Page 1

Dissolved
Dissolved
Oxygen
Oxygen
Water Quality Test Kit
Instruction Manual
Code 5860-01
Page 2

Introduction ............................................................................ 3
Dissolved Oxygen
General Safety Precautions
Use Proper Analytical Techniques
Kit Contents
Test Procedure
...................................................................4
...................................................... 8
............................................. 9
........................................................................... 10
....................................................................... 11
Part 1: Collecting a Water Sample ........................................... 11
Part 2: Adding the Reagents ..................................................... 12
Part 3: Titration ........................................................................ 13
Percent Saturation .................................................................. 15
BOD
...................................................................................... 16
EPA Compliance
Short Form Instructions
.................................................................... 17
......................................................... back
WARNING! This set contains chemicals
that may be harmful if misued. Read
cautions on individual containers
carefully. Not to be used by children
except under adult supervision.
Page 3

INTRODUCTION
Aquatic animals need dissolved oxygen to live.
Fish, invertebrates, plants, and aerobic bacteria
all require oxygen for respiration. Oxygen
dissolves readily into water from the atmosphere
until the water is saturated. Once dissolved in the
water, the oxygen diffuses very slowly and
distribution depends on the movement of the
aerated water. Oxygen is also produced by aquatic
plants, algae, and phytoplankton as a by-product
of photosynthesis.
This test kit uses the azide modifi cation of the
Winkler method for determining dissolved oxygen.
3
Page 4
DISSOLVED OXYGEN,
PERCENT SATURATION & BOD
Oxygen is critical to the survival of aquatic plants and animals, and a shortage of
dissolved
species are more sensitive to oxygen depletion than others, but some general
guidelines to consider when analyzing test results are:
Because of its importance to the fi sh’s survival, aquaculturists, or “fi sh farmers,” and
aquarists use the dissolved oxygen test as a primary indicator of their system’s ability
to support healthy fi sh.
WHERE DOES THE OXYGEN COME FROM?
The oxygen found in water comes from many sources, but the largest source is oxygen
absorbed from the atmosphere. Wave action and splashing allows more oxygen to be
absorbed into the water. A second major source of oxygen is aquatic plants, including
algae; during photosynthesis plants remove carbon dioxide from the water and replace
it with oxygen.
Absorption
Oxygen is continuously moving between the water and surrounding air. The direction
and speed of this movement is dependent upon the amount of contact between the
air and water. A tumbling mountain stream or windswept, wave-covered lake, where
more of the water’s surface is exposed to the air, will absorb more oxygen from the
atmosphere than a calm, smooth body of water. This is the idea behind aerators: by
creating bubbles and waves the surface area is increased and more oxygen can enter
the water.
oxygen is not only a sign of pollution, it is harmful to fi sh. Some aquatic
5–6 ppm Suffi cient for most species
<3 ppm Stressful to most aquatic species
<2 ppm Fatal to most species
Photosynthesis
In the leaves of plants, one of the most important chemical processes on Earth is
constantly occurring: photosynthesis. During daylight, plants constantly take carbon
dioxide from the air, and in the presence of water convert it to oxygen and carbohydrates,
which are used to produce additional plant material. Since photosynthesis requires
light, plants
the photosynthesis
do not photosynthesize at night, so no oxygen is produced. Chemically,
reaction can be written as:
Light + nCO2 + nH2O (C2HO)n + nO
2
Light + Carbon + Water Carbohydrate + Oxygen
Dioxide
4
Page 5

WHERE DOES THE OXYGEN GO?
Once in the water, oxygen is used by the aquatic life. Fish and other aquatic animals
need oxygen to breathe or respire. Oxygen is also consumed by bacteria to decay, or
decompose, dead plants and animals.
Respiration
All animals, whether on land or underwater, need oxygen to respire, grow and survive.
Plants and animals respire throughout the night and day, consuming oxygen and
producing carbon dioxide, which is then used by plants during photosynthesis.
Decomposition
All plant and animal waste eventually decomposes, whether it is from living animals
or dead plants and animals. In the decomposition process, bacteria use oxygen to
oxidize, or chemically alter, the material to break it down to its component parts.
Some aquatic systems may undergo extreme amounts of oxidation, leaving no oxygen
for the living organisms, which eventually leave or suffocate.
PERCENT SATURATION
The oxygen level of a water system is not only dependant on production and consumption.
The potential dissolved oxygen capacity of water is limited by atmospheric pressure
(altitude), salinity, and temperature. These factors determine the highest DO level
possible. The percent saturation value expresses the quantity of dissolved oxygen in
the sample as a percent of the theoretical potential.
When water holds all of the dissolved oxygen that it can hold at a given altitude,
temperature, and salinity, it is said to be 100% saturated. If it holds a quarter as much as it
could possibly hold under those conditions it is 25% saturated. It is possible to get percent
saturation values over 100% when water becomes highly aerated by tumbling over
rapids and dams. It can also become supersaturated on a sunny day when dense areas
of plants or algae produce oxygen through photosynthesis.
Low atmospheric pressure found at higher altitudes slightly decreases the solubility of
oxygen in water so the dissolved oxygen value must be corrected for altitude.
The various minerals dissolved in water lower the capacity of the water to hold oxygen.
A correction factor can also be applied to dissolved oxygen measurements in saline
waters. In fresh water, where the salinity is very low, this effect is insignifi cant when
compared to the effect of temperature. Therefore, a correction for salinity is not
incorporated into the calculation.
Cold water can hold more oxygen than warm water. That is why fi sh that require higher
levels of oxygen, like trout, are found in cold water and dissolved oxygen concentrations
are usually higher in the winter than they are in the summer at the same location. The
percent saturation concentration can be corrected for water temperature.
Percent saturation levels from 80 to 120 percent are considered to be excellent. Levels
between 60 and 79 percent are adequate. Above 125 percent and below 60 percent
saturation, levels are poor. Fish and invertebrates that can move will leave areas with
low dissolved oxygen and move to areas with higher levels. Slow moving, trapped
or non-mobile aquatic animals may perish if levels become too low. Extremely high
dissolved oxygen concentrations are harmful to fi sh even for very short periods of
time. Gas bubble disease, which is characterized by the rupturing of capillaries in the
gills due to supersaturated water, is usually fatal.
5
Page 6
MEASURING BOD (BIOCHEMICAL OXYGEN DEMAND)
Biochemical oxygen demand is determined by measuring the dissolved oxygen
concentration in a freshly collected water sample and comparing it to the dissolved
oxygen level in a sample that was collected at the same time but incubated under
specifi c conditions for a specifi c length of time. The difference between the two
oxygen levels represents the amount of oxygen required for the decomposition of
organic material and the oxidation of chemicals in the water during the storage period,
a measurement known as the BOD.
Unpolluted, natural waters will have a BOD of 5 ppm or less. Raw sewage may have
levels of 150 to 300 ppm. Wastewater treatment plants must reduce BOD to levels
specifi ed in their discharge permits, usually between 8 and 150 ppm BOD.
TESTING DISSOLVED OXYGEN
The fi rst step in a DO titration is the addition of Manganous Sulfate Solution (4167) and
Alkaline Potassium Iodide Azide Solution (7166). These reagents react to form a white
precipitate, or fl oc, of manganous hydroxide, Mn(OH)2. Chemically, this reaction can
be written as:
MnSO4 + 2KOH Mn(OH)2 + K2SO
4
Manganous + Potassium Manganous + Potassium
Sulfate Hydroxide Hydroxide Sulfate
Immediately upon formation of the precipitate, the oxygen in the water oxidizes
an equivalent amount of the manganous hydroxide to brown-colored manganic
hydroxide. For every molecule of oxygen in the water, four molecules of manganous
hydroxide are converted to manganic hydroxide. Chemically, this reaction can be
written as:
4Mn(OH)2 + O2 + 2H2O 4Mn(OH)
3
Manganous + Oxygen + Water Manganic Hydroxide
Hydroxide
After the brown precipitate is formed, a strong acid, such as Sulfamic Acid Powder
(6286) or Sulfuric Acid, 1:1 (6141) is added to the sample. The acid converts the
manganic hydroxide to manganic sulfate. At this point the sample is considered
“fi xed” and concern for additional oxygen being introduced into the sample is reduced.
Chemically, this reaction can be written as:
2Mn(OH)3 + 3H2SO4 Mn2(SO4)3 + 6H2O
Manganic + Sulfuric Manganic + Water
Hydroxide Acid Sulfate
6
Page 7
Simultaneously, iodine from the potassium iodide in the Alkaline Potassium Iodide
Azide Solution is oxidized by manganic sulfate, releasing free iodine into the
water. Since the manganic sulfate for this reaction comes from the reaction between
the manganous hydroxide and oxygen, the amount of iodine released is directly
proportional
to the amount
of oxygen present in the original sample. The release of
free iodine is indicated by the sample turning a yellow-brown color. Chemically, this
reaction can be written as:
Mn
(SO4)
2
+ 2KI 2MnSO
3
4
+ K2SO
+ I
4
2
Manganic + Potassium Manganous + Potassium + Iodine
Sulfate Iodide Sulfate Sulfate
The fi nal stage in the Winkler titration is the addition of sodium thiosulfate. The
sodium
thiosulfate reacts with the free iodine to produce sodium iodide. When all of the
iodine has been converted the sample changes from yellow-brown to colorless. Often a
starch indicator
is added to enhance the fi nal endpoint. Chemically, this reaction can be
written as:
2Na2S2O3 + I2 Na2S4O6 + 2NaI
Sodium + Iodine Sodium + Sodium
Thiosulfate Tetrathionate Iodide
7
Page 8

GENERAL SAFETY PRECAUTIONS
1. 2.
Store the test kit in a
cool dry area.
3. 4.
Material
Safety
Read the labels on
all reagent bottles.
Note warnings and
fi rst aid information.
Read all Material
Safety Data Sheets.
Avoid contact between reagent
chemicals and skin, eyes, nose,
and mouth.
Data
Sheet
6.5.
Read all instructions
and note precautions
before performing
the test procedure.
Instruction
Manual
Keep all
equipment
and reagent
chemicals
out of the
reach of
young children.
Wear safety glasses when
performing test procedures.
7.
In the event of an accident or suspected poisoning, immediately call
the Poison Center phone number in the front of your local telephone
directory or call a physician. Additional information for all LaMotte
reagents is available in the United States, Canada, Puerto Rico, and
the US Virgin Islands from Chem-Tel by calling 1-800-255-3924.
For other areas, call 813-248-0585 collect to contact Chem-Tel’s
International access number. Each reagent can be identifi ed by
the four digit number listed on the upper left corner of the
reagent label, in the contents list and in the test procedures.
8
Page 9

USE PROPER ANALYTICAL TECHNIQUES
1. 2.
Use test tube caps or
stoppers, not your
fi ngers, to cover tubes
during shaking or
mixing.
3. 4.
Wipe up any reagent
chemical spills
immediately.
Tightly close all
containers
immediately
after use. Do not
interchange caps
from containers.
Hold dropper bottles
vertically upside-down, and not
at an angle,
a reagent. Squeeze
the bottle gently to
dispense the
reagent one drop
at a time.
6.5.
when dispensing
Thoroughly rinse test
tubes before and after
each test.
Avoid prolonged exposure
of equipment
and reagents
to direct
sunlight. Protect
reagents from
extremes of
temperature.
9
Page 10

DISSOLVED OXYGEN
CODE 5860-01
QUANTITY CONTENTS CODE
30 mL *Manganous Sulfate Solution *4167-G
30 mL *Alkaline Potassium Iodide Azide *7166-G
30 mL *Sulfuric Acid, 1:1 *6141WT-G
60 mL *Sodium Thiosulfate, 0.025N *4169-H
30 mL Starch Indicator Solution 4170WT-G
1 Direct Reading Titrator 0377
1 Test Tube, 5-10-12.9-15-20-25 mL, glass, w/cap 0608
1 Water Sampling Bottle, 60 mL, glass 0688-DO
*WARNING: Reagents marked with an * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specifi ed code number.
Kit Diagram
0688-DO
0608
4167-G
4170WT-G
10
7166-G
4169-H
614WT-G
0377
0377
Page 11

DISSOLVED OXYGEN TEST PROCEDURE
Part 1 - Collecting the Water Sample
1. 2.
Rinse the Water
Sampling Bottle
(0688-DO) with
the sample water.
Tightly cap the bottle, and
submerge it to the desired depth.
3. 4.
Remove the cap and allow
the bottle to fi ll.
Replace the cap while the
bottle is still submerged.
Tap the sides of the bottle to
dislodge any air bubbles.
6.5.
Retrieve the bottle
and make sure that
no air bubbles are
trapped inside.
11
Page 12
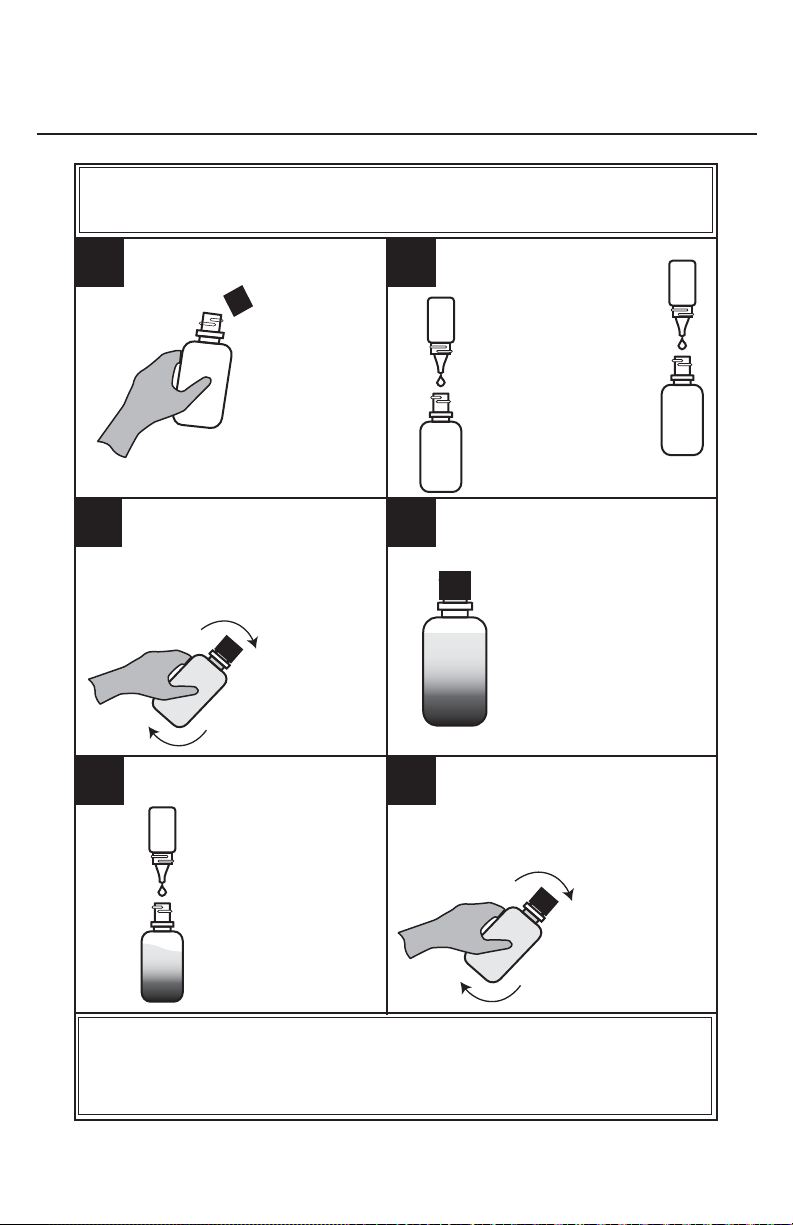
DISSOLVED OXYGEN TEST PROCEDURE
Part 2 - Adding the Reagents
NOTE: Be careful not to introduce air into the sample while adding
the reagents.
1. 2.
Immediately add 8
drops of *Manganous
Sulfate Solution (4167)
Remove the
cap from the
bottle.
and Add 8 drops of
*Alkaline Potassium
Iodide Azide (7166).
3. 4.
Cap the bottle and mix by
inverting several times.
A precipitate will form.
Allow the precipitate
to settle below the
shoulder of the bottle.
Cap and gently invert the bottle
6.5.
to mix the contents until the
precipitate and the reagent have
Add 8 drops of
*Sulfuric Acid, 1:1
(6141WT).
NOTE: At this point the sample has been “fi xed” and contact between the
sample and the atmosphere will not affect the test result. Samples may be
held at this point and titrated later.
totally dissolved. The solution
will be clear
yellow to
orange if the
sample contains
dissolved oxygen.
12
Page 13

DISSOLVED OXYGEN TEST PROCEDURE
Part 3 - The Titration
1. 2.
0
0
0.1
Fill the titration tube
(0608) to the 20 mL line
with the fi xed sample.
Cap the tube.
0.1
0.2
0.2
Depress plunger of
0.3
0.3
0.4
0.4
the Titrator (0377).
0.5
0.5
0.6
0.6
0.7
0.7
0.8
0.8
0.9
0.9
1.0
1.0
3. 4.
0
1
.
0
0
2
.
3
.
0
0
4
.
5
.
Insert the Titrator into
the plug in the top of the
*Sodium Thiosulfate,
0.025N (4169) titrating
solution.
0
0
.
6
7
.
0
0
8
.
9
.
0
0
1
.
Invert the bottle and
slowly withdraw the
plunger until the large
ring on the plunger is
opposite the zero (0)
line on the scale.
NOTE: If small air bubbles appear in the titrator barrel, expel them by
partially fi lling the barrel and pumping the titration solution back into the
reagent container. Repeat until bubble disappears.
5.
Turn the bottle
upright and remove
the Titrator.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
NOTE: If the sample
is a very pale yellow,
go to Step 9.
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
13
continued . . .
Page 14

DISSOLVED OXYGEN TEST PROCEDURE continued ...
6. 7.
Insert the tip of the Titraror
into the opening of the
titration tube cap.
8. 9.
Carefully remove the
Titrator and cap. Do
not disturb the Titrator
plunger.
11.10.
Cap the titration tube.
Insert the tip of the
Titrator into the opening
of the titration tube cap.
Slowly depress the
plunger to dispense the
titrating solution until
the yellow-brown color
changes to a very pale
yellow. Gently swirl the
tube during the titration
to mix the contents.
Add 8 drops of Starch
Indicator Solution
(4170WT). The sample
should turn blue.
Continue titrating until the blue
color disappears and the solution
becomes colorless.
NOTE: If the plunger ring
reaches the bottom line on
the scale (10 ppm) before the
endpoint color change occurs,
refi ll the Titrator and continue
the titration. Include
of the original
reagent dispensed
when recording the test result.
the value
amount of
(10 ppm)
Read the test result directly from the scale
12.
where the large ring on the Titrator meets
the Titrator barrel. Record as ppm Dissolved
Oxygen. Each minor division on the Titrator
scale equals 0.2 ppm.
NOTE: When testing is complete, discard the
titrating solution in the Titrator. Rinse Titrator
and titration tube thoroughly. DO NOT remove
plunger or adapter tip.
14
Page 15
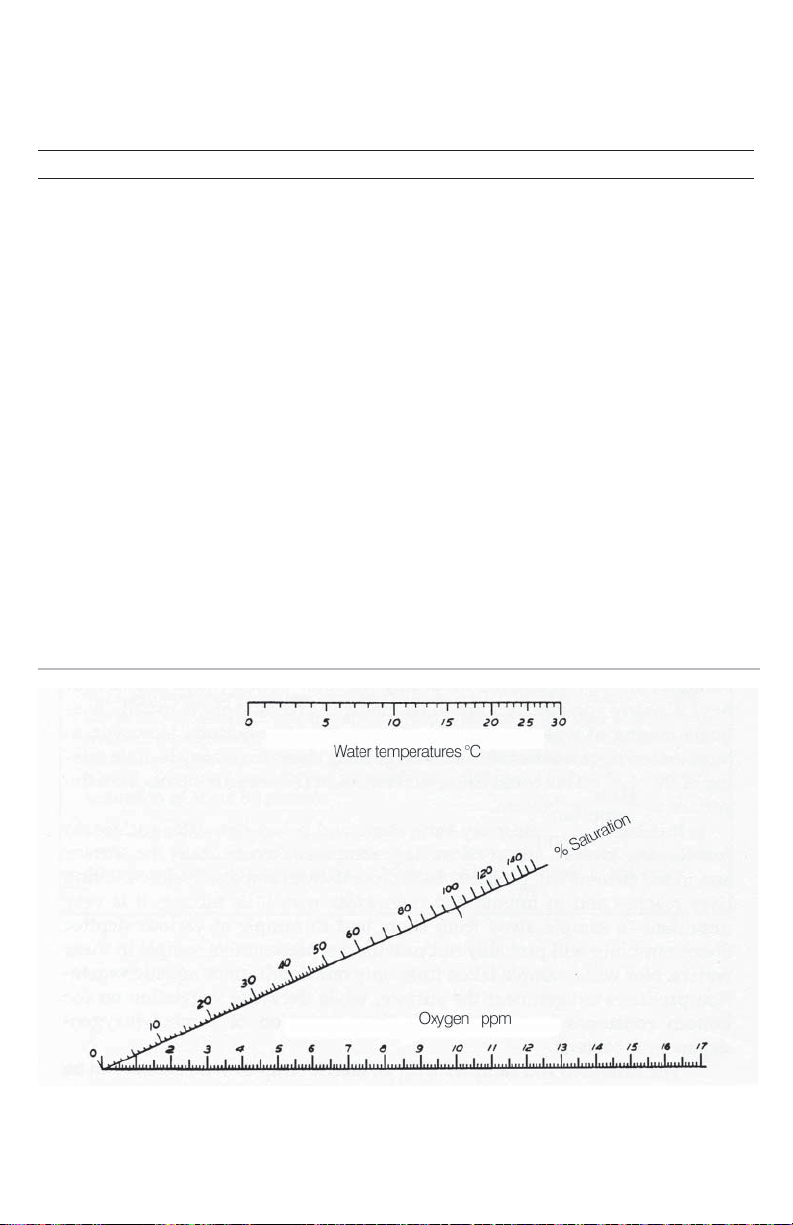
PERCENT SATURATION
Use the atmospheric pressure reading from a barometer or the local altitude to determine
the correction factor from the chart below. Multiply the dissolved oxygen test result (ppm)
by the correction factor to obtain the corrected dissolved oxygen value.
Atmospheric Pressure (mmHg)
a
775 540 1.02
760 0 1.00
745 542 0.98
730 1094 0.96
714 1688 0.94
699 2274 0.92
684 2864 0.90
669 3466 0.88
654 4082 0.86
638 4756 0.84
623 5403 0.82
608 6065 0.80
593 6744 0.78
578 7440 0.76
562 8204 0.74
547 8939 0.72
532 9694 0.70
517 10,472 0.68
Equivalent Altitude (ft) Correction Factor
To determine the percent saturation, locate the temperature (°C) of the water sample on
the top scale. Locate the corrected dissolved oxygen concentration (ppm) on the bottom
scale. Draw a straight line between the two points. Read the % saturation where the line
crosses the % saturation scale.
15
Page 16

BOD
1. 2.
Collect two samples
according to
Part 1 – Collecting
the W ater Sample.
1
3. 4.
Cover the bottle containing
the second sample completely
with aluminum foil to ensure
complete darkness. This will
prevent changes in
concentration caused by
photosynthesis
that may be present in
the sample.
the oxygen
in algae
2
Test one sample immediately
by following the procedures in
Part 2 – Adding the Reagents
and Part 3 – The Titration.
1
Incubate the second sample,
holding the temperature at
20 °C for fi ve days.
After fi ve days, test the incubated
sample by following the
procedures in Part 2 – Adding
the Reagents and Part 3 – The
Titration.
2
5.
Subtract the second dissolved
oxygen reading from the initial
dissolved oxygen reading to
obtain BOD in units of ppm.
16
Page 17

EPA COMPLIANCE
To qualify as an EPA accepted test, and to achieve the greatest accuracy, the Sodium
Thiosulfate Solution, 0.025N (4169) must be standardized daily. This procedure follows
Standard Methods for the Examination of Water and Wastewater. Numbers in ( ) are for
LaMotte products. These products are not included in this kit but can be ordered from
LaMotte Company by using the specifi ed code number.
1.
Use a 10 mL graduated
cylinder (0416) to add 15
mL of Deionized Water
(5115) to the titration tube
(0608).
3.
Add 2 drops of Sulfuric
Acid, 5N (8517WT).
Swirl to dissolve.
Solution will turn
yellowish brown.
2.
Use a Direct Reading
Titrator, 0-1 Range (1.0
mL capacity) (0376) to
add 2 mL of Potassium
Bi-iodate (7346).
4.
Use the 0.1 g spoon
(0699) to add 0.2 g
Potassium Iodide
Crystals (6809).
6.5.
Fill another Direct Reading
Titrator (0376) with Sodium
Thiosulfate Solution, 0.025N
(4169).
17
continued . . .
Page 18

EPA COMPLIANCE continued ...
7. 8.
While gently swirling the
tube, add Sodium
Thiosulfate, 0.025N until
the color fades to pale
yellow. It will be
necessary to refi ll the
Direct Reading Titrator.
9.
Continue adding
Sodium Thiosulfate,
0.025N until the blue
color disappears and
the solution is colorless.
10.
Read the test result directly from the scale
where the large ring on the Titrator meets the
Titrator barrel. Include the value of the original
amount dispensed (1 mL). If the reading is 2.0
+/- 0.1 mL, the Sodium Thiosulfate, 0.025N
(4169) is satisfactory. If not, discard and replace
with new reagent
Add 3 drops of
Starch Indicator
Solution (4170WT).
The solution will
turn blue.
18
Page 19

a
Page 20

SHORT FORM INSTRUCTIONS
Read all instructions before performing test. Use this guide as a quick reference.
1. Fill Water Sampling Bottle (0688-DO).
2. Add 8 drops of *Manganous Sulfate Solution (4167).
3. Add 8 drops of *Alkaline Potassium Iodide Azide (7166).
4. Cap and mix.
5. Allow precipitate to settle.
6. Add 8 drops of Sulfuric Acid, 1:1 (6141WT).
7. Cap and mix until reagent and precipitate dissolve.
8. Fill test tube (0608) to the 20 mL line.
9. Fill Titrator with *Sodium Thiosulfate, 0.025N (4169).
10. Titrate until sample color is pale yellow. DO NOT DISTURB TITRATOR.
11. Add 8 drops of Starch Indicator (4170WT).
12. Continue titration until blue color just disappears and solution is colorless.
13. Read result in ppm Dissolved Oxygen.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
65860-01-MN • 9/11
 Loading...
Loading...