Page 1

DHA-3000
L
Mott
DIGITAL pH METER
CODE 1706-02
TABLE OF CONTENTS
WhatispH?................................................ 4
GeneralInformation......................................... 5
MeterSpecifications......................................... 6
MeterAccessories........................................... 7
MeterPreparation........................................... 7
Standardizing............................................... 8
MeasuringpH .............................................. 9
MeasuringthepHofSoil ..................................... 9
MeasuringTemperature...................................... 10
Measuring Millivolts ........................................ 10
Meter Care............................................... 11
WarrantyInformation....................................... 12
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
3
SM
Page 2

WHAT IS pH?
pH is one of the most common analyses in soil and water testing. An
indication of the sample’s acidity, pH is actually a measurement of the
activity of hydrogen ions in the sample.
pH measurements run on a scale from 0 to 14, with 7.0 considered neutral.
Those solutions with a pH below 7.0 are considered acids, and those
between 7.0 and 14.0 are designated bases. The pH scale is logarithmic, so
a one unit change in pH actually reflects a ten fold change in the acidity.
For instance, orange juice, pH 4, is ten times more acidic than cottage
cheese, which has a pH of 5.
Many industries rely heavily on pH for their process to work properly, or
to maintain expensive equipment. Breweries maintain the pH between
4.2 and 4.6 to keep infectious bacteria from breeding during the
fermentation process. In many industrial applications if the pH is too low
the water may corrode metal equipment, but if it is too high scaling may
result.
pH can be measured visually or electronically. Visual comparisons use pH
indicators where color changes reflect the pH, which are then matched to
color standards. pH meters, such as the DHA-3000, simplify the pH test.
An electrode is placed in the sample, and the pH read directly from the
meter.
While the meter is very easy to use, the electronics within the meter are
more complex. After the pH electrode measures the millivolts of potential
between the reference electrode and the pH electrode, the meter converts
this reading to pH units using the Nernst Equation:
E=Ex+2.3RT
log(ai)
k
nF
where Ex= constant depending upon reference electrode
R = constant
= absolute temperature
T
k
n = charge of the ion (including sign)
F = constant
= activity of the ion
a
i
For actual use in converting pH readings to millivolts, this equation can
be simplified to:
(pH - 7.00) x (-0.198) = mV
4
Page 3

GENERAL INFORMATION
The DHA-3000 pH meter consists of three major components: the pH
electrode, the temperature probe and the meter.
The pH electrode consists of a glass, hydrogen-ion selective electrode, and
a reference electrode, combined into a single unit. The glass electrode is
specially treated to measure only hydrogen ions, while the reference
electrode is silver surrounded by silver chloride, and it provides a “zero” or
reference point for the measurement. This “zero” point means any change
in potential measured at the glass electrode is attributed to hydrogen ions,
and therefore expressed as pH.
The temperature probe serves a dual purpose, and may be connected to
the pH electrode. When the temperature probe is in the sample at the
same time as the pH electrode, it measures the temperature and allows the
meter to automatically compensate the temperature to 25°C. Secondly, it
allows the user to measure the actual temperature of the sample in °C.
When the temperature probe and pH electrode are immersed in the
sample, the meter measures the difference in potential between the glass
electrode and the reference electrode. This electronic measurement is
converted from millivolts to pH units, and the result appears on the
display.
If, for some reason, the temperature probe can not be used during the pH
measurement, the meter must be manually compensated for temperature.
To do this standardize the meter with buffers which are the same
temperature as the sample, and use the “Slope” knob to standardize meter
to the proper pH.
5
Page 4

METER SPECIFICATIONS
Range 0.00-14.00 pH units
±1999 mV
0.0 to 50.0°C
Readout 3
1
/2digit LCD
Readout Resolution 0.01 pH units
1mV
0.1°C
Controls pH
millivolts
°C Temperature
On/Off
Standardize
Slope
Temperature Compensation Automatic by separate probe
Electrode pH: Combination gel-filled epoxy body,
Ag/AgCl with BNC connector, 3’ cable
Probe Temperature: 2.5 mm cable jack
connector, 3’ cable
Power Alkaline (9V) DC battery;
3.5 mm jack for optional adapter
Size 5
7
/8”Lx31/4”Wx13/4”H
6
Page 5

METER ACCESSORIES
DESCRIPTION CODE
DHA-3000 pH meter with electrodes, buffers & case 1706-02
pH Electrode 1904
Temperature Probe 1771
pH Buffer, 4.0, 100 mL 2866-J
pH Buffer, 7.0, 100 mL 2881-J
pH Buffer, 10.0, 100 mL 2896-J
Beakers, plastic, 50 mL 0944
AC Adapter, 9 volt (120V/60Hz) 1744
or AC Adapter, 9 volt (220V/50Hz) 1778
METER PREPARATION
The DHA-3000 is shipped ready for standardization and use. See Meter
Care (p. 9) for information regarding any repairs which may be required.
The pH electrode and temperature probe can be connected together with
a rubber band for easier use.
STORING THE PROBE
To protect the pH electrode it should always be stored in the
accompanying soaker bottle (0668), with the lid tightened to prevent
leakage. The soaker bottle contains a dilute mixture of potassium chloride
and buffer.
7
Page 6

STANDARDIZING
The DHA-3000 pH meter should be standardized daily , or as operating
conditions are altered during use, for instance if changes occur in the
unknown or temperature. pH buffers should be replaced every three to six
months to assure accurate standardization. If the temperature probe is not
used during standardization, the buffers must be the same temperature as
the sample.
1. Press button labeled “pH”.
NOTE: If “BAT” appears in the lower left corner, the battery is low
and should be replaced. See Battery Replacement (p. 9) for
instructions.
2. Remove pH electrode from soaker bottle. Rinse pH electrode and
temperature probe with deionized or tap water.
3. Pour a small amount of pH 7.0 Buffer (2881) into a small flask or
beaker. Place pH electrode and temperature probe in beaker. Wait
until display stabilizes.
NOTE: The tip of the pH electrode must be immersed in the buffer
solution.
4. Adjust upper knob labeled “STANDARDIZE” until display reads
7.00.
5. Remove electrode and probe from buffer. Rinse with deionized or tap
water.
6. Select a second buffer based on expected pH to complete
standardization procedure.
NOTE: For solutions with an estimated pH below 7.0 use pH 4.0
Buffer (2866). For solutions with an estimated pH above 7.0 use pH
10.0 Buffer (2896).
7. Pour a small amount of selected buffer into a small flask or beaker.
Place pH electrode and temperature probes in beaker. Wait until
display stabilizes.
NOTE: The tip of the pH electrode must be immersed in the buffer
solution.
8. Adjust lower knob labeled “SLOPE” until pH of selected buffer is
displayed.
9. Repeat Steps 3 through 8 if necessary to check calibration.
10. Remove electrode and probe. Rinse electrode with deionized or tap
water. Place in soaker bottle for storage or proceed to Measuring pH
(p. 7).
11. Discard buffers.
NOTE: Do not disturb these adjustments.
8
Page 7

MEASURING pH
NOTE: If necessary standardize the DHA-3000. See Standardizing (p. 6)
for instructions.
1. Press button labeled “°C”.
NOTE: If “BAT” appears in the lower left corner, the battery is low
and should be replaced. See Battery Replacement (p. 9) for
instructions.
2. Remove pH electrode from soaker bottle. Rinse pH electrode and
temperature probes with deionized or tap water.
3. Place pH electrode and temperature probe in unknown sample. Wait
until display stabilizes.
NOTE: The tip of the pH electrode must be immersed in the sample.
4. Press button labeled “pH”. Record reading as pH.
5. Press button labeled “OFF” when finished testing.
6. Remove electrode and probe. Rinse with deionized or tap water. Place
pH electrode in soaker bottle for storage.
MEASURING THE pH OF SOIL
1. Place a 1 to 1 ratio of soil and distilled water in a small beaker.
NOTE: For most analyses 20 grams of soil and 20 mL of distilled
water is sufficient.
2. Wait 15 minutes, stirring occasionally with a stirring rod.
3. Stir sample. Immediately place pH electrode and temperature probe
in sample.
4. Press button labeled “°C” on DHA-3000. Wait until display stabilizes.
NOTE: If “BAT” appears in the lower left corner, the battery is low
and should be replaced. See Battery Replacement (p. 9) for
instructions.
5. Press button labeled “pH”. Record result as pH.
6. Press button labeled “OFF” when finished testing.
7. Remove electrode and probe. Rinse with deionized or tap water. Place
pH electrode in soaker bottle for storage.
9
Page 8

MEASURING TEMPERATURE
1. Rinse temperature probe with deionized or tap water.
2. Place temperature probe in unknown sample.
3. Push button labeled “°C”. Wait until display stabilizes.
4. Record result as temperature in “°C”.
5. Press button labeled “OFF” when finished testing.
6. Remove probe. Rinse with deionized or tap water.
MEASURING MILLIVOLTS
1. Disconnect pH electrode from DHA-3000.
2. Connect ion specific electrode or ORP probe to BNC connector.
3. Place ion specific electrode or ORP probe in sample.
4. Push button labeled “mV”. Wait until display stabilizes.
5. Record result as millivolts.
6. Press button labeled “OFF” when finished testing.
7. Remove ion specific electrode or ORP probe. Rinse with deionized or
tap water.
10
Page 9
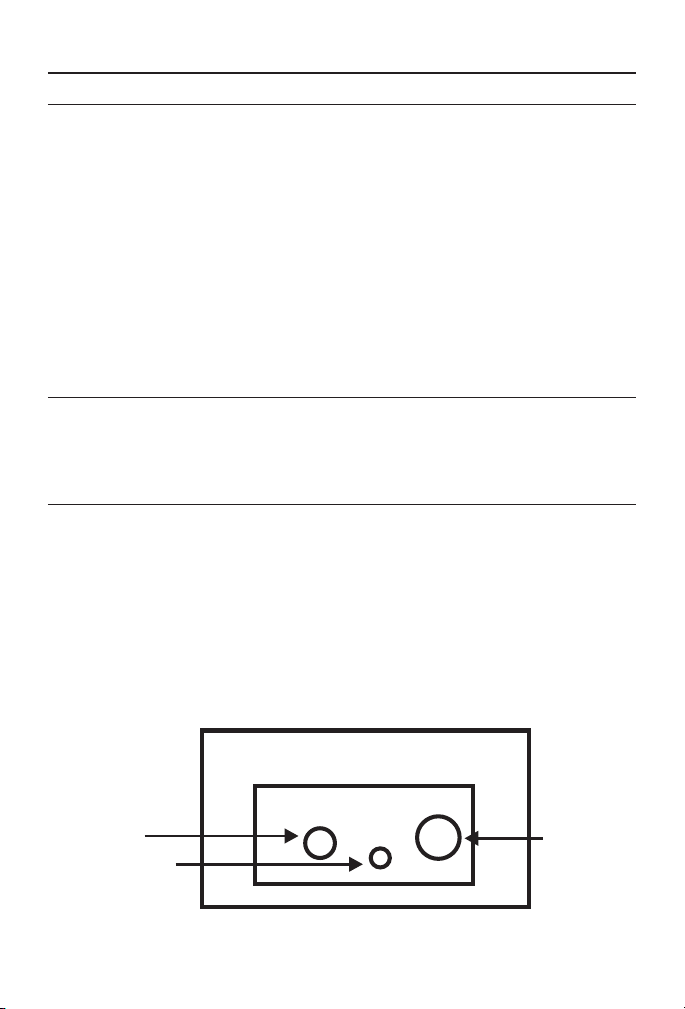
METER CARE
FIGURE A
BNC
Connector
AC Adapter
Connector
Temperature
Probe Connector
BATTER Y REPLACEMENT
When “Bat” appears on the display, the battery should be replaced. The
temperature reading will be the first to be affected by the low battery.
1. Use a #1 Phillips head screwdriver to remove four screws on the back
of the meter case.
2. Gently lift back panel from meter.
3. Lift battery from bottom of meter. Remove battery from connector.
4. Snap new battery onto connector.
NOTE: The DHA-3000 uses an Alkaline (9V) DC battery.
5. Lower the battery back into the meter.
6. Replace back panel on meter. Replace screws.
AC ADAPTER
An AC adapter is available for use with the DHA-3000. Order as
LaMotte Code 1744 (120V/60Hz) or 1778 (220V/50Hz).
CONNECTING THE PROBES
A. pH Electrode
1. Slide BNC connector over receptacle on meter, making sure the pin
on the meter connector is in the lower end of the slot. Turn outer ring
on BNC connector until the pin slides to upper end of the slot.
B. Temperature Probe
1. Slide connector pin into smallest hole on meter.
2. Hold probes next to each other. Slide rubber band to top of probes to
hold them together.
11
Page 10

WARRANTY INFORMATION
REPAIRS
If it is necessary to return the instrument for repair, contact LaMotte
Company Technical Service at 1-800-344-3100 for a return authorization
number.
INSTRUMENT GUARANTEE
This instrument, excluding the electrode, is guaranteed to be free of
defects in material and workmanship for 12 months from original
purchase. If in that time it is found to be defective, it will be repaired
without charge, except for transportation expenses. This guarantee does
not cover the batteries.
This guarantee is void under the following circumstances:
operator’s negligence
•
improper application
•
unauthorized servicing.
•
LIMITS OF LIABILITY
Under no circumstances shall LaMotte Company be liable for loss of life,
property, profits or other damages incurred through the use or misuse of
their products.
PA CKAGING AND DELIVERY
Experienced packaging personnel at LaMotte Company assure adequate
protection against normal hazards encountered during shipping. After the
product leaves the manufacturer, all responsibility for its safe delivery is
assured by the transporter. Damage claims must be filed immediately with
the transporter to receive compensation for damaged goods.
12
Page 11

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
SM
10/02
 Loading...
Loading...