Marguette Solar8000M Service manual

Solar8000M
Patient Monitor
Service Manual
2000701-123 Revision C

127(Due to continuing product innovation, specifications in this manual are subject to change without
notice.
Listed below are the GE Medical Systems Informatio n Technologies trademarks used in this document. All
other trademarks contained herein are the property of their respective owners.
APEX, CD TELEMETRY, CRG PLUS, DASH, MUSE, RAC, RAMS, RSVP, SAM, SOLAR, TRAM,
TRAM-NET, TRAM-RAC, TRIM KNOB, and UNITY NETWORK are trademarks of GE Medical Systems
Information Technologies registered in the United States Patent and Trademark Office.
CD TELEMETRY
®
-LAN, CENTRALSCOPE, EK-Pro, MENTOR, Octanet, Prism, SMART-PAC,
SMARTLOOK, and UNITY are trademarks of GE Medical Systems Information Technologies.
© GE Medical Systems Information Technologies, 2002, 2003. All rights reserved.
T-2 Solar 8000M Patient Monitor Revision C
2000701-123 17 February 2003

Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
Manual Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Revision History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Manual Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
Responsibility of the Manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
Warnings, Cautions, and Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-5
Equipment Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-6
Service Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
Service Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-7
Equipment Identification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-7
2 Equipment Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
System Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Solar 8000M Patient Monitoring System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
Solar 8000M Patient Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-3
UnityView Remote Display Controller . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Tram-rac Housing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-4
Connectivity Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
PRN 50/PRN 50-M Digital Writer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-5
Laser Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
Remote Control/Keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-6
Remote Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7
Tram-net Interface Adapter . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-7
Device Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Acquisition Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Peripheral Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Unity Network Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-10
Interfaces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-12
Revision C Solar 8000M Patient Monitor i
2000701-123

Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-15
Solar 8000M Patient Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-15
Tram-rac 2 and 4A Module Housings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
Tram Modules and Solar Parameter Functionality . . . . . . . . . . . . . . . . . . . . . . . .2-19
Dual Temperature Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-26
Capnostat Mainstream CO2 Module . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-27
SvO2 Module Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-29
Masimo SET Module Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . .2-30
BIS/EEG Module Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-34
Solar 8000M Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-36
Purchaser’s Responsibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-36
Medical-Grade Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-36
Computer-Grade Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-37
Isolation Transformers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-37
Required Specifications for Analog Flat Panel or CRT Displays . . . . . . . . . . . . .2-38
Recommended Specifications for Computer-Grade CRT Displays . . . . . . . . . . .2-39
Required Specifications for Digital Flat Panel Displays . . . . . . . . . . . . . . . . . . . .2-40
Recommended Specifications for Computer-Grade Digital Flat
Panel Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-41
3 Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Back Panel Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Front Panel Connectors and Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-7
Power Up . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-10
Tram-net Communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-11
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-11
Internal Hub . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-11
Ethernet Communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-12
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Twisted Pair . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Concentrator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Thin-net/Thick-net . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Node . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
Segment and Branch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
Repeater . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
Bridge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
Twisted Pair Cabling (10BaseT) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-14
ii Solar 8000M Patient Monitor Revision C
2000701-123

4 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Maintenance Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Manufacturer Recommendations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Manufacturer Responsibility . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Preventive Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-3
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Cleaning Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-5
Exterior Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-5
Electrical Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
Recommendations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
Power Outlet Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
Ground (Earth) Integrity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
Ground (Earth) Wire Leakage Current Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Enclosure Leakage Current Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Patient (Source) Leakage Current Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-13
Patient (Sink) Leakage Current Test (Mains Voltage on the Applied Part) . . . . .4-14
Test Completion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-15
Checkout Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-16
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Required Tools/Special Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Completion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-20
PM Form . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-20
Repair Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-21
5 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Terms Used . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Abort (Main Code) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Boot Loader or Boot Code . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Cold Start . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Continue (Main Code) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Monitor Memory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Protected Memory . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Power Cycle or Reboot . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Service Mode (Main Code) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Service Menu (Boot Code) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Warm Start (Boot Code) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Country Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Set Language . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-4
Revision C Solar 8000M Patient Monitor iii
2000701-123

Service Menus (Boot Code) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Boot Code Service Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-5
Main Code SERVICE MODE Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
General Fault Isolation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-11
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-11
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-12
AC Line Voltage Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-13
120 VAC, 50/60 Hz . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-13
240 VAC, 50/60 Hz . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-13
Troubleshooting Procedure . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-14
Before Replacing the Processor pcb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-14
After Replacing the Processor pcb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-15
Problems and Solutions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-16
LED Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-20
Troubleshooting Software Updates - Problems and Solutions . . . . . . . . . . . . . . 5-23
Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-24
6 Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-1
Configuring a Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-3
Set Unit Name . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-4
Set Bed Number . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-4
Patient-Monitor Type . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 -5
Set Graph Locations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
Admit Menu . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-7
Set Line Frequency . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
Set Defib Sync Voltage and Pulse Width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
Set Country Selection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
Set Language . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
Calibrate Touchscreen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
Completion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-10
Set Time and Date . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-10
Change Software Level . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-11
Enable Options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-12
Transfer Monitor Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-12
Change Ethernet Address . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-15
Set Internet Address . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-16
Power Cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-18
Reviewing Error/Event Logs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-18
Transferring Error Logs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-23
iv Solar 8000M Patient Monitor Revision C
2000701-123

7 Upper Level Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
Theory of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
Processor Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-4
Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-15
Speaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-19
Block Diagram of Internal Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-20
Input/Output Connectors and Signals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-21
VGA VID 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-21
VGA VID 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-22
DFP VID 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-22
DFP VID 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-23
RS-232 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-23
RS-232 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-24
TRAM-NET 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-24
TRAM-NET 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-24
ETHERNET . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-25
M-Port 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-25
M-Port 2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-25
M-Port 3 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-26
M-Port 4 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-26
Keypad . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-26
Disassembly Guidelines . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-27
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-27
Opening the Unit for Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-27
Ordering Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-30
Field Replaceable Units . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7-30
Solar 8000M PN 418713-001 Rev E *PN 418713-002 Rev D . . . . . . . . . . . . . . . . . 7-31
Keypads/Remote Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-33
Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-36
Power Supply PN 422811-001 Rev B . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-37
8 PCB Assemblies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
Processor PCB Parts List PN 801586-001 Rev C . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3
Processor PCB Parts Location PN 801586 Rev C . . . . . . . . . . . . . . . . . . . . . . . . . . 8-7
Processor PCB Schematic PN SD801586-001 Rev C . . . . . . . . . . . . . . . . . . . . . . . 8-8
Revision C Solar 8000M Patient Monitor v
2000701-123

Power Supply PCB Parts List PN 801674-001 Rev A . . . . . . . . . . . . . . . . . . . . . . 8-32
Power Supply PCB Parts Location PN 801674 Rev A . . . . . . . . . . . . . . . . . . . . . 8-36
Power Supply PCB Schematic PN SD801674-001 Rev A . . . . . . . . . . . . . . . . . . . 8-37
Processor PCB Parts List PN 2008705-001 Rev B . . . . . . . . . . . . . . . . . . . . . . . . 8-41
Processor PCB Parts Location PN 2008705 Rev B . . . . . . . . . . . . . . . . . . . . . . . 8-45
Processor PCB Schematic PN 2008706-001 Rev A . . . . . . . . . . . . . . . . . . . . . . . 8-46
vi Solar 8000M Patient Monitor Revision C
2000701-123

1 Introduction
Revision C Solar 8000M Patient Monitor 1-1
2000701-123

For your notes
1-2 Solar 8000M Patient Monitor Revision C
2000701-123
Manual Informatio n
Revision History
Each page of the document has the document part number and revision
letter at the bottom of the page. The revision letter changes whenever
the document is updated.
Revision Date Comment
A 14 October 2002 Initial release of this manual, corresponding
B 4 December 2002 Document revised to correct certain technical
C 17 February 2003 Document revised to reflect changes to field
Introduction: Manual Information
to software version 4.
specifications.
replaceable units.
Manual Purpose
Intended Audience
This manual supplies technical information for service representatives
and technical personnel so they can maintain the equipment to the
assembly level. Use it as a guide for maintenance and electrical repairs
considered field repaira ble. Where necessary the manual identifies
additional sources of relevant information and technical assistance.
See the operator’s manual for the instructions necessary to operate the
equipment safely in accordance with its function and intended use.
This manual is intended for service representatives and technical
personnel who maintain, troubleshoot, or repair this equipment.
Revision C Solar 8000M Patient Monitor 1-3
2000701-123

Introduction: Safety Information
Safety Information
Responsibility of the Manuf acturer
GE Medical Systems Information Technologies is responsible for the
effects of safety, reliability, and performance only if:
Assembly operations, extensions, readju stments, modifications, or
repairs are carrie d out by persons aut horized b y GE Medica l Systems
Information Technologies.
The electrical installation of the relevant room complies with the
requirements of the appropriate regulations.
The equipment is used in accordance with the instructions for use.
General
This device is intended for use under the direct supervision of a licensed
health care practitioner.
This device is not intended for home use.
Federal law restricts this device to be sold by or on the order of a
physician.
Contact GE Medical Systems Information Technologies for information
before connecting any devices to the equipment that are not
recommended in this manual.
Parts and accessories used must meet t he requireme nts of t he appli cable
IEC 601 series safety standards, and/or the system configuration must
meet the requirements of the IEC 60601-1-1 medical electrical systems
standard.
Periodically, and whenever the integrity of the device is in doubt, test all
functions.
The use of ACCESSORY equipment not complying with the equivalent
safety requirements of this equipment may lead to a reduced level of
safety of the resulting system. Consideration relating to the choice shall
include:
use of the accessory in the PATIENT VICINITY; and
evidence that the safety certification of the ACCESSORY has been
performed in accordance to the appropriate IEC 60601-1 and/or IEC
60601-1-1 harmonized national standard.
If the installation of the equipment, in the USA, will use 240V rather
than 120V, the source must be a center-tapped, 240V, single-phase
circuit.
1-4 Solar 8000M Patient Monitor Revision C
2000701-123

Introduction: Safety Information
Warnings, Cautions, and Notes
The terms danger, warning, and caution are used throughout this
manual to point out hazards and to designate a degree or level or
seriousness. Familiarize yourself with their definitions and significance.
Hazard is defined as a source of potential injury to a person.
DANGER indicates an imminent hazard which, if not avoided, will
result in death or serious injury.
WARNING indicates a potential hazard or unsafe practice which, if not
avoided, could result in death or serious injury.
CAUTION indicates a potential hazard or unsafe practice which, if not
avoided, could result in minor personal injury or product/property
damage.
NOTE provides application tips or other useful information to assure
that you get the most from your equipment.
Revision C Solar 8000M Patient Monitor 1-5
2000701-123
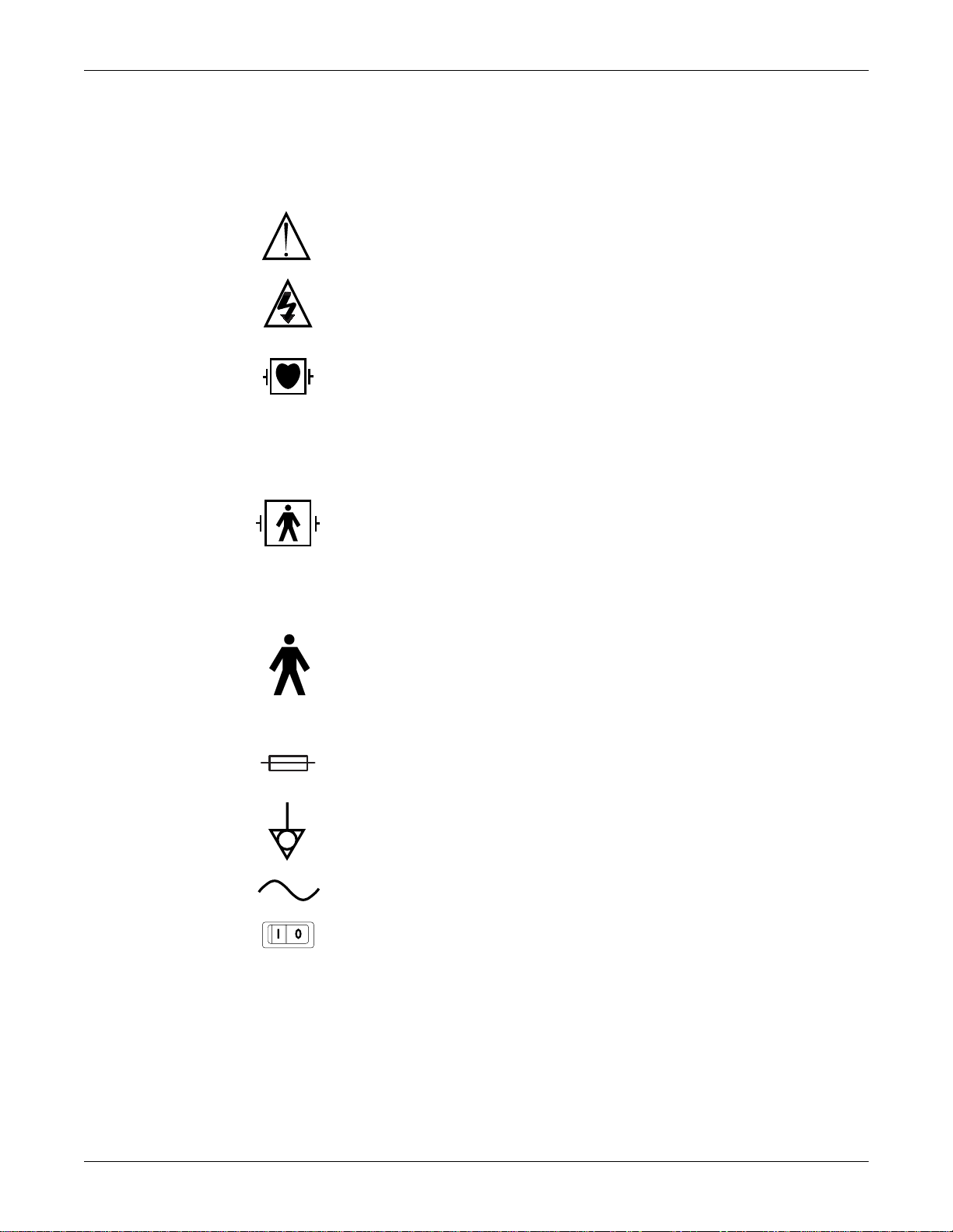
Equipment Symbols
Introduction: Safety Information
127(
Some symbols may not appear on all equipment.
ATTENTION: Consult accompanying documents.
CAUTION: To reduce the risk of electric shock, do NOT remove cover. Refer servicing to
qualified service personnel.
127(
The rating of
protection against
electric shock
(indicated by
symbol for CF or
BF) is achieved only
when used with
patient applied
parts recommended
by GE Medical
Systems
Information
Technologies.
TYPE CF APPLIED PART: Isolated (floating) applied part suitable for intentional external and
internal application to the patient including direct cardiac application. “Paddles” outside the
box indicate the applied part is defibrillator proof.
[Medical Standard Definition:] F-type applied part (floating/isolated) complying with the
specified requirements of IEC 60601-1/UL 2601-1/CSA 601.1 Medical Standards to provide a
higher degree of protection against electric shock than that provided by type BF applied parts.
TYPE BF APPLIED PART: Isolated (floating) applied part suitable for intentional external and
internal application to the patient excluding direct cardiac application. “Paddles” outside the
box indicate the applied part is defibrillator proof.
[Medical Standard Definition:] F-type applied part (floating/isolated) complying with the
specified requirements of IEC 60601-1/UL 2601-1/CSA 601.1 Medical Standards to provide a
higher degree of protection against electric shock than that provided by type B applied parts.
TYPE B APPLIED PART: Non-isolated applied part suitable for intentional external and
internal application to the patient excluding direct cardiac application.
[Medical Standard Definition:] Applied part complying with the specified requirements of IEC
60601-1/UL 2601-1/CSA 601.1 Medical Standards to provide protection against electric
shock, particularly regarding allowable leakage current.
Fuse
Equipotential
Alternating current (AC)
Power; I = ON; O = OFF
1-6 Solar 8000M Patient Monitor Revision C
2000701-123
Service Information
Service Requirements
Follow the service requirements listed below.
Introduction: Service Information
Refer equipment servicing to GE Medical Systems Information
Technologies’ authorized service personnel only.
Any unauthorized attempt to repair equipment under warr anty voids
that warranty.
It is the user’s responsibility to report the need for service to GE
Medical Systems Information Technologies or to one of their
authorized agents.
Failure on the part of the responsible individual, hospital, or
institution using this equipment to implement a satisfactory
maintenance schedule may cause undue equipment failure and
possible health hazards.
Regular maintenance, irrespective of usage, is essential to ensure
that the equipment will always be functional when required.
Equipment Identification
Every GE Medical Systems Information Technologies device has a
unique serial number for identification. A sample of the information
found on a serial number label is shown below.
D 0 XX 0005 G XX
Month
Manufactured
A = January
B = February
C = March
D = April
E = May
F = June
G = July
H = August
J = September
K = October
L = November
M = December
Year
Manufactured
0 = 2000
1 = 2001
2 = 2002
(and so on)
Product Code
Two-character
product descriptor
Product Sequence
Number
Manufacturing
number (of total units
manufactured)
Division
F = Cardiology
G = Monitoring
Device Characteristics
One or 2 letters that further
describe the unit, for example:
P = prototype not conforming to
marketing specification
R = refurbished equipment
S = special product
documented under Specials
part numbers
U = upgraded unit
Revision C Solar 8000M Patient Monitor 1-7
2000701-123

For your notes
Introduction: Service Information
1-8 Solar 8000M Patient Monitor Revision C
2000701-123

2 Equipment Overview
Revision C Solar 8000M Patient Monitor 2-1
2000701-123

For your notes
2-2 Solar 8000M Patient Monitor Revision C
2000701-123

Equipment Overview: System Components
System Components
Solar 8000M Patient Monitoring System
The Solar 8000M patient monitoring system consists of the following
standard components:
Solar 8000M processing unit
Display
Keypad and/or remote control
Tram-rac® housing with acquisition module(s)
Additional, option al components include:
Tram-net interface adapter (TIA)
Clinical Information Center (central station)
Remote display, VGA and DFP
Printer PRN 50/PRN 50-M
Octanet® or Unity Networ k® ID connectivity device
Solar 8000M Patient Monitor
The patient monitor consists of a Solar 8000M processing unit with
compatible display purchased from GE Medical Systems Information
Technologies or another vendor.
The processing unit is the center of the Solar 8000M patient monitoring
system. It provides the user controls, the process ors to communicate with
various patient monitoring modules, and it analyzes patient data. It can
display up to eight different waveforms at one time . System software
may be updated using a laptop computer connected to the Solar 8000M
processing unit or the Unity Network or from a Clinical Information
Center (CIC) on the Unity Network.
Revision C Solar 8000M Patient Monitor 2-3
2000701-123
Equipment Overview: System Components
UnityView Remote Display Controller
The UnityView remote display controller consists of a remote display
controller with a compatible display purchased from GE Medical
Systems Information Technologies or another vendor. The controller
connects to the Unity Network and may be configured to display any
patient waveforms broadcasted on the network for better visibility as a
remote full-view display, or as an in-room telemetry display. System
software may be updated using a laptop computer connected to the
UnityView remote display controller or the Unity Network or from a
Clinical Information Center (CIC) on the Unity Network.
Tram-rac Housing
The Tram-rac housing (remote acquisition case) acquires patient data for
the patient monitor. Th ere are two Tram-rac housings available for the
monitor:
Tram-rac 2 housing — h olds a single Tram module.
Tram-rac 4A housing — holds a Tram module and two additional
single-high modules.
See the Tram-rac Housing Service Manual for additional information.
Shown below is a Tram-rac 4A housing with a Tram module and two
single parameter modules inserte d .
2-4 Solar 8000M Patient Monitor Revision C
2000701-123

Connectivity Devices
Equipment Overview: System Components
The Octanet or the Unity Network ID connectivity device ac quires digita l
data from eight individually isolated serial ports. The data is collected
from up to eight peripheral devices (not necessarily manufactured by GE
Medical Systems Information Technologies), then the device transmi ts
the formatted da ta to the Solar 8000M patient monitor. See the
appropriate connectivity device service manual for additional
information.
PRN 50/PRN 50-M Digital Writer
The PRN 50/PRN 50-M digital writer thermally records patient data on a
paper strip. Any parameter or trace that can be monitored on a monitor
can be graphed by the writer. Graphs initiate automatically when an
alarm is activated, or they can be initiated manually from the monitor.
127(
The PRN 50-M digital writer is an M-Port device. To make an
AutoPort device (such as PRN 50) M-Port compatible, use the
AutoPort to M-Port adapter, pn 2001973-001. The adapter is not
required if connecting to an Octanet.
Revision C Solar 8000M Patient Monitor 2-5
2000701-123

Laser Printer
Equipment Overview: System Components
An optional laser printer can be connected directly to the monitor via one
of the M-Ports. The laser printer must have a serial port, and an
interface adapter is required for the cable between the laser printer and
the monitor. Refer to the Interface to a Laser Printer from a Solar 8000M
Patient Monitor Installation Instructions, pn 2013626-001, for details on
the interface adapter and installing a serial card in a laser printer.
:$51,1*
SHOCK HAZARD. Laser printers are UL 60950/IEC
60950 certified equipment, which may not meet the
leakage current requirements of patient care equipment.
This equipment must not be located in the patient
vicinity unless the medical system standard IEC
60601-1-1 is followed.
Do not connect a laser printer to a multiple portable
socket outlet (MPSO) supplying patient care equipment.
The use of an MPSO for a system will result in an
enclosure leakage current equal to the sum of all the
individual earth leak age currents of the s ystem if th ere is
an interruption of the MP SO protective earth conductor.
Remote Control/Keypad
The remote control/keypad provides all patient monitor controls on a
portable component with a TRIM KNOB control, and allows the user to
operate the patient monitor from across a room. Eighteen hard keys are
configured for adult, neonatal, or operating room applications. The
keypad is ‘fixed mounted’ on the front of the Solar 8000M patient monitor
or on a separate holster that has various mounting configurations.
2-6 Solar 8000M Patient Monitor Revision C
2000701-123
Equipment Overview: System Components
Remote Displays
Depending on your Solar 8000M configuration, there are up to two VGA
(CRT/analog flat panel) ports and two DFP (digital flat panel) ports for
remote viewing.
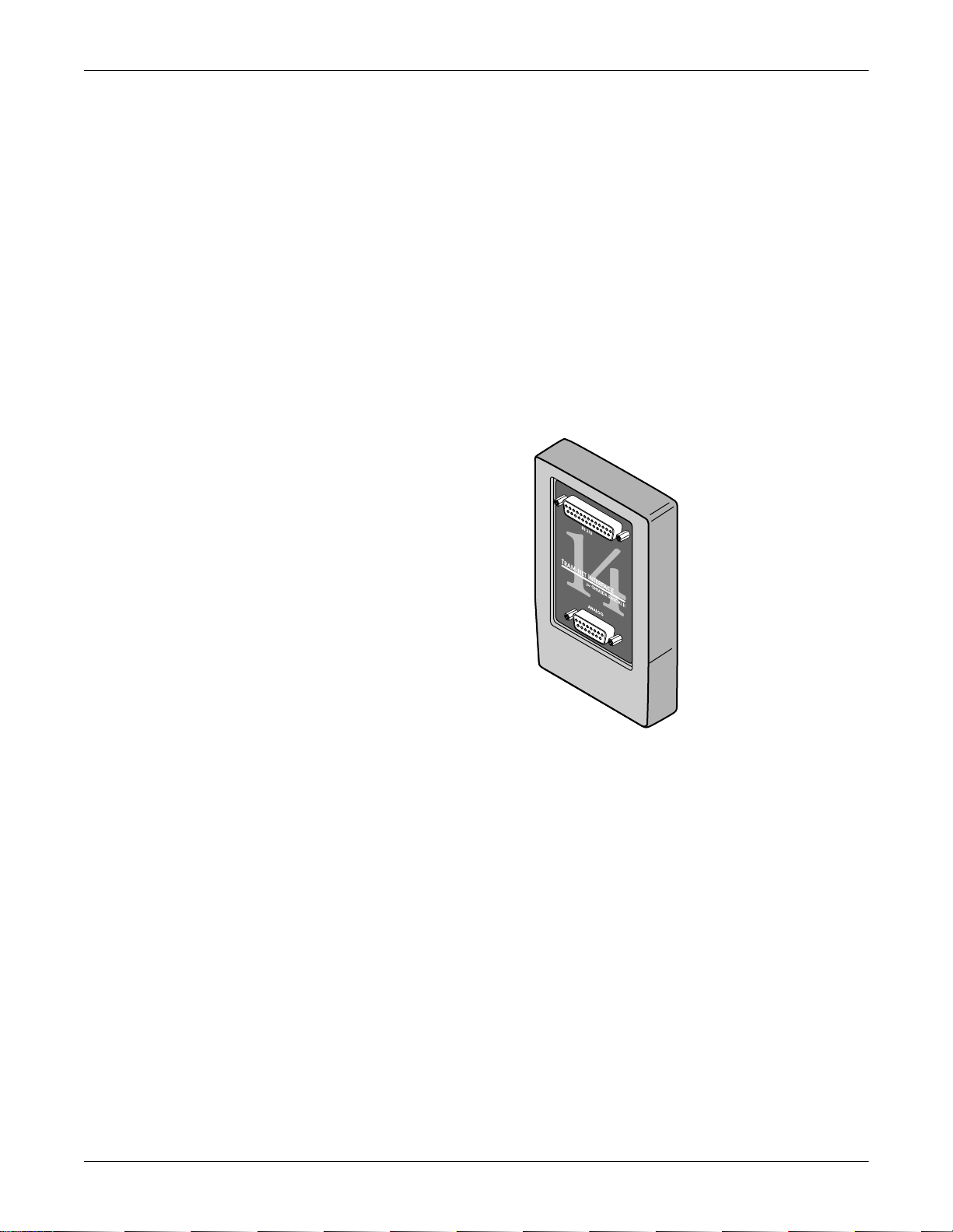
Tram-net Interface Adapter
The Tram-net interface adapter connects a specific device to the Solar
8000M patient monitor using Tram-net communication. Each adapter is
preprogrammed at the factory to interface with a specific device
manufactured by a company other than GE Medical Systems
Information Technologies. For more details about the Tram-net interface
adapter, refer to the Modular Patient Monitor Accessories Service
Manual.
Revision C Solar 8000M Patient Monitor 2-7
2000701-123

Equipment Overview: Device Compatibility
Device Compatibility
The tables in this section are current as of the publication date of this
manual and are subject to change. For current information, contact your
Service or Sales Representative.
Acquisition Devices
The Solar 8000M patient monitor is compatible with the following
acquisition modules.
406132-001 SvO2 Module
9399-003 Dual Temp Module, YSI 700
9399-004 SURG Dual Temp Module, YSI 700
96064-004, TT400=A Dual Temp Module, YSI 400
96064-005, TT400S=A SURG Dual Temp Module, YSI 400
Part Number Description
96064-010 BP/Dual Temp Module
96064-011 Surgical BP/Dual Temp Module
BPBPNIC=A Dual BP Module
BPBPNICS=A Surgical BP/BP Module
BPCONIC=A BP/CO Module
BPNIC=A BP Module
BPNICS=A Surgical BP Module
BPTT=A, BPTT400=A BP/Dual Temp Module
BPTTS=A, BPTTS400=A Surgical BP/Dual Temp Module
NBPDA=A NIBP Module - Adult
NBPDN=A NIBP Module - Neonatal
NBPDP=A NIBP Module - Pediatric
SLRECG=A ECG/Resp Module
SLRECGD=A ECG/Resp/Defib Sync Module
SLRECGSL=A ECG/Resp/12SL Module
SLRECGSLD=A ECG/Resp/12SL/Defib Sync Module
SLRSPO2=A SpO2 Module
MSN=A Capnostat Mainstream EtCO2 Module
MSSS=A Dual CO2 Module
SAM=A SAM Module
SAM80=A SAM80 Module
SS=A Side-Stream EtCO2 Module
2-8 Solar 8000M Patient Monitor Revision C
2000701-123

Equipment Overview: Device Compatibility
Part Number Description
T200=A Tram Module w/ECG, Resp, CO, 2 BP, NIBP, SpO2
T250=A Tram Module w/ECG, Resp, CO, 2 BP, NIBP, SpO2
T400=A Tram Module w/ECG, Resp, CO, 3 BP, NIBP, SpO2
T450=A Tram Module w/ECG, Resp, CO, 3 BP, NIBP, SpO2
T451=X Tram Module w/ECG, Resp, CO, 3 BP, NIBP, SpO2
(GEMS-IT)
T451N=X Tram Module w/ECG, Resp, CO, 3 BP, NIBP, SpO2
(Nellcor)
T451M=X Tram Module w/ECG, Resp, CO, 3 BP, NIBP, SpO2
(Masimo)
T600=A Tram Module w/ECG, Resp, CO, 4 BP, SpO2
T650=A Tram Module w/ECG, Resp, CO, 4 BP, SpO2
T800=A Tram Module w/ECG, Resp, CO, NIBP, SpO2
T800SL=A Tram Module w/ECG, Resp, CO, NIBP, SpO2
T850=A Tram Module w/ECG, Resp, CO, NIBP, SpO2
T850SL=A Tram Module w/ECG, Resp, CO, NIBP, SpO2
T851=X Tram Module w/ECG, Resp, CO, SpO2 (GEMS-IT)
T851N=X Tram Module w/ECG, Resp, CO, SpO2 (Nellcor)
T851M=X Tram Module w/ECG, Resp, CO, SpO2 (Masimo)
7030AAX, ABX etc. tcpO2/pCO2 Module
REMCH=A Respiratory Mechanics Module
ICGMOD=XXX Impedance Cardiograph Module
BISMOD=XXX, EEG/
BIS/EEG Module
BISMOD=XXX
SLRSPO2MAS Masimo Sp02 Module
Revision C Solar 8000M Patient Monitor 2-9
2000701-123

Peripheral Devices
Equipment Overview: Device Compatibility
The Solar 8000M patient monitor is compatible with the following
peripheral devices.
Product Software Interface
Solar 8000M RMT 1A M-Port or M-Port compatible Octanet
Solar 8000M Keypad 1A M-Port or M-Port compatible Octanet
PRN 50 1A, 2A Octanet or M-Port with M-Port compatible
PRN50
RAC 4A Comm 6C Tramnet
RAC 4A DAS 6C Tramnet
RAC 2 N/A Tramnet
Octanet 2B Tramnet
TIA 1C Tramnet
Unity Network ID 1A M-Port
Unity Network Devices
RM Module O3 Octanet or M-Port with M-Port compatible RM
module
Polled Data Services 1A Serial Port #1
Serial download N/A Serial Port #1
Elo Touchscreen N/A Serial Port #2
RAMS 1C, 1D Octonet or TIA
Remote Alarm N/A M-Port
Laser printer N/A M-Port
The Solar 8000M patient monitor is compatible with the following Unity
Network devices.
Product Software
ADU/Pager LAN 3G, 3H
ApexPro 1.1 and later
CDT-LAN 5H, 6A, 6C, 6D
Centralscope: CS 12 10A, 10B, 10C, 10D
CIC 1.5, 2.2 and later
Dash 2000 2A
Dash 3000/4000 2B and later
Eagle 3000 3A, 3B, 4A
Eagle 4000 5B, 6A, 6B, 6C, 6D, 6F, 6G
2-10 Solar 8000M Patient Monitor Revision C
2000701-123

Equipment Overview: Device Compatibility
Product Software
HL7 3.0, 4.0
ICMMS/Service Web 3.0, 4.0
Impact Pager 2.53, 3.10
Managed Care 1C
MARS-CRS 4.0a, 4.1
MUSE / MUSE NT 4B, 5A, 5B, 5C
Octacomm 2B, 2C, 2D, 2E
Octanet 2B
QS 5.03.0, 5.05.0, 5.06.0
RSVP 2.0, 3.0, 4.0
Solar 7000/8000 3C, 4B, 4C (Special), 5B, 5D, 5E, 6A, 7A, 7B, 7C
Solar 9000/9500 S9500-1A, 2A and later
ST Guard 4B
Tramscope 12 7D, 17F, 17G, 17H (Special)
Auto View 2.0, 3.0, 4.0
TRAM XX0 9B, 10A, 10B, 11A
CO2 Module Cap Combo: 1.2 & 1.4, Cap MS: 1.2 & 1.4
Pryon SS: 3.0 & 3.1
ECG/RESP Module 1A
Resp Mech Module Novametrix Release 1A
SAM Module 3B, 4D
SpO2 Module 1A
SvO2 Module SYS-08.01/ANLG-05.02
Masimo Sp02 Module 1A and later
Transcutaneous Module 1B
Revision C Solar 8000M Patient Monitor 2-11
2000701-123
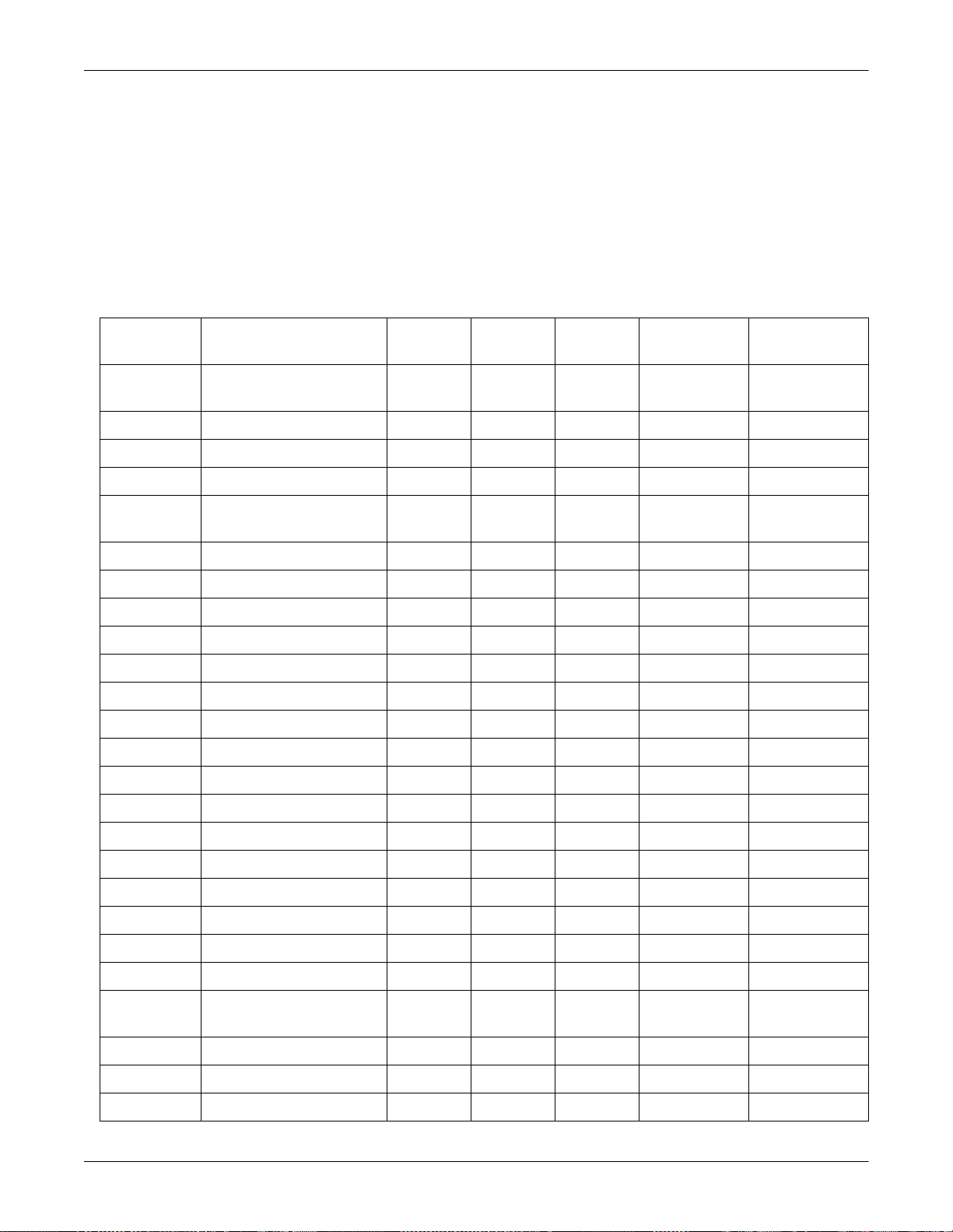
Interfaces
Equipment Overview: Device Compatibility
The Solar 8000M patient monitor supports the following interfaces
through an Octanet connectivity device, Unity Netwo r k ID connectivity
device, or TIA.
127(
Although this list was accurate at the time of publishing, it may no
longer be comprehensive. Contact your sales repres entative to obtain
current information.
Product Mfg Model TIA Octanet
Nellcor PB 7200E/SPE/AE X X X TIAPB7200AE=X420915-001
Siemens SV 900C/D/E X X X TIASS900CD=X 420915-002
Engstrom EAS 9000/9010/9020 X X TIAE9010=X 420915-003
Datex Capnomac Ultima X X X TIADU=X 420915-004
Allied Health
Care
Hamilton Veolar/Amadeus X X X TIAHV= X 420915-007
Nellcor PB Infant Star 500/950 X X X TIAIS=X 420915-008
Nellcor PB Adult Star 1500/2000 X X X TIAAS=X 420915-009
Siemens SV 300 X X X TIASS300=X 420915-011
GEMS IT RAMS X X TIARAMS=X 420915-012
GEMS IT Tauras/Xpar/Comm X 420915-013
Ohmeda Rascal II Anes Gas X X X TIAOHRASII=X 420915-014
Ohmeda 5250 RGM: Resp Gas X X X TIAOH5250=X 420915-015
N Amer Drager Narkomed 2B/2C/3/4/GS X X X TIANARKO=X 420915-016
Bear 1000 X X X TIAB1000=X 420915-005
Unity
Network ID
TIA PN DIDCA PN
Drager Babylog 8000 X X X TIABBL8000=X 420915-017
Taema Alys X 420915-018
Ohmeda 7800/7810 X X 420915-019
Bird 8400ST/6400ST/VIP X X X TIABIRD=X 420915-020
Drager Cato X X 420915-021
Novametrix 840/860 (TCO2M) X X X TIANOVA840=X 420915-022
Radiometer TINA™ (TCM3) X X X TIATINA=X 420915-023
Baxter
Edwards
Abbott Q-Vue/Q2 X X X TIAQVUE=X 420915-025
Abbott LifeCare 5000 X X 420915-026
Baxter Flowgard 6201/6301 X X 420915-027
2-12 Solar 8000M Patient Monitor Revision C
Vigilance X X X TIAVIGILANCE=X420915-024
2000701-123
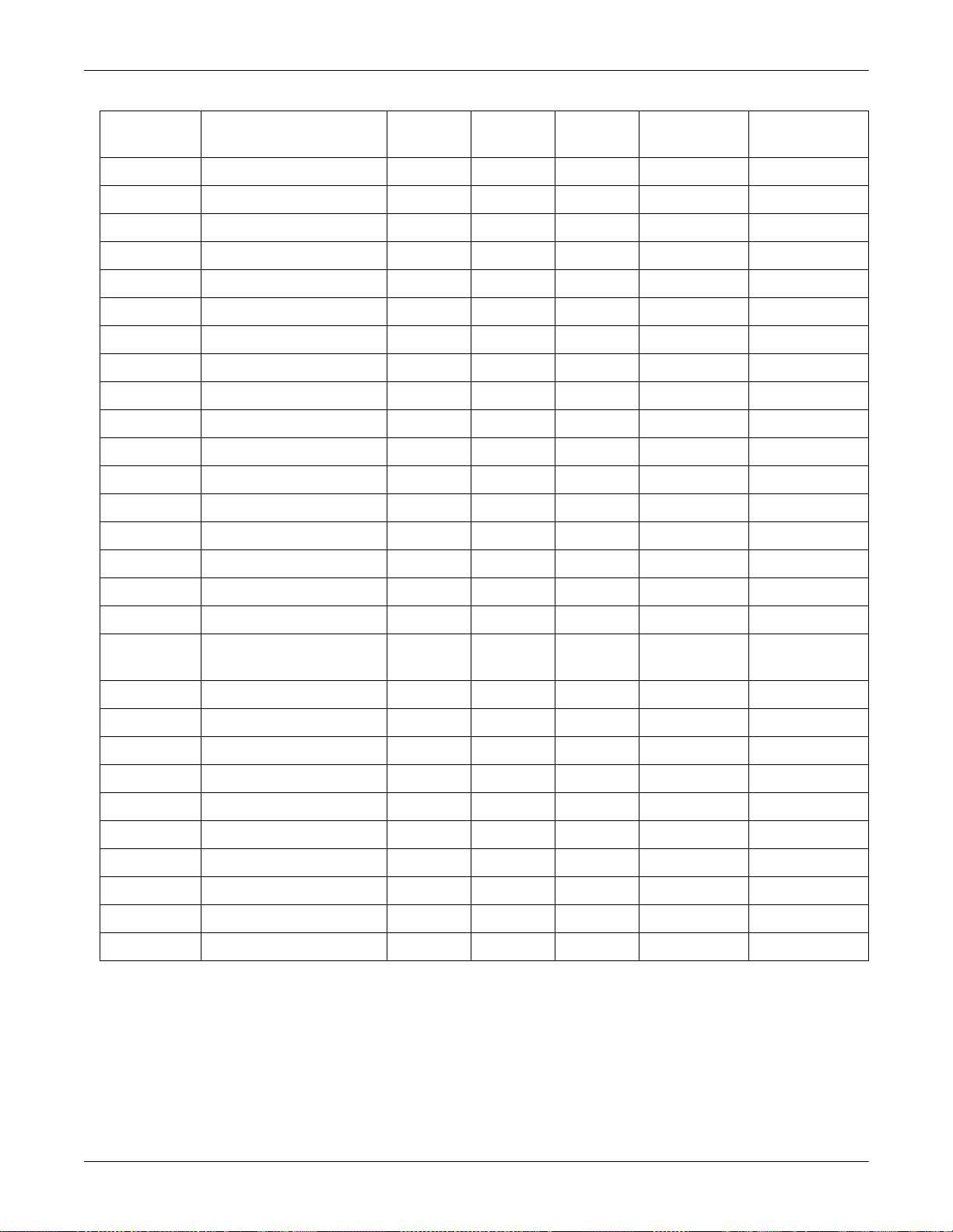
Equipment Overview: Device Compatibility
Product Mfg Model TIA Octanet
Unity
Network ID
TIA PN DIDCA PN
Alaris Medical 560M/570 X X 420915-028
Alaris Medical Gemini PC1/PC2/PC2TX/PC4 X X 420915-029
Bard CritiCore X X 420915-030
GEMS IT Test DIDCA X X 420915-031
Hellige SMU EVO X 420915-032
Nellcor PB N-200 X X 420915-033
Nellcor PB N-1000/N-2500 X 420915-034
Siemens SC 9000 X 420915-035
Drager Cicero PM 8060 (25 pin) X X 420915-036
Drager Cicero B/C X 420915-037
Drager Julian X X 420915-038
Drager Cicero EM (25 pin) X X 420915-039
Drager Evita X X X TIAEVITA=X 420915-040
Drager Evita 2 X X X TIAEVITA2=X 420915-041
Drager Evita 2 dura X X X TIAEVITA2D=X 420915-042
Drager Evita 4 X X X TIAEVITA4=X 420915-043
Drager Cicero EM (9 pin) X X 420915-044
GEMS IT Respiratory Mechanics X X TIARMECH=X (internal) 420915-
048
Ohmeda 7900 X X 420915-049
Ohmeda Aestiva 3000 X X 420915-050
Drager Cicero PM 8060 (9 pin) X X 420915-051
Aspect A-2000 BIS X 420915-056
Diametrics IRMA X 420915-057
Novametrix NICO X 420915-058
MIE Kestrel X 420915-059
Hamilton Galileo X X 420915-060
Puritan-Bennett PB840 X 420915-063
Nellcor PB N-395 X 420915-069
Revision C Solar 8000M Patient Monitor 2-13
2000701-123
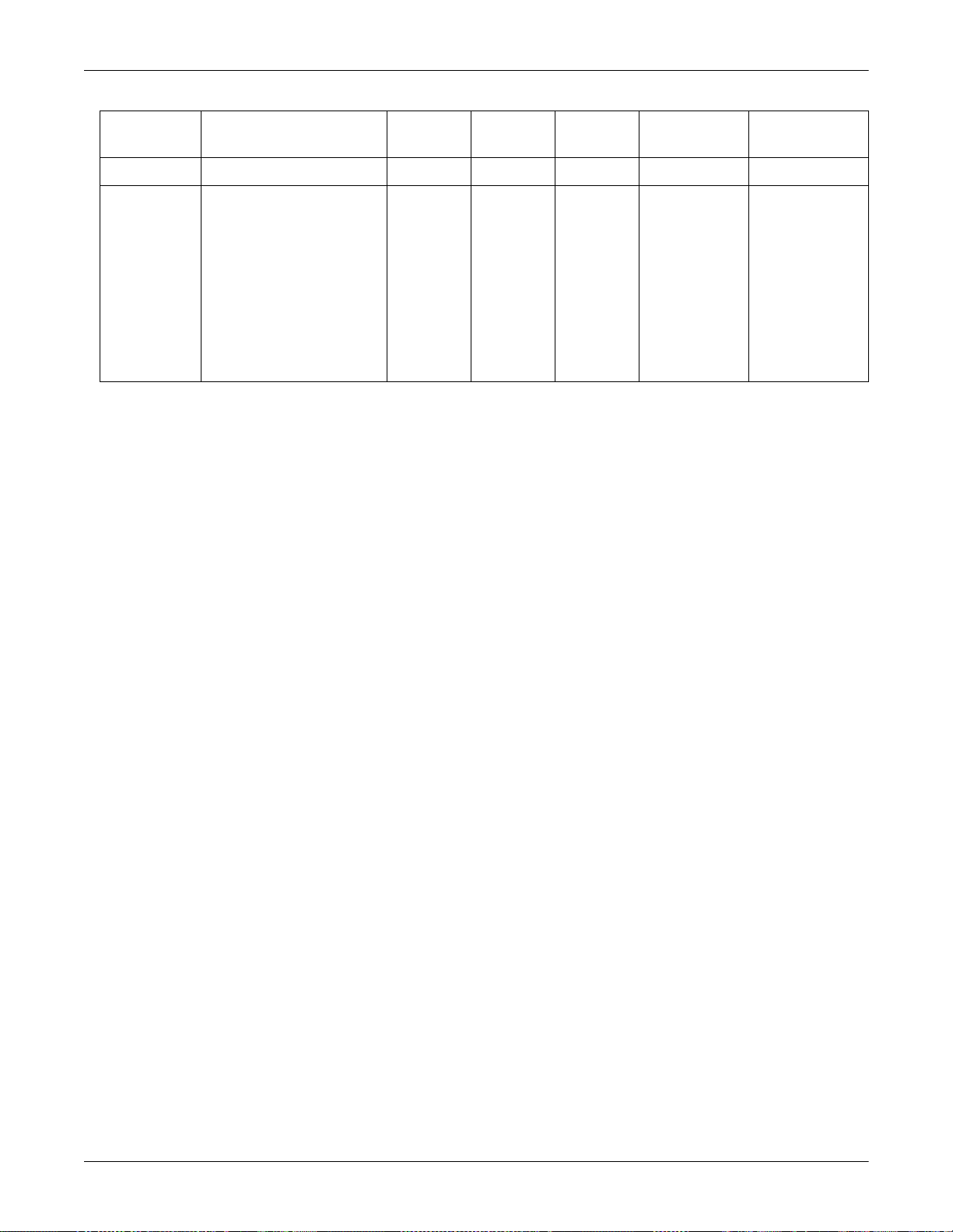
Equipment Overview: Device Compatibility
Product Mfg Model TIA Octanet
Unity
Network ID
TIA PN DIDCA PN
Drager Evita XL X 420915-070
GEMS IT PRN50
X (internal)
Solar 8000M Remote Control
(Adult)
Solar 8000M Remote Control
(OR)
Solar 8000M Remote Control
(Neo)
Solar 8000M Keypad (Adult)
Solar 8000M Keypad (OR)
Solar 8000M Keypad (Neo)
2-14 Solar 8000M Patient Monitor Revision C
2000701-123
 Loading...
Loading...