Page 1

605100, 605102, 655100, 655102
USER’S MANUAL
Page 2
Important user information
All users must read this entire manual to fully
understand the safe use of EMMA.
Declaration of conformity
0413
Complies with 93/42/EEC Medical Device Directive.
FDA Approval reference number K072813 and
K063167.
Safety notices
This user manual contains Warning notices and
Caution notices. These notices shall be followed.
WARNING! Warnings indicate a potential harmful
condition that can possibly lead to injury or death.
CAUTION! Cautions indicate conditions which may
lead to the damage or malfunction of the device.
NOTE! Alert the user to relevant facts and
conditions.
Liability
Masimo Sweden AB shall in no event be liable for any
direct, indirect, special or consequential damages,
including without limitation, loss of profits, income,
information, or use of the product, business interruption,
other related damages, however caused, arising from
the use of the product described in this manual.
Disclaimer
Masimo Sweden AB guarantees that the product
delivered has been tested to ensure that it meets its
published specifications.
Warranty
Please contact your local distributor for details
regarding warranty and product returns.
Use of the product for other than its intended use, or if it
is repaired by anyone except Masimo Sweden AB or a
Masimo authorized service center, or altered or
modified or used without following the instructions
provided with the product, voids the warranty.
MEDICAL – GENERAL
MEDICAL EQUIPMENT AS TO
ELECTRICAL SHOCK, FIRE
AND MECHANICAL HAZARDS
ONLY IN ACCORDANCE WITH
ANSI/AAMI ES60601-1 (2005)
and CAN/CSA-C22.2 No. 60601-
1 (2008)
3JSV
Patents
Masimo holds the following patents regarding
products described in this manual: SE519766;
SE519779; SE523461; SE524086. Other patents
pending.
Trademarks
Masimo EMMA and Masimo XTP Windows are
trademarks of Masimo Corporation.
Copyright
This document contains proprietary information
that is protected by copyright. All rights are
reserved. No part of this document may be
photocopied, reproduced or translated to another
language without prior written consent of Masimo
Sweden AB.
All Rights Reserved. © 2017 Masimo Sweden AB
Contact information
Masimo Sweden AB
Svärdvägen 15
SE-182 33 Danderyd
Sweden
Telephone: +46 8 544 98 150
Fax: +46 8 544 98 169
Web site: www.masimo.com
The information in this document is subject to
change without notice.
Article no: 0001-6984
Edition: 02
Released: March 2017
Revision history
Edition Date Description
01 September 30th 2016 Revised for EMMA Capnograph with Bluetooth functionality.
02 March 20th 2017 Ch.2.3: Added statement that EMMA Bluetooth complies with Canada RSS standards.
2 (34)
Page 3

Contents
1 INTENDED USE .................................................................................................................................. 4
2 SAFETY INFORMATION .................................................................................................................... 5
2.1 WARNINGS ..................................................................................................................................... 5
2.2 CAUTIONS ...................................................................................................................................... 6
2.3 NOTES ........................................................................................................................................... 6
2.4 SYMBOL DESCRIPTION ..................................................................................................................... 8
3 DEVICE DESCRIPTION .................................................................................................................... 10
3.1 EMMA CAPNOGRAPH OVERVIEW................................................................................................... 10
3.2 PRINCIPLE OF OPERATION ............................................................................................................. 11
3.2.1 EMMA Airway Adapter ........................................................................................................ 12
4 PREPARATIONS FOR USE ............................................................................................................. 13
4.1 SETTING UP .................................................................................................................................. 13
4.2 STARTING UP ................................................................................................................................ 14
4.3 SWITCHING OFF ............................................................................................................................ 15
4.4 CONNECTING THE EMMA CAPNOGRAPH TO A TUBE OR A MASK ....................................................... 15
5 USER INTERFACE ........................................................................................................................... 16
5.1 CONTROLS ................................................................................................................................... 16
5.2 BLUETOOTH (OPTIONAL) ................................................................................................................ 16
5.3 MONITORING ................................................................................................................................ 17
5.3.1 ETCO2 ................................................................................................................................. 17
5.3.2 Respiratory Rate ................................................................................................................. 17
5.2.3 Capnogram .......................................................................................................................... 17
5.4 INDICATORS AND ALARMS .............................................................................................................. 18
5.4.1 Alarm signals ....................................................................................................................... 18
5.4.2 Default limits for alarms ....................................................................................................... 19
5.4.3 Alarm silence ....................................................................................................................... 19
5.4.4 Battery Status Indicator ....................................................................................................... 20
5.4.5 Adjusting the ETCO2 alarm limits ........................................................................................ 20
6 EMMA AND ACCESSORIES ............................................................................................................ 22
7 MAINTENANCE AND SERVICE ...................................................................................................... 23
7.1 BATTERY REPLACEMENT ............................................................................................................... 23
7.2 CLEANING .................................................................................................................................... 23
7.3 EMMA AIRWAY ADAPTER ............................................................................................................. 23
7.4 ZEROING PROCEDURE ................................................................................................................... 24
7.5 GAS SPAN CHECK ......................................................................................................................... 24
7.6 TROUBLESHOOTING ...................................................................................................................... 26
7.7 SERVICE AND PRODUCT RETURN REQUIREMENTS ........................................................................... 26
8 TECHNICAL SPECIFICATIONS ....................................................................................................... 27
8.1 GENERAL SPECIFICATIONS ............................................................................................................ 27
8.3 ELECTROMAGNETIC COMPATIBILITY (EMC) .................................................................................... 29
8.4 COMPLIANCE ................................................................................................................................ 33
8.5 BLUETOOTH LE WIRELESS TECHNOLOGY INFORMATION ................................................................. 33
8.6 CLASSIFICATIONS ......................................................................................................................... 33
3 (34)
Page 4

1 Intended use
EMMA measures, displays and monitors carbon dioxide partial pressure and respiratory rate during
anesthesia, recovery and respiratory care. It may be used in the operating suite, intensive care unit,
patient room, clinic, emergency medicine and emergency transport settings for adult, pediatric and infant
patients.
4 (34)
Page 5

2 Safety information
Adhere to the following warnings, cautions and notes for safe operation of EMMA.
2.1 Warnings
WARNING! EMMA should only be used for the purpose and in the manner described in this
manual.
WARNING! EMMA is intended for use by authorized health care professionals only.
WARNING! EMMA must not be used with flammable anesthetic agents.
WARNING! Use only EMMA Airway Adapters manufactured by Masimo.
WARNING! No modification of the EMMA probe or the EMMA Airway Adapters is allowed.
WARNING! EMMA Airway Adapters shall not be reused. Reuse of single use Adapters can
cause cross infection. Used Airway Adapters shall be disposed of in accordance with local
regulations for medical waste.
WARNING! Do not use the EMMA Adult/Pediatric Airway Adapter with infants as the Adapter
adds 6 ml dead space to the patient circuit.
WARNING! Do not use the EMMA Infant Airway Adapter with adults as this may cause
excessive flow resistance.
WARNING! Measurements can be affected by mobile phones and RF communications
equipment. It should be assured that EMMA is used in the specified electromagnetic
environment.
WARNING! EMMA is intended only as an adjunct in patient assessment. It shall be used in
conjunction with the assessment of clinical signs and symptoms.
WARNING! If EMMA is used with a respirator or with harmful gases such as N
perform a pre-use tightness check of the patient circuit.
WARNING! Light transmission can be affected by secretions and moisture pooling on the
EMMA Airway Adapter XTP
paid to position the Airway Adapter in a vertical position and to change Airway Adapter if
necessary.
WARNING! Do not use EMMA with nebulized medications as this may affect the light
transmission of the EMMA Airway Adapter windows.
WARNING! Audible alarm of any monitor may not be heard in some loud environments, such
as when sirens are in use and the care provider is more distant from the alarm source. Alarm
volume should be tested with the extremes of your noise environment to confirm ability or
limitations to hear an alarm in all circumstances of the environment.
TM
windows. When using heated humidifiers special care should be
O, always
2
5 (34)
Page 6

WARNING! Replace batteries immediately when the Battery Status Indicator starts blinking.
Remaining battery time depends on battery type and other circumstances and cannot be
reliably predicted.
WARNING! Lithium batteries may present a fire or chemical burn hazard if mistreated. Do not
disassemble, heat above 100C (212F) or incinerate. Dispose of used cell promptly. Keep
away from children.
WARNING! Use only Alkaline batteries or Energizer Ultimate Lithium L92 batteries. Use of
other Lithium batteries may present a risk of fire or explosion.
2.2 Cautions
CAUTION! If EMMA is used in a manner other than that for which it was intended, unpredictable
behavior could result.
CAUTION! The EMMA Airway Adapters are non-sterile devices. Do not autoclave the devices as this
will damage them.
CAUTION! Never sterilize or immerse EMMA in liquid.
CAUTION! Do not operate EMMA at ambient temperatures less than -5°C (23°F) or greater than
50°C (122°F).
CAUTION! Federal law restricts this device to sale by or on the order of a physician.
CAUTION! Remove batteries if EMMA is not likely to be used for a period of time longer than 90 days.
2.3 Notes
NOTE! Throughout this User’s Manual:
EMMA Airway Adapter refers to both Airway Adapter Adult/Pediatric and Airway Adapter Infant if not
otherwise mentioned.
NOTE! A trained medical professional must determine the proper EMMA Airway Adapter model for
each patient application. No hardware or software configuration changes result from the EMMA
Airway Adapter model selected.
NOTE! The alarm limits will be reset to default values after power off.
NOTE! Always carry spare batteries in the EMMA pouch.
NOTE! The presence of ambient air (0% CO
for a successful Zeroing. Special care should be taken to avoid breathing near the EMMA Airway
Adapter before or during the Zeroing procedure.
NOTE! EMMA Bluetooth device complies with part 15 of the FCC rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and (2) this device must
accept any interference received, including interference that may cause undesired operation.
Changes or modifications not expressly approved by the manufacturer could void the user’s authority
to operate the equipment.
) in the EMMA Airway Adapter is of crucial importance
2
6 (34)
Page 7
NOTE! EMMA Bluetooth complies with Industry Canada license-exempt RSS standard(s). Operation
is subject to the following two conditions: (1) This device may not cause harmful interference, and (2)
this device must accept any interference received, including interference that may cause undesired
operation.
7 (34)
Page 8
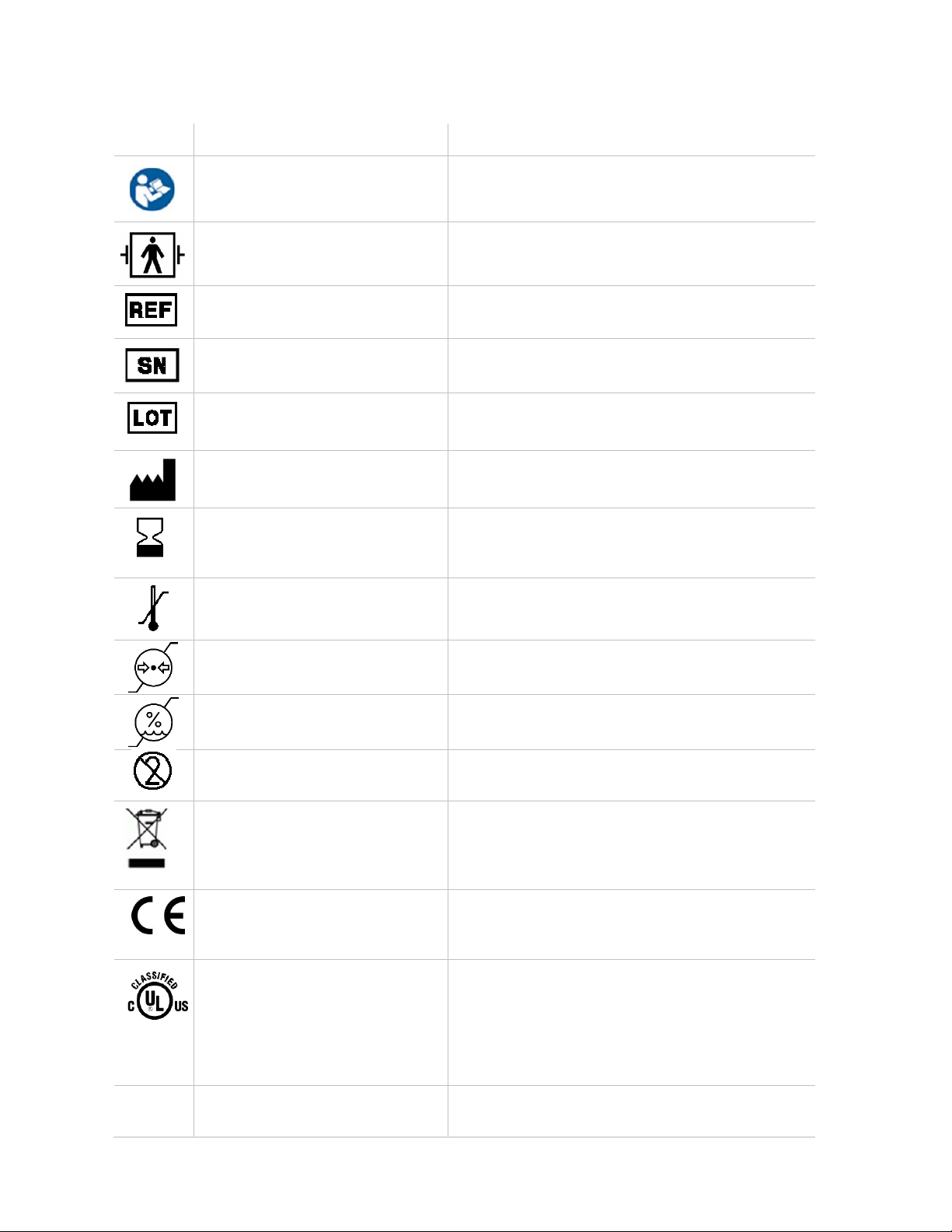
2.4 Symbol description
Symbol Title Explanation
Catalog number
Serial number
Batch code
Manufacturer
Use by date [YYYY-MM-DD] Indicates that the device should not be taken into
Temperature limitation
Follow instructions for use This symbol replaces, and has the same meaning,
as the previously used symbol ISO7000-0434.
Defibrillation-proof type BF applied
part
Accompanied by the name and address of the
manufacturer.
operation after the date accompanying the symbol
(EMMA Airway Adapters).
Pressure limitation
Humidity limitation
Do not re-use Intended for single patient use (EMMA Airway
Adapters).
For EU only:
Waste Electrical and Electronic
Equipment (WEEE)
Conformité Européenne Complies with 93/42/EEC Medical Device
0413
UL classification mark Classified by Underwriters Laboratories Inc. for
3JSV
IP33
IP classification indicating degree
of protection against water and
For EU only:
Electrical and electric equipment shall be collected
and recycled in accordance with Directive
2002/96/EC.
Directive.
Canada and US with respect to electrical shock,
fire and mechanical hazards in accordance with
ANSI/AAMI ES60601-1 (2005) and
CAN/CSA-C22.2 No. 60601-1 (2008)
3JSV = Control number assigned by UL.
IP33 =“Spray-proof” and “Tool-proof”.
8 (34)
Page 9
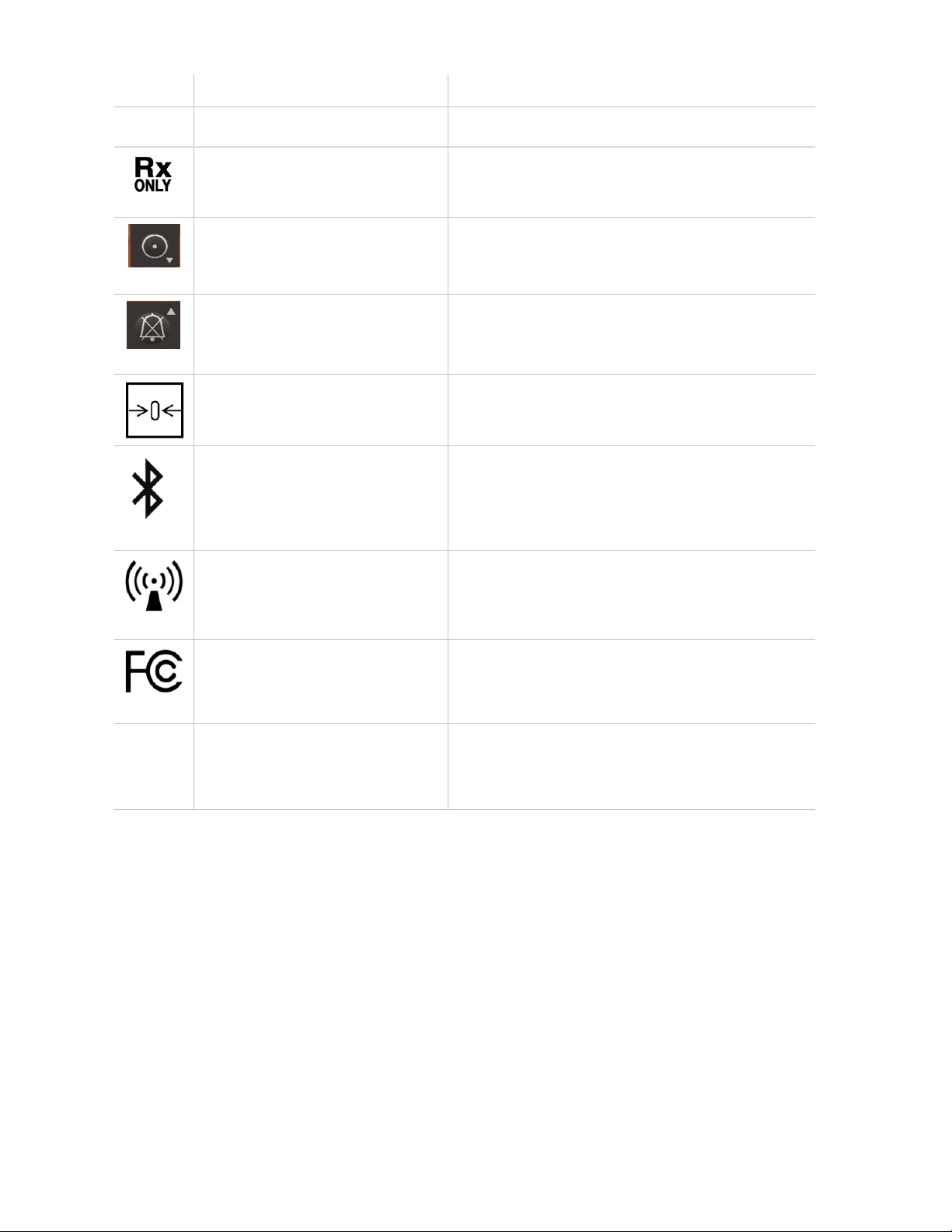
Symbol Title Explanation
solid foreign objects.
Rx only Caution (U.S.): Federal law restricts this device to
sale by or on the order of a licensed healthcare
practitioner.
Power on button
Alarm silence button
Zero-point adjustment
Bluetooth
Radio transmitter
Indicating that an offset in gas readings is
discovered and performing zeroing is required.
See ch 7.4.
Device equipped with Bluetooth.
Indicates that the device has a radio transmitter.
Federal Communications Commission (FCC)
licensing.
FCC ID,
IC Model
Identifies unit has been registered as a radio
device.
IC
9 (34)
Page 10

3 Device description
3.1 EMMA Capnograph overview
The EMMA Capnograph is a quantitative mainstream carbon dioxide monitor comprised of a Sensor
Body that fits on top of a disposable EMMA Airway Adapter.
Alarm Silence button
Battery Cover
Carry strap
ETCO
2
Capnogram
Power On
button
Battery Cover
release button
Value
Bluetooth Indicator (optional)
EMMA Sensor Body
Battery Status Indicator
Respiratory Rate Value
Alarm Status Indicator
EMMA Airway Adapter
Figure 1. EMMA Capnograph
10 (34)
Page 11
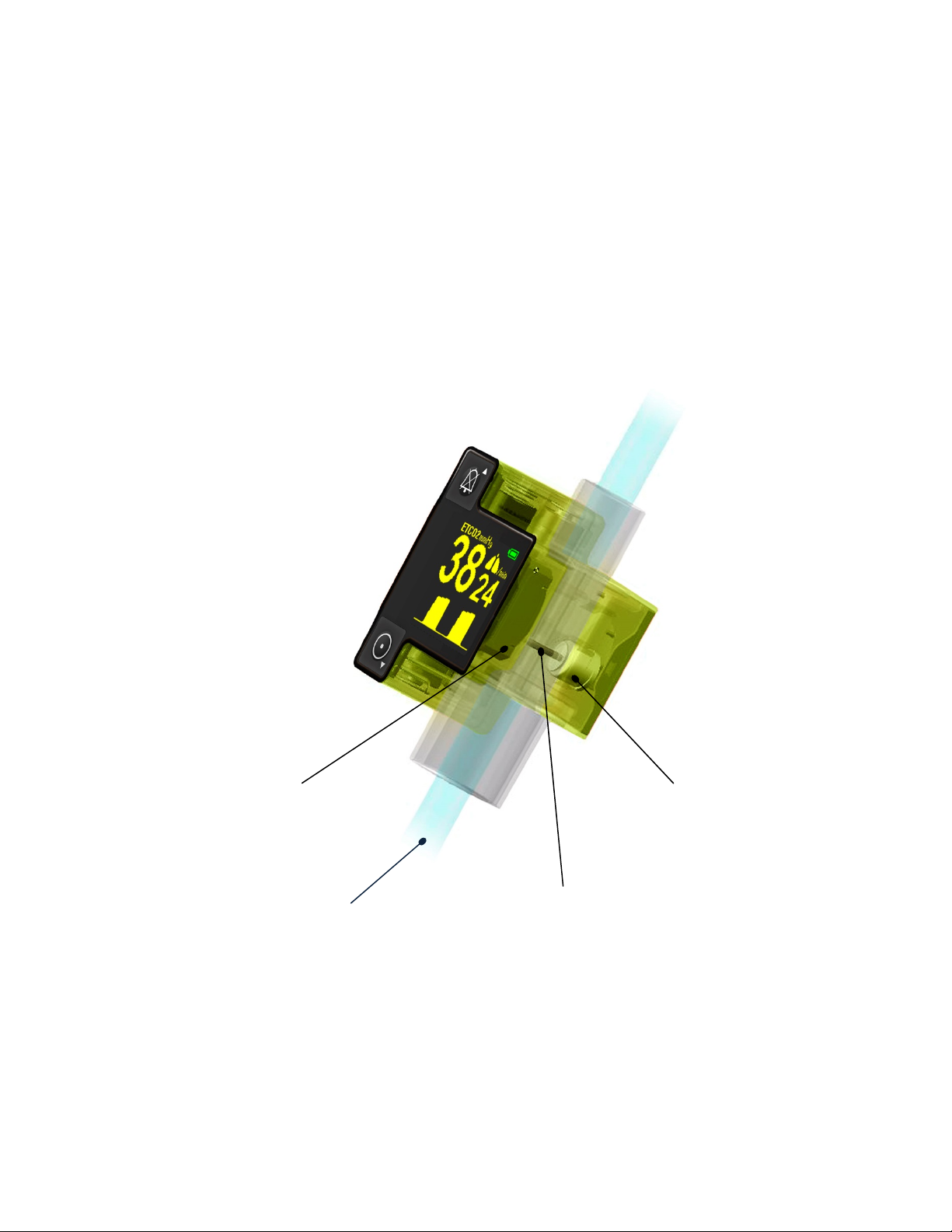
3.2 Principle of operation
The measurement of CO2 in the breathing gas mixture is based on the fact that different gas
components absorb infrared light at specific wavelengths. A beam of invisible infrared light is directed
through the respiratory gas flow in the EMMA Airway Adapter. As the beam passes through the EMMA
Airway Adapter, some of the light is absorbed by the gas mixture. The amount of absorbed light is
measured by a miniaturized two channel spectrometer positioned to receive the infrared light beam.
The spectrometer incorporates a filter wheel fitted with two different optical "color" filters. The
wavelength ranges of these filters are chosen such that one filters out colors where carbon dioxide has
very strong absorption and the other filters out colors where carbon dioxide has no absorption.
The spectrometer also incorporates an infrared detector that converts the light beam to an electrical
signal. The electrical signal is converted to a digital value that is fed to a microprocessor. The ratio of the
light measured through the two filters is then used by the microprocessor to calculate the carbon dioxide
concentration in the breathing gas mixture.
Figure 2. Principle of operation
Spectrometer with
optical filter wheel and
infrared detector
Respiratory gas
Infrared light source
Infrared light beam
11 (34)
Page 12

3.2.1 EMMA Airway Adapter
Respiratory gas measurements are, as described in the previous section, obtained by continuously
measuring the infrared light absorption through the EMMA Airway Adapter. The EMMA Airway Adapter
is fitted with optical XTP™ windows that are transparent to light in the wavelength ranges of interest. The
EMMA Airway Adapter may, for example, be inserted between the endotracheal tube and the
resuscitation bag or between the resuscitation bag and the patient mask.
The EMMA Airway Adapter is available in two models: Adult/Pediatric (Figure 3a) and Infant (Figure 3b).
EMMA operates to specification with either EMMA Airway Adapter model when used with its appropriate
patient population.
XTP™ window
Figure 3a. EMMA Airway Adapter Adult/Pediatric
Figure 3b. EMMA Airway Adapter Infant
XTP™ window
NOTE! A trained medical professional must determine the proper EMMA Airway Adapter model for each
patient application. No hardware or software configuration changes result from the EMMA Airway
Adapter model selected.
12 (34)
Page 13

4 Preparations for use
4.1 Setting up
Unpack and inspect the EMMA Capnograph for external damage. Please contact your local distributor in
case of damage.
1. Press the Battery Cover release button into the EMMA Sensor Body until the Battery Cover pops
off.
Figure 4. Releasing the Battery Cover
2. Open the battery compartment and insert two (2) AAA batteries. Make sure the batteries are
fitted according to the indicated polarity. After battery installation, snap the Battery Cover back
into place.
Figure 5. Inserting batteries
13 (34)
Page 14

4.2 Starting up
1. Snap the EMMA Airway Adapter into the EMMA Capnograph. It will click into place when
properly inserted.
2. Press the Power On button.
3. When the EMMA Capnograph is ready the ETCO
value is zero.
2
The audible alarm sound may be checked by detaching the EMMA Airway Adapter to generate a “No
Adapter” alarm.
When the EMMA Capnograph is ready the ETCO
Value indicates “0” and the Respiratory Rate Value
2
indicates “- -“.
If the ETCO
Value is non-zero, ensure that there has not been an accumulation of CO2 between the
2
EMMA Sensor Body and the EMMA Airway Adapter by removing and reattaching the EMMA Airway
Adapter. If the ETCO
Value still displays a non-zero value after this procedure, perform a Zeroing
2
procedure as described in chapter 7.4 prior to using the EMMA Capnograph with a patient.
14 (34)
Page 15

4.3 Switching off
The EMMA Capnograph switches off automatically during following conditions:
After 2 minutes if no breath is detected.
After 2 minutes if No Breath condition is detected and the Alarm Silence is activated.
After 15 seconds if the EMMA Airway Adapter is removed.
4.4 Connecting the EMMA Capnograph to a tube or a mask
The EMMA Capnograph can be connected to a patient in different ways. The following pictures illustrate
two methods of connection.
Figure 7. EMMA Capnograph connected to an endotracheal tube.
Figure 8. EMMA Capnograph connected to a mask
15 (34)
Page 16

5 User interface
5.1 Controls
The EMMA Capnograph has one Power On and one Alarm Silence button. These buttons may also be
used for adjusting the Low and High ETCO
5.2 Bluetooth (optional)
The EMMA Bluetooth (REF: 655100 and 655102) provides a Bluetooth Low Energy (LE) wireless option
to allow connection to a compatible smart device or host. EMMA Bluetooth can only communicate to a
single smart device at a time to minimize the risk of unauthorized access. Pressing the Power ON button
twice when EMMA Bluetooth device is ON will enable the LE Bluetooth.
Bluetooth enabled Bluetooth disabled
alarm limits up and down.
2
Bluetooth symbol
Connectivity indicator
Unique Bluetooth
adress (EMMA_serial
number)
Bluetooth may be enabled/disabled by repeatedly pressing the ON button. Bluetooth will as default be
disabled and must be enabled each time by the user. The unique Bluetooth address contains the
product name plus the serial number of the device and will be transmitted to the smart device or host.
Confirm the correct connection by controlling that the appropriate serial number is displayed on the
smart device or host.
If no button has been activated for a short period of time, the EMMA Bluetooth will automatically resume
normal operation. If the Bluetooth is enabled the Bluetooth indicator will appear on the screen.
Normal operation with
Bluetooth enabled
16 (34)
Page 17

5.3 Monitoring
The EMMA Capnograph is fitted with a graphic OLED-display that shows the ETCO2 Value, the
Respiratory Rate Value and a CO
5.3.1 ETCO2
The EMMA Capnograph is available in versions displaying ETCO2 either in mmHg (0 - 99 mmHg) or kPa
(0.0 - 9.9 kPa). ETCO
breath.
values are displayed after one breath and the averaged value is updated every
2
5.3.2 Respiratory Rate
Respiratory Rate (RR) is displayed as breaths per minute (3 - 150 bpm). RR is displayed after two
breaths and the value is updated every breath.
5.2.3 Capnogram
The Capnogram is displayed as a filled graph with a 14.4 sec horizontal sweep and a fixed 0-53
mmHg/0-7 kPa scale.
waveform (the capnogram).
2
ETCO2 value
Capnogram
Figure 9. EMMA Capnograph display
If the CO
indicate that the capnogram is saturated.
level reaches or exceeds 53 mmHg/7 kPa, a horizontal dashed line will be displayed to
2
Respiratory
Value
Saturated Capnogram
17 (34)
Page 18

5.4 Indicators and alarms
The EMMA Capnograph is equipped with an Alarm Status Indicator and an audible alarm that may be
silenced for a period of 2 minutes.
5.4.1 Alarm signals
When an alarm is triggered, an Alarm Satus Indicator in the lower right corner of the display is lit with a
steady or blinking yellow light depending on alarm priority, together with an audible alarm beep
according to the following table:
((()))
Alarm
No Breath
Low ETCO2
High ETCO2
Clogged Adapter
at t = 0
Alarm Priority: Low
((()))
((()))
at t = 20
Alarm Priority: Low
((())) ((()))
t = 40, 60, 80, ...
Alarm Priority: Medium
((())) ((())) ((()))
No Adapter
n/a
n/a
((()))
Zero point
adjustment
((()))
18 (34)
Page 19

Note: t = 0 is defined as the time when the alarm condition first is indicated. t = 40, 60, 80, ... shall be
interpreted as "40 sec later than t = 0", "60 sec later than t = 0", "80 sec later than t = 0" etc.
Active alarms are further displayed according to the following table:
Alarm Screen ETCO2 Value RR Value
No Breath NORMAL value steady 1) "- -" flashing 2)
Low ETCO2 NORMAL value flashing value steady
High ETCO2 NORMAL value flashing value steady
Clogged Adapter ADAPTER n/a n/a
No Adapter ADAPTER n/a n/a
Zero point adjustment 3) NORMAL value steady value steady
Note 1: ETCO2 value shows momentary CO2 during No Breath.
Note 2: RR value will show "- -" steady if no breath at all detected from power on.
Note 3: Perform Zeroing procedure as described in chapter 7.4.
5.4.2 Default limits for alarms
The default factory settings for the No Breath and the Low/High ETCO2 alarms are as follows:
RR (No Breath)
Low/High ETCO2
5.4.3 Alarm silence
The audible alarm can be muted for 2 minutes by pressing the Alarm
Silence button. When the audible alarm is muted the yellow silence alarm
indicator in the bottom right corner of the display, i.e. the Alarm Status
Silence Indicator, will be lit.
Pressing the Alarm Silence button again during the 2 minutes mute period
will reactivate the audible alarm.
If a No Breath alarm is muted by pressing the Alarm Silence button, the
EMMA Capnograph will automatically switch off after 2 minutes provided
that no new breaths are detected.
If the alarm disappears when the audible alarm is muted, the alarm icon
will turn green. Pressing the Alarm Silence button during no alarm will also
show a green silence alarm indicator in the bottom right corner of the
display.
Lower Limit Upper Limit
3 bpm (20 s) -
OFF 50 mmHg (7.0 kPa)
19 (34)
Page 20

5.4.4 Battery Status Indicator
The Battery Status Indicator is normally lit with a steady green light in the upper right corner of the
display (Battery OK). When batteries are low, the Battery Status Indicator starts blinking.
Battery OK
Battery low
(Blinking)
There will be an audible tone beep repeated every 80 seconds when batteries are low.
The terminal voltage of alkaline batteries recovers when the batteries are not in use. The remaining time
prediction is thus unreliable during the first period after power on. Nearly depleted batteries may still be
able to provide a voltage above the threshold for battery low indication, even if the internal battery
resistance is too high to provide sufficient current to start up the device next time the power on button is
activated.
To extend battery life time the EMMA display has an automatic brightness control which will be activated
during stable conditions. Any change in displayed vital parameters, alarm or pressing any button will
return the EMMA display to normal brightness.
WARNING! Replace batteries immediately when the Battery Status Indicator starts blinking.
Remaining battery time depends on battery type and other circumstances and cannot be reliably
predicted.
WARNING! Lithium batteries may present a fire or chemical burn hazard if mistreated. Do not
disassemble, heat above 100C (212F) or incinerate. Dispose of used cells promptly. Keep away
from children.
WARNING! Use only Alkaline batteries or Energizer Ultimate Lithium L92 batteries. Use of other
Lithium batteries may present a risk of fire or explosion.
5.4.5 Adjusting the ETCO2 alarm limits
5.4.5.1 Adjusting the High ETCO2 alarm limit
1. Press and hold the Alarm Silence button until the display shows the “Hi ETCO2 Screen” and the
ETCO
2. Release the button.
3. To adjust the alarm limit: press the Alarm Silence button (▲) to increase, or the Power On
button (▼) to decrease the value. It is possible to switch off the high ETCO
the limit above 99 mmHg (9.9 kPa). The EMMA Capnograph will indicate this setting by showing
"- -" on the ETCO
If no button has been activated for a short period of time, the EMMA Capnograph will automatically
resume normal operation.
display shows the current high ETCO2 alarm limit.
2
display during the adjustment routine.
2
alarm by adjusting
2
20 (34)
Page 21

Figure 10. Adjusting the High and Low ETCO
alarm limits
2
5.4.5.2 Adjusting the Low ETCO
alarm limit
2
1. Press and hold the Power On button until the display shows the “Lo ETCO2 Screen” and the
ETCO
display shows the current low ETCO2 alarm limit.
2
2. Release the button.
3. To adjust the alarm limit: press the Alarm Silence button (▲) to increase, or the Power On
button (▼) to decrease the value. It is possible to switch off the low ETCO
alarm by adjusting
2
the limit down to 0. The EMMA Capnograph will indicate this setting by showing "- -" on the
ETCO
display during the adjustment routine.
2
If no button has been activated for a short period of time, the EMMA Capnograph will automatically
resume normal operation.
5.4.5.3 Alarm limit adjustment ranges
The adjustment ranges for the ETCO
alarm limits are as follows:
2
ETCO2 displayed in mmHg
ETCO2 displayed in kPa
Lower range Upper range
OFF; 1 – 89 mmHg 11 – 99 mmHg; OFF
OFF; 0.1 – 8.9 kPa 1.1 – 9.9 kPa; OFF
If the high ETCO
limit is decreased close to the low ETCO2 limit, the low limit will be automatically
2
adjusted in order to maintain a minimum difference of 10 mmHg (1.0 kPa) between the high and low
alarm limit. Similarly, if the low ETCO
limit is increased close to the high ETCO2 limit, the high limit will
2
be automatically adjusted to maintain a minimum difference of 10 mmHg (1.0 kPa) between the high and
low alarm limit.
NOTE! The alarm limits will be reset to default values after power off.
21 (34)
Page 22

6 EMMA and accessories
Below is a list of device models, versions and approved accessories. For an up to date list of
accessories visit www.masimo.com
Catalog
number
(REF)
EMMA and accessories Description
605100
605102
655100
655102
606100
606102
3678
3639
4270
4271
EMMA (kPa)
EMMA (mmHg)
EMMA Bluetooth (kPa)
EMMA Bluetooth (mmHg)
EMMA Capnometer (kPa)
EMMA Capnometer (mmHg)
Kit EMMA (kPa)
Kit EMMA (mmHg)
Kit EMMA Bluetooth (kPa)
Kit EMMA Bluetooth (mmHg)
EMMA Emergency Capnograph (full-alarm, display
in kPa). Color: green
EMMA Emergency Capnograph (full-alarm, display
in mmHg). Color: green
EMMA Emergency Capnograph with Bluetooth
(full-alarm, display in kPa). Color: green
EMMA Emergency Capnograph with Bluetooth
(full-alarm, display in mmHg). Color: green
EMMA Emergency Capnometer (full-alarm, display
in kPa). Color: orange
EMMA Emergency Capnometer (full-alarm, display
in mmHg). Color: orange
Kit including EMMA Emergency Capnograph
(kPa), EMMA Pouch and EMMA Lanyard.
Kit including EMMA Emergency Capnograph
(mmHg), EMMA Pouch and EMMA Lanyard.
Kit including EMMA Emergency Capnograph with
Bluetooth (kPa), EMMA Pouch and EMMA
Lanyard.
Kit including EMMA Emergency Capnograph
Bluetooth (mmHg), EMMA Pouch and EMMA
Lanyard.
9633
9632
100620
100660
100681
100682
100685
100684
Kit EMMA Capnometer (kPa)
Kit EMMA Capnometer (mmHg)
EMMA Airway Adapter Adult/Pediatric,
box of 25
EMMA Airway Adapter Infant,
box of 10
EMMA Pouch, Green
EMMA Pouch, Orange
EMMA Lanyard, Green, 10 pcs
EMMA Lanyard, Orange, 10 pcs
Kit including EMMA Emergency Capnometer
(kPa), EMMA Pouch and EMMA Lanyard.
Kit including EMMA Emergency Capnometer
(mmHg), EMMA Pouch and EMMA Lanyard.
EMMA Airway adapter is needed in order for
EMMA to provide readings.
EMMA Airway adapter is needed in order for
EMMA to provide readings.
Easy to carry pouch for safe storage of EMMA.
Lanyard attaches to EMMA to be carried around
your neck.
22 (34)
Page 23

7 Maintenance and service
7.1 Battery replacement
WARNING! Replace batteries immediately when the Battery Status Indicator starts blinking.
Remaining battery time depends on battery type and other circumstances and cannot be reliably
predicted.
WARNING! Lithium batteries may present a fire or chemical burn hazard if mistreated. Do not
disassemble, heat above 100C (212F) or incinerate. Dispose of used cell promptly. Keep away from
children.
WARNING! Use only Alkaline batteries or Energizer Ultimate Lithium L92 batteries. Use of other
Lithium batteries may present a risk of fire or explosion.
To replace the batteries:
1. Open the battery compartment by pressing the release button.
2. Gently remove the depleted batteries.
3. Insert two new AAA type batteries into the battery compartment. Make sure that the batteries are
fitted according to the polarity marking.
4. When the batteries are properly fitted, gently snap the battery cover back into place.
NOTE! Always carry spare batteries in the EMMA pouch.
7.2 Cleaning
1. Remove the batteries before cleaning.
2. The EMMA Capnograph can be cleaned using a cloth moistened with 70% isopropyl alcohol.
CAUTION! DO NOT immerse EMMA in any liquid.
7.3 EMMA Airway Adapter
The EMMA Airway Adapters are intended for single patient use. They are disposable and shall
not be re-used. Reuse of single patient use Adapters can cause cross infection.
EMMA Airway Adapters shall be disposed of in accordance with local regulations for bio
hazardous waste.
23 (34)
Page 24

7.4 Zeroing procedure
Zeroing is recommended after 500 hours of operation or whenever an offset in gas readings is
discovered. Zeroing of the EMMA Capnograph is performed by the following procedure:
NOTE! The presence of ambient air (0% CO
successful Zeroing. Special care should be taken to avoid breathing near the EMMA Airway Adapter
before or during the Zeroing procedure.
1. Start the EMMA Capnograph by pressing the Power On button.
2. Make sure that a new EMMA Airway Adapter is properly fitted.
3. Press and hold down simultaneously the Power On and Alarm Silence button until the Service
Screen display the Service code “C0” and the Service value “10”. Keep both buttons depressed
while the Service value starts "counting down" i.e. displaying "9" - "8" - "7" etc. until "0" is
displayed.
4. When the Service value “0” is shown, Zeroing of the EMMA Capnograph is completed.
) in the EMMA Airway Adapter is of crucial importance for a
2
Service value
Service code
The EMMA Capnograph will return to normal measuring mode when the Service value has reached "0"
or if any of the buttons are released.
7.5 Gas span check
The EMMA Capnograph does not require any routine calibration. A gas span check is recommended at
regular intervals to make sure the measurement is within accuracy levels. The suggested interval for gas
span check is once every year.
To perform a gas span check of EMMA you will need:
1. A gas flow regulator with a plastic tube and a 15M
connector
2. Calibration gas (5% CO
3. Two EMMA Airway Adapters
, 21% O2, Balance N2)
2
24 (34)
Page 25

Directions
Attach the flow regulator to the calibration gas cylinder. Ensure that the valve is shut off completely.
1. Attach a new EMMA Airway Adapter to the EMMA Capnograph.
2. Turn on the EMMA Capnograph and ensure that the ETCO
reading is zero. Otherwise conduct
2
a Zeroing procedure according to chapter 7.4 above before proceeding.
3. Insert the 15M connector into one end of the EMMA Airway Adapter, and connect a second
EMMA Airway Adapter to the other end (see picture).
4. Turn on the regulator flow.
5. After 30 seconds, record the ETCO
reading.
2
6. Turn off the flow.
7. Determine and record an estimated ambient atmospheric pressure in mmHg.
8. Use the following table to determine if the unit is reading within specified limits.
Barometric pressure
[mmHg]
EMMA Capnograph ETCO2 readings should be between
5% CO2 [mmHg] 5% CO2 [kPa]
660-679 31-36 4,1-4,8
680-699 32-37 4,3-4,9
700-719 33-38 4,4-5,1
720-739 34-39 4,5-5,2
740-759 35-40 4,6-5,4
760-779 36-41 4,8-5,5
780-799 37-42 4,9-5,6
If the unit is reading within the above range then your EMMA Capnograph has been successfully
verified.
If the unit is not reading within the above range, disconnect the EMMA Airway Adapter from the gas
cylinder and perform a Zeroing procedure according to the instructions in chapter 7.4 above and then
repeat the Gas span check procedure. If verification still fails, contact your local distributor for further
instructions.
25 (34)
Page 26

7.6 Troubleshooting
Error Possible causes Recommended Solutions
The unit does not complete
the turn on sequence
The unit does not turn on No battery
The measured values of
ETCO
accuracy
Numbers appear dim Automatic brightness control is
Measurement does not display
on the smart device or host
using optional Bluetooth
are out of specified
2
Low battery Replace batteries
Low battery
Incorrect zero reference Perform a Zeroing procedure
activated
Exposed to bright lights or
sunlight
Bluetooth not connected
Smart device, host or EMMA
Bluetooth out of range
Compatible app not installed on
smart device
Smart device damaged
EMMA Bluetooth damaged
Replace batteries
and verify the measurement with
reference gas
Pressing any button will return
the EMMA display to normal
brightness
Confirm Bluetooth is on for the
EMMA Bluetooth and the smart
device or host
Move the EMMA Bluetooth, smart
device or host closer to each
other
Check that EMMA Bluetooth is
paired to the correct smart device
or host
Confirm a compatible app is
installed on the smart device
Close and re-launch the
compatible app on the smart
device
Contact Masimo Technical
Services
7.7 Service and product return requirements
Please contact Technical Services with any questions or assistance you may need regarding this
product. Local contact information can be found at http://service.masimo.com.
26 (34)
Page 27

8 Technical specifications
8.1 General specifications
Description Compact, battery powered, quantitative capnograph for mainstream
CO
monitoring of adult, pediatric and infant patients.
2
Measurements
Models CO2 displayed in kPa or mmHg
Warm up In operation and full accuracy within 15 s.
Calibration No routine calibration required
Certifications CE marked per 93/42/EEC, FDA 510(k) and UL/CSA 60601-1
Dimensions EMMA Capnograph: 52 x 39 x 39 mm (2.1 x 1.6 x 1.6 inches)
Weight <65 g (2.1 oz) with batteries
Mechanical robustness Withstands repeated 1 m drops.
(1)
The CO2 partial pressure is measured based on a 2 channel NDIR type
gas analyzer at 4–5 µm with data acquisition rate at 10 kHz (sample
rate 20 Hz / channel).
EMMA Bluetooth: 52 x 44 x 39 mm (2.1 x 1.8 x 1.6 inches)
Meets the shock and vibration requirements for transport of EN ISO
80601-2-55:2011 clause 201.15.3.5.101.2 and EN 1789:2007 clause
6.3.4.2 and 6.4.1.
Operating conditions Temperature: -5 - +50°C (23 to 122°F)
Humidity: < 40 hPa H
Atmospheric pressure: 60 - 120kPa
O (non-condensing) (95% RH at 30 °C)
2
(1)
(i.e. Altitude up to 4000 m)
At ambient temperature Surface temperature
Highest surface temperature
23°C / 73°F 30°C / 86°F
50°C / 122°F 57°C / 135°F
Storage conditions Temperature: -30 - +70°C (-22 to 158°F)
Humidity: 5 - 100% RH (condensing) at a water vapor partial pressure
not exceeding 74 hPa (100 %RH at 40 °C)
Atmospheric pressure: 50 - 120 kPa
Display 96 x 96 pixel RGB OLED-display
ETCO
(1)
0 - 99 mmHg (0 - 9.9 kPa)
2
(2)
ETCO2 will be within specification for respiration rates up to 150 bpm
CO2 accuracy
(3)
0-40 mmHg ± 2 mmHg, 41-99 mmHg 6% of reading
0-5.3 kPa ± 0.3 kPa, 5.4-9.9 kPa 6% of reading
during standard conditions.
Total system response time < 0.5 s
Drift of measurement
No drift
accuracy
Recovery time after
Unaffected
defibrillator test
(4)
Respiratory rate 3 - 150 bpm
Respiratory rate accuracy ± 1 bpm
Breath detect Adaptive threshold, minimum 1 kPa CO2 change
Adult/Pediatric Dead space 6 ml, Flow resistance < 0,3 cm H2O (@ 30 LPM)
27 (34)
Page 28

Infant Dead space 1 ml, Flow resistance < 1,3 cm H2O (@ 10 LPM)
Alarms No Breath, Low ETCO2, High ETCO2, Clogged Adapter, No Adapter,
Zero point adjustment, Low Battery
Sound Intensity Level ≥ 57 dB(A); ≤ 67 dB(A)
Batteries Two AAA Cell batteries (2x1.5VDC):
Alkaline IEC:LR03 or
Energizer Ultimate Lithium L92 batteries
(5)
. Use of other Lithium
batteries may present a risk of fire or explosion.
Battery life time EMMA Capnograph:
Duracell Plus Alkaline: ~5 hours
Energizer Ultimate Lithium L92: ~10 hours
EMMA Bluetooth:
Duracell Plus Alkaline: ~4 hours
Energizer Ultimate Lithium L92: ~8 hours
Notes:
(1)
. The EMMA Capnograph displays CO2 in partial pressure units (kPa or mmHg) and compensates the
displayed value for the actual barometric pressure. The ETCO
value is the max partial CO2 pressure
2
measured within a breath and the displayed value is:
– the latest ETCO2 values i.e. if ΔETCO
– the average of up to four ETCO
2
≥ 25% or
2
values measured within 30s given ΔETCO2 <25%.
(2)
Gas reading showing actual partial pressure at current humidity level. Partial pressure of CO2 in the
alveoli, where the breathing gas is saturated with water vapor at body temperature (BTPS), is typically
6% lower than the corresponding CO
(3)
To include quantitative effect on gas reading from variations in environment conditions (outside STP,
partial pressure after removal of all water vapor (ATPD).
2
electromagnetic disturbances) and presence of Halothane, Ethanol, Isopropyl alcohol, He, Acetone
and Methane, the CO
reading whichever is the greater. In addition the following interference effects on CO
- 60 vol% of N
- 60 vol% of O
influence from 21% O
- 5 vol% of ENF, ISO, SEV typically increases CO
- 15 vol% of DES typically increases CO
- 80% Xe typically decreases CO
- 50% He typically decreases CO
(4)
ETCO2 was measured at I/E ratio 1:1 using breath simulator according to the test setup in EN ISO
80601-2-55 fig. 201.101. The measured ETCO
accuracy range should be increased to ± 4 mmHg/ 0.5 kPa or 10% of
2
O typically increases CO2-readings by 10%
2
typically decreases CO2-readings by 4% (EMMA compensates CO2-values for
2
as default)
2
-readings by 8%
-readings by 12%
2
-readings by 10%
2
-readings by 6%.
2
was within the accuracy range for all respiration rates
2
2
readings exists:
2
up to 150 bpm.
(5)
www.energizer.com
28 (34)
Page 29

8.3 Electromagnetic compatibility (EMC)
Guidance and Masimo’s declaration – electromagnetic emissions
The EMMA gas analyzer is intended for use in the electromagnetic environment specified below. The
customer or the user of the EMMA gas analyzer should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11
EN 55011
RF emissions
CISPR 11
EN 55011
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Group 1
Class B The EMMA gas analyzer is suitable for use in all
Not applicable
Not applicable
The EMMA gas analyzer uses RF energy only for its
internal function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
establishments including domestic establishments and
those directly connected to the public low-voltage power
supply network that supplies buildings used for domestic
purposes.
29 (34)
Page 30

The EMMA gas analyzer will function according to general specification in chapter 8.1 and will not cause
any safety hazard or false alarm when exposed to the following immunity test levels.
Guidance and Masimo’s declaration – electromagnetic immunity
The EMMA gas analyzer is intended for use in the electromagnetic environment specified below. The
customer or the user of the EMMA gas analyzer should assure that it is used in such an environment.
Immunity test IEC 60601
test level
Electrostatic
discharge (ESD)
6 kV contact
8 kV air
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for
power supply
lines
1 kV for
input/output
lines
Surge
IEC 61000-4-5
1 kV line(s) to
line(s)
2 kV line(s) to
earth
Voltage dips, short
interruptions and voltage
variations on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in
U
T) for 0,5
cycle
40 % U
T
(60 % dip in
UT) for 5 cycles
70 % U
T
(30 % dip in
U
T) for 25
cycles
<5 % U
T
(>95 % dip in
UT) for 5 sec
Compliance
level
8 kV contact
15 kV air
Electromagnetic environment –
guidance
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30 %.
Not applicable Not applicable
Not applicable Not applicable
Not applicable Not applicable
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m 30 A/m Power frequency magnetic fields
should be at levels characteristic of a
typical location in a typical commercial
or hospital environment.
Note: UT is the a.c. mains voltage prior to application of the test level.
30 (34)
Page 31

Guidance and Masimo’s declaration – electromagnetic immunity
The EMMA gas analyzer is intended for use in the electromagnetic environment specified below. The
customer or the user of the EMMA gas analyzer should assure that it is used in such an environment.
Immunity test IEC 60601 test
level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
Radiated RF
IEC 61000-4-3
20 V/m, 80%
AM at 1kHz
3 Vrms
150 kHz - 80
MHz
3 V/m
80%AM@2Hz
80 MHz - 2,5
GHz
20 V/m
80%AM@1kHz
80 MHz - 2,5
GHz
field strength
is defined in
EN-ISO 806012-55 in
202.6.2.3.1
and
ETSI EN
301 489-1
Compliance
level
Not applicable
3 V/m
80%AM@2Hz
80 MHz - 2,7
GHz
20 V/m
80%AM@1kHz
80 MHz - 2,7
GHz
Electromagnetic environment – guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the EMMA gas analyzer including
cables than the recommended separation
distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance
80 MHz to 800 MHz
Pd 17,1
800 MHz to 2,7 GHz
Pd 33,2
80 MHz to 800 MHz
Pd 18,0
800 MHz to 2,7 GHz
Pd 35,0
where P is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in meters
(m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site
survey,
level in each frequency range.
a
should be less than the compliance
b
Interference
may occur in the vicinity of equipment
marked with the following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be
predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF
transmitters, an electromagnetic site survey should be considered. If the measured field strength in the
location in which the EMMA gas analyzer is used exceeds the applicable RF compliance level above,
the EMMA gas analyzer should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as reorienting or relocating the EMMA gas
31 (34)
Page 32

analyzer.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communications
equipment and the EMMA gas analyzer
The EMMA gas analyzer is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the EMMA gas analyzer can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the EMMA gas analyzer as recommended below,
according to the maximum output power of the communications equipment.
Rated maximum
output power of
Separation distance according to frequency of transmitter
[m]
transmitter
[W]
150 kHz to 80 MHz
Pd 17,1
80 MHz to 800 MHz
Pd 0,18
800 MHz to 2.7 GHz
Pd 0,35
0,01 0,12 0,02 0,04
0,1 0,37 0,06 0,11
1 1,17 0,18 0,35
10 3,70 0,57 1,11
100 11,70 1,80 3,50
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the
transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
Note 3: In the frequency band 80 MHz to 2.7 GHz the separation distance is based on the higher field
strength 20 V/m 80%AM@1kHz.
WARNING! Measurements can be affected by mobile phones and RF communications equipment. It
should be assured that EMMA is used in the electromagnetic environment specified.
32 (34)
Page 33

8.4 Compliance
EN 60601-1:2006, Amendment 1 (2012)
EN 60601-1-2:2007, C1:2010
EN 60601-1-8:2007, C1:2010, A1:2013
EN 1789:2007, A1:2010
EN 13718-1:2008
EN ISO 80601-2-55:2012
EN ISO 5356-1:2004
EN ISO 14971:2012
EN ISO 15223-1:2012
8.5 Bluetooth LE Wireless Technology information
Bluetooth LE Wireless Technology Information
Modulation Type GFSK
Max. Output Power -1 dBm
Frequency Range 2402-2480 MHz
Antenna Peak Gain -7 dBi
Recommended Range ~10 feet (~3 meters) line-of-sight
Radio Compliance
Radio Modes Bluetooth LE
USA FCC ID: VKF-EMMABT
FCC parts 15.207 and 15.247
Canada IC-7362A-EMMABT
RSS-247
Europe EN 300 328
ETSI EN 301 489-1
ETSI EN 301 489-17
R&TTE 1999/5/EC
Japan Japanese Radio Law, Item 19 of
Article 2-1
8.6 Classifications
According to the type of protection against electric shock
INTERNALLY POWERED EQUIPMENT (Battery power)
According to the degree of protection against electric shock
DEFIBRILLATION-PROOF TYPE BF APPLIED PART
According to the degree of protection provided by enclosures
IP33 (spray proof and tool proof EQUIPMENT)
According to the mode of operation
CONTINUOUS OPERATION
According to sterility
No part of EMMA is sterile
33 (34)
Page 34

Masimo Sweden AB
Svärdvägen 15
182 33 Danderyd
Sweden
www.masimo.com
All Rights Reserved. © 2017 Masimo Sweden AB
The information in this document is subject to change without notice.
34 (34)
 Loading...
Loading...