
Luna-FL™ Automated Cell Counter
User Manual
Catalog No. : L20001
Document No. : L24001
Revision Date : July 15, 2013
Version : 1.0.0
1

IMPORTANT
Luna-FL™ Automated Cell Counter is a Laboratory Electrical Instrument for
Scientific Research Use Only. It is NOT A MEDICAL or IN VITRO DIAGNOTICS
DEVICE.
Table of Contents
Safety Information -------------------------------------------------------------------------------------------- 4
General Guidelines ------------------------------------------------------------------------------------------- 6
Environment Conditions ------------------------------------------------------------------------------------- 7
Chapter 1 – Introduction --------------------------------------------------------------------------- 8
1.1 Product Overview ------------------------------------------------------------------------------------- 8
1.2 Product Contents -------------------------------------------------------------------------------------- 11
1.3 Product Specifications ------------------------------------------------------------------------------- 12
1.4 Product Description ----------------------------------------------------------------------------------- 13
Chapter 2 – Setting up ------------------------------------------------------------------------------- 16
2.1 Installation ---------------------------------------------------------------------------------------------- 16
2.2 Start-Up Screen --------------------------------------------------------------------------------------- 17
2.3 Settings -------------------------------------------------------------------------------------------------- 19
2.4 Setting the Date and Time -------------------------------------------------------------------------- 20
2.5 Save Options ------------------------------------------------------------------------------------------ 20
2.6 Counting Options ------------------------------------------------------------------------------------- 21
2.7 Result/Printer/Calculator Options ----------------------------------------------------------------- 23
Chapter 3 – Bright Field Counting Cells -------------------------------------------------- 24
3.1 Sample Preparation ---------------------------------------------------------------------------------- 24
3.2 Loading Samples into Slides ----------------------------------------------------------------------- 24
3.3 Counting Cells ----------------------------------------------------------------------------------------- 25
3.4 Using the “Tag” Function ---------------------------------------------------------------------------- 29
3.5 Cell Size and Number Distribution ---------------------------------------------------------------- 30
3.6 Using the Dilution Calculator ----------------------------------------------------------------------- 34
3.7 Saving Images and Generating a PDF Report of the Current Count--------------------- 36
3.8 Printing the Counting Results ---------------------------------------------------------------------- 38
2

Chapter 4 – Fluorescence Cell Counting ------------------------------------------------- 41
4.1 Sample Preparation ---------------------------------------------------------------------------------- 41
4.2 Loading Samples into Slides ----------------------------------------------------------------------- 41
4.3 Counting Cells ----------------------------------------------------------------------------------------- 42
4.4 Using the “Tag” Function ---------------------------------------------------------------------------- 48
4.5 Cell Size and Number Distribution --------------------------------------------------------------- 50
4.6 Using the Dilution Calculator ----------------------------------------------------------------------- 54
4.7 Saving Images and Generating a PDF Report of the Current Count --------------------
4.8 Printing the Counting Results ----------------------------------------------------------------------
Chapter 5 – Reviewing Saved Count Results -----------------------------------------
5.1 Importing Previous Counts ------------------------------------------------------------------------- 59
5.2 Reviewing Previous results ------------------------------------------------------------------------ 60
5.3 “Previous Count” button ----------------------------------------------------------------------------- 61
56
58
59
Chapter 6 – Setting Up the Protocol for Counting --------------------------------- 63
6.1 Adjusting Parameters for Bright Field Counting ---------------------------------------------- 63
6.2 Adjusting Parameters for Fluorescence Counting --------------------------------------------
6.3 Managing Protocols ----------------------------------------------------------------------------------
Chapter 7 – Maintenance and Troubleshooting -----------------------------------
7.1 Cleaning ------------------------------------------------------------------------------------------------- 67
7.2 Changing the Battery --------------------------------------------------------------------------------- 68
7.3 Calibrating the Counter ------------------------------------------------------------------------------ 69
65
66
67
7.4 Updating Firmware -----------------------------------------------------------------------------------
7.5 Troubleshooting ---------------------------------------------------------------------------------------
Chapter 8 – Ordering Information ------------------------------------------------------------
3
75
76
79

Safety Information
For the best results, users of the Luna-FL™ automated cell counter (or, hereinafter “Luna-FL™”)
must follow the instructions below in addition to the general precautions for using electrical
instruments.
1. Users must be careful to avoid electric shock while operating the instrument. Do not touch it or
its components with wet hands. Do not place it in a humid environment such as an incubator.
For operating environment, see page 7.
2. Trypan blue stain, acridine orange, propidium iodide, and other reagents are known as
hazardous materials. While handling the solution, always wear gloves to avoid exposure.
3. Before use, make sure that the input voltage is compatible with the instrument’s power supply
voltage.
4. For optimal operations, place the instrument on a flat bench and avoid any vibration.
5. Turn on the instrument only after connecting both ends of its power cord to the wall outlet as
well as the instrument. Always turn off the instrument before disconnecting the power cord
and/or moving the instrument.
6. Ensure that the power cord is firmly plugged into the power inlet, the wall outlet and AC adapter.
7. When the instrument is operating for a long time, its temperature can become too high. Please
be careful that the instrument’s temperature does not become too high during long and
continuous operation times. When operating, leave enough space around the instrument so
there is enough room for air circulation and cooling.
8. Do not disassemble the instrument in any event. If the instrument is out of order or dropped or
broken, please contact a service person. Disassembling the instrument invalidates its
warranty.
9. Use only authorized components (adaptor, power cord, and USB drive).
10. If the instrument emits smoke, disconnect the power cord immediately from the wall outlet and
contact a service person.
11. Used counting slides must be disposed as biohazard waste.
4

<Symbols used in this User Manual>
The WEEE (Waste Electrical and Electronic Equipment) symbol indicates that
users of this instrument have the responsibility of returning and disposing of
WEEE in an ecologically friendly manner. Follow waste ordinances of your region
for proper disposal provisions.
The CE mark indicates that this instrument conforms to all applicable European
Community provisions for which this marking is required. Users must be aware of
and follow the conditions described in this manual for operating the instrument.
The protection provided by the instrument may be impaired if the instrument is
used in a manner not specified by this manual.
Protective earth (Ground)
5

General Guidelines for Using the Luna-FL™ Automated Cell Counter
In order to achieve the best results with the Luna-FL™ automated cell counter, users must follow
the instructions below carefully.
1. The instrument must be operated in compliance with the environmental conditions described on
page 7. In particular, the temperature and humidity conditions are important.
2. Samples must be handled in an appropriate way, depending on user’s requirements.
3. Hold the Luna-FL™ cell counting chamber slide by the edges to avoid touching its optical
surface. Make sure that no damage or contamination occurs on the optical surfaces of the slide.
4. After mixing the cell sample with the supplied reagents, perform cell counting within 1-3
minutes for accurate cell viability measurements. Optimally, count your sample at least twice
(duplicate readings) to obtain better results.
5. Since the Luna-FL™ is calibrated before shipping, users generally do not need to re-calibrate
before use. However, if re-calibration is needed, for example due to long-term use, please refer
to Section 7.3 Calibrating the Counter.
6. Do not touch trypan blue solution and other reagents with bare hands, as they are hazardous
chemicals. After using chamber slides, dispose of them as hazardous waste. Do not reuse the
slides.
6

Environmental Conditions
Operating Power 100~240 VAC, 1.5A
Frequency 50/60 Hz
Electrical Input 12 VDC, 3.5A
Installation Site Indoor use only
Operating Temperature
Maximum Relative Humidity 20~80%
Altitude
Pollution Degree 2
10~35℃
≤2,000 m
7

Chapter 1 – Introduction
1.1 Product Overview
The Luna-FL™ automated cell counter is a small, fast, and affordable image-based cell counting
device, containing bright field and dual fluorescence optics, that automatically counts various kinds
of cells for research use.
The Luna-FL™ helps measure the number as well as the viability of cells (live, dead, total cells) with
sophisticated optical components and advanced image analysis algorithms. Due to the innovations
introduced by Logos Biosystems, the Luna-FL™ provides a state of the art cell counting device and
eliminates the tedium and subjectivity of manual cell counting.
The Luna-FL™ can be used in a very simple procedure. For example, in order to accomplish dual
fluorescence counting, first, mix 18 l of the cell sample with 2 l of the AO/PI (Acridine Orange and
Propidium Iodide) from the cell viability kit (in case that there is not enough cell sample, 9 l of the
cell sample with 1 l of the AO/PI solution can be used to load the total 10 l in the counting
chamber). Second, load 10-12 l of the mixed cell suspension into the Luna-FL™ cell counting slide.
Third, insert the slide into the slide port of the instrument and adjust the focus knob to get an
appropriate cell image. Last, press the “Count” button and the results of cell count and viability will
be displayed on the screen. The counting image can be downloaded onto a USB drive in a TIF
format for future analysis.
<AO/PI Stained Cell Image>
Assay principle: AO stains all cells, and PI stains dead cells only. If AO and PI exist together, AO
fluorescence is mostly quenched by PI. Therefore, AO fluorescence (green) is found in live cells
only, and PI fluorescence (red) is found in dead cells only. Cell viability analysis using AO/PI double
staining is a proven and accepted method.
8

The Luna-FL™ automated cell counter provides the data below.
Number of live and dead cells and their concentrations
Number of total cells and their concentrations
Viability percentage (% live cells to total cells)
Cell images (showing live cells as green circles and dead cells as red circles)
Histogram of cell size distribution and size gating
The Luna-L™ cell counting chamber slide is disposable and specifically designed for the Luna-FL™.
Each counting slide has two chambers, labeled A and B, one slide can be used for the same sample
reading in duplicate or it can be used for two different samples if preferred.
Key features of the Luna-FL™ are as follows:
Key features Description
Bright field & dual fluorescence
optics
Small footprint
Accuracy & precision
Easy-to-operate user interface
Shortest time-to-results
The Luna-FL™ integrates bright field, as well as dual
fluorescence optical components to provide advanced cell
counting functionalities. The cell image acquired from each
channel (bright, green, and red) can be merged directly on the
screen. The brightness of each color can be adjusted
independently for accurate monitoring.
Due to its minimal size (22 cm x 21 x 9 cm) & weight (1.8 kg),
it can be used on a clean bench or stored in a biosafety
cabinet for convenience.
Due to its sophisticated optical components and counting
algorithm, Luna-FL™ cell counter provides reproducible
results every time.
The intuitive user interface based on a touch screen enables
simple and easy operation.
Results for most cell lines are available within 10-30 seconds
of pressing the ‘Count’ button.
Innovative counting slide
Luna-FL™ cell counter adopts an innovative counting slide
made with “T-bond” technology without using hazardous
organic solvents.
9

Measurements can be made for cells at concentrations within
Cell concentration & viability range
Cell size gating
Easy-to-use dilution calculator
Set up & maintenance
Counting image transfer
Individual protocol saving
Automatic reporting
the 1x104 to 1x107 (preferably 5x104 to 1x107) cells/ml range
and for cells within the 1-90m (preferably, 5-60m) diameter
range.
After counting, users can set the gating parameter for cell
size.
Dilution concentration can easily be calculated using the built-
in calculator.
Just plug in and it is ready for use, with virtually no
maintenance time or costs.
The counting image can be downloaded onto a USB drive in
TIF (Tag Image File) format for later use or review.
Each user can set or adjust counting parameters and save the
counting protocol for later use.
Luna-FL™ cell counter provides a counting report based on
the count results in PDF (Portable Document Format)
immediately after performing a cell count.
10

1.2 Product Contents
The Luna-FL™ automated cell counter includes following components.
Component Quantity
Luna-FL™ automated cell counter 1 unit
Power cord (including an adapter) 1 unit
Luna™ cell counting chamber slides 1 box of 50 slides
AO/PI cell viability kit 2 X 0.5 ml
Trypan blue stain (0.4 %) 2 x 1 ml
Luna-FL™ calibration beads 0.5 X 1 ml
Luna™ USB drive (4GB) 1 unit
When you receive the product package, please check that all the components listed above are
included and that no damage has occurred during transit. The warranty does not cover damage that
may occur during the shipping and handling process. Any damage claims must be filed with the
carrier.
11

1.3 Product Specifications
1.3.1. Luna-FL™ cell counter specifications
Instrument Type Bench-top cell counter
Dimensions (WxDxH) 22 cm x 21 cm X 9 cm
Weight 1.8 kg (without the power cord/adapter)
Light Source LED
Excitation Wave Length 470 +/- 20 nm (Blue)
Emission Wave Length 525 +/- 25 nm (Green), 600 LP (Red)
Cell Concentration Range 1x104 ~ 1x107 (optimally 5x104 ~ 1x107) cells/ml
Cell Diameter Range
Cell Circularity Range 30~100%
Cell Viability Range 0~100%
Image Resolution CMOS camera (5 MP)
Image Type TIF
Reporting PDF report, .CSV file
LCD Display 7 inches (800 x 480 pixels)
Processing Time Approx. 7 seconds for bright field & approx. 30 seconds for
1.3.2 Luna-FL™ cell counter counting slide specifications
Dimensions (WxDxH) 25 mm X 75 mm X 2.4 mm
Chamber Depth
Chamber Volume
1~90 (optimally 5~60) m
dual fluorescence (may depend on cell type and
concentration)
100 m
10 l
1.3.3 Luna-FL™ software
Cell Counting Bright field cell counting
Dual fluorescence cell counting
12

1.4 Product Description
1.4.1 Front view of the Luna-FL™ automated cell counter
The front view of the Luna-FL™ automated cell counter shows a wide touch screen. This interface
contains buttons for all functions and displays results.
<Front View>
1.4.2 Rear view of the Luna-FL™ automated cell counter
The rear view of the Luna-FL™ automated cell counter shows a power button to turn on or off the
instrument and a power inlet. Connect the instrument to an electrical outlet with the power cord and
plug provided in the product package. Be sure to check the electrical outlet configuration in your
country.
<Rear View>
13

1.4.3 Right side view of the Luna-FL™ automated cell counter
The right side view of the Luna™ automated cell counter shows a focus knob and a slide port. Use
the focus knob to get optimized cell images by adjusting contrast between live cells with bright
centers and dead cells with dark centers. The slide port is used to insert the counting chamber slide
loaded with the sample into the instrument.
<Right Side View>
Focus knob
Counting slide port
1.4.4 Left side view of the Luna™ automated cell counter
On the left side of the cell counter, there is a USB drive port into which a USB drive is inserted for
transferring and saving data. One USB drive is supplied in the product package. Any standard USB
drive can be used.
<Left Side View>
USB drive port
14

1.4.5 Luna-FL™ cell counting chamber slide
The plastic disposable cell counting chamber slide consists of two chambers, labeled as A and B,
that can be used for the same sample as duplicates or for two different samples. The depth of the
counting chamber is 100 m. The counted cell volume is about 0.5 l, almost the same as counting
five (1 mm x 1 mm) squares in a standard hemocytometer.
Sample loading chamber (marked as A and B)
Note: 0.4% trypan blue solution is blue and the AO/PI solution is pink (or orange).
Sample loading port
15

Chapter 2 – Setting up
2.1 Installation
2.1.1 Upon receiving the product package, unpack it carefully and ensure that every component is
included and no damage has occurred.
2.1.2 Place the Luna-FL™ automated cell counter on a flat, stable surface.
2.1.3. Insert one end of the power cord into the instrument and plug the other end of the power cord
into an electrical outlet after checking the outlet configuration in your local area.
2.1.4 Turn on the instrument using the power button located on the back of the cell counter.
2.1.5 The Start-Up screen will display on the touch screen as shown below in 2.2.
16

2.2 Start-Up Screen
2.2.1 Home Screen
When the Luna-FL™ is turned on, the Start-Up screen will display two options: one for the Bright
Field Cell Counting and the other for Dual Fluorescence Cell Counting. Users can choose one of
them.
<Start-Up Screen of the Luna-FL™ Automated Cell Counter>
2.2.2 Bright Field Counting Screen
17

2.2.3 Fluorescence Counting Screen
2.2.3.1 Green Fluorescence Counting Screen
2.2.3.2 Red Fluorescence Counting Screen
18

2.3 Settings
Generally, users do not need to change the “Settings” of the instrument since they are preset at the
time of manufacture. If users need to reset the date, time, or other options, users can adjust or
change the options/parameters from the “Settings” menu described below.
2.3.1 After turning on the instrument, go to one of the counting screen options.
2.3.2 Press the “Settings” button ( ) located on the upper right corner of the counting screen. Then,
the following “Settings” screen appears. From here, users can change or adjust various preferences
or options.
<Settings>
2.3.3 The “Settings” menu allows you to perform the following:
Background calibration of the instrument (described in Section 7.3 Calibrating the Counter)
Firmware update for the installation of new versions of the firmware that will be released
from time to time (described in Section 7.4 Updating the Firmware)
Date and time set up
Other options such as “Save”, “Counting”, and “Result/Printer/Calculator”
19

2.4 Setting the Date and Time
2.4.1 On the “Settings” screen, select the date and time buttons.
2.4.2 After selecting the field, erase the number using the “Backspace” button ( ), set the date or
time using the number buttons, and press the “Apply” button ( ) to save the changes. .
2.5 Save Options
2.5.1 The “Save Options” screen will be seen as below, after selecting the “Save Options” button.
As a default, only the “Analyzed Image” button is activated (On), while the “Raw Image” and “Report”
button are inactivated (Off).
<Save Options>
2.5.2 In the “Default Image Save” menu, users can choose their options to save the raw image,
PDF report, or both. Each button can be switched on and off.
Saved Items Description
Analyzed image The image contains the counting results and the tagging of live and
dead cells.
Raw image The image contains only the captured image for counting. In the
bright field cell counting mode, the bright field image will be
20

displayed.. In the fluorescence cell counting,, bright field, green,
and red images will be displayed.
Report The PDF report containing counting results and histograms
2.5.3 The “Auto-header” which is red-lined in the above “Save Options” image is the newly created
folder name or file name that all the images start with during the saving process. After saving, users
can see a folder or a file name, containing the saved item, which begins with “Auto-header” in the
root directory of the USB drive. In case users want to have another default name, the “Auto-header”
can be customized by editing the default value of “Logos Biosystems-”.
2.6 Counting Options
2.6.1 The “Counting Options” applies only to the bright field cell counting. Before selecting an option,
please make sure that the “Bright Field Cell Counting” is selected from the options on the Home
Screen.
2.6.2 In the “Counting Options”, users can choose one of the trypan blue staining options as below.
<Staining Options>
Staining Options Description
With trypan blue (1:1) This option can be used for normal bright field counting, when cell
samples are mixed with trypan blue dyes in a 1:1 ratio. This option can
generate cell viability data.
21

Without trypan blue When samples do not contain trypan blue dye, turn on this button and
follow the directions in the message boxes. These instructions set the
“Dilution Factor”. For samples with trypan blue dye, the dilution factor must
be set to value “2”. For samples without trypan blue dye, the dilution factor
must be set to value “1”.
<Dilution Factor Check>
Custom staining In this option, cell samples can be stained with other dyes (or even the
culture media) for counting. Before choosing this option, the instrument
must be re-calibrated with users’ own stains or culture media. For re-
calibration, please refer to Section 7.3 Calibrating the Counter. When
using this option, make sure that the dilution is 1:1 (cell sample vs.
staining solution) and the “Dilution Factor” must be set to value 2.
22

2.7 Result/Printer/Calculator Options
2.7.1 This option shows three different menus, as below.
<Result/Printer/Calculator Options>
Option Description
Result The default value is “off.” When it is turned on, the counting result box
displays only the total cell number.
Dilution Calculator The default value is “Total Cells (on).” In order to use the “live cells” in
the Dilution Calculator, please turn the “live cells” on.
Printer When this button is on, the print out of the results has the details of the
parameters of counting.
23

Chapter 3 – Bright Field Cell Counting
3.1 Sample Preparation
3.1.1 Prepare the following materials in order to count cells.
Cell sample
Luna™ cell counting slides
Trypan blue
3.1.2 If desired, insert a USB drive to save data and results.
3.1.3 Mix 10 l of the cell sample with 10 l of trypan blue stain gently in a microfuge tube by
pipetting up and down.
Note: The cell counting parameters of the “Bright Field Cell Counting” mode of Luna-FLTM is
optimized with the use of trypan blue. Although the counting “Without Trypan Blue” option is
provided in the protocol menu (section 2.6.2) to meet the various needs of users, it is NOT
recommended to count cells without trypan blue staining. Low contrast due to the lack of the trypan
blue staining may sometimes cause abnormal results.
3.2 Loading Samples into Slides
3.2.1 Holding the edge of the slide, load 10-12 l of the mixed cell sample into the inlet of one
chamber of the counting slide. For easy and accurate loading, tilt the pipette around 45~60 degrees
as shown below.
<Sample Loading>
24

Note: Be careful not to over-load or under-load the sample into the chamber.
3.3 Counting Cells
3.3.1 Insert the loaded slide into the slide port of the instrument, ensuring that the loaded chamber
is inserted first. The instrument analyzes only the first inserted chamber. Gently insert the counting
slide to the end.
Note: After inserting the slide, Luna-FL™ only reads the first chamber. To read the second chamber,
it must be taken out, rotated, and inserted again. Make sure that the counting slide is not inserted
upside-down. This may lead to sample spilling and could severely damage the counter.
3.3.2 Select the “Bright Field Cell Counting” button on the home screen.
<Home Screen>
Note: After this step, the real image of the cell sample will be displayed on the screen. The cell
image can be navigated by touching the screen and moving the finger or stylus pen on the image.
Please note that the touch screen of the Luna-FL
TM
automated cell counter is a resistive touch
screen, which needs a bit of pressure in order to get a response.
25

3.3.3 The “Preview screen” will appear as shown in the image below. When necessary, adjust the
focus with the focus knob on the right side of the instrument to make sure that live cells have bright
centers and dark edges, and dead cells have uniform dark color all over them.
<Preview Screen>
Note: To get the best focus, the image can be magnified by using the“Zoom-in”button located under
the“Count”button. Initially, the “Zoom-in” button is set to a 1x image. When pressed once, a 2x
image appears. This 2x image may be the best zoom-in status for focusing. When pressed again, a
4x image is shown. Pressing the button again takes the screen back to a 1x image.
Note: The current staining option is displayed on the left side of the screen as marked by the red
box in the image above. The staining option can be changed in the settings menu (section 2.6.2).
Selecting the wrong staining option will result in an under-exposed preview image as shown below
in which the “Without Trypan Blue” option is selected while trypan blue is present.
26

<Wrong Staining Option Selected: Too Dark Preview Image>
Note: Default preview image is set to a grey scale. To change the preview images to a color mode,
press the “Color” button once, and the preview image will turn to a color image and the “Color
button” will change to blue. Regardless of which preview mode is selected, grey or color, grey
images will be used for calculating the cell number displayed in the cell counting result screen.
27
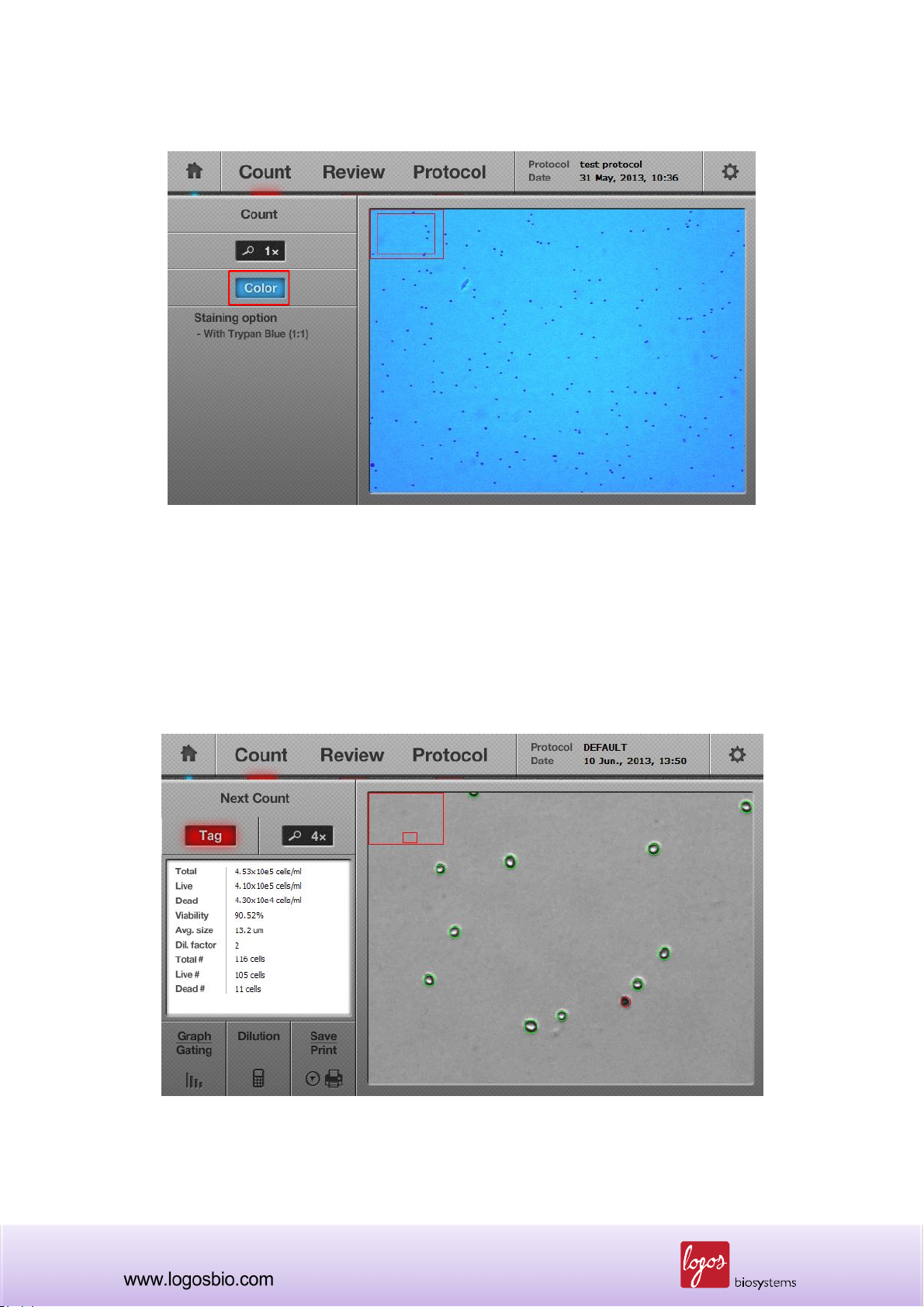
<Color Preview Screen>
3.3.4 Press the “Count” button, which is located on top of the “Zoom-in” button.
3.3.5 Within approximately 7 seconds (depending on cell concentration), the image of the cell
sample and data/results (Total, Live, and Dead cell concentrations, Viability, Avg. Cell Size, Actual
number of Total, Live and Dead cells) will be displayed on the screen.
<Cell Counting Results>
28

3.4 Using the “Tag” Function
3.4.1 After performing the counting function, the “Tag” button can be used to verify the counting
results.
<Tagged Cell Image>
Green circle – live cell Red circle – dead cell
Press
“Tag” button
Note: This “Tag” function is one of the distinct tools of the Luna-FL™ automated cell counter
because it allows the user to review data to determine the accuracy of the counting on-site without
depending on a computer.
3.4.2 When touching the “Tag” button, the image on the screen will show objects surrounded by
green or red circles. The green circles indicate live cells and the red circles indicate dead cells.
3.4.3 After reviewing the accuracy of the image analysis, “Tag” button can be pressed again to
remove the green and red circles.
29

3.5 Cell Size and Number Distribution
3.5.1 To obtain more data on the distribution of cell size and number in graphical representation,
press the “Graph/Gating” button on the lower left side of the screen. A histogram will appear with
more details on the size and number of the cell sample as shown below. Live cells are displayed as
a green bar and dead cells are displayed as a red bar.
<Graphical Representation of Cell Size Distribution>
Functional buttons in the menu
“Count Graph/Gating”
1) “Total, Live or Dead” button
2) “Cluster Map” button
3) “Cell number or /ml” button
* The above buttons can be used
in combination and the details are
described in next pages.
When the sizes of the cells are out of the range, they are displayed in grey color.
<Combination: Total and Cell Number>
Combination of “Total” and “Cell
number” shows the distribution of
cell size vs. cell number for total
cells.
Note: When the “Total” button is pressed, it toggles to the “Live” button, which shows only live cells
as shown below. When the button is pressed again, it toggles to the “Dead” button to show only
dead cells as in the next figure.
30

<Combination: Live Cells and Cell Number>
The combination of “Live” and
“Cell number” shows the
distribution of cell size vs. cell
number for only live cells.
<Combination: Dead Cells and Cell Number>
The combination of “Dead” and
“Cell number” shows the
distribution of cell size vs. cell
number for only dead cells.
Note: When the “Cell Number” button is pressed, it toggles to the “/ml” button which shows the cell
concentration (i.e., the Y axis of the histogram). When pressed again, it toggles to the “Cell Number”
button.
31

<Combination: Total Cells and Concentration>
The combination of “Total” vs.
“Cell concentration” shows the
distribution of cell size vs. cell
concentration for total cells.
<Combination: Live Cells and Concentration>
The combination of “Live” vs.
“Cell concentration” shows the
distribution of cell size vs. cell
concentration for only live cells.
<Combination: Dead Cells and Concentration>
The combination of “Dead” vs.
“Cell concentration” shows the
distribution of cell size vs. cell
concentration for dead cells.
32

3.5.1.1 Graphical representation of live and dead cells
When the “Total” button icon is clicked, the histogram is expressed as a “Stacked Column Chart.”
<Graphical Representation of Live and Dead Cells>
Live cells
Dead cells
3.5.2 “Cluster Map” button
3.5.2.1 When the “Cluster Map” button is pressed, the percentage distribution of the clustered cells
are displayed. “1-cell” means single cell and “2-cell” means 2 clustered cells as a unit. When the
“Cluster Map” button is on, the “Total” and “Cell Number” buttons become inactivated.
<Cluster map>
3.5.2.2 Press the “Graph/Gating” button again to go back to the previous screen.
33

3.6 Using the Dilution Calculator
3.6.1 The Luna-FL™ automated cell counter provides a built-in dilution calculator that helps easily
calculate adjustments to obtain a desired concentration.
<Built-in Dilution Calculator>
3.6.2 The dilution calculator initially shows the current concentration. Put the appropriate numbers
into the blanks of the “Desired Concentration” and “Final Volume” that you want to obtain.
Users can choose one of “Total”, “Live” or
“Dead” cells for calculating their dilution.
By clicking this button, the “Current
Concentration” displays the concentration of the
total, live or dead cells.
Before calculating the dilution factor, please
check this option to match your requirements.
34

Users can also calculate their custom dilution.
3.6.3 Press the “Calculate” button and then a dilution instruction will appear in the message box.
35
3.7 Saving Images and Generating a PDF Report of the Current Count
3.7.1 To save data and/or generate a report, insert a USB drive into the USB port on the left side of
the instrument.
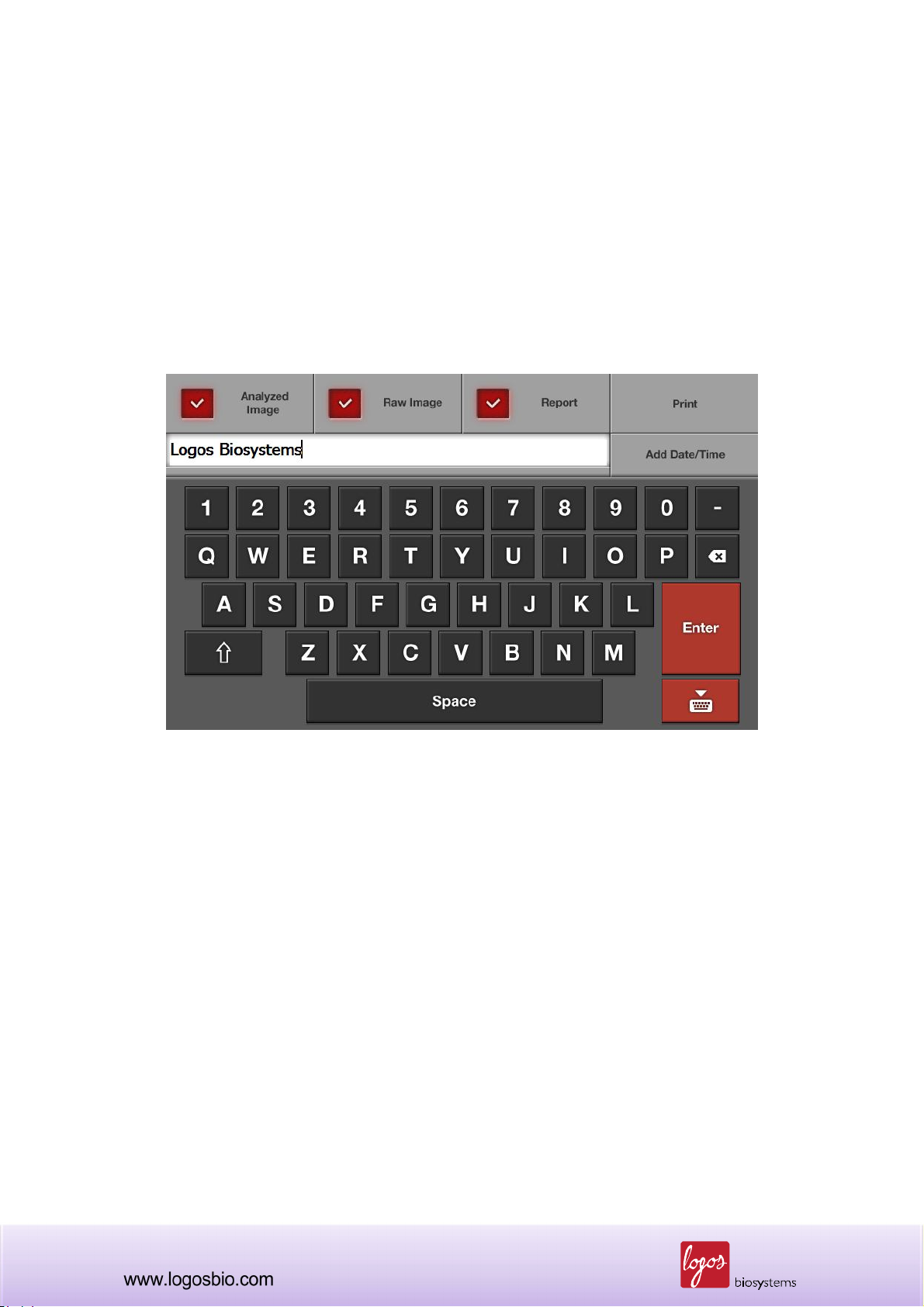
3.7.2 Press the “Save” button located on the lower left part of the screen and a new “Save” window
will pop up on the screen as shown below.
<Save and Report Screen>
Note: This “Save” window contains three check boxes as follows:
Analyzed Image: Check this box to save an analyzed image. Live cells are tagged in green
and dead cells are tagged in red for further analysis. If not checked, users can not use the
“Review” function with the saved image. So, please make sure that this must be checked if
users want to review the saved image on the instrument later.
Raw Image: This box must always be checked in order to save the image in TIF format.
The saved image can be re-analyzed on the instrument later if necessary.
Report: Check this box to generate a printed report as a PDF. This report contains all the
information of the cell counting session. This report can be read and printed with a
personal computer (PC). Please un-check if a report is not necessary.
36

3.7.3 Enter the file name using the keyboard buttons on the screen. The date and time can be
added by pressing the “Date/Time” button.
Note: Make sure that the cursor is located in the file name box before entering the file name.
Once the file name is verified, press the “Enter” button to save the image and/or report into the USB
drive. The saved files can be opened after transferring the images/report via USB drive to a PC with
the appropriate software.
Note: With the Luna-FL™ software, additional graphical representations of the data and results are
provided in the PDF report, as shown below.
<Two page PDF report generated by Luna-FL>
<1st page- Basic Data and Results>
Cell counting data
Protocol used
Cell images (tagged and zoomed)
<2nd page- Analytical Histograms>
By cell number (Total/Live/Dead)
By cell concentration (Total/Live/Dead)
By cell clustering
37

3.8 Printing the Counting Results
3.8.1 Users can print the counting results with the Luna™ Printer (Cat No.: P10001) and Luna™
Printer Paper (Cat No.: P12001). The Luna™ Printer is a mobile printer, which can be charged with
the battery pack before use.
<Luna™ Printer>
USB Connection Port
Note: The Luna™ Printer and Paper are specially designed for the Luna-FL™ and must be
purchased only from Logos Biosystems or its local distributors.
Note: For more information on the Luna™ Printer, please see the User Manual of the Luna™
Printer.
3.8.2 First, connect the Luna Printer to the Luna-FL™ via the supplied USB connector (The Luna
Printer can be connected at any time.) Then turn on the Luna Printer by pressing the “Power” button
for 1~2 sec (To turn off, press the “Power” button for 2~3 sec.).
3.8.3 Go to the “Save and Report” screen and then click the “Print” button.
38

<Print Button>
3.8.4 The counting results and its protocol will be printed out as below.
<Printed Results>
Note: The “Print” button prints only the latest counting results.
39

Note: If the “Protocol” part of the print is not necessary, users can turn off the option in the
“Results/Printer/Calculator” Option in the “Settings” menu, as shown below.
<Result/Printer/Calculator Options>
40

Chapter 4 – Fluorescence Cell Counting
4.1 Sample Preparation
4.1.1 Prepare the following materials to count cells
Cell sample
Luna™ cell counting slides (cat# L12001, L12002, L12003 or L12004)
AO/PI cell viability kit (cat# F23001)
4.1.2 If desired, insert a USB drive to save data and results,
4.1.3 Transfer 2 l of the AO/PI staining solution to a new 1.5 ml microfuge tube.
4.1.4. Add 18 l of the cell sample to the tube and mix by pipetting up and down or flicking the
bottom of the tube while holding with the other hand.
4.2 Loading Samples into Slides
4.2.1 Holding the edge of the slide, load 10 l of the mixed cell sample into the inlet of one chamber
of the counting slide. For easy and accurate loading, tilt the pipette around 45~60 degrees as
shown below.
<Sample Loading>
41

<Slide Inserting>
4.2.2 The remaining 10 l of the sample can be used for another counting to average the counting
results.
Note: Be careful not to over-load or under-load the sample into the chamber.
4.3 Counting Cells
4.3.1 Wait for about 1 min in order for the cells to settle down.
Note: Skipping this step will result in poor counting accuracy. Optimal waiting time should be
determined empirically. If cells are still moving during the preview step (step 4.3.4), a longer waiting
time is required.
Note: In case that the cells do not settle down, the captured images from bright field and green/red
fluorescence do not align exactly, as shown below.
42

<Unsettled Cells>
4.3.2 Insert the loaded slide into the slide port of the instrument, ensuring that the loaded chamber
is inserted first into the slide port. The instrument analyzes only the first inserted chamber. Gently
insert the counting slide to the end.
Note: After inserting the slide, Luna-FL only reads the first chamber. To read the second chamber, it
must be taken out, rotated, and inserted again.
Note: Make sure that the counting slide is not inserted upside-down. This may lead to sample
spilling and could severely damage the counter.
4.3.3 Select the “Fluorescence Cell Counting” button on the home screen.
<Home Screen>
43

Note: After this step, the real image of the cell sample will be displayed on the screen. The cell
image can be navigated by touching the screen and moving the finger or stylus pen on the image.
Please note that the touch screen of the Luna-FL
TM
automated cell counter is a resistive touch
screen, which needs a bit of pressure in order to get a response.
4.3.4 The “Preview” screen in the bright field mode will appear as shown in the image below. Make
sure that users have the best focus. When necessary, adjust the focus with the focus knob on the
right side of the instrument.
<Bright Field Mode in Fluorescence Counting>
Note: Make sure that the bright field mode is selected to adjust the focus.
<Adjusting the Focus>
Note: To get the best focus, the image can be magnified by using the “Zoom-in” button located
44

under the “Count” button. Initially, the “Zoom-in” button is set to a 1x image. When pressed once,
a 2x image appears. This 2X image may be the best zoom-in status for focusing. When pressed
again, a 4x image will be shown. Pressing the button again takes the screen back to a 1x image.
Note: Check if all cells in the preview screen are immobile. If some cells are still moving, wait for all
cells in the preview screen to stop moving.
4.3.5 Press “BF/GF/RF selection button” once to preview the green fluorescence (GF) image.
Increase the GF intensity value if the preview image is too dim so that only some cells are
visualized. Decrease the GF intensity value if the preview image is too bright so that background
fluorescence starts to come up. The optimal GF intensity value should be determined empirically.
The factory set value for mammalian cells is 5.
Note: During the preview step, if cells are irradiated for a long period of time, their fluorescent
intensities will quickly decrease. This bleaching phenomenon is accelerated when a higher level of
fluorescence is used. Always try to use the shortest preview time and the lowest fluorescence level
when the GF or RF preview is selected.
<Green Fluorescence Mode in Fluorescence Counting>
45

4.3.6 Press “BF/GF/RF selection button” one more time to preview the red fluorescence (RF) image.
Adjust RF intensity value as needed. The factory set value for mammalian cells is 5.
Note: During the preview step, if cells are irradiated for a long period of time, their fluorescent
intensities will quickly decrease. This bleaching phenomenon is accelerated when a higher level of
fluorescence is used. Always try to use the shortest preview time and the lowest fluorescence level
when the GF or RF preview is selected.
<Red Fluorescence Mode in Fluorescence Counting>
4.3.7 Press the “Count” button.
4.3.8 Within approximately 30 seconds (depending on cell concentration), the image of the cell
sample and data/results (Total, Live, Dead, Viability, avg. size, total number, live number and dead
number) will be displayed on the screen.
46

<Fluorescence Cell Counting Results>
47

4.4 Using the “Tag” Function
4.4.1 To verify counting results after performing the counting function, press “Images” and “Tag”
buttons as shown below.
Press
Images button
<Tagged Cell Image>
Press
Tag button
Green circle – live cell Red circle – dead cell
48

Note: This “Tag” function is one of the distinct tools of the Luna-FL™ automated cell counter
because it helps users review and determine the accuracy of the counting on-site without
depending on a computer.
4.4.2 When touching the “Tag” button, the image on the screen will show objects (mostly cells)
surrounded by green or red circles. The green circles indicate live cells and the red circles indicate
dead cells.
Note: In the “Fluorescence Cell Counting” mode, non-cellular debris contained a cell culture
medium can be easily identified and automatically excluded from cell counting because it does not
have any nucleic acids.
Non-cellular debris
4.4.3 After reviewing the accuracy of the image analysis, the “Tag” button can be pressed again to
remove the green and red circles.
49

4.5 Cell Size and Number Distribution
4.5.1 To obtain more data on the distribution of cell size and number in graphical representation,
press the “Graph/Gating” button on the lower left side of the screen. A histogram will appear with
more details on the size and number of the cell sample as shown below. Live cells are displayed as
a green bar and dead cells are displayed as a red bar.
<Graphical Representation of Cell Size Distribution>
Functional buttons in the menu
“Count Graph/Gating”
1) “Total, Live or Dead” button
2) “Cluster Map” button
3) “Cell number or /ml” button
* The above buttons can be used
in combination and the details are
described in next pages.
When the sizes of the cells are out of the range, they are displayed in grey color.
<Combination: Total and Concentration>
Combination of “Total” and “Cell
number” shows the distribution of
cell size vs. cell number for total
cells.
Note: When the “Total” button is pressed, it toggles to the “Live” button, which shows only live cells
as shown below. When the button is pressed again, it toggles to the “Dead” button to show only
dead cells as in the figure below.
50

<Combination: Live Cells and Cell Number>
The combination of “Live” and
“Cell number” shows the
distribution of cell size vs. cell
number for only live cells.
<Combination: Dead Cells and Cell Number>
The combination of “Dead” and
“Cell number” shows the
distribution of cell size vs. cell
number for only dead cells.
Note: When the “Cell Number” button is pressed, it toggles to the “/ml” button which shows the cell
concentration (i.e., the Y axis of the histogram). When pressed again, it toggles to the “Cell Number”
button.
51

<Combination: Total and Concentration>
The combination of “Total” vs.
“Cell concentration” shows the
distribution of cell size vs. cell
concentration for total cells.
<Combination: Live and Concentration>
The combination of “Live” vs.
“Cell concentration” shows the
distribution of cell size vs. cell
concentration for only live cells.
<Combination: Dead and Concentration>
The combination of “Dead” vs.
“Cell concentration” shows the
distribution of cell size vs. cell
concentration for dead cells.
52

4.5.1.1 Graphical representation of live and dead cells
When the “Total” button icon, the histogram is expressed as a “Stacked Column Chart.”
<Graphical representation of live and dead cells>
Live cells
Dead cells
4.5.2 “Cluster Map” button
4.5.2.1 When the “Cluster Map” button is pressed, the percentage distribution of the clustered cells
are displayed. “1-cell” means single cell and “2-cell” means 2 clustered cells as a unit. When the
“Cluster Map” button is on, the “Total” and “Cell Number” buttons become inactivated.
<Cluster Map>
4.5.2.2 Press the “Graph/Gating” button again to go back to the previous screen.
53

4.6 Using the Dilution Calculator
4.6.1 The Luna-FL™ automated cell counter provides a built-in dilution calculator that helps easily
calculate adjustments to obtain a desired concentration.
<A Built-in Dilution Calculator>
4.6.2 The dilution calculator initially shows the current concentration. Put the appropriate numbers
into the blanks of the “Desired Concentration” and “Final Volume” that you want to obtain.
Users can choose one of “Total”, “Live” or
“Dead” cells for calculating their dilution.
By clicking this button, the “Current
Concentration” displays the concentration of the
total, live or dead cells.
Before calculating the dilution factor, please
check this option to match your requirements.
54

Users can also calculate their custom dilution.
4.6.3 Press the “Calculate” button and then a dilution instruction will appear in the message box.
55

4.7 Saving the Image and Generating a Report of the Current Count
4.7.1 To save data and/or generate a report, insert a USB drive into the USB port on the left side of
the instrument.
4.7.2 Press the “Save” button located on the lower left part of the screen and then a new “Save”
window will pop up on the screen as shown below.
<Save and Report Screen>
Note: This “Save” window contains three check boxes as follows:
Analyzed Image: Check this box to save an analyzed image. Live cells are tagged in green
and dead cells in red for further analysis.
Raw Image: This box must always be checked in order to save the image in TIF format.
The saved image can be re-analyzed on the instrument later if necessary.
Report: Check this box to generate a printed report as a PDF. The report contains all the
information of the cell counting session. This report can be read and printed by a personal
computer (PC). Please un-check if a report is not necessary.
56

4.7.3 Enter the file name using the keyboard buttons on the screen. The date and time can be
added by pressing the “Date/Time” button.
Note: Make sure that the cursor is located in the file name box before entering the file name.
4.7.4 Once the file name is verified, press the “Enter” button to save the image and/or report into the
USB drive. Now, the saved files can be opened after transferring them via USB drive to a PC with
the appropriate software.
Note: With the Luna-FL software, additional graphical representations of the data and results are
provided in the PDF report, as shown below.
<Two page PDF report generated by Luna-FL>
<1st page- Basic Data and Results>
Cell counting data
Protocol used
Cell images (tagged and zoomed)
<2nd page- Analytical Histograms>
By cell number (Total/Live/Dead)
By cell concentration (Total/Live/Dead)
By cell clustering
57

4.8 Printing the Counting Results
Please see Section 3.8 (Page 38) to print the counting results.
58

Chapter 5 – Reviewing Saved Count Results
The Luna-FL™ automated cell counter provides an easy-to-use review function using the previous
count results saved in the USB drive for further review.
5.1 Importing Previous Counts
5.1.1 Insert the USB drive containing the previous results into the USB port of the instrument.
5.1.2 Press the “Review” menu located at the top of the screen. A list of files on the USB drive is
displayed on the left side of the screen as shown below.
<Review Screen>
Note: If there are several saved files in the USB drive, the scroll bar can be used to navigate the file
list.
59

5.2 Review previous results
5.2.1 Press the file name, and the previous result will start to be imported. Both the previous image
and the counting results will be displayed onto the instrument screen.
<Reviewing the previous result>
5.2.2 The previous image can be viewed at 1X, 2X or 4X by pressing the “Zoom-in” button and
navigated by touching and dragging the image window.
5.2.3 Press the “X (close)” button to review other previous results. Or press the “Count” menu
located at the top of the screen, and the instrument will be ready to count again.
60

5.3 “Previous Count” button
Whenever samples are counted, results are automatically saved to the internal memory of the
instrument. These data can be exported as a “.CSV” file to the USB drive for further analysis.
5.3.1 Pressing the “Previous Count” button on the left side of the “Review” menu will bring up a new
window. The “Previous Count” window contains up to 1,000 previous counting results that have
been saved in the memory of Luna-FL.
<”Previous Count” Button>
<”Previous Count” Window>
61

In the window of the “Previous Count,” there are 3 buttons:
1. “Export to USB (.CSV)” – Users can save the data of all previous counts into a USB memory
drive, which can be handled in PC software.
2. “Erase all” – All data can be removed from the memory.
3. “Close” – Close this window.
62

Chapter 6 – Setting Up the Protocol for Counting
Users can have unique protocols for each and apply them to specific cell types. When the “Protocol”
button is pressed on the main screen, a list of protocols will show up in the protocol list box.
Note: Initially, there are two protocols named as “DEFAULT” and “New protocol” in the instrument.
To create a customized protocol, select “New protocol” first and edit parameters, then save as
another name by pressing the “Save as” button. Because the protocol named, “DEFAULT” cannot
be edited, it must be saved as another name before parameters can be adjusted.
Note: Each counting mode has its own counting parameter set.
6.1 Adjusting Parameters for Bright Field Counting
When counting is performed, the DEFAULT parameters or one of the previously set protocols will be
applied to the counting.
<Bright Field Counting Parameters on the Protocol Menu>
63

The adjustable parameters are described as follow:
Parameter Range Default Description
The default value for the dilution factor is “2”.
Users can modify this value according to the dilution of
the original samples. If diluted for “A” times, users set
this variable to “2xA”. For example, 10 times diluted
samples should have the dilution factor of 20 (2 x10).
Dilution factor can be adjusted either by a scale of 1 or a
scale of 10 between 2 to 10 and 10-100 respectively.
Dilution
Factor
Noise
Reduction
1~100 2
0~10 5
Dilution factor corrects the actual number of cells; thus
the analyzed results are generated for the original
samples.
Dilution factor will help the users during repeated
counting of the samples of high cell density such as
fermented CHO cells.
Note: In any case, users must combine the diluted cell
samples with an equal volume of trypan blue solution
(1:1 ratio) before counting.
This refers to the decrease of the background for
counting. Higher noise reduction will not detect faint
signals and weakly stained objects. Lower noise
reduction means increasing the sensitivity of the objects
and detecting faint signals. Adjust the noise reduction to
the appropriate level. It is also useful when cells are
over-stained or under-stained with trypan blue stain.
Live Detection
1~10 5
Sensitivity
Roundness 30~100 60
”Live Detection Sensitivity” was designed to detect the
live cell having a various level of bright center. Increased
“Live Detection Sensitivity” will detect more live cells.
Decreased “Live Detection Sensitivity” will only detect
very bright centered cells as live cells.
This refers to the roundness of the objects on the image.
Increasing the value requires more roundness of the
objects to be included for the measurement. Meanwhile,
decreasing the value includes objects that are less
rounded for counting. Use this parameter when there are
64

cell types that are not generally circular or are irregularly
shaped.
Minimum
1-90 5
Cell Size
Maximum
1~90 60
Cell Size
Use this parameter to set the minimum cell size for
inclusion. The unit is 1 micrometer.
Use this parameter to set the maximum cell size for
inclusion. The unit is 1 micrometer.
6.2 Adjusting Parameters for Fluorescence Counting
<Fluorescence Counting Parameters on the Protocol Menu>
Parameter Range Default Description
The default value for the dilution factor is “1.11”.
In the protocol of the “Fluorescence Cell Counting”
mode, 2 l of the AO/PI cell viability kit (cat # F23001) is
added to 18 l of the cell sample. The dilution factor in
Dilution
1~10 1.11
Factor
this case is calculated as 1.11 (20/18). In case, 4 l of
the AO/PI cell viability kit is added to 16 l of the cell
sample, the dilution factor is 1.25 (20/16). When the
amount of the AO/PI cell viability kit used is increased by
2 l, the dilution factor changes from 1.11(2 l of the
AO/PI cell viability kit used), 1.25 (4 l), 1.42 (6 l), 1.66
65

(8 l), 2.00 (10 l), 2.50 (12 l), 3.33 (14 l), 5.00 (16 l)
to 10.00 (18 l).
Dilution factor corrects the actual number of cells; thus
the analyzed results are generated for the original
samples.
Dilution factor will help the users during repeated
counting of the samples of high cell density such as
fermented CHO cells.
Minimum
Cell Size
Maximum
Cell Size
Green
Fluorescence
Threshold
Red
Fluorescence
Threshold
1-90 5
1~90 60
1~10 5
1~10 5
Use this parameter to set the minimum cell size for
inclusion. The unit is 1 micrometer.
Use this parameter to set the maximum cell size for
inclusion. The unit is 1 micrometer.
Green and Red Fluorescence Threshold will determine
the level of threshold during the image processing.
Increased threshold will detect less cells by subtracting
the background more stringently. Decreased threshold
can detect more cells, but will increase the number of
noise signal.
6.3 Managing Protocols
To manage protocols, use the buttons described below.
Button Description
Load
Edit
Delete After selecting one of the protocols on the screen, press this button to remove it
Use this button to load one of the saved protocols to apply for counting or
adjusting the parameters.
After selecting a protocol, parameters can be adjusted by pressing this button.
Arrow buttons located over and under the parameter will be activated. Press the
arrow buttons to change the values for each parameter.
When no protocol is selected, this button is inactivated.
If you “Load” a new protocol after you “Edit” a protocol, the changed parameters
will be automatically saved to the same protocol name.
66

from the instrument.
When no protocol is chosen or loaded, this button is inactivated.
Save as
Note: The Luna-FLTM automated cell counter is provided with a factory setting protocol named
“DEFAULT”. This “DEFAULT” protocol cannot be edited or deleted. The parameters in the
“DEFAULT” protocol have been tested with many different cell types and can be used for a wide
variety of cells without changing parameters.
After adjusting the parameters, press this button to save a new protocol.
After saving, users can load the protocol for future counting.
67

Chapter 7 – Maintenance and Troubleshooting
7.1 Cleaning
Generally, the Luna-FL™ automated cell counter does not require regular maintenance for
appropriate operation. However, if the instrument is used for long periods of time and continuously,
it may need to be cleaned or decontaminated to remove any dirt or dust on the surface of the Luna-
FL™ cell counter. Be sure to turn off the Luna-FL™ cell counter and disconnect the power cable
before cleaning and performing any other maintenance. Ensure that water and other solutions do
not enter any part of the instrument during cleaning.
7.1.1 Cleaning the case
With a soft, damp cloth, wipe the surface of the instrument. Use some distilled water or alcohol for
dampening the cloth. After cleaning, immediately dry the cell counter with a dry cloth. Do not wet the
instrument by pouring or spraying water or other liquids directly on the instrument. In particular,
power-related parts should never become wet in order to avoid electrical shock or damage.
7.1.2 Cleaning the touch screen
Gently wipe off the touch screen with a soft cloth lightly moistened with an authorized LCD
cleansing detergent. Since excessive force or pressure on the touch screen can cause damage, be
gentle and cautious during cleaning. Wipe the touch screen dry immediately.
7.1.3 Decontaminating with alcohol
When the instrument needs to be decontaminated, use a soft cloth lightly moistened with 70%
alcohol to wipe the outer case. Never pour or spray alcohol or any other solution directly onto the
instrument; this can cause severe damage to the instrument or give an electric shock to users.
Note: Do not use an abrasive or a bleach solution that can cause scratches on the outer case or the
touch screen.
68

7.2 Changing the Battery
The battery of the Luna-FL™ automated cell counter is expected to last for 10 years. However, if
the date/time changes or slows with unknown causes, it may indicate that the battery is weakening
or running out. To change the battery, contact service personnel of the instrument supplier in your
country. Do not attempt to change the battery in any event. Disassembling the instrument voids the
product warranty.
69

7.3 Calibrating the Counter
In general, recalibration of the Luna-FL™ automated cell counter is not necessary since it is pre-
calibrated during manufacture. However, if needed for the installation or operation qualification,
please follow the procedure below.
7.3.1 Turn on the instrument to show the Start-Up screen on the display. If the instrument is already
turned on, please turn off and on, and then go to the “Start-Up” screen. Select the “Bright Field Cell
Counting” or “Fluorescence Cell Counting” mode. The calibration can be performed at either mode.
Note: Be sure to focus the instrument properly before calibration. Use the calibration bead to for the
focus adjustment.
7.3.2 Go to the “Settings” menu. Press the “Calibration” button and a message box will appear as
shown below.
<Recalibration>
Note: The recalibration consists of 3 steps for bright field calibration, custom staining calibration,
and fluorescence calibration. Depending on the requirements, each step can be performed or
skipped.
7.3.3 “Calibration Step 1” is necessary for bright field calibration. It can be performed by pressing
the “Start” button, or skipped by pressing the “Skip” button. When you click the “Exit” button, you
70
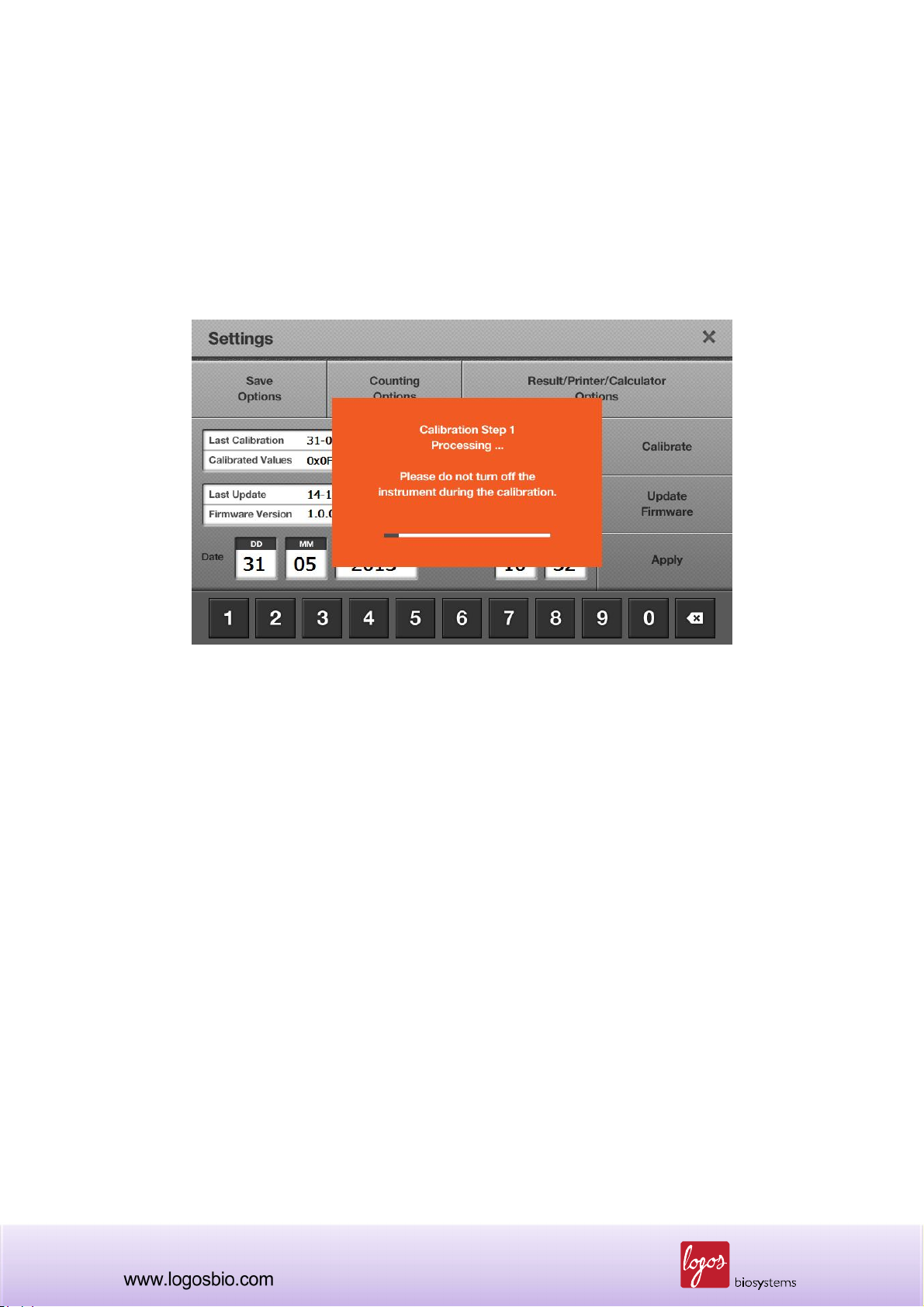
can quit this procedure at any time.
Note: It is very important to remove the counting slide prior to start the calibration step 1. If the
calibration step 1 is performed without removing the counting slide, cell counting result will be
inaccurate.
<Calibration Step 1>
Note: Never turn off the Luna-FL™ cell counter during calibration, which could lead to a significant
technical failure.
7.3.4 “Calibration Step 2” is necessary for the “Custom Staining Calibration.” From this custom
calibration, users can use their own stains for cell counting. This is related to Section 2.6 Counting
Options.
71

<Calibration Step 2-1>
Note: To perform the “Custom Staining Calibration”, users must be careful in preparing the staining
solution. For example, when users want to count cells under 0.1% trypan blue concentration, first,
mix 5 l of 0.4% trypan blue solution with 5 l of PBS to prepare 10 l of 0.2% concentration.
Second, mix 10 l of 0.2% concentration with another 10 l of PBS, to prepare 20 l of 0.1%
concentration, from which 10 l of 0.1% is loaded into one counting chamber to perform 0.1%
Custom Staining Calibration. To count cells under 0.1% trypan blue concentration, users can mix
cells with 0.2% trypan blue solution at a 1:1 ratio.
<Calibration Step 2-2>
Note: Never turn off the Luna-FL™ cell counter during calibration, which could lead to a significant
technical failure.
72

7.3.5 “Calibration Step 3” is necessary for “Fluorescence Counting Calibration.” Fluorescence
standard beads are supplied by Logos Biosystems or local distributors. Load 10 l of fluorescence
calibration beads into one of the counting chambers and wait for about 1 min in order for cells to
completely settle down. Insert the counting slide and then press the “Start” button. Make sure to
load the original standard solution - do not dilute it.
<Calibration Step 3-1>
<Calibration Step 3-2>
Note: Never turn off the Luna-FL™ cell counter during calibration, which could lead to a significant
technical failure.
73

7.3.6 When the three calibration steps are done successfully, users will see the message box as
below.
<Successful Calibration>
74

7.4 Updating Firmware
7.4.1 If the instrument is already turned on, please turn off and on, and then go to the “Start-Up”
screen. Press the “Settings” button and then the information of the last update and current firmware
version will be shown as below.
<Settings>
7.4.2 Check the firmware version and download the latest version from the Luna-FL™ website at
www.logosbio.com. if necessary. Save the file in a USB drive.
Note: The firmware, consisting of 1 file, must be saved into the root directory of the USB drive.
7.4.3 Insert the USB drive into the USB port on the instrument. Then press the “Update Firmware”
button.
7.4.4 When the display asks you to update, press the “Start” button. The process will take a few
minutes for completion.
7.4.5 When the update is finished, press the “Restart” button to reboot the instrument.
Note: The firmware update does not remove the user-created “Protocols” previously saved in the
instrument. Thus, after the update, all previous protocols are available for cell counting.
Note: Re-calibration is not necessary after a firmware update.
75

7.5 Troubleshooting
Problem Possible Cause Solution
Ensure that the cell image is appropriately focus
by using the focus knob. You may use the “Zoom-
Inaccurate cell
count
Unfocused cell image
Clumped cells
Cell concentration range
Counting slide insertion
Sample loading
in” button when adjusting the cell image. Make
sure that live cells have bright centers and dead
cells have dark/blue centers.
Make sure that cells are not clumped. The more
single cells, the better counting results.
The cell concentration range of the instrument is
preferably 5x104 ~ 1x107 cells/ml. Make sure that
the sample is in this range. The sample may
need to be concentrated or diluted.
Ensure that the counting slide is inserted
completely into the instrument. When the slide is
inserted, a soft click can be heard.
If the counting slide is over- or under-loaded with
the sample, it may affect counting results. The
optimal amount of sample is 10 ~12 l of cell
suspension.
Malfunction of optical
components
Damaged counting surface
Floating cells
Omitted dyes
Too low or too high
fluorescence setting
Any of the optical components may be damaged.
Or, the objective lens may be dirty due to dust,
spilled samples, or unknown causes. Please
contact your local supplier.
Make sure that the counting area of the slide is
transparent before loading the sample. Wear
gloves while handling the slide.
After loading the sample in the counting chip,
wait for about 1 min to allow the cells to settle
down. Time required for each cell type can vary
and should be determined empirically.
In the “Fluorescence Cell Counting” mode, cells
cannot be counted without fluorescent nucleic
acid binding dyes, e.g., AO and PI.
Make sure fluorescence settings in the preview
screen are in a reasonable range.
76

Every Luna-FL instrument has been pre-
Data transfer
and saving
Inaccurate calibration
Low precision range
Incorrect USB drive
Too many files in the USB
drive
calibrated during the manufacturing process. In
the rare event when calibration is wrong, perform
re-calibration as explained in Section 7.3.
The precision of cell counting results is
dependent on the number of cells actually
counted. The more cells that are counted, the
more precise the result. Although Luna-FL can
count as low as 5x104 cells/ml, cell concentration
higher than 2x105 cells/ml is required for 10% or
lower Coefficient for variation (CV).
Use the USB drive supplied with the instrument.
Or, make sure that your USB drive is compatible
with the instrument. The version of the USB drive
must be 2.0. Some types of USB drives are not
detected or incompatible with the instrument.
When there are too many saved files on the USB
drive, reading and writing by the counter may
slow down.
Errors during
updating and
calibrating the
instrument
Freezing during calibration
Incorrect USB drive
More than one firmware
version
Incorrectly saved or
damaged firmware
Generally, re-calibration takes several minutes.
However, it may take more time, depending on
the extent of background adjustment. If the
calibration takes more than 10 minutes, reset the
system by turning off and on using the power
button located rear side of the instrument. Please
contact service engineer if the calibration fails
repeatedly.
Use the USB drive supplied with the instrument.
Or, make sure that your USB drive is compatible
with the instrument. The version of the USB drive
must be 2.0. Some types of USB drives are not
detected or compatible with the instrument.
Delete previous versions from the USB drive
before downloading new firmware.
First, make sure that the USB drive works well
and is compatible with the instrument.
Second, download the file again onto the USB
77

drive. The file should be located in the root
directory.
Third, ensure that the USB drive is inserted
correctly.
Last, try the update again.
If the problem continues, contact your local
supplier.
78

Chapter 8 - Ordering Information
The following products can be ordered from your regional supplier or the website
(www.logosbio.com).
Cat # Product Quantity
L20001 Luna-FL™ Automated Fluorescence Cell Counter 1ea
L12001 Cell Counting Slides, 50 Slides (100 Counts) 1 box
L12002 Cell Counting Slides, 500 Slides (1,000 Counts) 10 boxes
L12003 Cell Counting Slides, 1,000 Slides (2,000 Counts) 20 boxes
B13101
F23001 AO/PI Cell Viability Kit (500 tests) 2 X 0.5ml
F23002 AO Cell Viability Kit (500 tests) 2 X 0.5ml
F23003 PI Cell Viability Kit (500 tests) 2 X 0.5ml
F23102 Luna-FL™ Calibration Bead (100 tests) 1 x 0.5ml
F23202 Yeast Viability Kit 1 TBD
F23203 Yeast Viability Kit 2 TBD
F24004 Luna-FL™ IQ/OQ Protocol 1 copy
T13001 Trypan Blue Stain 0.4% (200 tests) 2 X 1.0ml
U10004 Luna™ USB Drive 4GB 1ea
P10001 Luna™ Printer 1ea
Luna™ Standard Bead (Bright Field)
2 X 1.0ml
*Concentration: 1.0 X 10e6/ml *Size: 10 um
P12001 Luna™ Printer Paper 1ea
P13001 Luna™ Printer Cleaning Pen 1ea
79

Contact Information
For more information or technical support, please call, write, fax, or email. Our regional suppliers
are listed on our web page (www.logosbio.com).
Logos Biosystems, Inc.
Address: STE 930 Doosan Venturedigm, 126-1 Pyungchon-Dong, Dongan-Gu, Anyang-City,
Gyunggi-Do, Republic of Korea (Zip code: 431-755)
Tel: +82-31-478-4185
Fax: +82-31-478-4184
E-mail: sales@logosbio.com
80
 Loading...
Loading...