Page 1

Portable Ultrasonic Nebulizer
Model 6700
User Manual
Read this manual before operating the nebulizer. Save this manual for future reference.
Info: The most current version of this manual can be found online at www.grahamfield.com.
Federal Law (USA) restricts this device to sale by or on the order of a physician. This device should not be used unless the
operator has been instructed by a qualified healthcare professional.
6700-INS-LAB-RevC14
Page 2

Contents
English..........................................Page 3
Español ........................................Page 21
GF Health Products, Inc. is not responsible for typographical errors. All illustrations, specifications, packaging and
warranties contained in this literature are based on the latest product information available at the time of printing.
The most current product information, including the most current version of this manual, can be found online at
www.grahamfield.com.
Graham-Field, Lumiscope, and Lumiscope For The Quality Of Life are registered trademarks of GF Health Products,
Inc. © 2008, GF Health Products, Inc.
2 6700-INS-LAB-RevC14
Page 3
CONTENTS
6700 PARTS .......................................................................................................................................................................4
OPERATION & INDICATION ..................................................................................................................................................4
INTRODUCTION ...................................................................................................................................................................5
IMPORTANT: BEFORE USE, READ THE INSTRUCTIONS CAREFULLY. USE THIS DEVICE ONLY AS INDICATED. .............................5
IMPORTANT SAFETY PRECAUTIONS .....................................................................................................................................5
SETUP ................................................................................................................................................................................8
METHOD OF POWERING ....................................................................................................................................................10
OPERATION ......................................................................................................................................................................12
MAINTENANCE .................................................................................................................................................................13
STORAGE ..........................................................................................................................................................................16
SAFETY PROTECTIONS ......................................................................................................................................................16
6700 TECHNICAL SPECIFICATIONS ....................................................................................................................................17
ACCESSORIES INCLUDED ..................................................................................................................................................17
LIMITED WARRANTY .........................................................................................................................................................18
6700-INS-LAB-RevC14 3..
Page 4
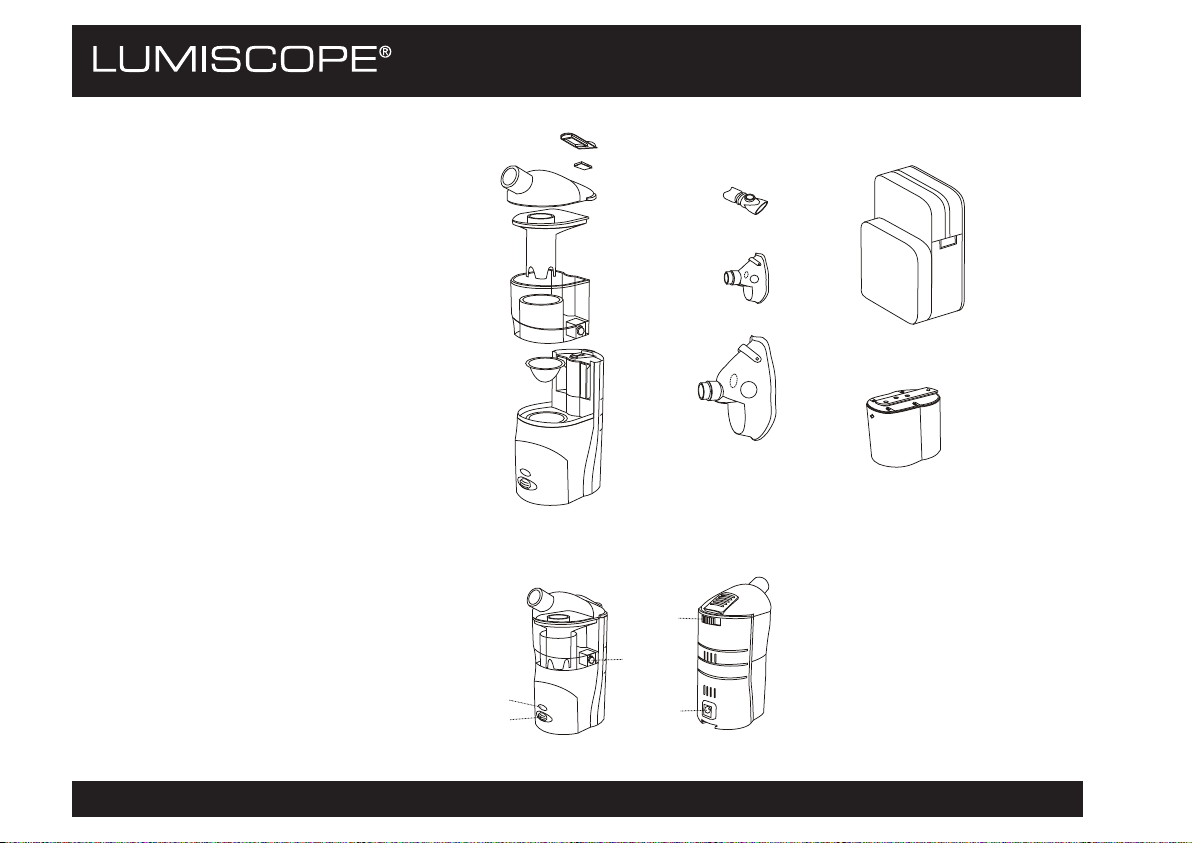
6700 PARTS
1) Air Filter Holder
2) Air Filter
3) Top Cover
4) Nebulization Chamber
5) Air Flow Chamber
6) Medication Cup
7) Main Device
8) Mouthpiece
9) Pediatric Mask
10) Adult Mask
11) AC/DC Adapter (not shown)
12) Carrying Bag
13) Optional: Rechargeable Battery
14) Optional: Car Adapter (Cigarette
Lighter) (not shown)
OPERATION & INDICATION
1) Power Indicator Light
2) On/Off Button
3) Air-Flow Chamber Locking Knob
4) Flow Rate Controller
5) AC/DC Power Socket
1
2
3
4
5
6
7
4
3
1
2
5
8
9
12
10
13
4 6700-INS-LAB-RevC14
Page 5
INTRODUCTION
Thank you for choosing the Lumiscope 6700 portable ultrasonic nebulizer for aerosol therapy. The 6700 is designed
and manufactured according to the most advanced technology. It is also characterized by a high operation speed (up
to .7ml/minute) and is extremely quiet (noise level below 40dB). The 6700 ultrasonic nebulizer is equipped with a
two-valve system which ensures maximum efficiency of inhalation, reducing any medication waste to the minimum.
Intended use
The 6700 is designed to be used by an adult or pediatric patient, under a physician's prescription, to produce
medicated aerosols for inhalation therapy. Indications for therapy include asthma, chronic bronchitis, infection of the
upper respiratory tract, chronic obstructive pulmonary disease, and other respiratory disorders in accordance with a
physician's prescription. Do not use this product for any other purpose than the preceding.
IMPORTANT: BEFORE USE, READ THE INSTRUCTIONS CAREFULLY. USE THIS DEVICE ONLY AS INDICATED.
IMPORTANT SAFETY PRECAUTIONS
When using electrical products, especially when children are present, always follow basic safety precautions,
including the following:
DANGER: Indicates a potential hazard situation or unsafe practice that, if not avoided, will result in death or serious
personal injury.
WARNING: Indicates a potential hazard situation or unsafe practice that, if not avoided, could result in death or serious
personal injury.
CAUTION: Indicates a potential hazard or unsafe practice that, if not avoided, could result in minor or moderate personal
injury.
s NOTICE: Indicates a potential hazard or unsafe practice that, if not avoided, could result in product or property damage.
Info: Provides application recommendations or other useful information to ensure that you get the most from your product.
6700-INS-LAB-RevC14 5..
Page 6

DANGER: To reduce the risk of electrocution:
1. Always unplug this product immediately after use.
2. Do not use this product while bathing, showering, washing dishes, or close to water sources of any kind.
3. Do not place or store device where it can fall or be pulled into a tub or sink.
4. Do not place device in or drop into water or other liquid.
5. Do not reach for a device that has fallen into water. Unplug it immediately.
WARNING: To reduce the risk of burns, electrocution, fire or personal injury:
1. Never leave this device unattended when plugged in.
2. This product contains small parts that may present a choking hazard to small children. Always use close supervision
when this device is used by or near children or those who require close supervision.
3. Children under the age of 3, or any patient who is unable to use a mouthpiece properly under supervision, should use a
mask.
4. Use this device only as intended and described in this manual. Do not use attachments or accessories not recommended
by GF Health Products, Inc.
5. Never operate this product if:
a. it has a damaged cord or plug,
b. it is not working properly,
c. it has been dropped or damaged, or
d. it has been dropped into water.
Return the product to your Graham-Field distributor for examination and repair.
6. Keep the cord away from heated surfaces.
6 6700-INS-LAB-RevC14
Page 7
7. Never block the air openings of this product or place it on a soft surface, such as a bed or couch, where the air openings
may be blocked. Keep the air openings free of lint, hair and the like.
8. Never drop or insert any object into any openings or hose.
9. Never use this device while drowsy or sleeping.
10. Do not use this device outdoors or operate where aerosol (spray) products are being used.
11. Do not use this device in the presence of flammable anesthesia or where oxygen or nitrous oxide is being administered.
This device has no AP or APG protection.
12. Connect this device to a properly grounded outlet only.
13. Unplug the device before filling or cleaning the nebulizer.
14. This device is oil-free. Do not lubricate.
15. Do not remove the nebulizer while the device is operating.
16. Do not leave medication in the nebulizer when it is not in use.
17. Use the nebulizer intermittently; the maximum operating period is 45 minutes. The nebulizer must stand unused for 45
minutes between uses.
18. The mouthpiece and mask are for use by one person only; in order to avoid infection, do not share them.
19. Notice for California Customers- California Proposition 65 WARNING: This product contains a chemical known to the
State of California to cause cancer and reproductive or developmental harm.
WARNINGS FOR MEDICATION USE
Always follow the prescribing physician's instructions regarding the type of medication to be used, the dosage,
the frequency, and duration of the inhalations. The 6700 has been designed for use with aqueous solutions
of medication; medication that is too dense or oily will not be nebulized correctly. If a medication has not
been nebulized correctly, dilute it with an equal quantity of physiological solution for inhalations (add 2 ml of
physiological solution to 2 ml of oily medication).
6700-INS-LAB-RevC14 7..
Page 8
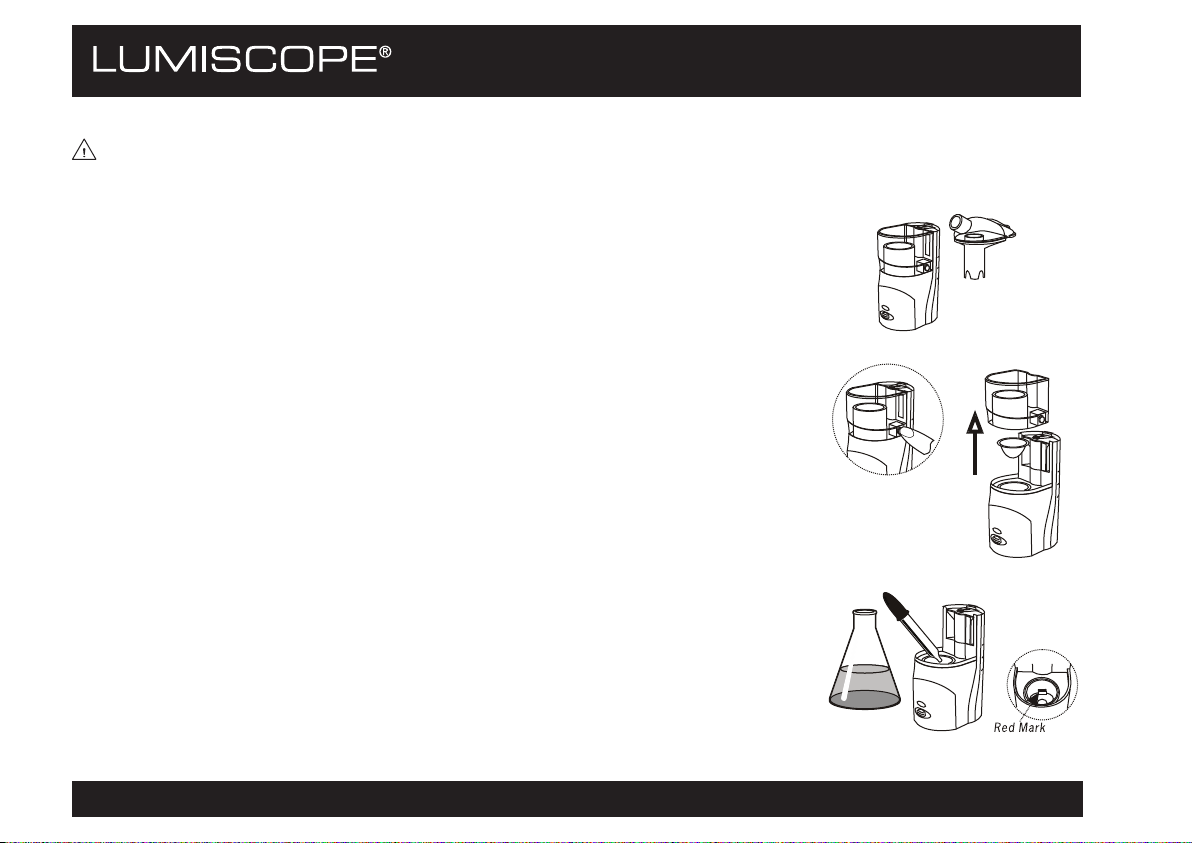
SETUP
WARNING: Before using the device, ensure that it has been cleaned as described in
MAINTENANCE/Cleaning the Nebulizer After Each Use.
1. Remove the top cover and nebulization chamber from the device.
2. Simultaneously press the button on each side of the air-flow chamber to remove
the air-flow chamber and medication cup from the device.
3. Fill the reservoir of the device with distilled water until it reaches exactly the
level marked in red (approximately 4 ml). This amount of water serves as a
liquid conductor, to conduct the ultrasound waves to the medication, and will
never be nebulized.
s NOTICE: The nebulizer will not operate if the chamber is not filled with water.
8 6700-INS-LAB-RevC14
Page 9
4. Carefully insert the medication cup as shown, taking care not to damage it.
5. Pour the physician-prescribed medication into the medication cup. If the dose to
be inhaled is larger than the maximum capacity of the medication cup, divide it
into several separate inhalations.
6. Re-install the air-flow chamber, nebulization chamber, and top cover on the
device. Apply sufficient pressure when re-installing to ensure that they are
securely closed.
7. Insert the mouthpiece or one of the two provided masks into the front of the
device as shown.
6700-INS-LAB-RevC14 9..
Page 10
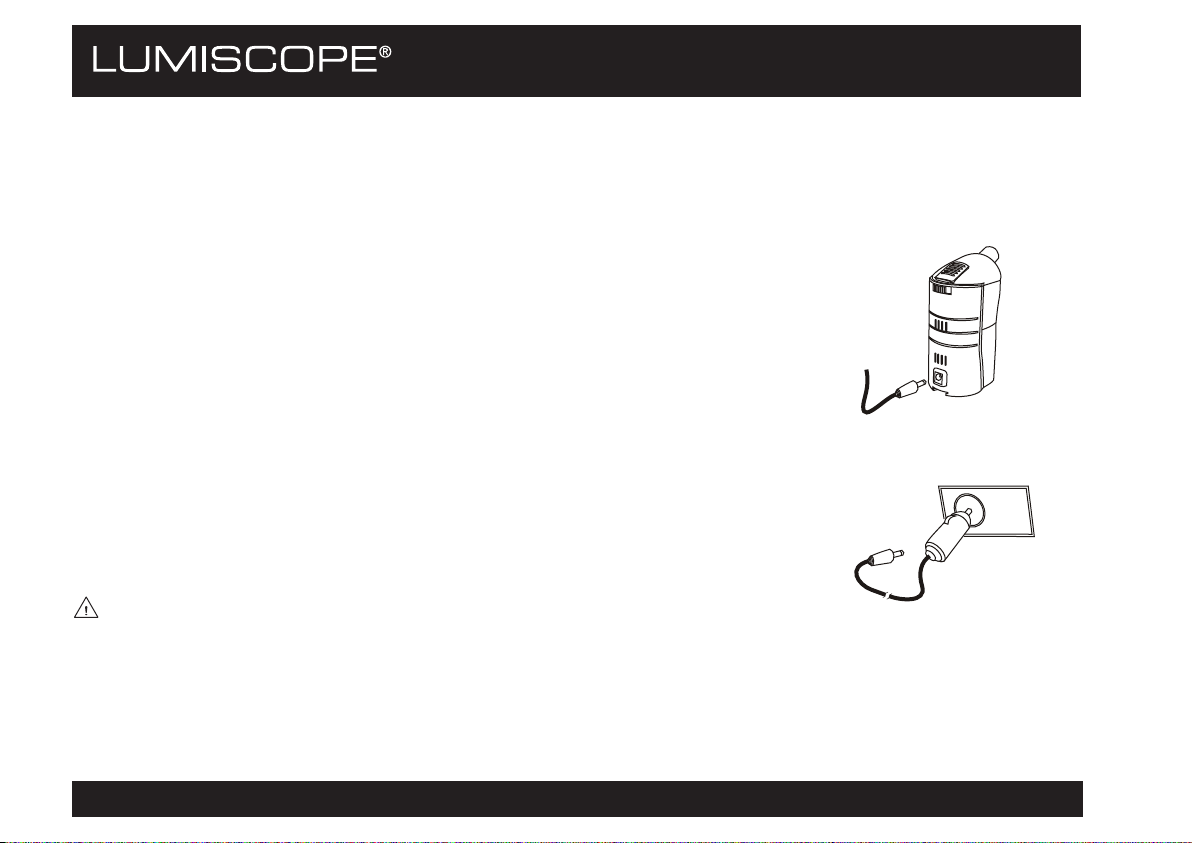
METHOD OF POWERING
After the preparation, connect the device to electrical power. The 6700 can be
powered in various ways:
With the AC/DC Adapter
This is designed for use in the home or wherever normal-voltage power supply is
available (different connections are available for different voltages).
1. Ensure that device power is OFF. Ensure that the voltage at the socket is the
same as that of the transformer, as indicated on the transformer specification
plate. Plug the power cord into a properly grounded electrical wall outlet.
2. Insert the transformer plug into the device's power socket.
With the Optional Car Adapter (Cigarette Lighter)
This method allows the patient to take the inhalations when traveling by car.
1. Ensure that the cigarette lighter socket in the car is designed for power of at
least 15 watts. Insert the car adapter plug into the cigarette lighter socket.
2. Insert the car adapter pin into the device's power socket.
WARNING: Never use the device while driving.
Cigarette
lighter
socket
10 6700-INS-LAB-RevC14
Page 11
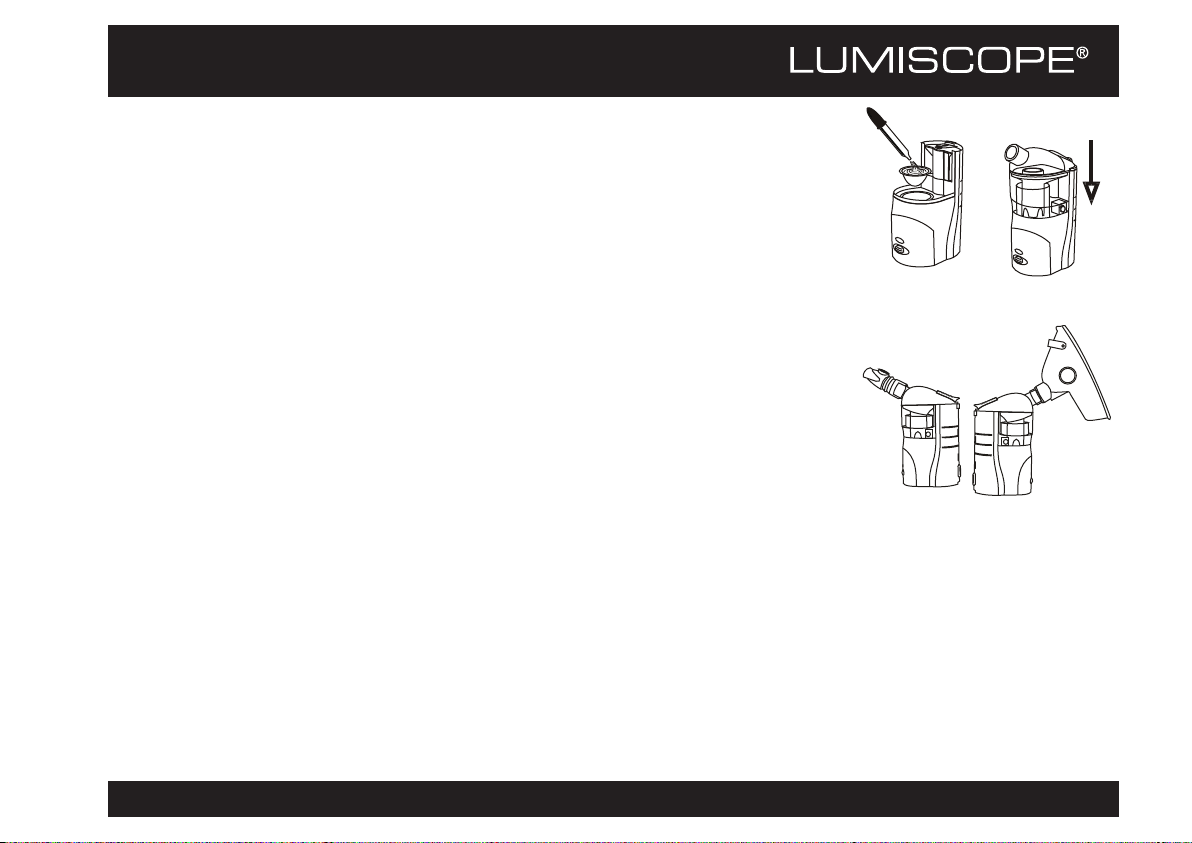
With the Optional Rechargeable Battery
One can also use the optional Ni-MH
rechargeable battery for the power input.
It provides approximately 80 minutes of
operation.
To install the battery: Match the device bottom
to the battery top as shown at near right. Slide
until you hear it click into place.
To remove the battery: Push down on the
battery lock as shown at far right and slide the
battery out from beneath the device.
To recharge the battery: Insert the AC/DC
adapter into the device's power socket. An
orange light indicates the battery is being
recharged. The orange light will de-illuminate
when charging is complete. The minimum
recharge time is 5~6 hours.
Info: The first charge of a new rechargeable battery
will require at least 8 hours.
Battery Installation Battery Removal
Battery Recharging
6700-INS-LAB-RevC14 11..
Page 12
OPERATION
Keep the device and accessories dry. Avoid exposure to direct sunlight, high moisture, and dust.
s NOTICE: The device's operating environment must be between 50°F and 104°F (10°C and 40°C) and 10% - 95% RH.
WARNING: Ensure that device has been cleaned as described in MAINTENANCE/Cleaning the Nebulizer After Each Use.
WARNING: Ensure that device has been prepared for operation as described in SETUP.
WARNING: Ensure that the chosen power source, as described in METHOD OF POWERING, is safely connected.
Inhalation Therapy
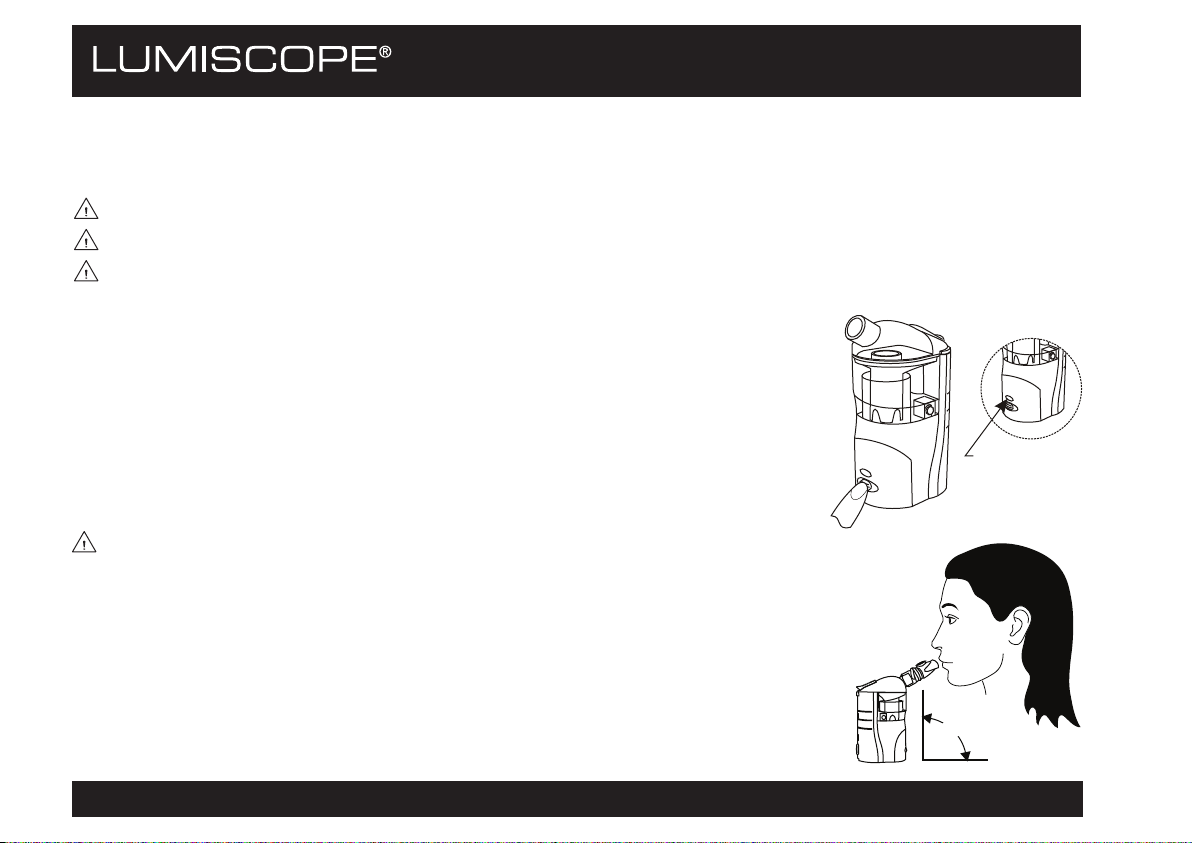
1. Press the ON/OFF button to start the device. The green power indicator light
illuminates to indicate the device is working normally.
s NOTICE: A light that alternates between red and green indicates a low battery
condition; the device can still function normally for a short time, but the battery
should be charged as soon as possible.
s NOTICE: A red light indicates a low battery condition; the device will turn off
automatically.
WARNING: To achieve correct function of the device and maximum effectiveness of the
treatment, always keep the device in a vertical position during inhalation therapy.
2. Inhale the medication as prescribed by your physician.
Info: Certain medications have different nebulization rates than others.
Power
Indicator
Light
90°
12 6700-INS-LAB-RevC14
Page 13

3. The aerosol flow rate can be regulated by adjusting the flow rate controller as shown
at right. The recommended practice, especially for children, is to set to the "Minimum"
position in order to promote the deposit of the nebulized aerosol directly in the
airways.
4. When the mist generated is no longer constant, the medication in the medication cup
has begun to run out. When this happens, tap the nebulizer cover gently several times
to collect dispersed liquid inside the nebulizer chamber. Then continue the therapy
until there is no aerosol at all.
Info: The unit will shut off automatically when the medication cup is nearly empty. At the end of
inhalation therapy, a small amount of the medication (about 0.4 ml) will remain in the medication
cup; this is normal. This portion of the medication, called the "residual volume", cannot be
nebulized.
5. Disconnect the plug from the device's power socket.
Automatic Valve System
The device's mouthpiece has a two-valve system to ensure maximum efficiency of the
inhalation and to minimize medication waste. In practice, the mouthpiece expiration valve
will open during expiration, allowing the patient's exhaled air to pass to atmosphere,
avoiding contamination of the device. During inspiration, the valve will close, maximizing
the inhalation amount.
MAINTENANCE
WARNING: To reduce the risk of burns, electrocution, fire, or personal injury:
1. Electrical shock hazard - do not remove the outer case of the device. All disassembly and
maintenance of the device must be performed by a qualified service technician. Refer service
to qualified service personnel.
2. This device does not require oil. Do attempt to lubricate any internal parts.
3. Unplug this device before cleaning. Do not submerge in water for cleaning.
6700-INS-LAB-RevC14 13..
Page 14

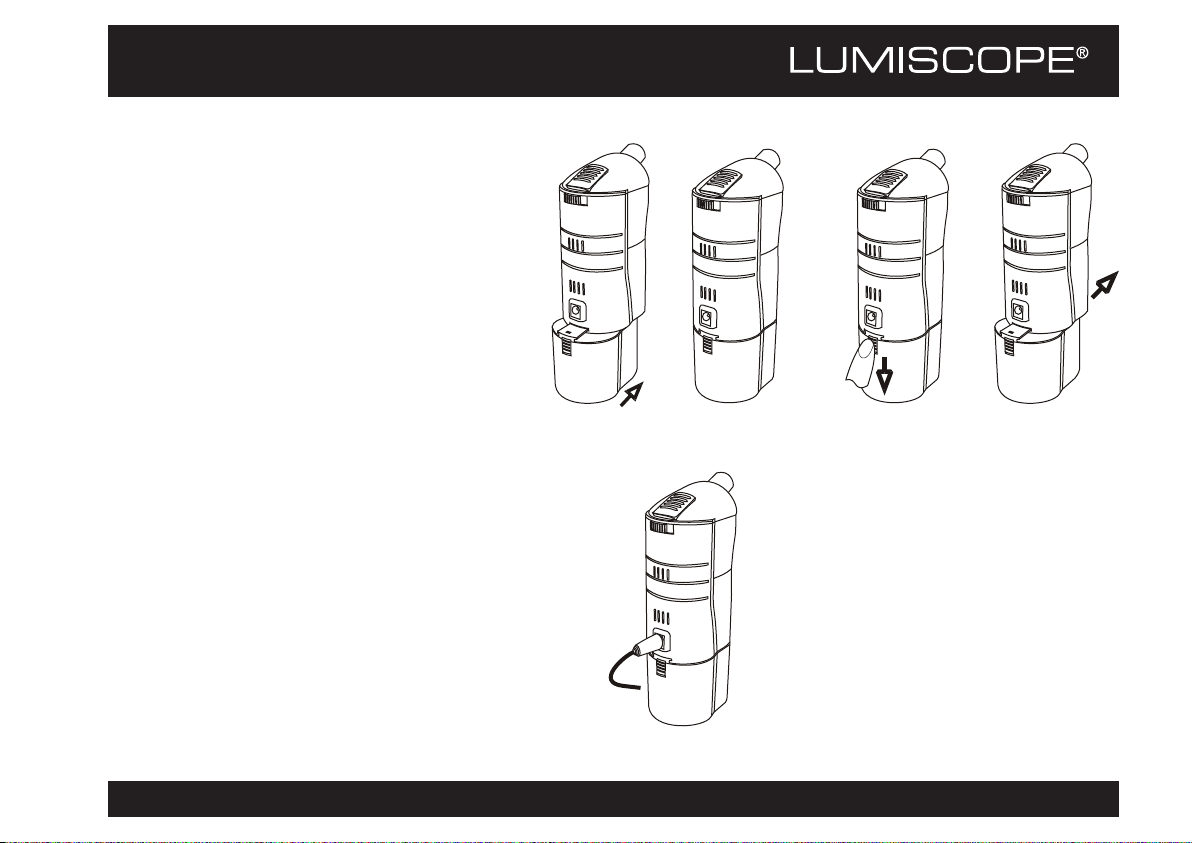
Cleaning the Nebulizer After Each Use
WARNING: To prevent possible risk of infection from contaminated
medication, clean the nebulizer as follows after each treatment.
1. Ensure the plug is disconnected from the device's power socket.
2. Remove the top cover, nebulization chamber, air-flow chamber, and
medication cup from the device. Remove the filter from the cover.
3. Completely empty the conduction liquid in the reservoir.
4. Wipe the main unit with a clean, soft, moistened cloth.
5. Wash the top cover, nebulization chamber, air-flow chamber,
mouthpiece, masks, and medication cup in warm, soapy water, and
rinse thoroughly. Allow to air dry. Store in a clean, dry location.
Cleaning the Nebulizer Daily
WARNING: To prevent possible risk of infection from contaminated
medication, clean the nebulizer as follows daily.
Once daily, after washing as above, soak the washed components in a
fresh solution consisting of one part warm water and one part white
vinegar for 30 minutes followed by a warm water rinse. Allow to air dry.
Store in a clean, dry location.
Replacing the Medication Cup
Replace the medication cup after every two treatments. There is a
package of 20 replacement medication cups supplied with the nebulizer.
Additional medication cups can be purchased from Graham-Field.
20 Replacement
Medication Cups
14 6700-INS-LAB-RevC14
Page 15

Replacing the Air Filter
The air filter prevents extraneous substances from entering the mist generated by the
device. Replace the air filter after about 20 hours of use, or when the filter turns gray.
There is a package of 5 replacement air filters supplied with the nebulizer. Additional
filters can be purchased from Graham-Field.
1. Remove the top cover from the device.
2. Lift the air filter holder from the top cover.
3. Remove the filter from the air filter holder.
4. Insert a new filter into the air filter holder.
5. Replace the air filter holder on the top cover.
s NOTICE: Do not use cotton or other material as a filter.
s NOTICE: Do not wash or clean the filter.
s NOTICE: Do not reuse a dirty filter. Do not operate the nebulizer without a filter; nebulizer damage can result.
6700-INS-LAB-RevC14 15..
Page 16

STORAGE
Keep the device and accessories dry. Avoid exposure
to direct sunlight, high moisture, and dust.
s NOTICE: If the device is to be stored long-term,
remove the battery.
s NOTICE: The battery's storage environment must be
between -4°F and 95°F (-20°C and 35°C).
s NOTICE: The device's storage environment must be
between -13°F and 140°F (-25°C and 60°C) and 10%
- 95% RH.
SAFETY PROTECTIONS
The nebulizer is equipped with protection against
several unsafe situations. If these situations occur, the
nebulizer will either (1) not power up or (2) turn off
automatically, as described below.
Protection against incorrect Power Supply
If an AC/DC adapter is used to power the device,
ensure the adapter meets local power regulations; if
not, the device may not power up (the Power Indicator
Light will not illuminate).
Protection Against Overheating
During normal operation, the Power Indicator Light will
illuminate GREEN. However, in the two following cases,
the device will indicate irregularity by turning off
automatically. The device then requires approximately
45 minutes at rest before restarting.
Protection Against Overuse: The electronic
components of the device are designed to function
continuously for approximately 40 minutes. Operating
the device for too long will trigger the heat-protection
mechanism and the device will turn off automatically.
Lack of Liquid in the Reservoir: In the event of lack or
insufficient liquid in the reservoir, the device will turn
off automatically.
16 6700-INS-LAB-RevC14
Page 17

6700 TECHNICAL SPECIFICATIONS
- Power Supply: AC/DC Adapter (240V, 50Hz;
12VDC 800mA)
- Power Consumption: ~ 12W (12V/1A)
- Sound Level: < 40dB
- Frequency: 2.8 Mhz
- Particle Range: 0.5 ~ 4.0 microns
- Medication Cup Capacity: 5 ml maximum
- Average Rate: 0.55 ~ 0.80 ml/min.
(NaCl0.9% Normal Saline)
- Intermittent Use: 45 min. on / 45 min. off
- Operation Conditions:
50°F ~ 104°F (10°C ~ 40°C), RH 10% ~ 95%
- Storage Conditions:
-13°F ~ 140°F (25°C ~ 60°C), RH 10% ~ 95%
- Dimensions:
~ 5.1 x 3.3 x 2.2 in. (~ 130 x 85 x 55 mm)
- Weight without batteries: ~ .44 lb (198 g)
ACCESSORIES INCLUDED
- 1 Pediatric Mask
- 1 Adult Mask
- 1 Mouthpiece
- 1 Carrying Bag
- 5 Spare Air Filters
- 20 Medication Cups
- 1 AC/DC Adapter
- Optional: 1 Rechargeable Battery Pack
- Optional: 1 Car Adapter
6700-INS-LAB-RevC14 17..
Page 18

LIMITED WARRANTY
Scope of Warranty
GF Health Products, Inc. (“GF”) warrants to the original
purchaser only that it will replace or repair components,
at GF’s sole discretion, that are defective in material
or workmanship under normal use and service. All
warranties are conditioned upon the proper use of the
products strictly in accordance with good commercial
practice and applicable GF instructions and manuals,
including proper use and maintenance. To the extent
that a component is warranted by a third party, GF
conveys all of its rights under that warranty to the
original purchaser, to the extent permitted.
This limited warranty shall only apply to defects that
are reported to GF’s customer service team within
the applicable warranty period and which, upon
examination by GF or its authorized representative,
prove to be a warranty item. This limited warranty is
not transferable.
The warranted components and time period are set
forth below:
6700 Portable Ultrasonic Nebulizer: one year
This warranty does not include batteries.
The applicable warranty period shall commence from
date of shipment to the original customer, unless there
is an expiration date on the component in which case
the warranty shall expire on the earlier of warranty
period or the expiration date.
Obtaining Warranty Service
A GF Customer Service Representative must authorize
warranty service. Please contact the GF Customer
Service department by calling 678-291-3207, sending a
fax request to 770-368-2386 or by e-mailing a request
to cs@grahamfield.com. Specific directions will be
provided by the Customer Service Representative.
Failure to abide by the specific directions will result in
denial of the warranty claim.
Exclusions
The warranty does not cover and GF shall not be liable
for the following:
1) Defects, damage, or
whole or in part, by misuse, abuse, negligence,
alteration, accident, freight damage, tampering or
failure to seek and obtain repair or replacement in a
timely manner;
2) Products which are not installed, used, or properly
cleaned and maintained as required in the official
manual for the applicable product;
3) Products considered to be of a non-durable nature
including, but not limited to: casters, filters, fuses,
gaskets, lubricants, and charts;
4) Accessories or parts not provided by GF;
5) Charges by anyone for adjustments, repairs,
replacement parts, installation or other work
performed upon or in connection with such
products which are not expressly authorized in
other conditions caused, in
18 6700-INS-LAB-RevC14
Page 19

writing, in advance, by GF;
6) Any labor or shipping charges incurred in the
replacement part installation or repair;
7) Costs and expenses of regular maintenance and
cleaning; and
8) Representations and warranties made by any person
or entity other than GF.
Entire Warranty, Exclusive Remedy and
Consequential Damages Disclaimer
THIS WARRANTY IS GF’S ONLY WARRANTY AND IS
IN LIEU OF ALL OTHER WARRANTIES, EXPRESS OR
IMPLIED. GF MAKES NO IMPLIED WARRANTIES OF
ANY KIND INCLUDING ANY IMPLIED WARRANTIES OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE.
IF ANY MODEL OR SAMPLE WAS SHOWN TO THE
CUSTOMER, SUCH MODEL OR SAMPLE WAS USED
MERELY TO ILLUSTRATE THE GENERAL TYPE AND
QUALITY OF THE PRODUCT AND NOT TO REPRESENT
THAT THE PRODUCT WOULD NECESSARILY CONFORM
TO THE MODEL OR SAMPLE IN ALL RESPECTS.
THIS WARRANTY IS LIMITED TO THE REPAIR OR
REPLACEMENT OF THE DEFECTIVE PARTS. GF SHALL
NOT BE LIABLE FOR AND HEREBY DISCLAIMS ANY
DIRECT, SPECIAL, INDIRECT, INCIDENTAL, EXEMPLARY
OR CONSEQUENTIAL DAMAGES, INCLUDING, BUT
NOT LIMITED TO: DAMAGES FOR LOSS OF PROFITS
OR INCOME, LOSS OF USE, DOWNTIME, COVER, OR
EMPLOYEE OR INDEPENDENT CONTRACTOR WAGES,
PAYMENTS AND BENEFITS.
The warranties contained herein contain all the
representations and warranties with respect to the
subject matter of this document, and supersede all
prior negotiations, agreements and understandings with
respect thereto. The recipient of this document hereby
acknowledges and represents that it has not relied on
any representation, assertion, guarantee, warranty,
collateral contract or other assurance, except those set
out in this document.
For additional information on this product or this
warranty, please contact a GF Customer Service
Representative.
Notes:
1) Additional terms and conditions may apply.
2) Freight claims must be notated on the Bill of
Lading and must be made with immediacy. The ICC
regulations govern specific requirements for freight
claims. Failure to abide by those regulation
result in a denial of the freight claim. GF will assist
you in filing the freight claim.
3) Claims for any short shipment must be made within
thirty (30) days of the invoice date.
s may
6700-INS-LAB-RevC14 19 ..
Page 20

Manufactured for:
GF Health Products, Inc.
2935 Northeast Parkway
Atlanta, Georgia 30360 USA
Telephone: 770-368-4700
Fax: 770-368-2386
www.grahamfield.com
Made in China
Page 21

Nebulizador Portable Ultrasonido
Modelo 6700
Manual Usuario
Lea antes de operar el nebulizador. Guarde este manual para referencia en el futuro.
Info: La más reciente versión de este manual podrá ser encontrado en www.grahamfield.com.
La Ley Federal (USA) restringe este aparato para ventas por la orden de su médico. Este aparato no debe ser utilizado si
el operador no ha sido adiestrado por profesional de cuido de salud cualificado.
6700-INS-LAB-RevC14
Page 22

Contenidos
English..........................................Page 3
Español ........................................Page 21
GF Health Products, Inc. no es responsable de errores tipográficos. Para obtener la información más actualizada y
vigente relativa a los envases, garantías, productos y especificaciones, incluyendo la versión más actualizada de estas
instrucciones, por favor visite nuestro sitio web en www.grahamfield.com.
Graham-Field, Lumiscope, y Lumiscope For The Quality Of Life son marcas registradas de GF Health Products, Inc.
© 2008 GF Health Products, Inc.
2 6700-INS-LAB-RevC14
Page 23

CONTENIDOS
6700 PARTES ..................................................................................................................................................................... 4
OPERACIÓN & INDICACIONES ..............................................................................................................................................4
INTRODUCCIÓN ..................................................................................................................................................................5
IMPORTANTE: ANTES DE USO, LEA LAS INSTRUCCIONES CUIDADOSAMENTE .........................................................................5
PRECAUCIONES DE SEGURIDAD IMPORTANTE ......................................................................................................................5
MONTAJE ...........................................................................................................................................................................8
MÉTODO DE PRENDIENDO .................................................................................................................................................10
OPERACIÓN ......................................................................................................................................................................12
MANTENIMIENTO ..............................................................................................................................................................13
ALMACENAJE ....................................................................................................................................................................16
PRECAUCIONES DE SEGURIDAD ........................................................................................................................................16
6700 ESPECIFICACIONES TÉCNICAS ..................................................................................................................................17
ACCESORIOS INCLUIDOS....................................................................................................................................................17
GARANTÍA LIMITADA .........................................................................................................................................................18
6700-INS-LAB-RevC14 3..
Page 24

6700 PARTES
1) Porta Filtro de Aire
2) Filtro de Aire
3) Tapa
4) Cámara de Nebulización
5) Cámara de Flujo de Aire
6) Taza de Medicamento
7) Aparato Principal
8) Boquilla
9) Máscara Pediátrica
10) Máscara Adulta
11) Adaptador de AC/DC (no mostrado)
12) Bolsa de Cargar
13) Opcional: Batería Recargable
14) Opcional: Adaptador de Encendedor
de Cigarillo (no mostrado)
OPERACIÓN & INDICACIONES
1) Luz Indicador de Electricidad
2) Botón de ON/OFF
3) Tornillo de Cerrar la Cámara de Flujo
de Aire
4) Control de Tasa de Flujo
5) Enchufe de Electricidad de AC/DC
1
2
3
4
5
6
7
4
3
1
2
5
8
9
12
10
13
4 6700-INS-LAB-RevC14
4..
Page 25

INTRODUCCIÓN
Le agradecemos por haber elegido el Lumiscope 6700 nebulizador portable ultrasonido para terapia de aerosol. El
6700 es diseñado y fabricado como un producto con soluciones técnicas muy adelantadas. Es caracteriza por una
velocidad elevada de funcionamiento (hasta 0.7 ml/ minuto) y es casi silencioso (nivel de rumor inferior a 40 dB).
El 6700 es equipado con un sistema de dos válvulas que asegura eficiencia máximo de inhalación, reduciendo cual
quiere gasta mínimo de medicamento.
Uso entendido
Es diseñado para uso por un paciente adulto y pediátrico, por receta de un médico, para producir aerosol medicado
para terapia de inhalación. Indicaciones para terapia incluye asma, bronquitis crónica, infección de las vías
respiratorias altas, enfermedad pulmonar obstructiva crónica (COPD) y otras enfermedades respiratorias en acuerdo
con la receta de médico. No debe usar este producto por cualquier otra razón lo de arriba.
IMPORTANTE: ANTES DE USO, LEA LAS INSTRUCCIONES CUIDADOSAMENTE. UTILICE ESTE APARATO
SOLAMENTE COMO INDICADO.
PRECAUCIONES DE SEGURIDAD IMPORTANTE
Cuando usando productos eléctricos, especialmente cuando están presentes niños, siempre sigue las precauciones
básicas de seguridad, incluyendo lo siguiente:
PELIGRO: Indica una situación de peligro o una práctica insegura que, si no es evitada, resultará en muerte o heridas
serias a la persona.
ADVERTENCIA: Indica una situación de peligro o una práctica insegura que, si no es evitada, puede resultar en muerte o
heridas serias a la persona.
PRECAUCIÓN: Indica una situación de peligro o una práctica insegura que, si no es evitada, puede resultar en herida leve
a la persona.
s AVISO: Indica una situación de peligro o una práctica insegura que, si no es evitada, puede resultar en daño al producto
o propiedad.
Info: Proporciona recomendaciones para la aplicación y otra información útil para asegurar que obtenga lo más que pueda del
producto.
6700-INS-LAB-RevC14 5..
Page 26

PELIGRO: Para reducir el riesgo de electrocución:
1. Siempre desenchufe este producto inmediatamente después de uso.
2. No utilice durante el baño, lavando platos, y cerca de cualquier fuente de agua.
3. No posicionar o almacenar donde pueda caer a la bañera o al lava manos.
4. No debe introducir en agua u otro liquido.
5. No debe recoger producto sumergido en agua. Desconecte inmediatamente.
ADVERTENCIA: Para reducir el riesgo de quemadura, electrocución, fuego o heridas a la persona:
1. Este producto nunca debería ser desatendido mientras esté conectado.
2. Este producto contiene piezas pequeñas que presenta riesgos de asfixia para niños, y la manga puede presentar riesgo
de estrangulación. Supervisión cercana es necesaria cuando este producto esté utilizado cerca de niños o personas con
impedimentos.
3. Niños menores de 3 años, y cualquier paciente quien no puede usar la boquilla correctamente bajo supervisión, deben
usar la máscara.
4. Utilice este producto solamente como indicado en el manual de usuario. No utilice aditamentos o accesorios no
recomendados por GF Health Products, Inc.
5. Nunca utilice este producto si:
a. el enchufe o el cable está dañado,
b. no funciona adecuadamente,
c. ha sufrido caída o daño, o
d. ha sido mojado o sumergido en agua.
Devuelve este producto al centro de servicio para examen y/o arreglos.
6. Mantenga el cable lejos de superficies calientes.
6
Page 27

7. Nunca obstruya entradas de aire del producto o utilice en una superficie suave tal como cama o sillón donde las
entradas del aire pueden ser bloqueadas. Mantenga las entradas libres de pelusa, cabello, o materiales de este tipo.
8. Nunca inserte objetos en cualquier entrada o manga.
9. Nunca utilice soñoliento o dormido.
10. No utilice al aire libre, ni opere donde se utilicen productos de aerosol.
11. No utilice en presencia de anestesia, oxígeno, oxido nítrico, o cualquier gas inflamable mientras estos estén siendo
administrado. Este aparato no tiene protección AP o APG.
12. Conecte este producto solamente en un enchufe de pared correcto.
13. Desconecte el aparato antes de llenar o limpiar el nebulizador.
14. Este aparato no utiliza aceite. No lubrique.
15. No debe remover el nebulizador de chorro cuando el aparato está operando.
16. No debe dejar medicamento en el nebulizador cuando no esté en uso.
17. Use el nebulizador por periodos: el periodo de operación máximo es 45 minutos. El nebulizador no debe ser usado por
45 minutos entre usos.
18. La boquilla y la máscara son para uso de una sola persona; para evitar infección no debe compartirlos.
19. Aviso para los clientes de California - Proposición 65 de California ADVERTENCIA: Este producto contiene una sustancia
química conocida en el estado de California por causar cáncer y daños reproductivos o del desarrollo.
ADVERTENCIA PARA EL USO DE MEDICAMENTOS
Seguir siempre las indicaciones del médico para el tipo de fármacos a utilizar, el dosis, la frecuencia y la duración
de las inhalaciones. El 6700 ha sido diseñado para funcionar con soluciones medicinales acuosas; medicamentos
demasiado densos u oleosos no serán nebulizado correctamente. En el caso que el medicamento no sea nebulizado
correctamente, ensayar de diluir con cantidad igual de solución fisiológica para inhalaciones (ejemplo: adjuntar a 2
ml. de fármaco oleoso otros 2 ml. de solución fisiológica).
7..
Page 28

MONTAJE
ADVERTENCIA: Antes de usar este aparato, asegure que está limpiado
como describido in MANTENIMIENTO/Limpiando el Nebulizador
Después de Cada Uso.
1. Remueve la tapa y cámara de nebulización del aparato.
2. Apreté simultáneamente el botón en cada lado de la cámara de flujo de aire
para remover la cámara de flujo de aire y taza de medicamento del aparato.
3. Llene la reserva del aparato con agua destilada hasta que llegue al nivel
marcado en rojo (aproximadamente 4 ml). La cantidad de agua sirve como un
conductor de líquido para conducir las ondas ultrasonidos del medicamento y
nunca será nebulizado.
s AVISO: El nebulizador no operará correctamente si la reserva no está llenada con
agua.
8 6700-INS-LAB-RevC14
8
Page 29

4. Cuidadosamente inserta la taza de medicamento como mostrado, tiendo
cuidado no dañarlo.
5. Ponga el medicamento de la receta de su médico en la taza de medicamento. Si
la dosis inhalada es más que la capacidad máximo de la taza de medicamento,
divídelo en inhalaciones separados.
6. Ponga de nuevo la cámara de flujo de aire, la cámara de nebulización, y la
tapa del aparato. Aplique presión suficiente cuando instalando de nuevo para
asegurar que están puestos seguramente.
7. Inserta la boquilla o uno de las dos máscaras provistas en la parte del frente del
aparato como mostrado.
6700-INS-LAB-RevC14 9..
9..
Page 30

MÉTODO DE PRENDIENDO
Después de preparación, conecte el aparato a electricidad. El 6700 puede
ser usado en varias maneras:
Con el Adaptador de AC/DC
Esto es diseñado para uso en la casa o donde haya voltaje normal de
suministro de electricidad (diferentes conexiones son disponible para
voltajes diferentes).
1. Asegure que el botón de ON/OFF esté en la posición OFF. Asegure
que el voltaje en el enchufe es el mismo que del transformador, como
indicado en el placa de especificaciones del transformador. Enchufe el
cordón eléctrico en una toma eléctrica apropiada.
2. Inserte el enchufe del transformador en el enchufe del aparato.
Con el Adaptador de Carro (Encendedor de Cigarillo) Opcional
Este método permite que el paciente puede tomar las inhalaciones cuando
viajando en el carro.
1. Asegure que el enchufe del encendedor de cigarrillo en el carro
diseñado para dar electricidad de 15 vatios. Inserte el adaptador de
carro en el enchufe del carro.
2. Inserte el clavija del adaptador de carro en el aparato.
Enchufe del
Encendedor
de Cigarrillo
ADVERTENCIA: Nunca utilice este aparato cuando manejando.
10 6700-INS-LAB-RevC14
10
Page 31

Con la Batería Recargable Opcional
Uno puede usar la batería recargable
opcional de Ni-MH para electricidad. Provee
aproximadamente 80 minutos de operación.
Para instalar la batería: Ajuste la parte de
abajo del aparato a la parte de arriba de la
batería como mostrado a la derecha. Deslice
hasta que oyes un “clic” que está puesto.
Para remover la batería: Apreté hacia abajo en
la cerradura de la batería como mostrado a la
derecha y deslice la batería hasta que no hasta
abajo del aparato.
Para recargar la batería: Inserte el adaptador
de AC/DC en el enchufe del aparato. Una
luz anaranjado indica que la batería se está
cargando. La luz anaranjado se apagará
cuando se ha terminado de cargar. El tiempo
mínimo de recargar es 5-6 horas.
Info: La primera carga de la batería recargable
nueve necesita por lo menos 8 horas.
Instalación de la Batería Retiro de la Batería
Recargo de la Batería
6700-INS-LAB-RevC14 11..
11..
Page 32

OPERACIÓN
Guarde este aparato y sus accesorios en un sitio seco. Evite exposición a luz del sol, humedad alta y polvo.
s AVISO: El ambiente donde se va operar este aparato tiene que estar 50°F y 104°F (10°C y 40°C) y 10% - 95% RH.
ADVERTENCIA: Asegure que el aparato está limpiado como describido in MANTENIMIENTO/Limpiando el Nebulizador
Después de Cada Uso.
ADVERTENCIA: Asegure que el aparato está preparado para operación como describido en MONTAJE.
ADVERTENCIA: Asegure que el enchufe eléctrico es suficiente, como describido en MÉTODO DE PRENDER, y que está
conectando seguramente.
Terapia de Inhalación
1. Apreté el botón de ON/OFF para prender el aparato. La luz verde de prender se
iluminará.
s AVISO: Una luz que se alterna de rojo a verde indica una condición de batería baja;
el aparato sigue a funcionar normalmente por poco tiempo, pero la batería debe ser
cargado lo más antes posible.
Luz de Indicador
de Electricidad
s AVISO: Una luz roja indica una condición de batería baja, el aparato se apagara
automáticamente.
ADVERTENCIA: Para que el aparato funcione correctamente y da efectividad máximo
de tratamiento, siempre guarde el aparato en una posición vertical durante terapia
de inhalación.
2. Inhale el medicación como en la receta de su médico.
Info: Algunas medicaciones tendrán velocidad de nebulización diferente que otros.
90°
12 6700-INS-LAB-RevC14
12
Page 33

3. La tasa de flujo de aerosol puede ser regulado por ajustando el control de la tasa de
flujo como mostrado a la derecha. La práctica recomendada, especialmente para niños,
es ponerlo en la posición “Mínimo” para promover el depósito de aerosol nebulizado
directamente a los respiraderos.
4. Una vez que la neblina generado no es constante, indica que el medicamento en la taza
de medicamento se ha cavado; pero continúe la terapia hasta que el aparato se apaga
automaticamente.
Info: El aparato se apagará automaticamente cuando la taza de medicamento está casi vacía. Al
final de la terapia de inhalación, una cantidad del medicamento (como 0.4ml) puede quedarse
en la taza de medicamento; esto es normal. La porción de medicamento, que se llama “volumen
residual”, que no puede ser nebulizado.
5. Desconecte el enchufe de la toma.
Sistema de Válvula Automático
La boquilla del aparato tiene un sistema de dos-válvula para asegurar efectividad
máximo de la inhalación y para reducir el gasto de medicamento. En práctica, la válvula
de expiración de la boquilla puede ser abierta durante caducidad, permitiendo que el
aire espirado del paciente al atmosfera, evitando la contaminación del aparato. Durante
inspiración, la válvula se cierre, maximizando la cantidad de inhalación.
MANTENIMIENTO
ADVERTENCIA: Para reducir el riesgo de quemadura, electrocución, fuego, o herida personal:
1. Peligro de descarga eléctrica – no debe remover el estuche del aparato. Todo el
mantenimiento del aparato debe ser hecho por un técnico de servicio cualificado. Refiere
servicio a una persona de servicio cualificado.
2. Este aparato no requiere aceite. No intente lubricar los partes internas.
3. Desenchufe el aparato antes de limpiar. No debe sumergir en agua para limpiar.
6700-INS-LAB-RevC14 13..
13..
Page 34

Limpiando el Nebulizador Después de Cada Uso
ADVERTENCIA: Para prevenir el riesgo posible de infección de
medicamento contaminado, limpie el nebulizador después de cada
tratamiento.
1. Asegure que el enchufe está desconectado del enchufe del aparato.
2. Remueve la tapa, la cámara de nebulización, cámara del flujo de aire
y taza de medicamento del aparato. Remueve el filtro de la tapa.
3. Vacié completamente el líquido de conducción en la reserva.
4. Seque el aparato con una toalla limpia y suave.
5. Lave la tapa, la cámara de nebulización, cámara del flujo de aire,
boquilla, máscara y taza de medicamento en agua de jabón tibia
y enjuague. Permita que se seca al aire. Guarde en un sitio seco y
limpio.
Limpiando el Nebulizador Al Diario
ADVERTENCIA: Para prevenir el riesgo posible de infección de
medicamento contaminado, limpie el nebulizador diario siguiendo lo
siguiente.
Una vez al día, después de lavar, deja remojar los componentes limpios
en una solución fresco consistiendo de una parte de agua tibia y una
parte vinagre por 30 minutos y un enjuague de agua tibia. Permita secar
al aire libre. Guarde en un sitio seco y limpio.
Reemplazando la Taza de Medicamento
Reemplace la taza de medicamento después de cada dos tratamientos.
Hay un paquete de 20 tazas de medicamento de reemplazo que viene
con cada nebulizador. Las tazas de medicamento adicional pueden ser
compradas por GF Health Products, Inc.
14 6700-INS-LAB-RevC14
14
20 Tazas de
Medicamento
de Reemplazo
Page 35

Reemplazando el Filtro de Aire
El filtro de aire previene que substancias entran la neblina generado entra por el aparato.
Reemplace el filtro de aire después de 20 horas de uso, o cuando el filtro cambia al
color gris. El nebulizador viene con un paquete de 5 filtros de aire de reemplazo. Filtros
adicionales pueden ser comprados por GF Health Products, Inc.
1. Remueve la tapa del aparato.
2. Levante el aguante de filtro de aire de la tapa.
3. Remueve el filtro del aguante de filtro.
4. Inserte un nuevo filtro en el aguante de filtro.
5. Reemplace el aguante de filtro en la tapa.
s AVISO:
s AVISO: No se lave o limpie el filtro.
s AVISO: No use de nuevo un filtro sucio. No debe operar el nebulizador sin un filtro; daño al nebulizador puede resultar.
6700-INS-LAB-RevC14 15..
No utilice algodón o otro material como el filtro.
Page 36

ALMACENAJE
Guarde el aparato y accesorios secos. Evite exposición
a luz del sol, humedad alta y polvo.
s AVISO:
s AVISO: El ambiente de almacenaje de la batería tiene
s AVISO: El ambiente de almacenaje del aparato tiene
Si el aparato va ser guardado por un periodo
de tiempo largo, remueve la batería.
que estar dentro de -4°F y 95°F (-20°C y 35°C).
que estar dentro de -13°F y 140°F (-25°C y 60°C) y
10% - 95% RH.
PRECAUCIONES DE SEGURIDAD
El nebulizador es equipado con protección sobre
situaciones inseguros. Si estas situaciones ocurren,
el nebulizador se va 1) no prenderse 2) apagar
automáticamente, como describido abajo.
Protección sobre Suministro de Electricidad
Incorrecto
Si el adaptador de AC/DC es usado para prender el
aparato, asegure que el adaptador sigue las regulaciones
de electricidad local; si no, el aparato puede no
prenderse (la Luz de Indicación de Electricidad no se
iluminará).
Protección Sobre Recalentarse
Durante operación normal, la Luz de Indicación
de Electricidad se iluminará VERDE. Pero, in los
siguientes dos casos, el aparato puede indicar
irregularidad en apagándose automáticamente. El
aparato requiere aproximadamente 45 minutos de
descanso antes de prender de nuevo.
Protección Sobre Usar en Exceso: Los componentes
electrónicos del aparato son diseñados de funcionar
continuamente 40 minutos. Operando el aparato por
más de este tiempo puede provocar el mecanismo
de protección de calor y el aparato se apaga
automáticamente.
Falta de Líquido en la Reserva: En el evento que falta
o hay insuficiente líquido en la reserva, el aparato se
apagará automáticamente.
16 6700-INS-LAB-RevC14
Page 37

6700 ESPECIFICACIONES TÉCNICAS
- Suministro de Electricidad; Adaptador AC/DC
(240V, 50Hz; 12VDC 800mA)
- Consumación de Electricidad ~ 12W (12V/1A)
- Nivel de Sonido: < 40dB
- Frecuencia: 2.8 Mhz
- Gama de Partícula: 0.5 ~ 4.0 micrómetros
- Capacidad de Taza de Medicamento: 5 ml máximo
- Promedio de Gama: 0.55 ~ 0.80 ml/min.
(NaCl0.9% Normal Salino)
- Uso Intermitente Utilice:
45 min. Prendido / 45 min. Apagado
- Condiciones de Operaciones:
50°F ~ 104°F (10°C ~ 40°C), RH 10% ~ 95%
- Condiciones de Almacenaje:
-13°F ~ 140°F (25°C ~ 60°C), RH 10% ~ 95%
- Dimensiones:
~ 5.1 x 3.3 x 2.2 in. (~ 130 x 85 x 55 mm)
- Peso sin las baterías: ~ .44 lb (198 g)
ACCESORIOS INCLUIDOS
- 1 Máscara Pediátrica
- 1 Máscara de Adulto
- 1 Boquilla
- 1 Bolsa de Cargar
- 5 Filtros de Aire Adicionales
- 20 Taza de Medicamento
- 1 Adaptador AC/DC
- Opcional: 1 Batería Recargable
- Opcional: 1 Car Adaptador
6700-INS-LAB-RevC14 17..
Page 38

GARANTÍA LIMITADA
Ambito de Garantía
GF Health Products, Inc. (“GF”) garantía al comprador
original o arrendatario (cual quiera es el “Cliente”)
solamente, que va reemplazar o reparar componentes,
a la decisión solo de GF, cuales son defectos en material
o calidad utilizado normalmente y con servicio. La
garantía es para partes solamente y no incluye fuerza
laboral o el costo de transporte. Esta garantía limitada
no es transferible. Todas garantías son condicionales
por el uso apropiado del producto estrictamente
en acuerdo con las instrucciones aplicables de GF,
incluyendo uso apropiado y mantenimiento. Producto
próvido a usuarios como réntales deben ser serviciados
por el proveedor y inspeccionado antes de entrega.
Cada usuario debe ser preparado en la operación
y seguridad de este producto antes de uso. Dentro
las reglas normales en este documento, el John
Bunn JB0112-061 Compresor Nebulizador y sus
componentes a estar libre de defectos de materiales o
mano de obra según listado debajo:
Nebulizador Portable Ultrasonido: un año
Esta garantía no incluye baterías.
El período de garantía se comienza en la fecha de
transporte al Cliente a menos que haya una fecha
de expiración en el componente en cual la garantía
se expira en la período de garantía o la fecha de
expiración.
Obteniendo Servicio de Garantía
La garantía limitada solamente aplica a defectos
reportados al Distribuidor de quien el Cliente compro
el producto y quienes ellos en turno notifica al equipo
de Servicio de Cliente de GF dentro el período de
garantía aplicable en cuanto será examinado por GF o
un representante autorizado para probar el producto
sobre garantía. Falta de seguir las instrucciones
específicas resultara en rechazo de la reclamación de
garantía. Si no hay Distribuidor, debes llamar a GF
directamente llamando 678-291-3207, mandando
fax a 770-368-2386 o por email a cs@grahamfield.
com. Direcciones específicos se van a dar por el
Representante de Servicio De Clientes.
Exclusiones
La garantía no cubre ni GF será responsable de lo
siguiente: 1) Defectos, daño u otras condiciones
causadas por mal uso, abuso, negligencia, alteración,
accidente, daño de transporte o falta de buscar
y obtener reparo o repuesto en manera puntual;
2) Productos con los números seriales quitados o
desfigurados; 3) Productos no instalados, utilizados
or mantenidos correctamente como requerido en
los manuales oficiales del Producto; y 4) Cargos
de Labor o transporte incurridos en el regreso o
instalación de partes de reemplace; 5) Productos
considerados ser consumibles como dicho en este
documento; 6) Asesorías o partes no próvidos por
GF; 7) Cargas por otras personas por ajustamientos,
18 6700-INS-LAB-RevC14
Page 39

reparaciones, partes de reemplace, instalación u
otro trabajo hecho en conexión con este Producto no
autorizado en escrito en avanza por GF; 8) Cargas
y gastos de mantenimiento y limpieza regular; y
9) Representaciones y garantías hechos por otras
personas o entidades no GF.
Garantía Entero, Remedio Exclusivo y Descargo
de Daños Consiguiente
ESTA GARANTIA ES LA UNICA GARANTIA DE GF Y ES
EN VEZ DE TODAS OTRAS GARANTIAS, EXPRESIDAS
O IMPLICITADAS. GF NO HACE GARANTIAS
ENTENTIDOS DE CUAL QUIER MANERA INCLUYENDO
GARANTIAS ENTENDIDOS DE COMERCIALIZACION
O IDONEIDAD PARA UN PROPOSITO PARTICULAR.
SI UN MODELO Y MUESTRA SE LE ENSENO A UN
CLIENTE, TAL MODELO O MUESTRA FUE UTILIZADO
SOLAMENTE PARA ILUSTRAR EL TIPO GENERAL Y
CALIDAD DEL PRODUCTO Y NO PARA REPRESENTAR
QUE EL PRODUCTO SE CONFORME CON ESE
MODELO Y MUESTRA EN TODOS RESPETOS.
TODA RESPONSABILIDAD DE GF POR CUAL QUIER
PRODUCTO Y SERVICIO PROVIDO ES LIMITADA AL
COSTO DEL PRODUCTO EN LA RECLAMACION. EN
NO EVENTO EN CONTRACTO, INDEMNIZACION,
GARANTIA, NEGLIGENCIA, RESPONSABILIDAD
ESTRICTA O OTRO QUE GF SERA RESPONSABLE POR
CUAL QUIER DANO DIRECTO, ESPECIAL, INDIRECTO,
INCIDENTAL, EJEMPLAR O CONSIGUIENTES
INCLUYENDO PERO NO LIMITADO A: DANOS FOR
PERDIDA DE BENEFICIO O GANANCIA, PERDIDA
DE USO, INACTIVO, O SALARIOS DE EMPLEADOS
O TRABAJADORES CONTRACTORES, PAGOS O
BENEFICIOS.
Esta garantía te da derechos legales específicos.
Podrás tener derechos adicionales que varían estado
por estado.
6700-INS-LAB-RevC14 19..
Page 40

Fabricado para:
GF Health Products, Inc.
2935 Northeast Parkway
Atlanta, Georgia 30360 USA
Telephone: 770-368-4700
Fax: 770-368-2386
www.grahamfield.com
Fabricado en China
 Loading...
Loading...