Page 1
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
Radius PPG™
-40 C
+70 C
5%-95% RH
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
5%-95% RH
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
Chip and Receiver
DIRECTIONS FOR USE
Reusable
Prior to using this product the user should read and understand the Operator’s Manual for the device/monitor, The
Directions for Use for the sensor, and this Directions for Use.
INDICATIONS
Masimo Radius PPG™ is intended for the non-invasive continuous monitoring of functional oxygen saturation of arterial
hemoglobin (SpO
Masimo Radius PPG is indicated for the continuous monitoring of functional arterial oxygen saturation of hemoglobin
(SpO
conditions and for patients who are well or poorly perfused in hospital, hospital-type facilities and home environments.
2), pulse rate (PR).
2) and pulse rate (PR) for use with adult, pediatric and neonatal patients during both no motion and motion
LATEX
Not made with natural rubber latex
Devices with Masimo technology are only to be used with Masimo sensors and cables.
CONTRAINDICATIONS
The Radius PPG is contraindicated for patients who exhibit allergic reactions to foam rubber products and/or adhesive tape.
DESCRIPTION
Radius PPG consists of three parts:
• Radius PPG wireless receiver
• Radius PPG reusable chip
• Radius PPG adhesive sensor (shipped seperately, see Directions for Use for the Radius PPG Adhesive Sensor)
Radius PPG is a wireless sensor for use with devices containing Masimo technology MX Version
individual device manufacturers for compatibility of particular device and sensor models. Each device manufacturer is
responsible for determining whether their device is compatible with each sensor model.
WARNINGS, CAUTIONS, AND NOTES
• Do not leave the sensor components unattended around children. Small items may become choking hazards.
• To ensure continued monitoring, routinely verify the wireless connection.
• When using multiple Radius PPG sensors, repeat pairing before monitoring to ensure proper wireless connection.
• When using Radius PPG, keep it within the recommended range from the connected host (see Wireless Technology
Information for details); moving outside of this range may cause a loss in connection with the host device.
• When using Radius PPG, relocate the devices away from sources that may inter fere with the Bluetooth connection.
The presence of other devices that may create radio frequency interference (RFI) may result in loss of Quality of
Service (see Specifications for details) of the Bluetooth connection. Devices that may cause RFI include but are not
limited to the following: electrocautery equipment, diathermy equipment, other cellular telephones, wireless PC
and tablets, pagers, RFID devices, MRI, and electromagnetic security systems.
INSTRUCTIONS
a) Initial setup
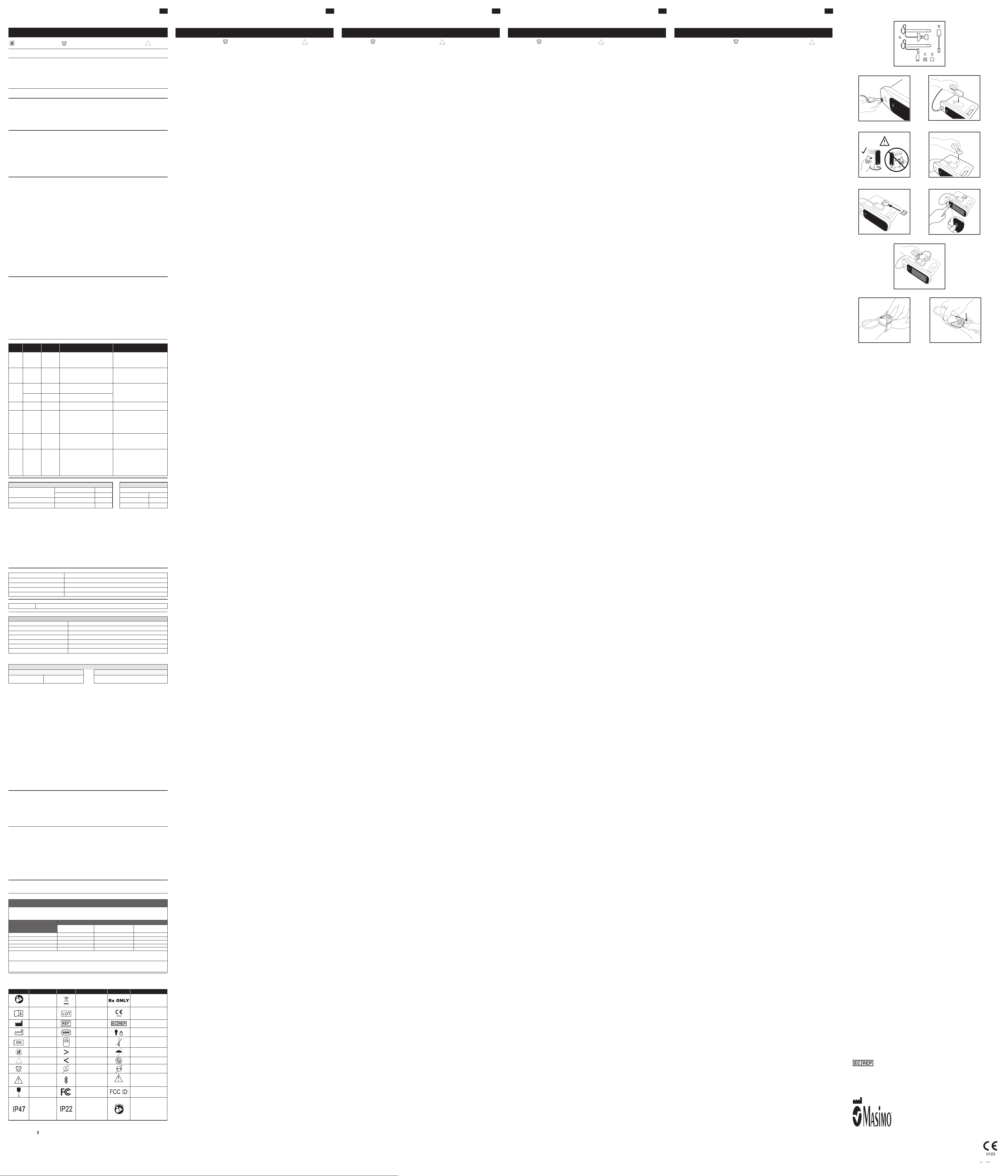
1. Verify you have all components. Refer to Fig. 1.
A. Radius PPG adhesive sensor B. Radius PPG wireless bluetooth receiver C. Radius PPG reusable chip D. Radius PPG reusable chip holder for device
2. Turn on the patient monitor.
3. Plug the cable into the patient monitor. Refer to Fig. 2. The light on wireless receiver will be white.
4. Attach the wireless receiver to the side of the patient monitor using the adhesive provided. Refer to Fig. 3.
5. Avoid covering speakers or holes used for mounting when attaching the module. Refer to Fig. 4.
6. Attach the chip holder near the receiver on the patient monitor. Refer to Fig. 5.
7. Verify that the wireless receiver is attached and plugged in to patient monitor. Verify that the chip holder is attached
to patient monitor. Refer to Fig. 6.
b) Pairing the reusable transmitter chip with the wireless receiver
1. Ensure the device is powered on. Refer to Fig. 7.
2. Hold the reusable chip to the indent on the wireless receiver until the Bluetooth symbol on the wireless receiver
turns green. Refer to Fig. 8.
3. Insert the reusable chip into the sensor attachment strap until there is a tactile or audible click of connection. Refer
to Fig. 9.
4. Verify the light on the wireless receiver turns blue. (See LIGHT INDICATOR GUIDE section.)
c) Disconnecting
1. Push down on the tab to release the reusable chip from the sensor. Refer to Fig. 10.
2. After cleaning, store the reusable chip in the chip holder attached to the patient monitor. Refer back to Fig. 6.
3. Discard the adhesive sensor and strap.
CLEANING
WARNING: Before cleaning, make sure the device is o and is not applied to a patient.
Thoroughly clean the Radius PPG reusable chip and wireless receiver before applying to a new patient.
To surface clean the reusable chip and wireless receiver:
1. Remove the sensor from the patient and disconnect the reusable chip.
2. Wipe all surfaces of the reusable chip and wireless receiver with one of the following:
a. 70% Isopropyl alcohol
b. 10% (1:10) chlorine bleach to water solution
c. Quaternary ammonium chloride solution
3. Allow the reusable chip and wireless receiver to dr y thoroughly before using again.
CAUTIONS:
• To avoid permanent damage to the reusable chip and wireless receiver, do not use undiluted bleach (5% - 5.25%
sodium hypochlorite) or any other cleaning solution not recommended.
• Do not immerse the reusable chip and wireless receiver in any liquid solution.
• Do not sterilize by irradiation, steam, autoclave or ethylene oxide.
LIGHT INDICATOR GUIDE
Wireless
Color
No light
White
Green
Blue
Purple
Orange
Red
(2 seconds)
(30 seconds)
Transmitter
receiver
solid --
solid
ashing
solid ashing • Successful pairing of receiver and chip
ashing ashing
ashing ashing • Low sensor battery
ashing ashing
-- --
ashing
(30 seconds)
chip
Description Next steps
• Wireless receiver cable is not
connected to host device with power
• Chip not connected to sensor with
battery
• Wireless receiver is connected to host
device with power ready to initiate
pairing with transmitter chip
• Paring search period has expired
-- • Chip and receiver are linked
• Pairing search period
• Battery seal tab has not been removed
to activate battery
• Battery is obstructed
• Depleted sensor battery
• Hardware or sensor failure, chip
blinking board failure code
• Turn on patient monitor and plug cable
into patient monitor
• See Instructions, section a) for set up
• Hold reusable chip to the indent on the
wireless receiver to initiate pairing
• See Instructions, section b) for pairing
• Insert reusable chip into sensor
attachment strap to complete pairing
• See Instructions, section b) for pairing
• Verify sensor attachment so host device
can receive data
• Remove tab to activate battery
• Refer to Directions for Use for the
Radius PPG Adhesive Sensor
• Disconnect reusable chip from sensor,
wait 30 seconds, insert chip into sensor
(Refer to Figs. 9 and 10.)
• Consider replacing sensor, do not
discard reusable chip
• See Instructions, section c) for
disconnecting
• Replace sensor, do not discard reusable
chip. If issue persists, replace reusable
chip
• See Instructions, section c) for
disconnecting
• Contact Masimo Technical Support, or
replace sensor and chip
ACCURACY SPECIFICATIONS Arms*
Oxygen Saturation (SpO2) Pulse Rate4 (PR) 25–240bpm
SpO2 Accuracy, No Motion
(70–100%)
SpO2 Accuracy, Motion
SpO
2 Accuracy, Low Perfusion
rms accuracy is a statistical calculation of the dierence between device measurements and reference measurements.
*NOTE: A
Approximately two-thirds of the device measurements fell within +/- Arms of the reference measurements in a controlled study.
1
The Masimo SET Technology has been validated for no motion accuracy in human blood studies on healthy adult male and female
volunteers with light to dark pigmented skin in induced hypoxia studies in the range of 70%-100% SpO2 against a laboratory co-oximeter.
2
The Masimo SET Technology has been validated for motion accuracy in human blood studies on healthy adult male and female volunteers
with light to dark pigmented skin in induced hypoxia studies while performing rubbing and tapping motions, at 2 to 4 Hz at an amplitude of
1 to 2 cm and a non-repetitive motion between 1 to 5 Hz at an amplitude of 2 to 3 cm in induced hypoxia studies in the range of 70%-100%
SpO2 against a laboratory co-oximeter.
3
The Masimo SET Technology has been validated for low perfusion accuracy in bench top testing against a Biotek Index 2 simulator and
Masimo’s simulator with signal strengths of greater than 0.02% and transmission of greater than 5% for saturations ranging from 70% to
100%.
4
The Masimo SET Technology has been validated for pulse rate accuracy for the range of 25-240 bpm in bench top testing against a Biotek
Index 2 simulator and Masimo’s simulator with signal strengths of greater than 0.02% and transmission of greater than 5% for saturations
ranging from 70% to 100%.
5
The saturation accuracy of the Neonate and Preterm sensors were validated on adult volunteers and 1% was added to account for the
properties of fetal hemoglobin.
1
2
Adults, Pediatrics, Infants 2% All patient populations
All patient populations 3% Motion 5 bpm
3
All patient populations 2% Low Perfusion 3 bpm
Neonates
5
3% No Motion 3 bpm
ENVIRONMENTAL SPECIFICATIONS
Storage/Transport Temperature -40°C - +70°C @ ambient humidity
Operating Temperature 0°C - 40°C @ ambient humidity
Storage/Transport Humidity 5% - 95% non-condensing
Operating Humidity 5% - 95% non-condensing
Atmospheric Pressure 540 to 1060 mBar @ ambient temperature and humidity
BATTERY LIFE
Battery Life 96 hours in typical continuous usage
WIRELESS TECHNOLOGY INFORMATION
Modulation Type GFSK
Max. Output Power +8 dBm
Frequency Range 2402 MHz - 2480 MHz
Antenna Peak Gain 1 dBi
Recommended Range 100 ft (~30 meters) line-of-sight
Quality of Service (QoS) Delay <30 seconds
Security Proprietary binary protocol
FCC ID are as follows: Chip: VKF-AIRTB01 Receiver: VKF-AIRDG01
IC IDs are as follows: Chip: 7362A-AIRTB01 Receiver: 7362A-AIRDG01
EN 301 489-17 V3.1.1
EN 301 489-3 V2.1.1
WARNING: Changes or modications not expressly approved by the party responsible for compliance could void the
user’s authority to operate the equipment.
WARNING: The frequency bands of this device (2.4 GHz) are only for indoor use, in accordance with international
telecommunication requirements.
CAUTION: Keep the Radius PPG away from electrical equipment that emits radio frequencies to minimize radio
interference. Radio interference may result in no or inaccurate readings.
Note: This device complies with part 15 of FCC Rules and Industry Canada’s license-exempt RSSs’. Operation is subject
to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any
interference received, including interference that may cause undesired operation.
Note: When using Radius PPG consideration should be taken to local government frequency allocations and technical
parameters to minimize the possibility of interference to/from other wireless devices.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to
part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a
residential installation. This equipment generates, uses, and can radiate radio frequency energy and, if not installed and
used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference
to radio or television reception, which can be determined by turning the equipment o and on, the user is encouraged
to try to correct the interference by one or more of the following measures:
RF Radiation Exposure Statement: This equipment has been exempted from FCC RF radiation exposure testing set
forth for an uncontrolled environment.
Note: Users are advised that high-power radars are allocated as primary users (i.e. priority users) of the bands 5250-5350
MHz and 5650-5850 MHz and that these radars could cause interference and/or damage to LE-LAN devices
RF Radiation Exposure Statement: Radius PPG Receiver has been exempted from IC RSS 102 RF radiation exposure
limits set forth for an uncontrolled environment.
WARRANTY
Masimo warrants to the initial buyer only that these products, when used in accordance with the directions provided with
the Products by Masimo, will be free of defects in materials and workmanship for a period of six (6) months. Single use
products are warranted for single patient use only.
THE FOREGOING IS THE SOLE AND EXCLUSIVE WARRANTY APPLICABLE TO THE PRODUCTS SOLD BY MASIMO TO BUYER.
MASIMO EXPRESSLY DISCLAIMS ALL OTHER ORAL, EXPRESS OR IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION
ANY WARRANTIES OF MERCHANTABILITY OR FITNESS FOR PARTICULAR PURPOSE. MASIMO’S SOLE OBLIGATION AND
BUYER’S EXCLUSIVE REMEDY FOR BREACH OF ANY WARRANTY SHALL BE, AT MASIMO’S OPTION, TO REPAIR OR REPLACE
THE PRODUCT.
WARRANTY EXCLUSIONS
This warranty does not extend to any product that has been used in violation of the operating instructions supplied
with the product, or has been subject to misuse, neglect, accident or externally created damage. This warranty does not
extend to any product that has been connected to any unintended device or system, has been modied, or has been
disassembled or reassembled. This warranty does not extend to products that have been reprocessed, reconditioned
or recycled.
IN NO EVENT SHALL MASIMO BE LIABLE TO BUYER OR ANY OTHER PERSON FOR ANY INCIDENTAL, INDIRECT, SPECIAL
OR CONSEQUENTIAL DAMAGES INCLUDING WITHOUT LIMITATION LOST PROFITS, EVEN IF ADVISED OF THE
POSSIBILITY THEREOF. IN NO EVENT SHALL MASIMO’S LIABILITY ARISING FROM ANY PRODUCTS SOLD TO BUYER
UNDER A CONTRACT, WARRANTY, TORT OR OTHER CLAIM EXCEED THE AMOUNT PAID BY BUYER FOR THE LOT OF
PRODUCTS INVOLVED IN SUCH CLAIM. IN NO EVENT SHALL MASIMO BE LIABLE FOR ANY DAMAGES ASSOCIATED
A PRODUCT THAT HAS BEEN REPROCESSED, RECONDITIONED OR RECYCLED. THE LIMITATIONS IN THIS SECTION
SHALL NOT BE DEEMED TO PRECLUDE ANY LIABILITY THAT, UNDER APPLICABLE PRODUCTS LIABILITY LAW, CANNOT
LEGALLY BE PRECLUDED BY CONTRACT.
NO IMPLIED LICENSE
PURCHASE OR POSSESSION OF THIS PRODUCT DOES NOT CARRY ANY EXPRESS OR IMPLIED LICENSE TO USE WITH
ANY DEVICE THAT IS NOT AN AUTHORIZED DEVICE OR SEPARATELY AUTHORIZED TO USE MASIMO PRODUCTS.
Radius PPG Receiver: Radius PPG Chip:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Consult the dealer or an experienced radio/TV technician for help.
Bluetooth LE Wireless Technology Information
EU Radio Equipment Directive (RED 2014/53/EU)
EN 300 328 V2.2.1
EN 300 330 V2.1.0
EN 301 489-17 V3.1.1
EN 300 328 V2.2.1
RECOMMENDED SEPARATION DISTANCES
RECOMMENDED SEPARATION DISTANCE BETWEEN PORTABLE AND
The ME Equipment is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The
customer or the user of the ME Equipment can help prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment (transmitters) and the ME Equipment as recommended below,
according to the maximum output power of the communication equipment.
RATED MAXIMUM OUTPUT
POWER OF TRANSMITTER (W)
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.17 1.17 2.33
10 3.7 3.7 7.37
100 11.7 11.7 23.3
For transmitters rated at a maximum output power not listed above, the recommended separation distance (d) in meters (m) can
be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of
the transmitter in watts (W) according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is aected by absorption and reection
from structures, objects and people.
CAUTION: FEDERAL LAW (U.S.A.) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
For professional use. See instructions for use for full prescribing information, including indications, contraindications,
warnings, precautions and adverse events.
The following symbols may appear on the product or product labeling:
MOBILE RF COMMUNICATION EQUIPMENT AND THE ME EQUIPMENT
SEPARATION DISTANCE ACCORDING TO FREQUENCY OF TRANSMITTER (m)
150 K Hz to 80 MHz
d = 1.17*√P
80 MHz to 800 MHz
d = 1.17*√P
SYMBOL DEFINITION SYMBOL DEFINITION SYMBOL DEFINITION
(blue background)
Patents: http://www.masimo.com/patents.htm
Radius PPG is a trademark of Masimo Corporation.
Masimo, SET, X-Cal, and are federally registered trademarks of Masimo Corporation.
Follow instructions for use
Consult instructions for use Lot code
Manufacturer
Date of manufacture
YYYY-MM-DD
Serial Number
Do not discard Greater than Keep dry
NON
Non-Sterile Less than Do not use if package is damaged
STERILE
Not made with natural
LATEX
rubber latex
Caution Bluetooth
Fragile, handle with care
Protected against solid
foreign objects of 1.0 mm
diameter and greater
and protected against
the eects of temporary
immersion in water.
Separate collection for electrical
and electronic equipment
(WEEE).
Catalogue number (model
number)
Masimo reference number Body weight
Light Emitting Diode (LED) LED
emits light when current ows
X
through
Storage humidity limitation Atmospheric pressure limitation
(yellow background)
Federal Communications
Commission (FCC) Licensing
Protected against solid foreign
objects of 12.5 mm diameter
and greater and protection
against vertically falling water
drops when enclosure is tilted
at 15 degrees.
NON
STERILE
7.14.8.x.
or higher. Consult
800 MHz a 2.5 GHz
d = 2.33*√P
Caution: Federal law (USA) restricts
this device to sale by or on the order
of a physician
Mark of conformity to
European Medical Device
Directive 93/42/EEC
Authorized representative in the
European community
Storage temperature range
Warning
Identies unit has been
registered as a radio device
Instructions/Directions for Use/
Manuals are available in electronic
format @ http://www.Masimo.com/
TechDocs
Note: eIFU is not available in all
countries.
en
Non-sterile
Radius PPG™
Chip and Receiver
Mode D’EMPLOI
LATEX
Ne contient pas de latex naturel
NON
STERILE
Non stérile
fr de
Radius PPG™
Chip and Receiver
GEBRAUCHSANWEISUNG
LATEX
Enthält keinen Latex aus Naturkautschuk
NON
STERILE
Nicht steril
Radius PPG™
Chip and Receiver
ISTRUZIONI PER L'USO
LATEX
Non contiene lattice di gomma naturale
NON
STERILE
Non sterile
it
Radius PPG™
Chip and Receiver
INSTRUCCIONES DE USO
LATEX
Fabricado sin látex de caucho natural
es
NON
STERILE
No estéril
Fig. 1
Fig. 2
Fig. 4
Fig. 6
Fig. 3
Fig. 5
Fig. 7
Fig. 8
Fig. 9
Fig. 10
Radius PPG™
Chip and Receiver
EU Authorized Representative for Masimo Corporation:
MDSS GmbH
Schiffgraben 41
D-30175 Hannover, Germany
Manufacturer:
Masimo Corporation
52 Discovery
Irvine, CA 92618
USA
www.masimo.com
© 2019 Masimo Corporation
300633/10353B
C-101219
Page 2

Radius PPG™
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
Chip and Receiver
BRUKSANVISNING
LATEX
Inte tillverkad med naturgummilatex
sv
Radius PPG™
Chip and Receiver
GEBRUIKSAANWIJZING
Bij de productie is geen latex van
NON
STERILE
Osteril
LATEX
natuurrubber gebruikt
NON
STERILE
Niet-steriel
Radius PPG™
Chip and Receiver
BRUGSANVISNING
LATEX
Ikke fremstillet med naturlig gummilatex
NON
STERILE
Ikke-steril
danl
Radius PPG™
Chip and Receiver
LATEX
INSTRUÇÕES DE UTILIZAÇÃO
NON
Não fabricado com látex de borracha natural
STERILE
Não esterilizado
pt
Radius PPG™
Chip and Receiver
LATEX
非天然乳胶制造
使用说明
zh
Radius PPG™
ja
Chip and Receiver
使用方法
NON
STERILE
未消毒
LATEX
天然ゴムは不使用
NON
STERILE
非殺菌
Page 3

Radius PPG™
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
STERILE
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
STERILE
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
Chip and Receiver
KÄYTTÖOHJEET
LATEX
Ei sisällä luonnonkumilateksia
fi
Radius PPG™
Chip and Receiver
BRUKSANVISNING
NON
STERILE
Ei-steriili
LATEX
Er ikke fremstilt med naturgummilateks
STERILE
NON
Ikke-steril
no ro
Radius PPG™
Chip and Receiver
POKYNY K POUŽITÍ
LATEX
Neobsahuje přírodní latex
NON
STERILE
Nesterilní
cs
Radius PPG™
Chip and Receiver
LATEX
HASZNÁLATI ÚTMUTATÓ
NON
Nem tartalmaz természetes latexgumit
STERILE
Nem steril
hu
Radius PPG™
Chip and Receiver
LATEX
INSTRUKCJA UŻYTKOWANIA
Produkt został wykonany bez
zastosowania lateksu naturalnego
NON
Niejałowe
p
l
Radius PPG™
Chip and Receiver
LATEX
INSTRUCIUNI DE UTILIZARE
Produs care nu conine latex din cauciuc
natural
NON
Nesteril
300633/10353BC-101219
Page 4

Radius PPG™
STERILE
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
STERILE
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
STERILE
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
-40 C
+70 C
+1060 hPa - +500 hPa
795 mmHg - 375 mmHg
Chip and Receiver
NÁVOD NA POUŽITIE
Pri výrobe sa nepoužil prírodný gumový
LATEX
latex
NON
Nesterilné
Radius PPG™
Chip and Receiver
KULLANIM KILAVUZU
Reusable
LATEX
Doğal kauçuk lateks ile üretilmemiştir
NON
STERILE
Steril değildir
tr
Radius PPG™
Chip and Receiver
LATEX
ΟΗΓΙΕΣ ΧΡΗΣΗΣ
εν έχει κατασκευαστεί ε φυσικό
ελαστικό λάτεξ
NON
Μη αποστειρωένοι
el
Radius PPG™
Chip and Receiver
LATEX
NON
ru
Radius PPG™
Chip and Receiver
LATEX
ar
kosk
Radius PPG™
Chip and Receiver
D
NON
STERILE
NON
STERILE
LATEX
 Loading...
Loading...