Mahe MSV-2010 Operator's Manual

MSV-2010
Digital Video Camera
Operator Manual


TABLE OF CONTENTS
Page Page
1 INTRODUCTION .....………………………………………………………………………..…....
2 SAFETY PRECAUTIONS – IMPORTANT ….………………………………………………...
3 SPECIFICATION…………………………………………………………………………………
4 OPERATING ELEMENTS, SYMBOLS AND FUNCTIONS………………………………….
4.1
4.2
4.3
VIDEO CAMERA………………………………………………………………………………….
KEYBOARD………………………………………………………………………………………..
MONITOR SCREEN DISPLAY………………………………………………………………...
5 INSTALLATION………………………………………………………………………………….
5
6
10
11
11
14
16
17
5.1 SETTING UP THE MSV-2010……………………………………………………………………. 17
5.2 CONNECTING THE CAMERA HEAD…………………………………………………………... 17
5.3 CONNECTING PERIPHERAL EQUIPMENT……………………………………………………. 17
5.4 CONNECTING POWER…………………………………………………………………………... 18
5.5 ASSEMBLING THE OPTO-MECHANICAL ADAPTER………………………………………... 19
6 OPERATION………………………………………………………………………………………
20
6.1 POWERING UP THE MSV-2010…………………………………………………………………. 20
6.2 FREE-SPIN/ORIENTATION MECHANISM……………………………………………………... 20
6.3 CENTERING MECHANISM……………………………………………………………………… 20
6.4 FUNCTION CONTROLS…………………………………………………………………………. 21
6.5 NORMAL OPERATION MODE 22
6.5.1 White Balance……….……………………………………………………………………………... 22
6.5.2 Gain Control……………………………………………………………………………………….. 22
6.5.3 Brightness Adjustment…………………………………………………………………………….. 22
6.6 MENU OPERATION MODE……………………………………………………………………... 23
6.6.1 Main Menu………………………………………………………………………………………… 23
6.6.2 Picture Setting Sub-Menu…………..……………………………………………………………… 23
6.6.2.1 Gain ………………………………………………………………………………………………... 23
6.6.2.2 Brightness…………………………………………………………………………………………... 24
6.6.2.3 Color Red and Blue………………………………………………………………………………… 24
6.6.2.4 Enhance…………………………………………………………………………………………….. 24
6.6.2.5 Shutter Window……………………………………………………………………………………. 24
6.6.2.6 Shutter Speed………………………………………………………………………………………. 25
6.6.2.7 Freeze Mode……………………………………………………………………………………….. 25
6.6.3 SCREEN SETTING SUB-MENU………………………………………………………………… 25
6.6.3.1 Patient Data………………………………………………………………………………………… 25
6.6.3.2 User Window………………………………………………………………………………………. 26
6.6.3.3 Clock Display……………………………………………………………………………………… 26
6.6.3.4 Copy Mode A……………………………………………………………………………………… 26
6.6.3.5 Copy Mode B……………………………………………………………………………………… 26
6.6.3.6 PIP Location……………………………………………………………………………………….. 26
6.6.3.7 Color Bars………………………………………………………………………………………….. 26
6.6.4 BUTTON SETTING SUB-MENU………………………………………………………………… 27
6.6.5 CLOCK SETTING SUB-MENU………………………………………………………………….. 27
6.6.5.1 Date Style………………………………………………………………………………………….. 27
6.6.5.2 Year, Month, Day, Hour, Minute…………………………………………………………………... 28
6.6.5.3 Stop Watch…………………………………………………………………………………………. 28
4
6.6.6 DEFAULT SETTING SUB-MENU……………………………………………………………….. 28

6.7 KEYBOARD OPERATION………………………………………………………………………. 29
6.7.1 Short Keys…..……………………………………………………………………………………... 29
6.7.2 Short Keys Combination………..…………………………………………………………………. 30
6.7.3 Entering Patient Data………………………………………………………………………………. 30
6.7.4 Entering User Window Data……………………………………………………………………….. 31
6.8 MENU SUMMARY……………………………………………………………………………….. 32
7
CLEANING………………………………………………………………………………………..
33
7.1 CAMERA CONTROL UNIT……………………………………………………………………… 33
7.2 CAMERA HEAD………………………………………………………………………………….. 33
7.3 OPTO-MECHANICAL ADAPTER………………………………………………………………. 33
7.4 GLASS WINDOW ON CAMERA HEAD AND OPTO-MECHANICAL ADPTER……………. 33
8
DEZINFRCTION AND STERILIZATION…………………………………………………….
34
8.1 DEZINFECTING THE MSV-2010………………………………………………………………. 34
8.2 STERILIZING THE CAMERA HEAD AND OPTO-MECHANICAL ADAPTER……………… 34
9
10
MAINTENANCE…………………………………………………………………………………..
TROUBLESHOOTING……………………………………………………………………………
35
36

5
1. INTRODUCTION
Congratulations on the purchase of your new MSV-2010 system.
The user-friendly MSV-2010 provides superior quality images. In addition it offers a variety of features such as:
• Capability to hook-up a keyboard directly to the video camera
• Capability to operate and adjust the video camera by on-screen menu
• Capability to display on-screen date and time and operate a stop watch
• Capability to enter and display a patient data (Patient name, ID, age and sex)
• Capability to enter and display additional user data (Comments, doctor name, etc).
• Capability to activate peripheral video equipment (Video printer, VCR, etc)
• Capability to freeze a picture while keeping a live picture-in-picture (PIP) image
• Capability to adjust the window used for automatic shutter control
• Capability to manually change shutter speed
• Capability to adjust image contrast (Enhancement)
• Capability to correct “bad” pixels
• Capability to present color bar chart image for monitor adjustment
• Capability of programmable buttons on the camera head
• Control of the camera functions from keyboard or camera head buttons
In short - you chose the best, and we would like to make sure that you receive the optimal results with the MSV2010, by using it correctly.
This user manual will help you to install the MSV-2010 and optimally integrate it with other components of your
system. It will also instruct you how to operate the MSV-2010, how to keep it clean, sterilize it, will give you
maintenance and service guidelines and recommendations, for best performance results.

6
2. SAFETY PRECAUTIONS – IMPORTANT !
The following precautions should be always be exercised with the use of all electro-medical equipment to ensure
safety to all involved parties – user(s), patient(s), etc.
2.1 TRAINING
This equipment should only be used under the supervision of a trained physician in a medical facility. Do not use in
other locations or for any other purposes than the intended application.
2.2 INSTALLATION
1. This equipment should NEVER be installed or used in areas where the unit could get wet or be exposed to any
environmental conditions such as high temperature, humidity, direct sunlight, dust, salt, etc., which could
adversely affect the equipment.
2. This equipment should NEVER be installed or used in the presence of flammable or explosive gases or
chemicals.
3. This equipment should NEVER be installed, used or transported in an inclined position nor should it be
subjected to impact or vibration.
4. For safety reasons, this equipment must be properly grounded. (This equipment should be connected to a three
(3)-prong hospital grade receptacle in U.S.A. or Canada).
5. Ensure that all power requirements are met and comfort to those specified on the rating place located on the
rear panel.
6. Do not block the air intake vent of this equipment.
7. Do not allow the power cord to became twisted, crushed or pulled taut.
8. When using an isolation transformer for any ancillary equipment, ensure the power requirements of the devices
do not exceed the capacity of the isolation transformer. For further information, contact your local MAHE
distributor.
! WARNING
Never drop this equipment or subject it to severe impact as it could compromise the functionality and/or safety of
the unit. Should this equipment be mishandled or dropped, do not use it. Return it to an authorized MAHE
service facility for inspection and repair.
CAUTION:
All devices connecting to the MSV-2010 must be Classified Medical Equipment. Additional information
processing equipment connected to the MSV-2010 form a Medical System and the operator must determine that
all equipment comply with the appropriate end-product standard (such as IEC 60950 or IEC 60065) and the
Standard for Medical System, IEC 60601-1-1.
2.3 PRIOR TO USE
1. Confirm that this equipment functions properly and check the operation of all switches, indicators, etc.
2. To prevent electrical shock when used with endoscopes, this equipment is insulated (type BF electro-medical
equipment). Do not allow it to be grounded to other electrical devices being used on the patient. Rubber gloves
should always be worn to prevent grounding through user(s).
3. Confirm that other devices used in conjunction with equipment function properly and that these other devices
will not adversely affect the operation or safety of this equipment. If any component of the endoscopic system
is not properly functioning, the procedure should not be performed.
4. To prevent any potential electro-magnetic interference, do not use any kind of cellular phone near the camera.
5. Check and confirm that all cords or cables are connected correctly and securely.

7
2.4 DURING USE
1. To prevent electrical shock, the endoscope and /or any other ancillary device should NEVER be applied
directly to the heart.
2. Make sure that contact is made between the patient and this equipment.
3. To avoid damage to the front panel keyboard do not press any keys with any sharp or pointed objects.
4. The light emitted by a Xenon or Metal Halide Light Source is extremely intense. Avoid looking directly at the
light exiting the endoscope and/or this equipment.
5. During clinical procedures, avoid unnecessary prolonged use, which could compromise patient/user safety.
6. Continually monitor this equipment and the patient for any signs of irregularities.
7. Do not connect or disconnect the camera head during operation. This may cause unrecoverable damage to the
camera head.
8. In the event that some type of irregularity is noted to the patient or this equipment, take the appropriate action
to ensure patient safety.
9. If the operation of any of the components of the endoscopic system fails during the procedure and the
visualization of the procedure is lost or compromised, place the endoscope in the neutral position and slowly
withdraw the endoscope.
10. This equipment should only be used according to the instruction and operating conditions described in this
manual. Failure to do so could result in compromised safety, equipment malfunction or instrument damage.
11. To prevent fire or electric shock, do not open or expose the camera control unit to rain or moisture. Refer all
servicing to qualified personnel only.
2.5 AFTER USE
1. Refer to the operating instructions supplied with all the components of the endoscopic system to establish the
right order in which components should be turned off. Some peripheral devices may have been turned off first
to avoid compromising their operation.
2. Wipe all surfaces clean with gauze slightly dampened with alcohol.
3. Be sure connector interfaces and ventilation ports are not allowed wet or splashed with liquids.
2.6 STORAGE
1. This equipment should NEVER be stored in areas where the unit could get wet or be exposed to any
environmental conditions such as high temperature, humidity, direct sunlight, dust, salt, etc., which could
adversely affect the equipment.
2. This equipment should NEVER be stored in the presence of flammable or explosive gases or chemicals.
3. This equipment should NEVER be stored or transported in an inclined position, nor should it be subjected to
impact or vibration.
4. Cords, accessories, etc., should be cleaned and neatly stored.
5. This equipment should be maintained in a clean condition during storage and be ready for subsequent use.
2.7 SERVICE
1. Alterations/modifications to the equipment should NEVER be made. Repairs should only be performed by an
authorized MAHE service facility.
2.8 MAINTENANCE
1. Periodically this equipment and any applicable accessories should be inspected for operation and safety.
2.9 DISPOSAL
1. The equipment should be returned for disposal to MAHE. Contact your local MAHE representative or service
facility.
8
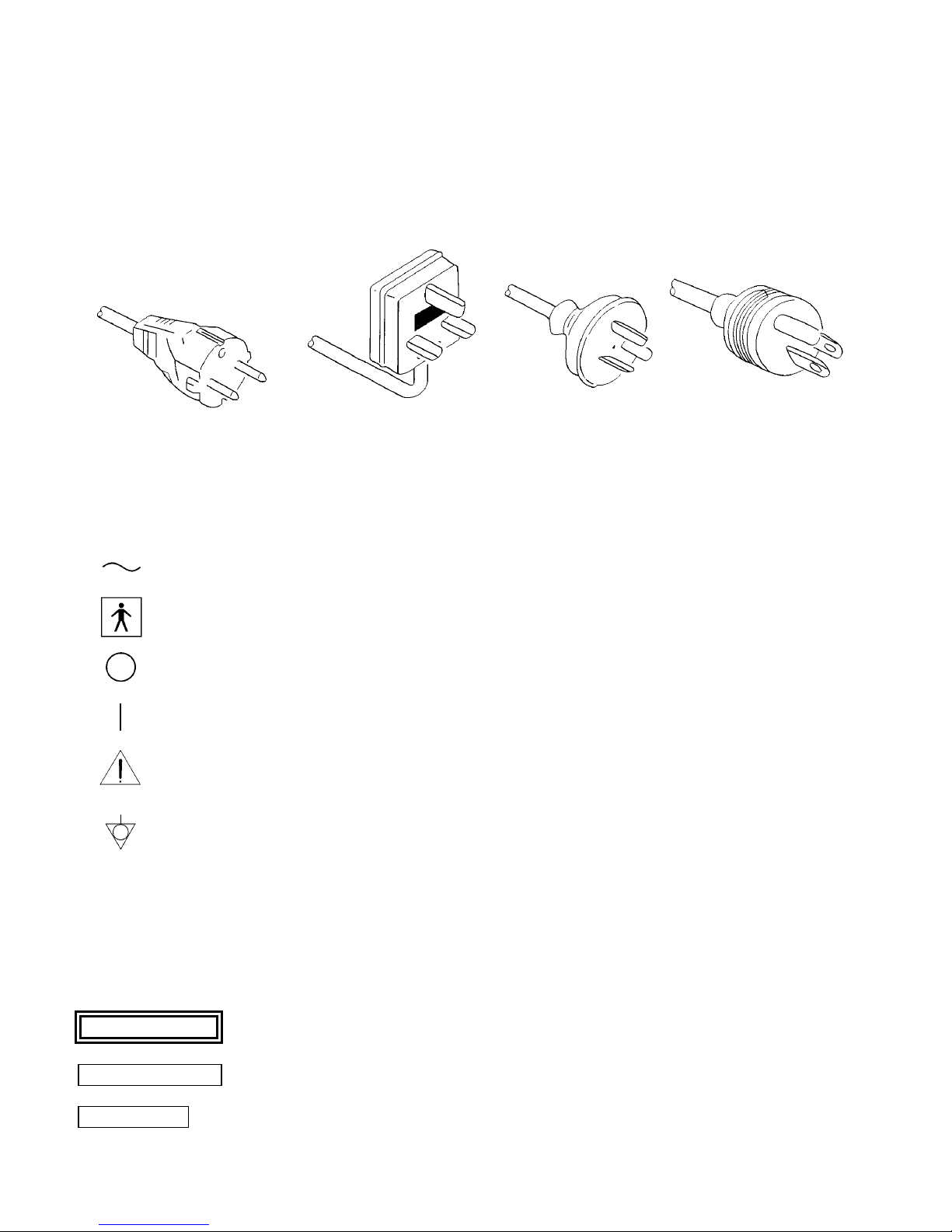
POWER REQUIREMENT
Check the standard power plug configurations that are used in your country. If the appropriate power cord is not
included in your product, notify your local MAHE distributor.
Continental Europe U.K. Australia U.S.A. and
(Use a SEV approved and Canada
plug for Switzerland) New Zealand
(Hospital Grade)
SYMBOLS ON MARKING
Alternating current
Type BF applied part (Safety degree specified by IEC 601-1)
OFF (Power: disconnection from the mains)
ON (Power: connection to the mains)
Attention, consult accompanying documents
Equipotentiality
CONVENTIONS
The following conventions have been established for the text of this manual to aid in the identification of potential
hazards of operation:
! WARNING
CAUTION
NOTE
: Could result in death or serious injury.
: May result in minor or moderate injury or property-damage.
: May result in property-damage. Also, advises owner/operator about important
information on the use of this equipment.
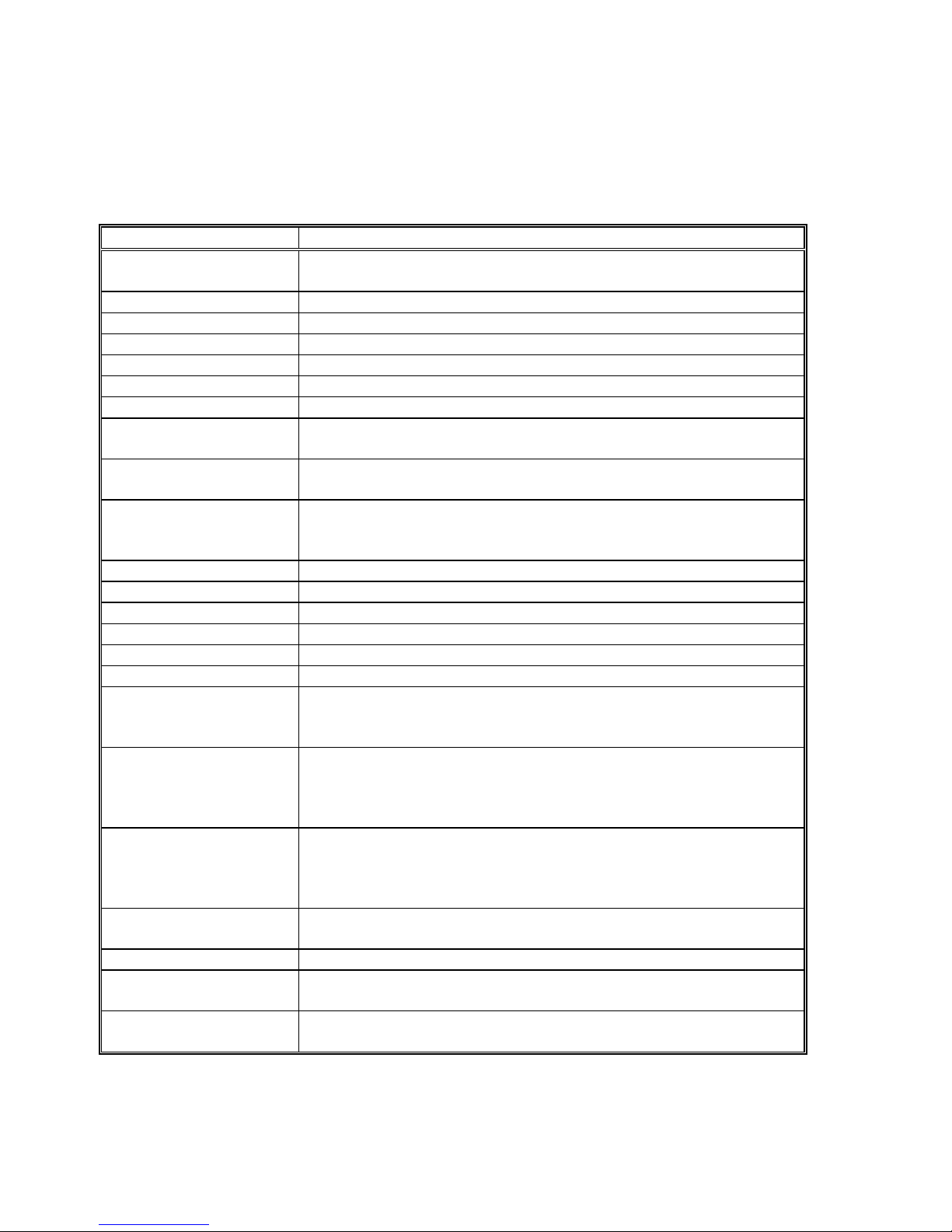
3. SPECIFICATIONS
Item Specification
Number of Pixels PAL 795 (H) x 596 (V), approximately 470,000 pixels
Pick-up Element Interline transfer CCD 1/3" image sensor
Scanning System 2:1 interlace
Minimum Illumination Less than 1.5 Lux @ F 1.2
Resolution 470 TV lines (horizontal)
Signal/Noise Ratio Greater than 46dB @ AGC off
Gain Control Automatic Gain Control and manual Gain boost
White Balance Fast auto white balance (less than 1.0 second)
Electronic Shutter Automatic windowed shutter
Video Outputs Composite video signal 1.0V ptp @ 75 Ohm
Power Supply 100-240VAC @ 50/60Hz automatic
Power Consumption Approximately 20 Watt
Optical Interface CS or C-mount (C-mount with extension ring)
Regulatory Approvals IEC 60601-1, IEC 60601-1-2
Equipment Class Class 1, camera head BF-type
Mode of Operation Continuous operation
Water Resistant Camera head connected to cable is fully soak able (Watertight
Operating Environment
Temperature
Relative Humidity
Air Pressure
Storage Environment
Temperature
Relative Humidity
Air Pressure
Camera Head
Dimensions
Camera Head Weight 40 g
Camera Control Unit
Dimensions
Camera Control Unit
Weight
9
NTSC 811 (V) x 508 (V), approximately 410,000 pixels
Manual RED and BLUE fine tuning
1/50 (PAL) or 1/60 (NTSC) to 1/250,000 seconds
Y/C (S-VHS) Y=1.0V, C(Burst)=0.3V ptp @ 75 Ohm
RGBS R,G,B=0.7V, Sync=2.1V ptp @ 75 Ohm
Equipment, Class IPX7), camera control unit - Not Protected
Equipment, Class IPX0
+10° to +40° C (50° to 104° F)
30 to 85%
700 to 1060 hPa
-20° to +60° C (-4° to 140° F)
0 to 95%
700 to 1060 hPa
33 x 33 x 46 mm
295 (W) x 75 (H) x 253 (D) mm
1.8 kg
10
4. OPERATING ELEMENTS, SYMBOLS AND FUNCTIONS
4.1 VIDEO CAMERA
The video camera consists of the camera control unit, camera head and opto-mechanical adapter.
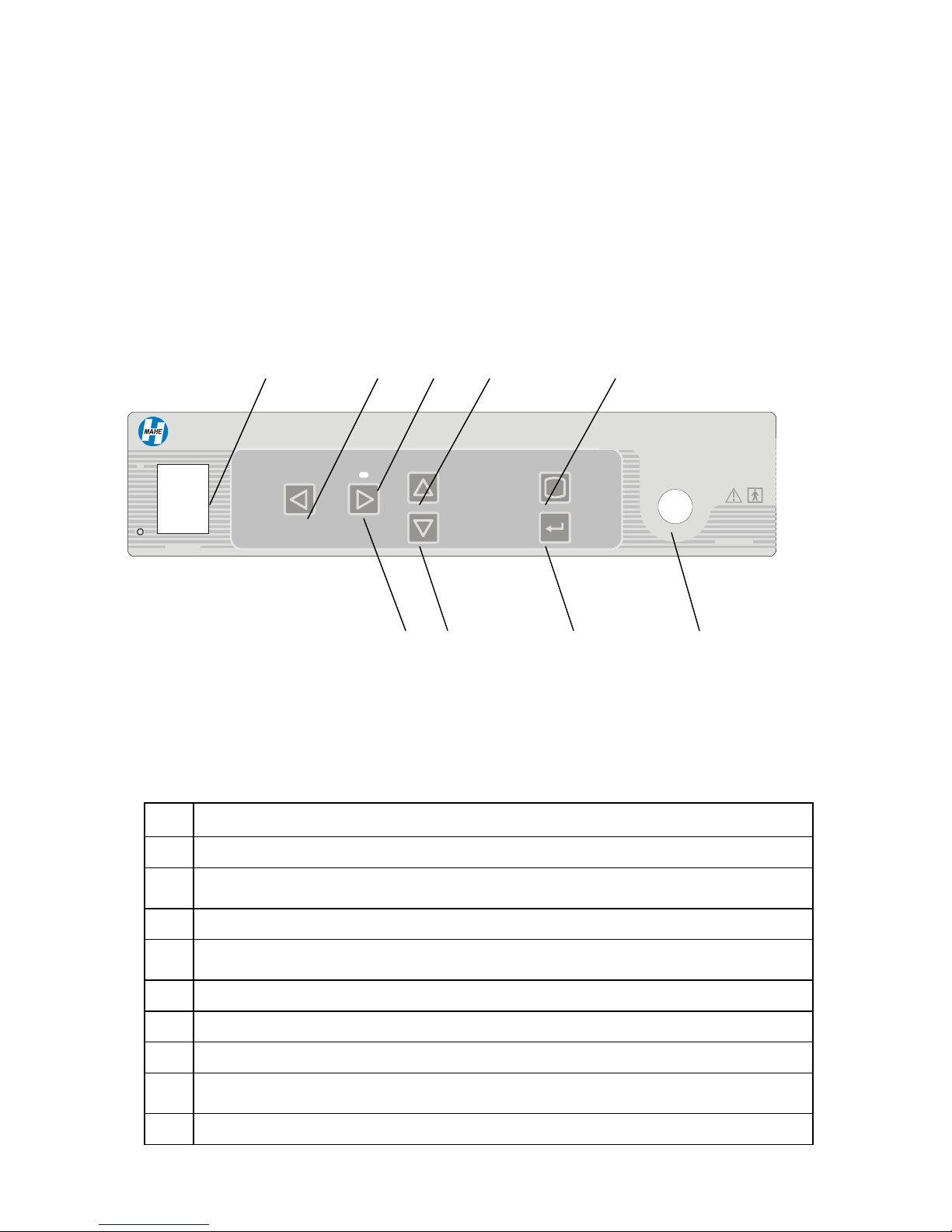
4.1.1 Camera control unit - front panel
1 2 3 4 5
MSV-2010
I
WHITE
BALANCE
GAIN BRIGHTNESS
MENU
Digital Video Camera
9 8 7 6
POWER
Fig.1 Camera control unit front panel
No Description
1 Main power switch with neon lamp indicator
2 White balance button for automatic white balance control / Left scroll button in Menu
mode
3 Green LED indicating activated Gain function
4 Brightness up button for manual brightness adjustment / Up scroll button in Menu
mode
5 Menu button for entering in Menu mode
CAMERA
6 Camera head cable connector
7 Enter button for entering in sub-Menu
8 Brightness down button for manual brightness adjustment / Down scroll button in
Menu mode
9 Gain on/off button for boosting low light images / Right scroll button in Menu mode
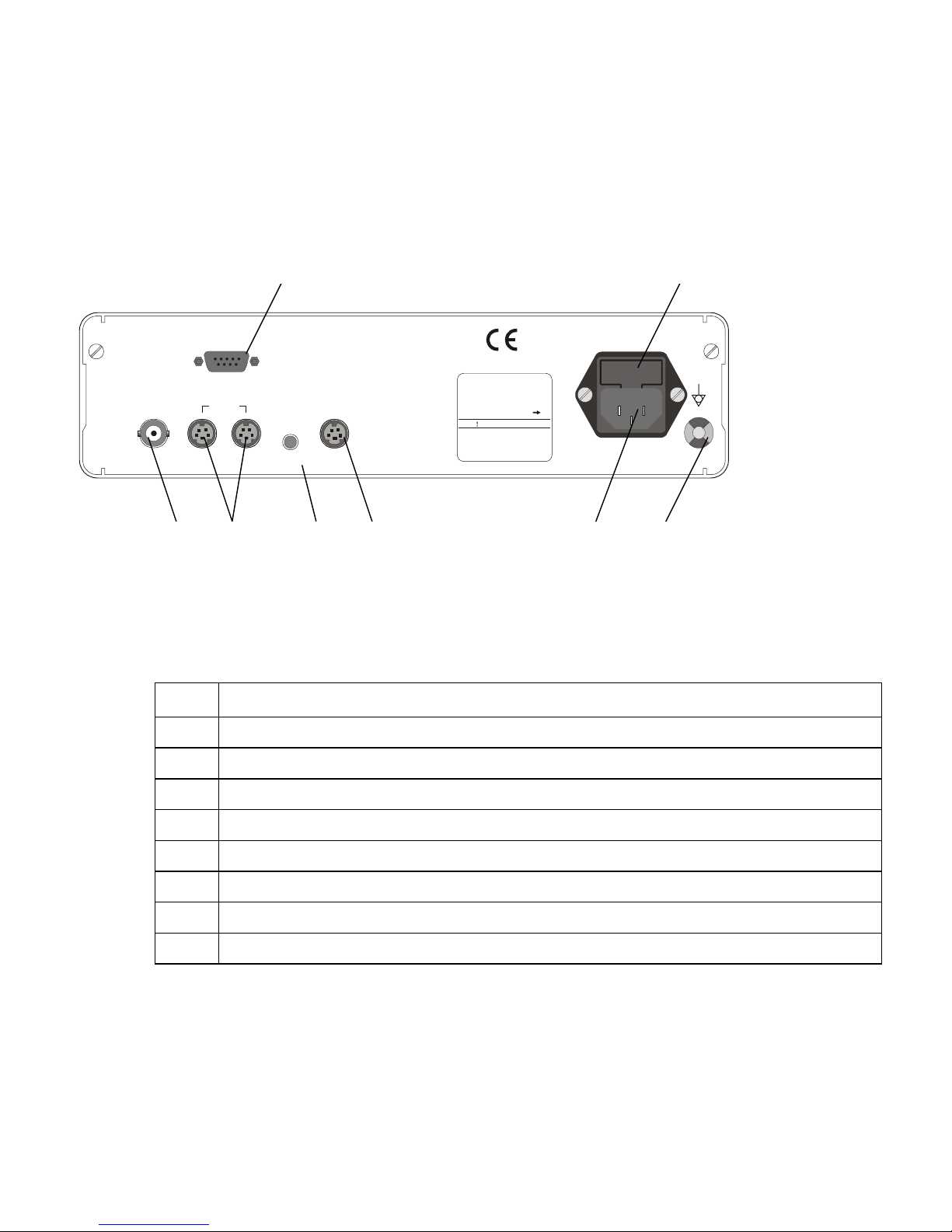
4.1.2 Camera control unit - rear panel
1 2
RGB
VIDEO
Y/C
REMOTE
KEYBOARD
11
INPUT 100-240V~ 50/60Hz
RATING 25VA MAX
SERIAL No.
FUSE 2xT2AL 250V
WARNING
For continued protection
against fire hazard
replace only with same
type and rating of fuse.
8 7 6 5 4 3
Figure 2. Camera control unit rear panel
No Description
1 RGBS video output connector (9-pin D-type)
2 Power fuses (type T2A, 250V)
3 Equipotentiality pin (grounding)
4 AC mains power inlet
5 Keyboard connector (6-pin mini DIN type connector)
6 Remote control connector (3.5mm jack) to operate peripheral devices
7 Y/C video outputs (2 x 4-pin mini DIN type connector)
8 Composite video output (BNC connectors)
 Loading...
Loading...