Software from the Product to any other device.
Purchaser may not disclose, publish, translate, release, distribute copies of, modify,
adapt, translate, reverse engineer, decompile, disassemble, or create derivative
works based on the Software or the written materials.
3. Transfer Restrictions: In no event may Purchaser transfer, assign, rent, lease, sell,
or otherwise dispose of the Product or the Software on a temporary basis.
Purchaser shall not assign or transfer this License, in whole or in part, by operation
of law or otherwise without Masimo's prior written consent; except that the
Software and all of Purchaser’s rights hereunder shall transfer automatically to any
party that legally acquires title to the Product with which this Software is included.
Any attempt to assign any rights, duties or obligations arising hereunder other
than as set forth in this paragraph shall be void.
4. U.S. Government Rights: If Purchaser is acquiring Software (including the related
documentation) on behalf of any part of the United State Government, the
following provisions apply: the Software and documentation are deemed to be
“commercial software” and “commercial computer software documentation,”
respectively pursuant to DFAR Section 227.7202 FAR 12.212, as applicable. Any
use, modification, reproduction, release, performance, display or disclosure of the
Software (including the related documentation) by the U.S. Government or any of
its agencies shall be governed solely by the terms of this Agreement and shall be
prohibited except to the extent expressly permitted by the terms of this
Agreement.
www.masimo.com 61 Masimo
Page 64

Page 65

Index
A
About This Manual • 5
Alerts • 45
Audible Alerts • 30
B
Basic Operation • 27
Battery Level Indicator • 22, 25, 30
Battery Replacement • 25, 35, 39, 57, 58
C
Chapter 1
Technology Overview • 15
Chapter 2
System Description • 21
Chapter 3
Setup • 25
Chapter 4
Operation • 27
Chapter 5
Messages • 27, 35
Chapter 6
Troubleshooting • 39
Chapter 7
Specifications • 43
Chapter 8
Service and Maintenance • 57
Citations • 48
Cleaning • 57, 58, 59
Cleaning and Service Warnings and
Cautions • 13
Compliance • 46
Compliance Warnings and Cautions • 14,
36, 57
Contacting Masimo • 59
Contraindications • 7
www.masimo.com 63 Masimo
Default Settings • 25, 29, 34, 40
Display Indicators • 46
Display Ranges • 44
E
Electrical • 44
Environmental • 45
Erasing Trend Memory • 33
Exclusions • 60
F
Front Panel • 21
Functional Oxygen Saturation (SpO2) •
16
G
General Description for Oxygen
Saturation (SpO2) • 16
General Description for Perfusion Index
(PI) • 16
General Description for Pulse Rate (PR) •
16
General Description for Total
Hemoglobin (SpHb) • 18
Guidance and Manufacturer's
Declaration - Electromagnetic
Emissions • 51
Guidance and Manufacturer's
Declaration - Electromagnetic
Immunity • 52
I
Indications for Use • 7
Initial Setup • 25
L
Limitation of Warranty • 60
Limited Warranty • 60
Low Battery Audible Alert • 41
D
Page 66

Pronto Index
Low Perfusion • 40
Low Signal I.Q. (Low SIQ) • 19, 22, 33, 35,
40
M
Masimo SET® DST • 16
Masimo SET® Parallel Engines • 15
Masimo Signal Extraction Technology
(Masimo SET®) • 15
Messages • 35
S
Safety Information, Warnings, and
Cautions • 9, 18, 39, 40
Safety Warnings and Cautions • 9
Sales & End-User License Agreement • 61
Service and Repair • 58
SpO2 and SpHb Measurements During
Patient Motion • 19
Successful Monitoring for SpHb • 18
Symbols • 49
N
Navigating the Menu • 22, 31, 40
P
Performance Specifications • 43
Performance Verification • 58
Performance Warnings and Cautions • 10
Physical Characteristics • 45
Power Requirements • 25
Product Description and Features • 7
Product Description, Features, and
Indications for Use • 7
Pulse CO-Oximetry vs. Drawn Whole
Blood Measurements • 18
R
rainbow Pulse CO-Oximetry Technology •
17
Rear Panel • 23
Recommended Separation Distances •
55
Repair Policy • 58
Resolution • 44
Restrictions • 61
Return Procedure • 25, 35, 39, 58, 59
T
Trend Data Format • 34
Trend Memory • 45
Trend Setup and Use • 33
TrendCom Utility Installation and
Operation • 33
Troubleshooting • 39
U
Unpacking and Inspection • 25
www.masimo.com 64 Masimo
Page 67

Page 68

www.masimo.com
32900/5052J-0616
 Loading...
Loading...