Page 1

Dissolved Oxygen
Oxígeno disuelto
Oxygène dissous
Water Quality Test Kit
Instruction Manual
Code 5860-01
Kit de análisis de la
calidad del agua
Manual de instrucciones
Código 5860-01
Kit d’analyse de la
qualité de l’eau
Manuel d’instructions
Code 5860-01
Page 2

2
Page 3

Dissolved Oxygen | Water Quality Test Kit
Short Form Instructions ...................................................................................................................... 3
Introduction ........................................................................................................................................... 4
Dissolved Oxygen, Percent Saturation, & BOD .............................................................................. 4
General Safety Precautions ................................................................................................................ 8
Use Proper Analytical Techniques ..................................................................................................... 9
Kit Contents .......................................................................................................................................... 10
Test Procedure ..................................................................................................................................... 10
Part 1: Collecting a Water Sample .......................................................................................... 10
Part 2: Adding the Reagents ..................................................................................................... 11
Part 3: Titration ............................................................................................................................ 12
Percent Saturation ............................................................................................................................. 14
Biochemical Oxygen Demand .......................................................................................................... 15
Warning! This set contains chemicals that may be harmful if misused. Read cautions on individual containers carefully. Not to
be used by children except under adult supervision.
SHORT FORM INSTRUCTIONS
Read all instructions before performing test. Use this guide as a quick reference.
1. Fill Water Sampling Bottle (0688-DO).
2. Add 8 drops of *Manganous Sulfate Solution (4167).
3. Add 8 drops of *Alkaline Potassium Iodide Azide (7166).
4. Cap and mix.
5. Allow precipitate to settle.
6. Add 8 drops of *Sulfuric Acid, 1:1 (6141WT).
7. Cap and mix until reagent and precipitate dissolve.
8. Fill test tube (0608) to the 20 mL line.
9. Fill Titrator with Sodium Thiosulfate, 0.025N (4169).
10. Titrate until sample color is pale yellow. DO NOT DISTURB TITRATOR.
11. Add 8 drops of Starch Indicator (4170WT).
12. Continue titration until blue color just disappears and solution is colorless.
13. Read result in ppm Dissolved Oxygen.
3
Page 4

INTRODUCTION
Aquatic animals need dissolved oxygen to live. Fish, invertebrates, plants, and aerobic
bacteria all require oxygen for respiration. Oxygen dissolves readily into water from
the atmosphere until the water is saturated. Once dissolved in the water, the oxygen
diff uses very slowly and distribution depends on the movement of the aerated water.
Oxygen is also produced by aquatic plants, algae, and phytoplankton as a by-product of
photosynthesis.
This test kit uses the azide modifi cation of the Winkler method for determining dissolved
oxygen.
DISSOLVED OXYGEN, PERCENT SATURATION & BOD
Oxygen is critical to the survival of aquatic plants and animals, and a shortage of dissolved
oxygen is not only a sign of pollution, it is harmful to fi sh. Some aquatic species are more
sensitive to oxygen depletion than others, but some general guidelines to consider when
analyzing test results are:
5–6 ppm Suffi cient for most species
< 3 ppm Stressful to most aquatic species
< 2 ppm Fatal to most species
Because of its importance to the fi sh’s survival, aquaculturists, or “fi sh farmers,” and
aquarists use the dissolved oxygen test as a primary indicator of their system’s ability to
support healthy fi sh.
4
Page 5

WHERE DOES THE OXYGEN COME FROM?
The oxygen found in water comes from many sources, but the largest source is oxygen
absorbed from the atmosphere. Wave action and splashing allows more oxygen to be
absorbed into the water. A second major source of oxygen is aquatic plants, including
algae; during photosynthesis plants remove carbon dioxide from the water and replace it
with oxygen.
Absorption
Oxygen is continuously moving between the water and surrounding air. The direction
and speed of this movement is dependent upon the amount of contact between the air and
water. A tumbling mountain stream or windswept, wave-covered lake, where more of the
water’s surface is exposed to the air, will absorb more oxygen from the atmosphere than
a calm, smooth body of water. This is the idea behind aerators: by creating bubbles and
waves the surface area is increased and more oxygen can enter the water.
Photosynthesis
In the leaves of plants, one of the most important chemical processes on Earth is
constantly occurring: photosynthesis. During daylight, plants constantly take carbon
dioxide from the air, and in the presence of water convert it to oxygen and carbohydrates,
which are used to produce additional plant material. Since photosynthesis requires
light, plants
photosynthesis
Light + nCO2 + nH2O (C2HO)n + nO
Light + Carbon Dioxide + Water Carbohydrate + Oxygen
do not photosynthesize at night, so no oxygen is produced. Chemically, the
reaction can be written as:
2
WHERE DOES THE OXYGEN GO?
Once in the water, oxygen is used by the aquatic life. Fish and other aquatic animals
need oxygen to breathe or respire. Oxygen is also consumed by bacteria to decay, or
decompose, dead plants and animals.
Respiration
All animals, whether on land or underwater, need oxygen to respire, grow and survive.
Plants and animals respire throughout the night and day, consuming oxygen and
producing carbon dioxide, which is then used by plants during photosynthesis.
Decomposition
All plant and animal waste eventually decomposes, whether it is from living animals or
dead plants and animals. In the decomposition process, bacteria use oxygen to oxidize, or
chemically alter, the material to break it down to its component parts.
Some aquatic systems may undergo extreme amounts of oxidation, leaving no oxygen
the living organisms, which eventually leave or suff ocate.
for
PERCENT SATURATION
The oxygen level of a water system is not only dependant on production and consumption.
The potential dissolved oxygen capacity of water is limited by atmospheric pressure
(altitude), salinity, and temperature. These factors determine the highest DO level
possible. The percent saturation value expresses the quantity of dissolved oxygen in the
sample as a percent of the theoretical potential.
5
Page 6

When water holds all of the dissolved oxygen that it can hold at a given altitude,
temperature, and salinity, it is said to be 100% saturated. If it holds a quarter as much as it
could possibly hold under those conditions it is 25% saturated. It is possible to get percent
saturation values over 100% when water becomes highly aerated by tumbling over rapids
and dams. It can also become supersaturated on a sunny day when dense areas of plants
or algae produce oxygen through photosynthesis.
Low atmospheric pressure found at higher altitudes slightly decreases the solubility of
oxygen in water so the dissolved oxygen value must be corrected for altitude.
The various minerals dissolved in water lower the capacity of the water to hold oxygen.
correction factor can also be applied to dissolved oxygen measurements in saline waters.
In fresh water, where the salinity is very low, this eff ect is insignifi cant when compared to
the eff ect of temperature. Therefore, a correction for salinity is not incorporated into the
calculation.
Cold water can hold more oxygen than warm water. That is why fi sh that require higher
levels of oxygen, like trout, are found in cold water and dissolved oxygen concentrations
usually higher in the winter than they are in the summer at the same location. The percent
saturation concentration can be corrected for water temperature.
Percent saturation levels from 80 to 120 percent are considered to be excellent. Levels
between 60 and 79 percent are adequate. Above 125 percent and below 60 percent
saturation, levels are poor. Fish and invertebrates that can move will leave areas with low
dissolved oxygen and move to areas with higher levels. Slow moving, trapped or nonmobile aquatic animals may perish if levels become too low. Extremely high dissolved
oxygen concentrations are harmful to fi sh even for very short periods of time. Gas
bubble disease, which is characterized by the rupturing of capillaries in the gills due to
supersaturated water, is usually fatal.
A
are
MEASURING BIOCHEMICAL OXYGEN DEMAND
Biochemical oxygen demand is determined by measuring the dissolved oxygen
concentration in a freshly collected water sample and comparing it to the dissolved
oxygen level in a sample that was collected at the same time but incubated under specifi c
conditions for a specifi c length of time. The diff erence between the two oxygen levels
represents the amount of oxygen required for the decomposition of organic material and
the oxidation of chemicals in the water during the storage period, a measurement known
as the BOD.
Unpolluted, natural waters will have a BOD of 5 ppm or less. Raw sewage may have
levels of 150 to 300 ppm. Wastewater treatment plants must reduce BOD to levels
specifi ed in their discharge permits, usually between 8 and 150 ppm BOD.
6
Page 7

TESTING DISSOLVED OXYGEN
The fi rst step in a DO titration is the addition of Manganous Sulfate Solution (4167) and
Alkaline Potassium Iodide Azide Solution (7166). These reagents react to form a white
precipitate, or fl oc, of manganous hydroxide, Mn(OH)2. Chemically, this reaction can be
written as:
MnSO4 + 2KOH Mn(OH)2 + K2SO
4
Manganous + Potassium Manganous + Potassium
Sulfate Hydroxide Hydroxide Sulfate
Immediately upon formation of the precipitate, the oxygen in the water oxidizes an
equivalent amount of the manganous hydroxide to brown-colored manganic hydroxide.
For every molecule of oxygen in the water, four molecules of manganous hydroxide are
converted to manganic hydroxide. Chemically, this reaction can be written as:
4Mn(OH)2 + O2 + 2H2O 4Mn(OH)
3
Manganous Hydroxide + Oxygen + Water Manganic Hydroxide
After the brown precipitate is formed, Sulfuric Acid 1:1 (6141) (a strong acid), is added
to the sample. The acid converts the manganic hydroxide to manganic sulfate. At this
point the sample is considered
into the sample is reduced.
“fi xed” and concern for additional oxygen being introduced
Chemically, this reaction can be written as:
2Mn(OH)3 + 3H2SO4 Mn2(SO4)3 + 6H2O
Manganic Hydroxide + Sulfuric Acid Manganic Sulfate + Water
Simultaneously, iodine from the potassium iodide in the Alkaline Potassium Iodide Azide
Solution is oxidized by manganic sulfate, releasing free iodine into the water. Since
the manganic sulfate for this reaction comes from the reaction between the manganous
hydroxide and oxygen, the amount of iodine released is directly proportional
amount
of oxygen present in the original sample. The release of free iodine is indicated
to the
by the sample turning a yellow-brown color. Chemically, this reaction can be written as:
Mn2(SO4)3 + 2KI 2MnSO4 + K2SO4 + I
2
Manganic + Potassium Manganous + Potassium + Iodine
Sulfate Iodide Sulfate Sulfate
The fi nal stage in the Winkler titration is the addition of sodium thiosulfate. The sodium
thiosulfate reacts with the free iodine to produce sodium iodide. When all of the iodine has
been converted the sample changes from yellow-brown to colorless. Often a starch indicator
is added to enhance the fi nal endpoint. Chemically, this reaction can be written as:
2Na2S2O3 + I2 Na2S4O6 + 2NaI
Sodium Thiosulfate + Iodine Sodium Tetrathionate + Sodium Iodide
7
Page 8

GENERAL SAFETY PRECAUTIONS
Store the test kit in
a cool dry area.
Read all instructions
and note precautions
before performing
the test procedure.
Instruction
Manual
Safety
Data
Read the labels on
Sheet
all reagent bottles.
Note warnings and
fi rst aid information.
Read all Safety Data
Sheets.
Avoid contact between reagent
chemicals and skin, eyes, nose,
and mouth.
*WARNING: Reagents marked with an * are considered to be
potential health hazards.
for these reagents go to www.lamotte.com. Search for the four digit
reagent code number listed on the reagent label, in the contents list
or in the test procedures. Omit any letter that follows or precedes
the four digit code number. For example, if the code is 4450WT-H,
search 4450. To obtain a printed copy, contact LaMotte by email,
phone or fax.
Emergency information for all LaMotte reagents is available from
Chem-Tel: US, 1-800-255-3924
International, call collect, 813-248-0585
Keep all equipment
and reagent
chemicals
out of the reach of
young children.
Wear safety glasses when
performing test procedures.
To view or print a Safety Data Sheet (SDS)
8
Page 9

USE PROPER ANALYTICAL TECHNIQUES
Use test tube caps or
stoppers, not your
fi ngers, to cover tubes
during shaking or
mixing.
Wipe up any reagent chemical
spills immediately.
Tightly close all
containers immediately
after use.
Do not interchange
caps from containers.
Hold dropper
bottles vertically
upside-down, and
not at an angle,
when dispensing
a reagent. Squeeze
the bottle gently to
dispense the reagent
one drop at a time.
Thoroughly rinse test tubes
before and after each test.
Avoid prolonged
exposure of
equipment and
reagents to direct
sunlight. Protect
reagents from
extremes of
temperature.
9
Page 10

DISSOLVED OXYGEN CODE 5860-01
QUANTITY CONTENTS CODE
30 mL *Manganous Sulfate Solution *4167-G
30 mL *Alkaline Potassium Iodide Azide *7166-G
30 mL *Sulfuric Acid, 1:1 *6141WT-G
60 mL Sodium Thiosulfate, 0.025N 4169-H
30 mL Starch Indicator Solution 4170WT-G
1 Direct Reading Titrator 0377
1 Test Tube, 5-10-12.9-15-20-25 mL, glass, w/cap 0608
1 Water Sampling Bottle, 60 mL, glass 0688-DO
*WARNING: Reagents marked with an * are considered to be potential health hazards. See page XX for further safety information.
To order individual reagents or test kit components, use the specifi ed code number.
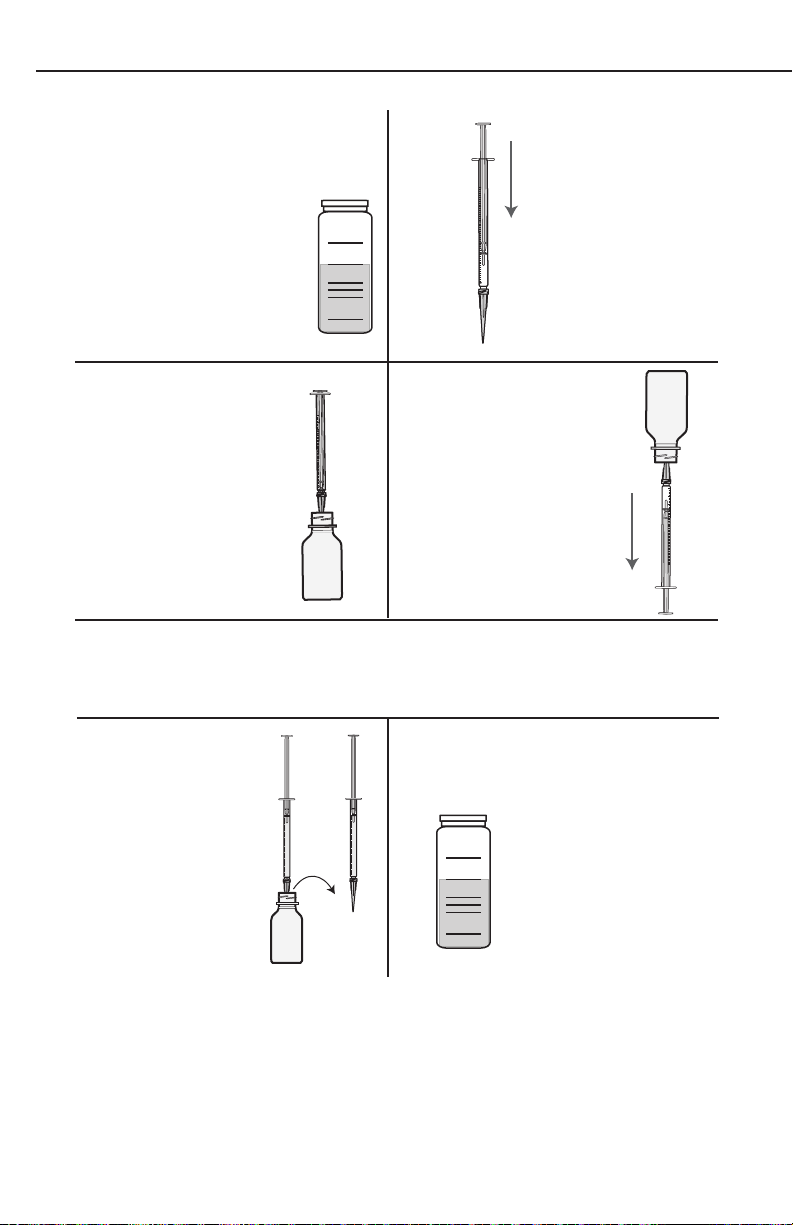
TEST PROCEDURE
Part 1 - Collecting the Water Sample
1. 2.
Rinse the Water
Sampling Bottle
(0688-DO) with
the sample water.
Tightly cap the bottle, and
submerge it to the desired depth.
3. 4.
Remove the cap and allow the
bottle to fi ll.
6.5.
Retrieve the bottle
and make sure that
no air bubbles are
trapped inside.
Replace the cap while the bottle
is still submerged.
10
Tap the sides of the bottle to
dislodge any air bubbles.
Page 11

Part 2 - Adding the Reagents
NOTE: Be careful not to introduce air into the sample while adding
the reagents.
12
1. 2.
Immediately add 8
drops of *Manganous
Sulfate Solution (4167Remove the
cap from the
bottle.
3. 4.
34
Cap the bottle and mix by
inverting several times.
A precipitate will form.
CN) and Add 8 drops of
*Alkaline Potassium
Iodide Azide (7166-
CN).
Allow the precipitate
to settle below the
shoulder of the bottle.
65
Cap and gently invert the bottle
6.5.
to mix the contents until the
precipitate and the reagent have
Add 8 drops of
*Sulfuric Acid, 1:1
(6141WT-CN).
NOTE: At this point the sample has been “fi xed” and contact between the
sample and the atmosphere will not aff ect the test result. Samples may be
held at this point and titrated later.
11
totally dissolved. The solution
will be clear
yellow to
orange if the
sample contains
dissolved oxygen.
Page 12

Part 3 - The Titration
12
1. 2.
0
0
0.1
Fill the titration tube
(0608) to the 20 mL line
with the fi xed sample.
Cap the tube.
34
3. 4.
Insert the Titrator into
the plug in the top of
the Sodium Thiosulfate,
0.025N (4169-CN)
titrating solution.
0
.
0
1
0
.
2
.
3
0
0
.
4
0
5
.
0
.
6
.
7
0
0
8
.
.
0
9
1
0
.
Invert the bottle and
slowly withdraw the
plunger until the large
ring on the plunger is
opposite the zero (0)
line on the scale.
0.1
0.2
0.2
Depress plunger of
0.3
0.3
0.4
0.4
the Titrator (0377).
0.5
0.5
0.6
0.6
0.7
0.7
0.8
0.8
0.9
0.9
1.0
1.0
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
NOTE: If small air bubbles appear in the titrator barrel, expel them by
partially fi lling the barrel and pumping the titration solution back into the
reagent container. Repeat until bubble disappears.
5.
5
Turn the bottle
upright and remove
the Titrator.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
12
NOTE: If the sample
is a very pale yellow,
go to Step 9.
Page 13

67
6. 7.
Insert the tip of the
Titraror into the opening
of the titration tube cap.
89
8. 9.
Slowly depress the
plunger to dispense the
titrating solution until
the yellow-brown color
changes to a very pale
yellow. Gently swirl the
tube during the titration
to mix the contents.
Carefully remove
the Titrator and cap.
Do not disturb the
Titrator plunger.
Cap the titration tube.
Insert the tip of the
Titrator into the
opening of the
titration tube cap.
12.
12
Read the test result directly from the scale
where the large ring on the Titrator meets
the Titrator barrel. Record as ppm Dissolved
Oxygen. Each minor division on the Titrator
scale equals 0.2 ppm.
Add 8 drops of Starch
Indicator Solution
(4170WT-CN). The
sample should turn blue.
1110
11.10.
Continue titrating until the blue
color disappears and the solution
becomes colorless.
NOTE: If the plunger ring
reaches the bottom line on
the scale (10 ppm) before the
endpoint color change occurs,
refi ll the Titrator and continue
the titration. Include
of the original
reagent dispensed
when recording the test result.
the value
amount of
(10 ppm)
NOTE: When testing is complete, discard the
titrating solution in the Titrator. Rinse Titrator
and titration tube thoroughly. DO NOT remove
plunger or adapter tip.
13
Page 14

PERCENT SATURATION
Use the atmospheric pressure reading from a barometer or the local altitude to determine
the correction factor from the chart below. Multiply the dissolved oxygen test result
(ppm) by the correction factor to obtain the corrected dissolved oxygen value.
a
Atmospheric Pressure (mmHg)
775 540 1.02
760 0 1.00
745 542 0.98
730 1094 0.96
714 1688 0.94
699 2274 0.92
684 2864 0.90
669 3466 0.88
654 4082 0.86
638 4756 0.84
623 5403 0.82
608 6065 0.80
593 6744 0.78
578 7440 0.76
562 8204 0.74
547 8939 0.72
532 9694 0.70
517 10,472 0.68
Equivalent Altitude (ft) Correction Factor
To determine the percent saturation, locate the temperature (°C) of the water sample on
the top scale. Locate the corrected dissolved oxygen concentration (ppm) on the bottom
scale. Draw a straight line between the two points. Read the % saturation where the line
crosses the % saturation scale.
14
Page 15

BIOCHEMICAL OXYGEN DEMAND
12
Collect two samples according
to Part 1 – Collecting the Water
Sample.
Test one sample immediately
by following the procedures in
Part 2 – Adding the Reagents
and Part 3 – The Titration.
1
2
1
34
Cover the bottle containing
the second sample completely
with aluminum foil to ensure
complete darkness. This will
prevent changes in the oxygen
concentration caused
by photosynthesis in
algae that may be
present in the sample.
Incubate the second sample, holding
the temperature at 20 °C for fi ve days.
After fi ve days, test the incubated
sample by following the procedures
in Part 2 – Adding the Reagents and
Part 3 – The Titration.
2
5
Subtract the second dissolved oxygen reading from the initial
dissolved oxygen reading to obtain BOD in units of ppm.
15
Page 16

16
Page 17

Oxígeno disuelto | Kit de análisis de la calidad del agua
Instrucciones abreviadas .................................................................................................................. 17
Introducción ......................................................................................................................................... 18
Oxígeno disuelto, porcentaje de saturation, & DBO ................................................................... 18
Precauciones generales de seguridad ........................................................................................... 22
Uso de técnicas analíticas adecuadas ........................................................................................... 23
Contenido del kit ..................................................................................................................................24
Procedimiento de análisis ...................................................................................................................24
Parte 1: recogida de muestra de agua ................................................................................... 24
Parte 2: adición de reactivos .................................................................................................... 25
Parte 3: valoración ...................................................................................................................... 26
Porcentaje de saturación .................................................................................................................. 28
Demanda bioquímica de oxígeno .................................................................................................... 29
¡ATENCIÓN! Este kit contiene productos químicos que pueden ser perjudiciales si no se utilizan correctamente. Lea con
atención las precauciones que se indican en cada envase. Prohibido su uso a menores sin la supervisión de un adulto.
INSTRUCCIONES ABREVIADAS
Lea todas las instrucciones antes de realizar el análisis. Utilice esta guía como referencia
rápida.
1. Llene el frasco para muestras de agua (0688-Oxígeno disuelto).
2. Añada 8 gotas de *Solución de sulfato manganoso (4167).
3. Añada 8 gotas de *Solución álcali-yoduro-azida de potasio (7166).
4. Tape y mezcle.
5. Deje que el precipitado se asiente.
6. Añada 8 gotas de *Ácido sulfúrico, 1:1 (6141WT).
7. Tape y mezcle hasta que el reactivo y el precipitado se disuelvan.
8. Llene el tubo de ensayo (0608) hasta la línea de 20 ml.
9. Llene el valorador con tiosulfato de sodio, 0,025N (4169).
10. Valore hasta que el color de la muestra sea amarillo pálido. NO AGITE EL
VALORADOR.
11. Añada 8 gotas del Indicador de almidón (4170WT).
12. Continúe la valoración hasta que el color azul desaparezca y la solución sea incolora.
13. Lea el resultado en ppm de oxígeno disuelto.
17
Page 18

INTRODUCCIÓN
Los animales acuáticos necesitan oxígeno disuelto para vivir. Los peces, los
invertebrados, las plantas y las bacterias aerobias requieren oxígeno para la respiración.
El oxígeno se disuelve fácilmente en el agua de la atmósfera hasta que el agua se satura.
Una vez disuelto en el agua, el oxígeno se difunde muy lentamente y su distribución
depende del movimiento del agua aireada. Las plantas acuáticas, las algas y el
fi toplancton también producen oxígeno como subproducto de la fotosíntesis.
Este kit de análisis utiliza la modifi cación de azida del método Winkler para determinar el
oxígeno disuelto.
OXÍGENO DISUELTO, PORCENTAJE DE SATURACIÓN Y DBO
El oxígeno es fundamental para la supervivencia de las plantas y los animales acuáticos,
y la falta de oxígeno disuelto no solo es un signo de contaminación, sino que además es
perjudicial para los peces. Algunas especies acuáticas son más sensibles al agotamiento
del oxígeno que otras, pero algunas pautas generales a tener en cuenta cuando se analizan
resultados de análisis son:
5–6 ppm Sufi ciente para la mayoría de especies
< 3 ppm Estresante para la mayoría de especies acuáticas
< 2 ppm Letal para la mayoría de especies
Debido a su importancia para la supervivencia de los peces, los acuicultores, o
«piscicultores», así como los acuaristas, utilizan la prueba de oxígeno disuelto como
indicador principal de la capacidad de su sistema para mantener una fauna acuática sana.
¿DE DÓNDE PROVIENE EL OXÍGENO?
El oxígeno que se encuentra en el agua proviene de muchas fuentes, pero la fuente
principal es el oxígeno absorbido de la atmósfera. La acción de las olas y las salpicaduras
permiten que el agua absorba más oxígeno. Una segunda fuente importante de oxígeno
18
Page 19

son las plantas acuáticas, incluidas las algas; durante la fotosíntesis, las plantas eliminan
el dióxido de carbono del agua y lo remplazan con oxígeno.
Absorción
El oxígeno se mueve continuamente entre el agua y el aire circundante. La dirección y
velocidad de este movimiento depende de la cantidad de contacto entre el aire y el agua.
Un arroyo de montaña revuelto o un lago azotado por el viento y cubierto de olas, donde
la mayor parte de la superfi cie del agua está expuesta al aire, absorberá más oxígeno
de la atmósfera que una masa de agua en calma y llana. Esta es la idea detrás de los
dispositivos de aireación: al crear burbujas y olas, la superfi cie aumenta y puede entrar
más oxígeno en el agua.
Fotosíntesis
En las hojas de las plantas ocurre constantemente uno de los procesos químicos
más importantes de la Tierra: la fotosíntesis. Durante el día, las plantas absorben
continuamente dióxido de carbono del aire, y en presencia de agua lo convierten en
oxígeno y carbohidratos, que se utilizan para producir material vegetal adicional. Debido
a que la fotosíntesis requiere luz, las plantas no se fotosintetizan durante la noche,
por lo que no se produce oxígeno. Químicamente, la reacción de la fotosíntesis puede
representarse como:
Luz + nCO2 + nH2O (C2HO)n + nO
Luz + Dióxido de carbono + Agua Carbohidratos + Oxígeno
2
¿A DÓNDE VA A PARAR EL OXÍGENO?
Una vez en el agua, el oxígeno es usado por los organismos acuáticos. Los peces y otros
animales acuáticos necesitan oxígeno para respirar. Las bacterias también consumen
oxígeno para descomponer plantas y animales muertos.
Respiración
Todos los animales, ya sea en tierra o bajo el agua, necesitan oxígeno para respirar, crecer
y sobrevivir. Las plantas y los animales respiran durante la noche y el día, consumiendo
oxígeno y produciendo dióxido de carbono, que luego es utilizado por las plantas durante
la fotosíntesis.
Descomposición
Todos los desechos de plantas y animales con el tiempo se descomponen, ya sean de
animales vivos o de plantas y animales muertos. En el proceso de descomposición,
las bacterias utilizan oxígeno para oxidar, o alterar químicamente, el material para
descomponerlo en sus distintos componentes. Algunos sistemas acuáticos pueden
someterse a una oxidación extrema, sin dejar oxígeno para los organismos vivos, que
fi nalmente se marchan o se asfi xian.
PORCENTAJE DE SATURACIÓN
El nivel de oxígeno de un circuito de agua no depende únicamente de la producción y el
consumo. La capacidad potencial de oxígeno disuelto del agua está limitada por la presión
atmosférica (altitud), salinidad y temperatura. Estos factores determinan el nivel de oxígeno
disuelto más alto posible. El valor porcentual de saturación expresa la cantidad de oxígeno
disuelto en la muestra como porcentaje del potencial teórico.
19
Page 20

Cuando el agua contiene todo el oxígeno disuelto que puede contener a una determinada
altitud, temperatura y salinidad, se dice que está 100 % saturada. Si tiene una cuarta parte
de lo que podría tener en esas condiciones, está saturado en un 25 %. Es posible obtener
valores porcentuales de saturación superiores al 100 % cuando el agua se airea mucho al
caer sobre rápidos y presas. También puede sobresaturarse en un día soleado cuando áreas
densas de plantas o algas producen oxígeno a través de la fotosíntesis.
La baja presión atmosférica que se encuentra a mayores altitudes disminuye ligeramente
la solubilidad del oxígeno en el agua, por lo que el valor de oxígeno disuelto debe
corregirse en función de la altitud.
Los diversos minerales disueltos en el agua reducen la capacidad del agua para retener
oxígeno. También se puede aplicar un factor de corrección a las mediciones de oxígeno
disuelto en aguas salinas. En agua dulce, donde la salinidad es muy baja, este efecto es
insignifi cante en comparación con el efecto de la temperatura. Por lo tanto, en el cálculo
no se incorpora una corrección por salinidad.
El agua fría puede contener más oxígeno que el agua caliente. Por ello los peces que
requieren mayores niveles de oxígeno, como la trucha, se encuentran en agua fría y las
concentraciones de oxígeno disuelto son generalmente más altas en invierno que en
verano en el mismo lugar. El porcentaje de concentración de saturación puede corregirse
en función de la temperatura del agua.
Los porcentajes de saturación del 80 % al 120 % se consideran excelentes. Los niveles
entre el 60 % y el 79 % son adecuados. Por encima del 125 % y por debajo del 60 %
de saturación, los niveles son malos. Los peces e invertebrados que puedan trasladarse
dejarán las áreas con bajo nivel de oxígeno disuelto y se trasladarán a áreas con niveles
más altos. Los animales acuáticos lentos, atrapados o inmóviles pueden perecer si los
niveles son demasiado bajos. Las concentraciones extremadamente altas de oxígeno
disuelto son perjudiciales para los peces, incluso durante períodos de tiempo muy cortos.
La enfermedad de la burbuja de gas, que se caracteriza por la ruptura de capilares en las
branquias debido al agua sobresaturada, suele ser letal.
MEDICIÓN DE LA DEMANDA BIOQUÍMICA DE OXÍGENO
La demanda bioquímica de oxígeno se determina midiendo la concentración de oxígeno
disuelto en una muestra de agua recién recogida y comparándola con el nivel de oxígeno
disuelto en una muestra recogida al mismo tiempo, pero incubada en condiciones
específi cas durante un período de tiempo determinado. La diferencia entre los dos
niveles de oxígeno representa la cantidad de oxígeno necesaria para la descomposición
de la materia orgánica y la oxidación de los químicos en el agua durante el período de
almacenamiento, una medida conocida como DBO.
Las aguas naturales no contaminadas tendrán una DBO de 5 ppm o menos. Las aguas
residuales no tratadas pueden tener niveles de 150 a 300 ppm. Las plantas de tratamiento
de aguas residuales deben reducir la DBO a los niveles especifi cados en sus permisos de
descarga, generalmente entre 8 y 150 ppm de DBO.
20
Page 21

ANÁLISIS DE OXÍGENO DISUELTO
El primer paso en una valoración de oxígeno disuelto es la adición de una solución
de sulfato manganoso (4167) y una solución álcali-yoduro-azida de potasio (7166).
Estos reactivos reaccionan para formar un precipitado blanco, o fl óculo, de hidróxido
manganoso, Mn(OH)2. Químicamente, esta reacción puede representarse como:
MnSO4 + 2KOH Mn(OH)2 + K2SO
Hidróxido + Sulfato Hidróxido + Sulfato
manganoso potásico manganoso potásico
Inmediatamente después de la formación del precipitado, el oxígeno en el agua oxida
una cantidad equivalente de hidróxido manganoso a hidróxido de manganeso de color
marrón. Por cada molécula de oxígeno en el agua, cuatro moléculas de hidróxido
manganoso se convierten en hidróxido de manganeso. Químicamente, esta reacción
puede representarse como:
4Mn(OH)2 + O2 + 2H2O 4Mn(OH)
Hidróxido de manganeso + Oxígeno + Agua Hidróxido de manganeso
Una vez formado el precipitado marrón, se añade a la muestra ácido sulfúrico 1:1
(6141) (un ácido fuerte). El ácido convierte el hidróxido manganoso en sulfato de
manganeso. En este punto, la muestra se considera «fi ja» y se reduce la preocupación por
la introducción de oxígeno adicional en la muestra. Químicamente, esta reacción puede
representarse como:
2Mn(OH)3 + 3H2SO4 Mn2(SO4)3 + 6H2O
Hidróxido manganoso + Ácido sulfúrico Sulfato de manganeso + Agua
Simultáneamente, el yodo del yoduro de potasio en la solución álcali-yoduro-azida de
potasio se oxida por el sulfato de manganeso, liberando yodo libre en el agua. Dado que
el sulfato de manganeso para esta reacción proviene de la reacción entre el hidróxido
manganoso y el oxígeno, la cantidad de yodo liberado es directamente proporcional a
la cantidad de oxígeno presente en la muestra original. La liberación de yodo libre se
indica por el color amarillo-marrón de la muestra. Químicamente, esta reacción puede
representarse como:
Mn2(SO4)3 + 2KI 2MnSO4 + K2SO4 + I
Sulfato + Yoduro de Sulfato + Yoduro + Yodo
manganoso potasio manganoso potásico
La etapa fi nal en la valoración de Winkler es la adición de tiosulfato de sodio. El
tiosulfato de sodio reacciona con el yodo libre para producir yoduro de sodio. Cuando se
ha convertido todo el yodo, la muestra cambia de amarillo-marrón a incoloro. A menudo
se añade un indicador de almidón para mejorar el resultado fi nal. Químicamente, esta
reacción puede representarse como:
2Na2S2O3 + I2 Na2S4O6 + 2NaI
Tiosulfato de sodio + Yodo Tetrationato de sodio + Yoduro de sodio
4
3
2
21
Page 22

PRECAUCIONES GENERALES DE SEGURIDAD
Instruction
Guarde el kit de
prueba en un lugar
fresco y seco.
Manual
Lea todas las
instrucciones y
tenga en cuenta
las precauciones
antes de realizar el
procedimiento de
análisis.
Safety
Lea las etiquetas de
todos los frascos de
Data
Sheet
reactivos. Tenga en
cuenta las advertencias
y la información de
primeros auxilios. Lea
todas las fi chas de datos
de seguridad.
Evite que los reactivos entren en
contacto con la piel, los ojos, la
nariz y la boca.
*¡ATENCIÓN!: Los reactivos marcados con un * se consideran
riesgos potenciales para la salud. Si quiere ver o imprimir una fi cha
de datos de seguridad de estos reactivos, visite www.lamotte.com.
Busque el código de cuatro dígitos del reactivo que aparece en la
etiqueta, en la lista de contenido o en los procedimientos de análisis.
Omita cualquier letra que siga o anteceda al código de cuatro dígitos.
Por ejemplo, si el código es 4450WT-H, busque 4450. Para obtener
una copia impresa, contacte con LaMotte por correo electrónico,
teléfono o fax.
Puede obtener información para casos de emergencia sobre todos
los reactivos de LaMotte en el teléfono: (EEUU, 1-800-255-3924)
(Internacional, a cobro revertido, 813-248-0585).
Mantenga el
equipo y los
químicos reactivos
fuera del alcance
de los niños.
Use gafas de seguridad
cuando realice procedimientos
de análisis.
22
Page 23

USO DE TÉCNICAS ANALÍTICAS ADECUADAS
Sostenga los
Use tapas o tapones
para tubos de ensayo,
no use los dedos,
para cubrir los tubos
mientras los agita o
mezcla.
frascos cuentagotas
verticalmente
boca abajo, y
no inclinados,
cuando dispense un
reactivo. Apriete el
frasco suavemente
para dispensar el
reactivo gota a gota.
Limpie inmediatamente
cualquier derrame de reactivos
químicos.
Cierre herméticamente
todos los recipientes
inmediatamente
después de su uso. No
intercambie los tapones
de los recipientes.
Enjuague minuciosamente
los tubos de ensayo antes y
después de cada prueba.
Evite la exposición
prolongada de
equipos y reactivos
a la luz solar
directa. Proteja
los reactivos de
temperaturas
extremas.
23
Page 24

OXÍGENO DISUELTO CÓDIGO 5860-01
CANTIDAD
30 mL
30 mL
30 mL
60 mL
30 mL
1
1
1
*¡ATENCIÓN!: Los reactivos marcados con un * se consideran riesgos potenciales para la salud. Consulte la página 8 para obtener
más información sobre seguridad.
CONTENIDO
*Solución de sulfato manganoso
*Solución álcali-yoduro-azida de potasio
*Ácido sulfúrico, 1:1
Tiosulfato de sodio, 0,025N
Solución del indicador de almidón
Valoración de lectura directa
Tubo de ensayo, 5-10-12.9-15-20-25 ml, vidrio, con tapón
Frasco de muestra de agua, 60 ml, vidrio
Si quiere pedir reactivos o componentes de kits de prueba individuales, use el código
especifi cado.
PROCEDIMIENTO DE PRUEBA DE OXÍGENO DISUELTO
Parte 1: recogida de muestra de agua
CÓDIGO
*4167-G
*7166-G
*6141WT-G
4169-H
4170WT-G
0377
0608
0688-DO
1.
Enjuague el frasco
para muestras de
agua (0688-DO) con
el agua de muestra.
3. 4.
Retire el tapón y deje que el
frasco se llene.
Reemplace el tapón mientras el
frasco aún esté sumergido.
2.
Tape bien el frasco y sumérjalo
hasta la profundidad deseada.
Golpee ligeramente los lados del
frasco para extraer cualquier burbuja
de aire.
6.5.
Saque el frasco y
asegúrese de que
no queden burbujas
de aire atrapadas en
su interior.
24
Page 25

Parte 2: adición de reactivos
NOTA: tenga cuidado de no introducir aire en la muestra mientras
añade los reactivos.
12
1. 2.
Inmediatamente
añada 8 gotas de
*Solución de sulfato
Retire el
tapón del
frasco.
3. 4.
34
Tape el frasco y mezcle
invirtiéndolo varias veces.
Se formará un precipitado.
manganoso (4167) y
8 gotas de *Solución
álcali-yoduro-azida de
potasio (7166).
Deje que el precipitado
se asiente por debajo
del hombro del frasco.
Tape e invierta suavemente el
65
6.5.
frasco para mezclar el contenido
hasta que el precipitado y el
reactivo se hayan disuelto por
Agregue 8 gotas de
*Ácido sulfúrico, 1:1
(6141WT).
NOTA: en este punto la muestra se ha «fi jado» y el contacto entre la
muestra y la atmósfera no afectará al resultado de la prueba. Pueden
tomarse muestras en este punto y valorarlas más tarde.
25
completo. La solución será de
color amarillo
claro a naranja
si la muestra
contiene
oxígeno
disuelto..
Page 26

Parte 3: la valoración
1. 2.
12
0
0
0.1
Llene el tubo de
valoración (0608) hasta
la línea de 20 ml con la
muestra fi jada. Cierre el
tubo.
34
3. 4.
Inserte el valorador en
el conector de la parte
superior del tiosulfato
de sodio, solución de
valoración 0,025N
(4169).
0
.
0
1
0
.
2
.
3
0
0
.
4
0
5
.
0
.
6
.
0
7
0
8
.
.
0
9
1
0
.
Invierta el frasco y
retire lentamente el
émbolo hasta que
la anilla grande del
émbolo quede opuesta
a la línea cero (0) de la
escala.
NOTA: si aparecen pequeñas burbujas de aire en el tambor del valorador,
expúlselas llenando parcialmente el tambor y devuelva la solución de
valoración al recipiente de reactivos. Repita hasta que la burbuja desaparezca.
0.1
0.2
0.2
Presione el émbolo
0.3
0.3
0.4
0.4
del valorador
0.5
0.5
0.6
0.6
0.7
0.7
(0377).
0.8
0.8
0.9
0.9
1.0
1.0
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
5.
5
Gire el frasco boca
arriba y retire el
valorador.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
es de color amarillo
pálido, continúe al
paso 9.
26
NOTA: si la muestra
Page 27

67
6. 7.
Inserte la punta del
valorador en la abertura
del tapón del tubo de
valoración.
89
8. 9.
Presione lentamente el
émbolo para dispensar la
solución de valoración
hasta que el color
amarillo-marrón cambie
a un amarillo muy
pálido. Gire suavemente
el tubo durante la
valoración para mezclar
el contenido.
Retire con cuidado
el valorador y el
tapón. No moleste
el émbolo del
valorador.
Tape el tubo de
valoración. Inserte la
punta del valorador
en la abertura del
tapón del tubo de
valoración.
12.
12
Lea el resultado de la prueba directamente
de la escala donde la anilla grande del
valorador se junta con el tambor del
valorador. Registre como ppm de oxígeno
disuelto. Cada división menor en la escala
de valoración es igual a 0,2 ppm.
NOTA: una vez fi nalizada la prueba,
deseche la solución de valoración en el
valorador. Enjuague bien el valorador y el
tubo de valoración. NO retire el émbolo ni
la punta adaptadora.
Añada 8 gotas de solución
indicadora de almidón
(4170WT). La muestra
debe volverse azul.
1110
11.10.
Continúe valorando hasta que
el color azul desaparezca y la
solución se vuelva incolora.
NOTA: si la anilla del émbolo
alcanza la línea inferior de la
escala (10 ppm) antes de que
se produzca el cambio de color
en el resultado fi nal, rellene
el valorador y continúe con la
valoración. Incluya el valor de
la cantidad original de reactivo
dispensado (10 ppm) al registrar
el resultado de la prueba.
Resultado
4.0 ppm
27
Page 28

PORCENTAJE DE SATURACIÓN
Use la lectura de presión atmosférica de un barómetro o la altitud local para determinar el
factor de corrección de la tabla de abajo. Multiplique el resultado de la prueba de oxígeno
disuelto (ppm) por el factor de corrección para obtener el valor corregido de oxígeno
disuelto.
a
Presión atmosférica (mmHg)
775 540 1.02
760 0 1.00
745 542 0.98
730 1094 0.96
714 1688 0.94
699 2274 0.92
684 2864 0.90
669 3466 0.88
654 4082 0.86
638 4756 0.84
623 5403 0.82
608 6065 0.80
593 6744 0.78
578 7440 0.76
562 8204 0.74
547 8939 0.72
532 9694 0.70
517 10,472 0.68
Altitud equivalente (pies) Factor de corrección
Temperatura (°C) de agua
Porcentaje de saturación
Oxígeno ppm
Para determinar el porcentaje de saturación, sitúe la temperatura (°C) de la muestra de
agua en la escala superior. Localice la concentración corregida de oxígeno disuelto (ppm)
en la escala inferior. Dibuje una línea recta entre los dos puntos. Lea el % de saturación
donde la línea cruza la escala de % de saturación.
28
Page 29

DEMANDA BIOQUÍMICA DE OXÍGENO
Recoja dos muestras de acuerdo
12
con la Parte 1: recogida de
muestra de agua.
1
2
34
Cubra completamente el frasco que
contiene la segunda muestra con
papel de aluminio para garantizar
una oscuridad total. Esto evitará
cambios en la concentración de
oxígeno causados por la
fotosíntesis en algas que
puedan estar presentes
en la muestra.
Analice una muestra
inmediatamente siguiendo los
procedimientos de la Parte 2:
adición de reactivos y de la
Parte 3: valoración.
1
Incube la segunda muestra,
manteniendo la temperatura a 20 °C
durante cinco días.
Transcurridos cinco días, analice
la muestra incubada siguiendo
los procedimientos de la Parte 2:
adición de reactivos y la Parte 3:
valoración.
2
5
Reste la segunda lectura de oxígeno disuelto de la lectura inicial
de oxígeno disuelto para obtener la DBO en unidades de ppm.
29
Page 30

30
Page 31

Oxygène dissous | Kit d’analyse de la qualité de l’eau
Instructions abrégées ........................................................................................................................ 31
Introduction ......................................................................................................................................... 32
Oxygène dissous, tax de saturation, & DBO ................................................................................. 32
Mesures de sécurité générales ........................................................................................................ 36
Utilisation des techniques d’analyse appropriées ...................................................................... 37
Contenu du kit ...................................................................................................................................... 38
Procédure d’essai ............................................................................................................................... 38
1ère phase : Prélèvement d’un échantillon d’eau ............................................................... 38
2e phase : Ajout des réactifs ..................................................................................................... 39
3e phase : Titrage ........................................................................................................................ 40
Taux de saturation .............................................................................................................................. 42
Demande biochimique d’oxygène ................................................................................................... 43
AVERTISSEMENT ! Ce kit contient des produits chimiques qui peuvent être nocifs s’ils sont utilisés de façon impropre. Lisez
avec attention les avertissements sur chaque récipient. Ce produit n’est pas destiné à être utilisé par des enfants, sauf sous la
surveillance d’un adulte.
INSTRUCTIONS ABRÉGÉES
Lisez toutes les instructions avant d’eff ectuer l’essai. Utiliser ce guide à titre de référence.
1. Remplir le fl acon d’échantillon d’eau (0688-Oxygène dissous).
2. Ajouter 8 gouttes *de la solution de sulfate de manganèse (4167).
3. Ajouter 8 gouttes *d’azoture d’iodure de potassium alcalin (7166).
4. Fermez l’éprouvette et mélangez.
5. Laisser reposer le précipité.
6. Ajouter 8 gouttes d’acide sulfurique, 1:1 (6141WT)
7. Fermer et mélanger jusqu’à complète dissolution du réactif et du précipité.
8. Remplissez le tube à essai (0608) jusqu’à la graduation de 20 ml.
9. Remplir le titrateur avec du thiosulfate de sodium, 0,025N (4169).
10. Titrer jusqu’à ce que la couleur de l’échantillon soit jaune pâle. NE PAS
PERTURBER LE TITRATEUR.
11. Ajouter 8 gouttes de la solution d’indicateur à amidon (4170WT).
12. Poursuivre le titrage jusqu’à ce que la solution varie de la couleur bleue à incolore.
13. Observer les résultats de l’oxygène dissous en ppm.
31
Page 32

INTRODUCTION
Les animaux aquatiques ont besoin d’oxygène dissous pour vivre. Les poissons, les
invertébrés, les plantes et les bactéries aérobies ont tous besoin d’oxygène pour respirer.
L’oxygène de l’atmosphère se dissout rapidement dans l’eau jusqu’à saturation de
l’eau. Une fois dissous dans l’eau, l’oxygène se diff use très lentement et sa répartition
dépend du mouvement de l’eau aérée. L’oxygène est également produit par les plantes
aquatiques, les algues et le phytoplancton comme élément dérivé de la photosynthèse.
Le kit d’analyse permet de déterminer le taux d’oxygène dissous en utilisant le facteur de
modifi cation de l’azoture de la méthode Winkler.
OXYGÈNE DISSOUS, TAUX DE SATURATION & DBO
Les plantes aquatiques et les animaux ont besoin d’oxygène pour survivre, et un manque
d’oxygène dissous indique non seulement la présence de pollution mais peut s’avérer
nuisible pour les poissons. Certaines espèces aquatiques sont plus sensibles que d’autres
à la raréfaction de l’oxygène, c’est pourquoi les résultats seront interprétés en prenant
compte les indications suivantes :
5–6 ppm Suffi sant pour la plupart des espèces
< 3 ppm Oppressant pour la plupart des espèces aquatiques
< 2 ppm Mortel pour la plupart des espèces
Sachant que l’oxygène dissous est essentiel à la survie des poissons, les aquaculteurs
ou « pisciculteurs » et les aquariophiles utilisent le test d’oxygène dissous comme un
indicateur principal déterminant si le système favorise la survie d’une faune aquatique
saine.
32
Page 33

D’OÙ VIENT L’OXYGÈNE ?
L’oxygène présent dans l’eau provient de plusieurs sources mais il est principalement
issu de l’atmosphère. L’action des vagues et des éclaboussures permet une plus grande
absorption de l’oxygène dans l’eau. La deuxième source d’oxygène se trouve dans les
plantes aquatiques, y compris les algues ; en eff et, au cours de la photosynthèse, les
plantes absorbent du dioxyde de carbone de l’eau et rejettent de l’oxygène.
Absorption
L’oxygène se déplace en permanence entre l’eau et l’air environnant. La direction et la
vitesse de ce mouvement dépend de la fréquence de contact entre l’air et l’eau. Un torrent
de montagne ou un lac balayé par le vent ou les vagues dans lesquels davantage d’eau de
surface est en contact avec l’air absorberont plus d’oxygène provenant de l’atmosphère
qu’une étendue d’eau paisible. C’est le principe des aérateurs : en stimulant la formation
de bulles et de vagues, la surface de contact est augmentée, permettant ainsi une plus
grande pénétration de l’oxygène dans l’eau.
La photosynthèse
L’une des plus importantes réactions chimiques de notre planète se produit dans les
feuilles des plantes : il s’agit de la photosynthèse. Pendant la journée, les plantes
absorbent en permanence du dioxyde de carbone provenant de l’air et en présence
d’eau, elles le transforment en oxygène et en glucides, qui sera par la suite utilisé pour
produire d’autres matériaux végétaux. La lumière est indispensable au phénomène de
la photosynthèse, les plantes ne photosynthèsent pas la nuit et ne produisent donc pas
d’oxygène. D’un point de vue chimique, la réaction de la photosynthèse s’écrit comme
suit :
Photons + nCO2 + nH2O (C2HO)n + nO
Photons + Dioxyde de carbone + Eau Glucides + Oxygène
2
OÙ VA L’OXYGÈNE ?
Une fois dans l’eau, l’oxygène est utilisé par les organismes aquatiques. Les poissons et
autres animaux aquatiques ont besoin d’oxygène pour vivre ou respirer. Les bactéries se
servent également de l’oxygène pour permettre la décomposition des plantes ou animaux.
Respiration
Tous les animaux vivant en surface ou dans l’eau ont besoin d’oxygène pour respirer,
grandir et survivre. Les plantes et les animaux respirent nuit et jour en consommant de
l’oxygène et en produisant du dioxyde de carbone, qui sera ultérieurement utilisé par les
plantes au cours de la photosynthèse.
Décomposition
Tous les déchets de plantes et d’animaux se décomposent à un moment donné, qu’ils
proviennent d’animaux vivants ou de plantes et d’animaux morts. Au cours de la phase de
décomposition, les bactéries utilisent l’oxygène pour oxyder ou modifi er chimiquement la
matière et la décomposer ainsi en ses éléments constituants. Certains systèmes aquatiques
subissent une forte oxydation, et ne laissent pas donc pas d’oxygène aux organismes
vivants, les obligeant à fuir ou les asphyxiant.
33
Page 34

TAUX DE SATURATION
Le taux d’oxygène d’un circuit d’eau ne dépend pas uniquement de la production et de
la consommation. La pression atmosphérique (altitude), la salinité et la température sont
des facteurs qui limitent la capacité de l’eau à produire de l’oxygène dissous. Ces facteurs
permettent de défi nir le taux d’oxygène dissous le plus élevé possible. Le taux de saturation
indique la quantité d’oxygène dissous présente dans l’échantillon comme pourcentage du
potentiel théorique.
Lorsque l’eau contient le maximum d’oxygène dissous possible à une altitude, à une
température et à un degré de salinité donnés, il est dit qu’elle est à 100 % de saturation. Si
dans les conditions indiquées, elle ne contient qu’un quart d’oxygène dissous, elle est à 25
% de saturation. Des taux de saturation dépassant les 100 % peuvent être atteints lorsque
l’eau est fortement aérée au contact de rapides ou de barrages. Une sursaturation de l’eau est
possible par temps ensoleillé lorsque de grandes étendues de plantes et d’algues produisent de
l’oxygène par photosynthèse.
La pression atmosphérique basse présente à plus haute altitude diminue sensiblement la
solubilité de l’oxygène dans l’eau, il convient donc d’adapter le taux d’oxygène dissous en
altitude.
Les diff érents minéraux dissous dans l’eau réduisent la capacité de l’eau à absorber de
l’oxygène. Les taux d’oxygène dissous dans des eaux salées pourront également être rectifi és.
Comme l’eau fraiche présente un taux de salinité faible, cela a peu d’impact en comparaison
avec les eff ets de la température. De plus, ce calcul n’intègre pas de correction de la salinité.
L’eau froide peut contenir plus d’oxygène que l’eau chaude. C’est pour cela que les poissons
nécessitant des taux d’oxygène plus élevés, comme par exemple les truites, se trouvent en
eaux froides et les concentrations d’oxygène dissous sont généralement plus élevées en hiver
qu’en été à un même endroit. Le taux de saturation peut être corrigé pour ce qui est de la
température de l’eau.
Des taux de saturation compris entre 80 % et 120 % sont jugés excellents. Des taux compris
entre 60 % et 79 % sont satisfaisants. Des taux supérieurs à 125 % et inférieurs à 60 % sont
jugés insuffi sants. Les poissons et les invertébrés mobiles quitteront les zones à faible taux
d’oxygène dissous pour rejoindre des zones présentant des taux plus importants. La faune
aquatique se déplaçant lentement, se trouvant prise au piège ou sédentaire peut disparaître
en cas de taux insuffi sants. De fortes concentrations d’oxygène dissous sont nocives pour les
poissons, y compris pour des durée de temps limitées. L’embolie gazeuse, qui se caractérise
par une rupture des capillaires des branchies causée par une eau sursaturée, est habituellement
mortelle.
MESURE DE LA DEMANDE BIOCHIMIQUE D’OXYGÈNE
La demande biochimique d’oxygène consiste à mesurer la concentration d’oxygène
dissous dans un échantillon d’eau récemment prélevé en la comparant au taux d’oxygène
dissous présent dans un échantillon prélevé au même moment mais dans des conditions
spécifi ques et pour une durée spécifi que. La diff érence entre les deux taux d’oxygène
correspond à la quantité d’oxygène nécessaire à la décomposition de matière organique
et à l’oxydation des produits chimiques dans l’eau au cours de la période de stockage, ce
calcul étant connu sous le nom de DBO.
La DBO d’eaux non-polluées et naturelles sera inférieure ou égale à 5 ppm. Le taux des
eaux usées sera compris entre 150 et 300 ppm. Les installations de traitement des eaux
34
Page 35

usées doivent réduire le niveau de la DBO conformément aux spécifi cations de leur
permis de rejet, ce niveau BDO est habituellement compris entre 8 et 150 ppm.
ANALYSE DE L’OXYGÈNE DISSOUS
La première étape d’une titration d’oxygène dissous consiste à ajouter une solution de
sulfate de manganèse (4167) et une solution d’azoture d’iodure de potassium alcalin
(7166). Ces réactifs agissent pour former un précipité blanc, ou fl oculat d’hydroxyde de
manganèse, Mn(OH)
MnSO4 + 2KOH Mn(OH)2 + K2SO
Hydroxyde de + Sulfate de Hydroxide de + Sulfate de
manganèse potassium manganèse potassium
Après la formation du précipité, l’oxygène dans l’eau oxyde une quantité équivalente
d’hydroxyde de manganèse pour former un hydroxyde manganique de couleur marron.
Pour chaque molécule d’oxygène dans l’eau, quatre molécules d’hydroxyde de
manganèse sont transformées en hydroxyde manganique. D’un point de vue chimique,
cette réaction s’écrit comme suit :
4Mn(OH)2 + O2 + 2H2O 4Mn(OH)
Hydroxyde de Manganèse + Oxygène + Eau Hydroxyde manganique
Après la formation du précipité marron, l’acide sulfurique 1:1 (6141) est ajouté à
l’échantillon. L’acide transforme l’hydroxyde manganique en sulfate manganique. À
ce stade, l’échantillon est « fi xé » et il existe peu de possibilités d’observer un surplus
d’oxygène dans l’échantillon. D’un point de vue chimique, cette réaction s’écrit comme
suit :
2Mn(OH)3 + 3H2SO4 Mn2(SO4)3 + 6H2O
Hydroxyde + Acide Hydroxyde de + Eau
manganique sulfurique manganèse
Dans le même temps, l’iode de l’iodure de potassium de la solution d’azoture d’iodure
de potassium alcalin est oxydé par le sulfate manganique, et libère de l’iode libre dans
l’eau. Étant donné que ce sulfate manganique est issu de la réaction entre l’hydroxyde
de manganèse et l’oxygène, la quantité d’iode libérée est proportionnelle à la quantité
d’oxygène présent dans l’échantillon d’origine. L’échantillon prend une couleur marronjaune lors de la libération d’iode libre. D’un point de vue chimique, cette réaction s’écrit
comme suit :
Mn2(SO4)3 + 2KI 2MnSO4 + K2SO4 + I
Sulfate + Iodure de Sulfate de + Sulfate de + Iode
manganique potassium manganèse potassium
L’étape fi nale du titrage de Winkler consiste à ajouter du thiosulfate de sodium. Le
thiosulfate de sodium entre en réaction avec l’iode libre et forme de l’iodure de sodium.
Après la transformation complète de l’iode, la couleur de l’échantillon varie de marronjaune à incolore. Un indicateur à l’amidon est souvent ajouté pour améliorer le résultat
fi nal. D’un point de vue chimique, cette réaction s’écrit comme suit :
2Na2S2O3 + I2 Na2S4O6 + 2NaI
Thiosulfate de sodium + Iode Tétrathionate de sodium + Iodure de sodium
. D’un point de vue chimique, cette réaction s’écrit comme suit :
2
4
3
2
35
Page 36

MESURES DE SÉCURITÉ GÉNÉRALES
Instruction
Stockez le kit
d’analyse dans un
lieu sec et frais.
Manual
Veuillez lire avec
soin les instructions
et mesures de
sécurité avant de
procéder à l’essai.
Veuillez lire avec
soin les étiquettes
des fl acons réactifs.
Prenez note des
Safety
Data
Sheet
mises en garde et
des mesures de
premier secours.
Lire toutes les
fi ches de données de
sécurité.
Évitez tout contact des réactifs
avec la peau, les yeux, le nez et
la bouche.
*AVERTISSEMENT : Les réactifs signalés par un astérisque * sont
considérés comme étant potentiellement dangereux pour la santé.
Pour affi cher ou imprimer les fi ches de données de sécurité (FDS)
de ces réactifs, rendez-vous sur www.lamotte.com. Identifi ez le code
à quatre chiff res du réactif indiqué sur l’étiquette du réactif, dans la
liste du contenu ou dans les procédures d’essai. Ignorez toute lettre
précédant ou suivant le code à quatre chiff res. Par exemple, si le code
est 4450WT-H, tenez compte uniquement de 4450. Pour obtenir une
version imprimée, contactez LaMotte par courriel, téléphone ou fax.
En cas d’urgence, des informations concernant les réactifs LaMotte
sont disponibles auprès de Chem-Tel : U.S. 1-800-255-3924 ou appel
international, en PCV, 813-248-0585.
Conservez tous
les réactifs et
équipements hors
de portée des
jeunes enfants.
Portez des lunettes de
sécurité lors de la procédure
d’essai.
36
Page 37

UTILISATION DE TECHNIQUES D’ANALYSE APPROPRIÉES
Lors de l’utilisation
Utiliser des capuchons
ou des bouchons de
tubes à essai et non
pas vos doigts pour
fermer les tubes
pendant les phases
d’agitation ou de
mélange.
du réactif, tenir
les fl acons avec
compte-gouttes en
position verticale à
l’envers, et non pas
en position inclinée.
Pressez délicatement
le fl acon pour verser
le réactif goutte par
goutte.
Essuyez immédiatement tout
réactif chimique répandu.
Fermer
hermétiquement tous
les récipients après
usage. Ne pas échanger
les capuchons des
récipients.
Rincer les tubes
soigneusement avant et
après chaque essai.
Éviter toute
exposition
prolongée des
accessoires et des
réactifs à la lumière
directe du soleil.
Protéger les réactifs
des écarts de
température.
37
Page 38

OXYGÈNE DISSOUS CODE 5860-01
QUANTITÉ CONTENU CODE
30 mL *Solution de sulfate de manganèse *4167-G
30 mL *Azoture d’iodure de potassium alcalin *7166-G
30 mL *Acide sulfurique, 1:1 *6141WT-G
60 mL Thiosulfate de sodium, 0,025N 4169-H
30 mL Solution d’indicateur à l’amidon 4170WT-G
1 Titrateur avec lecture directe 0377
1 Tube à essai, 5-10-12,9-15-20-25 ml, en verre, avec bouchon 0608
1 Flacon d’échantillon d’eau, 60 ml, en verre 0688-DO
*AVERTISSEMENT : Les réactifs signalés par un astérisque * sont considérés comme étant potentiellement dangereux pour la
santé. Reportez-vous à la page 8 pour obtenir davantage d’informations.
Pour commander à nouveau à l’unité des réactifs ou des composants du kit d’analyse,
utilisez le numéro de code indiqué.
PROCÉDURE D’ESSAI D’OXYGÈNE DISSOUS
1ère partie - Prélever l’échantillon d’eau
1. 2.
Rincer le fl acon de
l’échantillon d’eau
(0688 Oxygène
dissous) avec l’eau
de l’échantillon.
Fermer hermétiquement le fl acon,
et le plonger à la profondeur
souhaitée.
3. 4.
Ôter le capuchon et remplir le
fl acon.
6.5.
Replacez le capuchon pendant
que le fl acon est submergé.
38
Tapotez les côtés du fl acon pour
évacuer les bulles d’air.
Extraire le
fl acon de l’eau et
vérifi er l’absence
de bulles d’air.
Page 39

2e partie - Ajout des réactifs
REMARQUE : Prendre soin de ne pas introduire d’air dans
l’échantillon lors de l’ajout des réactifs.
12
1. 2.
Ajouter immédiatement
8 gouttes de solution de
sulfate de manganèse
Enlevez le
capuchon du
fl acon.
3. 4.
34
Fermer le fl acon et mélanger
en retournant le tube plusieurs
fois. Un précipité se formera
alors.
(4167) et ajouter 8
gouttes * d’azoture
d’iodure de potassium
alcalin (7166).
Laisser le précipité se
déposer en dessous du
niveau de l’épaule du
fl acon.
Fermer et retourner le fl acon
65
6.5.
délicatement pour mélanger
son contenu jusqu’à complète
dissolution du précipité et du
Ajouter 8 gouttes
d’acide sulfurique,
1:1 (6141WT)
REMARQUE : À ce stade, l’échantillon a été « fi xé » et tout contact entre
l’échantillon et l’atmosphère n’altèrera pas les résultats de l’essai. Les
échantillons peuvent alors être conservés et titrés plus tard.
réactif. La solution prendra une
teinte jaune clair
à orangée si
l’échantillon
contient de
l’oxygène
dissous.
39
Page 40
3e partie - Le titrage
12
1. 2.
Remplissez le tube
d’essai de titration
(0608) avec l’échantillon
fi xé jusqu’à la ligne de
remplissage marquant
20 ml. Fermez le tube
0
0
0.1
0.1
0.2
0.2
Débrayez le piston
0.3
0.3
0.4
0.4
du titrateur (0377).
0.5
0.5
0.6
0.6
0.7
0.7
0.8
0.8
0.9
0.9
1.0
1.0
d’essai avec le bouchon.
34
3. 4.
Insérer le titrateur dans
l’embout de la solution
de titration de thiosulfate
de sodium, 0,025N
(4169).
0
.
0
1
0
.
2
.
3
0
0
.
4
0
5
.
0
.
6
.
0
7
0
8
.
.
9
0
1
0
.
Renverser la bouteille
et retirer délicatement
le piston jusqu’à ce que
l’anneau large du piston
soit à l’opposé de la
marque du zéro (0) sur
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
la graduation.
REMARQUE : Si le cylindre du titrateur renferme des bulles d’air, les
expulser en le remplissant partiellement et en réinjectant la solution de titration
dans le récipient du réactif. Répéter l’opération jusqu’à la complète disparation
des bulles d’air.
5.
5
Remettre le fl acon
à l’endroit et ôter le
titrateur.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
l’échantillon a une
couleur jaune pâle,
voir l’étape 9.
40
REMARQUE : Si
Page 41

67
6. 7.
Insérer la tige
du titrateur dans
l’ouverture du bouchon
du tube de titration.
89
8. 9.
Appuyez délicatement
sur le piston pour libérer
la solution de titration
jusqu’à ce que la couleur
varie de marron-jaune à
jaune très pâle. Secouez
délicatement le tube
pendant le titrage pour
eff ectuer le mélange.
Ôter délicatement
le titrateur et le
bouchon. Ne pas
détériorer la seringue
du titrateur.
Fermez le tube
d’essai avec son
bouchon. Insérer la
pointe du titrateur
dans l’ouverture du
bouchon du tube de
titration.
12.
12
Le résultat de l’essai se lit directement sur la
graduation à l’endroit où l’anneau large du
titrateur coïncide avec le cylindre du titrateur.
Enregistrez la valeur d’oxygène dissous en ppm.
Toute sous-marque sur la graduation du titrateur
correspond à 0,2 ppm.
Ajouter 8 gouttes de la
solution d’indicateur
à amidon (4170WT)
L’échantillon doit prendre
une couleur bleue.
1110
11.10.
Poursuivre le titrage jusqu’à ce
que la couleur bleue disparaisse et
que la solution devienne incolore.
REMARQUE : Si l’anneau du
piston atteint la dernière marque
de la graduation (10 ppm) avant
le changement fi nal de couleur,
remplir de nouveau le titrateur
et poursuivre le titrage. Prendre
en compte la quantité initiale de
réactif employé (10 ppm) lors de la
saisie des résultats de l’essai.
Résultat
4.0 ppm
REMARQUE : À la fi n de l’essai, jeter la solution
de titration dans le titrateur. Rincer soigneusement
le titrateur et le tube d’essai. NE PAS secouer le
piston ou la pointe d’adaptateur.
41
Page 42

TAUX DE SATURATION
Utiliser la pression atmosphérique indiquée sur un baromètre ou l’altitude locale pour
défi nir les facteurs de correction du tableau ci-dessous. Multiplier les résultats de l’essai
d’oxygène dissous (ppm) par le facteur de correction pour obtenir le bon taux d’oxygène
dissous.
a
Pression atmosphérique (mmHg)
775 540 1.02
760 0 1.00
745 542 0.98
730 1094 0.96
714 1688 0.94
699 2274 0.92
684 2864 0.90
669 3466 0.88
654 4082 0.86
638 4756 0.84
623 5403 0.82
608 6065 0.80
593 6744 0.78
578 7440 0.76
562 8204 0.74
547 8939 0.72
532 9694 0.70
517 10,472 0.68
Altitude équivalente (m) Facteur de correction
Température (ºC) d’eau
Pourcentage de saturation
Oxygène ppm
Pour défi nir le pourcentage de saturation, indiquer la température (ºC) de l’échantillon
d’eau sur l’échelle du haut. Placer la concentration d’oxygène dissous rectifi ée (ppm) sur
l’échelle du bas. Tracer une ligne fi ne entre les deux points. Obtenir le % de saturation à
l’endroit où la ligne croise l’échelle du taux de saturation.
42
Page 43

DEMANDE BIOCHIMIQUE D’OXYGÈNE
12
Prélever deux échantillons
conformément à la 1ère partie –
Prélever l’échantillon d’eau.
Réaliser immédiatement l’essai
sur un échantillon en suivant les
instructions de la 2e partie – Ajout
des réactifs et de la 3e partie – Le
titrage.
1
2
1
34
Fermer hermétiquement le fl acon
contenant le deuxième échantillon
à l’aide d’une feuille d’aluminium
pour le protéger de la lumière. Cela
permettra d’éviter une quelconque
modifi cation de la concentration
d’oxygène causée par la
photosynthèse dans les
algues pouvant se trouver
dans l’échantillon.
Incuber le deuxième échantillon, en
maintenant la température à 20° C
pendant cinq jours.
jours, réaliser un essai sur l’échantillon
incubé en suivant les instructions de la
2e partie – Ajout des réactifs et de la 3e
partie – Le titrage.
Au bout de cinq
2
5
Soustraire la deuxième lecture d’oxygène dissous à la première
lecture d’oxygène dissous pour obtenir la DBO en ppm.
43
Page 44

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
65860-01-MN • 12.18
 Loading...
Loading...