Page 1

Page 2

Page 3

TABLE OF CONTENTS
Introduction..............................................4
Accessories...............................................4
TestMethods............................................. 5
UseoftheOcta-SlideViewer............................... 5
UseoftheDirectReadingTitrator........................... 6
SamplingDilutionTechniques ............................... 7
UnitsofMeasure .......................................... 8
Fertilizer Applications ...................................... 9
SoilSamplingProcedures .................................. 10
HowtouseaFunnelandFilterPaper ......................... 10
Test Procedures:
pH .................................................. 11
Extraction ............................................ 13
NeutralizationofSoilFiltrate ............................. 15
NitrateNitrogen ....................................... 16
Ammonia Nitrogen ..................................... 18
NitriteNitrogen ....................................... 19
Phosphorus ........................................... 20
PhosphorusinAlkalineSoils .......................... 21
Potassium ............................................ 22
Iron ................................................. 23
Sulfur................................................ 24
Copper .............................................. 26
Calcium&Magnesium .................................. 27
Chloride ............................................. 30
Aluminum............................................ 32
Manganese ........................................... 34
Humus............................................... 36
GreenPlantTissueTests ................................. 38
3
Page 4

INTRODUCTION
This instruction manual was written for use with LaMotte’s AST Series
Soil Test Kits. The Model AST-5 (5410) includes tests for pH, Nitrogen,
Phosphorus, Potassium, and Humus. The AST-15 (5412-01) contains all
of the tests included with Model AST-5, plus Calcium and Magnesium,
Ammonia Nitrogen, Manganese, Aluminum, Nitrite Nitrogen, Sulfur,
Chloride, Ferric Iron, and Copper. Instructions for all tests are included
in this manual.
ACCESSORIES
QUANTITY CONTENTS CODE
1 Brush, Test Tube 0514
1 Spoon, 0.5 g 0698
1 Demineralizer Bottle 1155
100 Soil Sample Bags 0615-J
2 x 50 Soil Test Report Forms 1626
1 AST Instruction Manual 36071
1 Improving Soil Sampling Accuracy 36150
1 A Study of Soil Science 1530
1 LaMotte Soil Handbook 1504
To reorder individual reagents or test kit components, use the specified
code numbers.
Read the Demineralizer Bottle Instruction Manual before proceeding.
This will be the source of all deionized water used in the tests.
4
Page 5
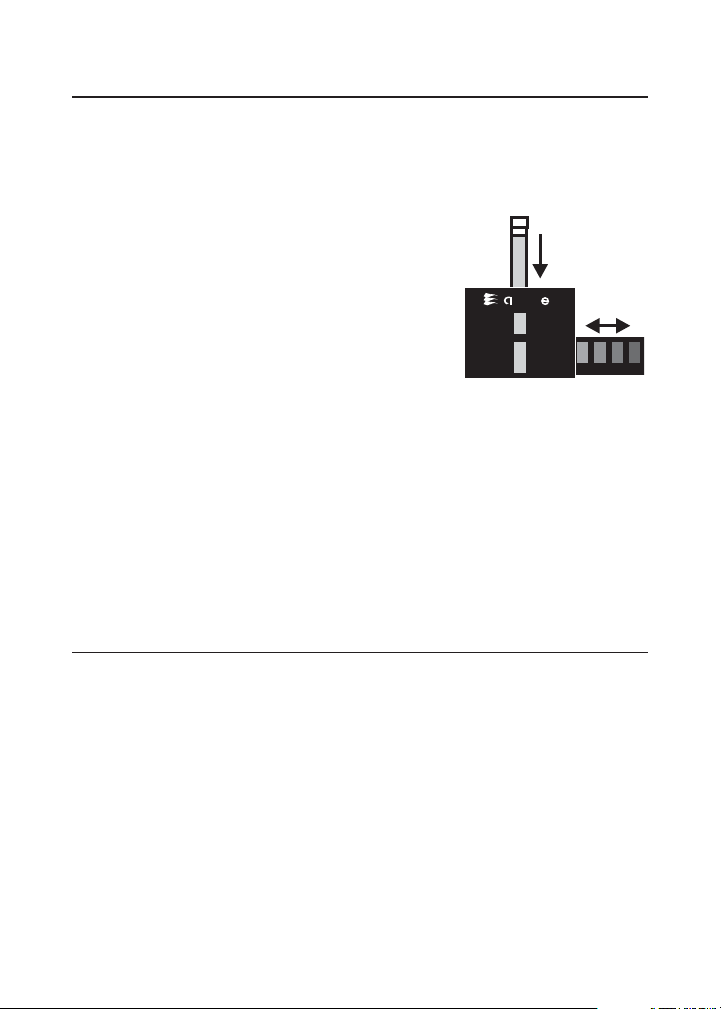
TEST METHODS
L
Mott
pH, Nitrate Nitrogen, Ammonia Nitrogen, Nitrite Nitrogen,
Phosphorus, Iron, and Sulfur test results are determined using an
Octa-Slide Viewer. In this method, the color or turbidity of the reacted
sample is matched to plastic color standards mounted in a black color
bar.
TheOcta-SlideViewershouldbeheldso
non-direct light enters through the back of the
viewer. With sample tube inserted at top, slide
the Octa-Slide bar through the viewer and
match with color standard.
Humus, Aluminum and Manganese test results
are determined using a color chart. After the
reaction is performed on a spot plate, the
resulting color is matched to a printed color
standard on a laminated chart.
The Copper test result is determined by a simple drop count. A standard
solution is added a drop at a time to an unreacted sample until it
matches the color of a reacted sample.
The Potassium test uses a Double Tube to measure the turbidity of the
sample formed by the reacted potassium.
Calcium, Magnesium, and Chloride test results are determined using a
Direct Reading Titrator, where small amounts of a titrant are added to
the sample until a specified color change occurs.
USE OF THE DIRECT READING TITRATOR
The Direct Reading Titrator consists of a plastic barrel, a plastic plunger,
and a plastic adapter tip. The adapter tip reduces the size of the drops
that are dispensed, increasing the precision of the test results. Detailed
instructions for the use of the Direct Reading Titrator are provided on
page 6.
see next page
5
Page 6
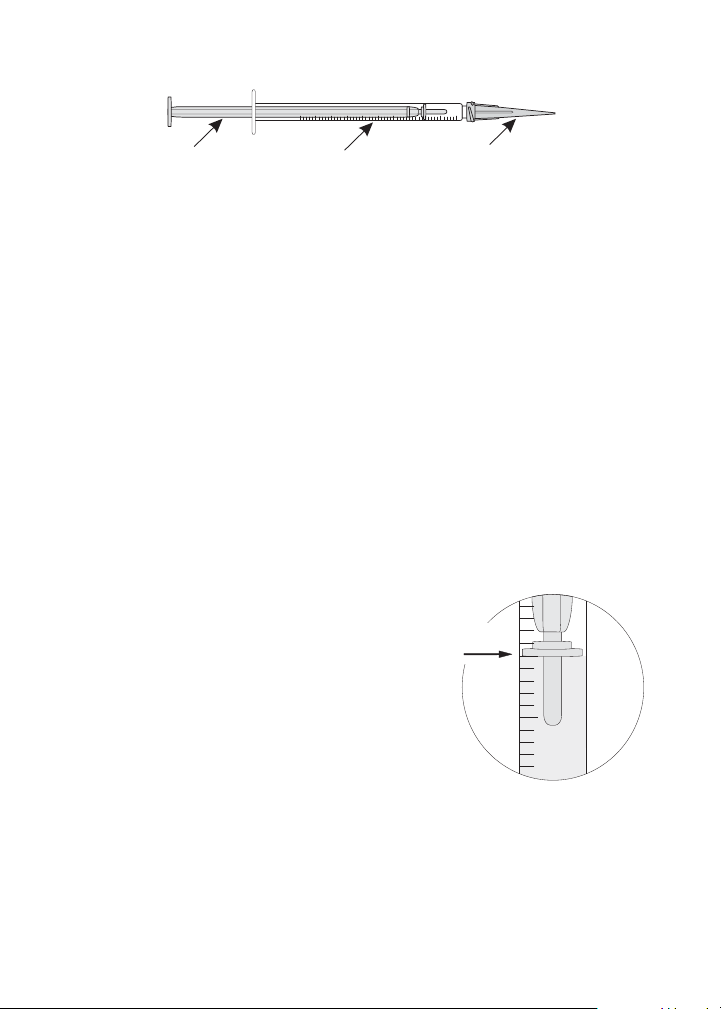
50
60
Result:
50 ppm
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
BarrelPlunger Adapter tip
1. Fill the test tube to the specified line with the water sample.
2. Add the reagents as specified in the instructions for the individual
test method. Cap the tube with the special test tube cap. Mix by
swirling gently.
3. Depress the plunger of the Titrator to expel air.
4. Insert the adapter tip into the special plastic plug in the titrating
solution bottle.
5. Invert the bottle. Hold the bottle and the Titrator firmly together.
Slowly pull out the plunger until the large ring on the plunger is
opposite the zero (0) line on the scale.
6. If an air bubble appears in the Titrator barrel or the adapter tip,
partially fill the barrel and pump the titration solution back into the
inverted reagent bottle to expel the bubble. Repeat this pumping
action until the bubble disappears.
7. Turn the bottle right-side-up and remove the Titrator.
8. Insert the adapter tip into the opening in the test tube cap. Slowly
depress the plunger to dispense the titrating solution. Gently swirl
tube to mix.
9. Continue adding the titrating solution
until the specified color change occurs. If
the color change has not occurred when
plunger reaches the bottom of the scale,
refill the Titrator to the zero (0) line.
Continue the titration until the color
change occurs.
10. Read the test result directly from the scale
where the large ring on the plunger meets
the Titrator barrel. If the Titrator was
refilled to reach the final color change, add the total amounts of
titrant used to determine the final test result.
11. If no additional tests are to be made, discard the titrating solution in
the Titrator. DO NOT return the titrating solution to the reagent
bottle. Thoroughly rinse the Titrator and the titration tube. DO
NOT remove the plunger or the adapter tip from the Titrator.
6
Page 7

SAMPLE DILUTION TECHNIQUES
In some tests the sample color may be darker than the darkest color
standard. When this occurs, the original sample must be diluted so an
accurate measurement can be made. Multiply the reading by the
appropriate dilution factor.
EXAMPLE: Measure 5 mL of the sample into a graduated cylinder.
Fill to the 10 mL line with deionized or distilled water. The sample
has been diluted by one-half, and the dilution factor is therefore 2.
Run the test procedure, and multiply the reading by 2 to obtain the
final result.
The following table provides dilution factors for several sample sizes:
Size
Of Sample
Distilled Water To Bring
Volume To 10 mL
Multiplication
Factor
10 mL 0 mL 1
5mL 5mL 2
2.5 mL 7.5 mL 4
1mL 9mL 10
0.5 mL 9.5 mL 20
NOTE: It is important to use pipets and graduated cylinders to make
accurate dilutions.
7
Page 8

UNITS OF MEASURE
All tests in the AST kits measure the concentration of the nutrients that
are available to the plants. The tests are conducted on soil extract, the
liquid formed by removing the nutrients from the soil. Since extraction
procedures remove varying amounts of nutrients, the test is dependent
upon the time and extracting solution used.
Test results are expressed in the following terms:
Parts Per Million
(ppm)
Pounds Per Acre
(lb/acre) Low To High
=
Calcium Nitrate Nitrogen Manganese
Magnesium Phosphorus Aluminum
Copper Potassium Humus
Sulfur Ammonia Nitrogen
Chloride Nitrate Nitrogen
Iron
=
Pounds per acre represents the number of pounds of soil in an acre to a
depth of 6 inches, or 2,000,000 pounds. Conversion from pounds to acre
to parts per million, or vice versa, can be accomplished using the
following equations:
ppm x 2 = lb/acre
lb/acre x 0.5 = ppm
8
Page 9
FERTILIZER APPLICATIONS
Test results should not be the only consideration when establishing a
fertilizer program. Soil composition, drainage, climate, previous fertilizer
programs, and the type of plant must also be considered when
determining the type and amount of fertilizer needed. The following
table offers quick-reference general guidelines to correlate soil test results
and fertilizer application rates. These guidelines can be supplemented by
the information in the LaMotte Soil Handbook (Code 1504). Consult
your local agricultural extension services for advice on establishing a
fertilizer program specific to your area.
General Guidelines For Fertilizer Application Rates
Nitrogen Phosphorus Potash (K2O)
Test
Result
Add
(lbs/acre)
Test
Result
Add
(lbs/acre)
Test
Result
Add
(lbs/acre)
10 220 10 260 100 180
20 180 25 220 140 150
40 130 50 180 180 130
60 110 75 150 220 110
100 90 100 130 300 90
150 40 150 90 400 70
9
Page 10
SOIL SAMPLING PROCEDURES
Detailed soil sampling procedures are described in the LaMotte Soil
Handbook (Code 1504) and in Improving Soil Sampling Accuracy (Code
36150).
The following procedure is recommended for sampling greenhouse soils.
1. Collect soil before watering.
2. Remove any mulch covering the soil. Use a soil sampling tube or
spoon to take a sample from the entire plant rooting surface, top to
bottom. Take 8 to 10 samples from the area.
3. Thoroughly mix the individual samples to form a composite sample.
Spread the mixed composite sample on a sheet of paper or plastic to
dry.
NOTE: A composite sample insures representative test results.
4. Sift the dried sample through a 10 mesh wire screen or 2 mm sieve.
Collect the soil. Discard particulate which remains on top of screen.
HOW TO USE A FUNNEL AND FILTER PAPER
A funnel and filter paper are used in the preparation of soil filtrate and
plant tissue extracts, and to filter the soil extract for the Phosphorus in
Alkaline Soils, Chloride, and Humus procedures.
1. Fold a piece of filter paper (0465) in half. Fold in half again.
2. With pointed end at the bottom, gently push corners together to
form a cone.
NOTE: There should be three layers on one side of the opening
and one layer on the opposite side.
3. Place in funnel (0459). Place funnel in container for collecting
filtrate.
4. Pour liquid through the funnel and filter paper to filter solution.
10
Page 11

pH
pH is a measure of alkalinity or acidity. The pH of soil ranges from 3.5 to
11.0, but research has found plants grow best in the range of 5.0 to 8.5.
In soils with low pH, some nutrients may reach toxic levels, and the
activity of soil microbes may be drastically reduced. Soils with a high pH
generally have a lower availability of micro-nutrients, and some
nutrients may not be present at sufficient levels.
A distilled water extraction procedure with a Flocculating Reagent
provides a clear liquid extract, to which *Wide Range Indicator is added.
The resulting color is matched to a color standard to determine the pH.
QUANTITY CONTENTS CODE
500 mL Tricon Flocculating Reagent 5941-L
30 mL *Wide Range Indicator *2218-G
1 Test tube, plastic, w/cap 0106
1 Spoon, 0.5 g, plastic 0698
1 Pipet, 1.0 mL, plastic 0354
1 Octa-Slide Viewer 1100
1 pH Wide Range Octa-Slide Bar 3424
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
11
Page 12

PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Fill a test tube (0106) to 5 mL line with Tricon Flocculating
Reagent (5941).
2. Use the 0.5 g spoon (0698) to add 3 level measures of the soil
sample. Cap and slowly invert back and forth for one minute to mix.
Wait for soil particles to settle.
3. Use a pipet (0354) to fill another tube (0106) to the 2.5 mL line
with the clear solution above the settled soil particles.
4. Add 6 drops of *Wide Range Indicator (2218). Cap and mix.
5. Insert test tube into Octa-Slide Viewer (1100). Slide the pH Wide
Range Octa-Slide Bar (3424) into the viewer. Match sample color
to a color standard. Record as pH.
NOTE: Liming tables are found in the LaMotte Soil Handbook
(Code 1504).
12
Page 13

EXTRACTION
The following procedures are used to extract the soil filtrate needed for
the Nitrate Nitrogen, Phosphorus, Potassium, Calcium, Magnesium,
Ammonia Nitrogen, Nitrite Nitrogen, Copper, Manganese, Iron, and
Aluminum tests. Separate extraction procedures are used for the
Chloride, Sulfur, pH, and Humus tests. Consult the LaMotte Soil
Handbook (Code 1504) for information on sampling and preparation of
soil for testing.
*Acid Extracting Solution (6361) is used to prepare Melich 1 extracting
solution. After dilution (performed during the extraction procedure) the
resulting solution is 0.05N Hydrochloric Acid and 0.025N Sulfuric
Acid.
NOTE: The Single Test Procedure will provide sufficient extract to do
one of each of the tests in the Model AST-5 (5410). The Multiple Test
Procedure should be used with the AST-15 (5412-01).
QUANTITY CONTENTS CODE
500 mL *Acid Extracting Solution *6361-L
60 mL Charcoal Suspension 5638-H
100 Filter Papers 0465
1 Funnel 0459
1 Pipet, 1.0 mL, plastic 0354
1 Test Tube, 5-10-15 mL, plastic, w/cap 0701
1 Spoon, 1 g, plastic 0697
1 Graduated Cylinder, glass, 100 mL 0419
1 Bottle, glass, 100 mL, w/cap 0990
1 Spoon, 0.5 g 0698
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
13
Page 14

SINGLE TEST PROCEDURE
1. Use the 1.0 mL pipet (0354) to add 1 mL of *Acid Extracting
Solution (6361) to the test tube (0701). Dilute to the 15 mL line
with deionized water.
2. Use the 1 g spoon (0697) to add 3 measures of soil. Add 0.5 mL of
Charcoal Suspension (5638). Cap and shake for five minutes.
3. Use funnel and filter paper to filter mixture (see How to Use a
Funnel and Filter Paper, page 10). Collect the filtrate. Use this
liquid as the extract for the Nitrate Nitrogen, Phosphorus,
Potassium, Calcium, Magnesium, Ammonia Nitrogen, Nitrite
Nitrogen, Copper, Manganese, and Aluminum tests.
MULTIPLE TEST PROCEDURE
1. Use the 1.0 mL pipet (0354) to add 5 mL of *Acid Extracting
Solution (6361) to the 100 mL graduated cylinder (0419). Dilute to
75 mL line with deionized water. Pour into 100 mL bottle (0990).
2. Use the soil measure (1165) to add 15 grams of soil. Add 2 mL of
Charcoal Suspension (5638). Cap and shake for 5 minutes.
3. Use funnel and filter paper to filter mixture (see How to Use a
Funnel and Filter Paper, page 10). Collect the liquid. Use this liquid
as the extract for the Nitrate Nitrogen, Phosphorus, Potassium,
Calcium, Magnesium, Ammonia Nitrogen, Nitrite Nitrogen,
Copper, Manganese, and Aluminum tests.
14
Page 15

NEUTRALIZATION OF SOIL FILTRATE
The extract used for the Calcium, Magnesium, Ammonia Nitrogen,
Copper and Iron tests must be neutralized before proceeding with the
test. Neutralization can be accomplished using the following procedure.
QUANTITY CONTENTS CODE
30 mL *Sodium Hydroxide Solution, 15% *7886WT-G
1 pH Hydrion Test papers, 4.5-7.5 2953
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
PROCEDURE
Add *Sodium Hydroxide Solution, 15% (7886) to the soil filtrate, one
drop at a time, until the pH test paper (2953) indicates that the pH is
between 6.0 and 7.0.
15
Page 16

NITRATE NITROGEN
Nitrogen, in the form of nitrate, is an important element in plant
growth. It is found in plant proteins, chlorophyll, nucleic acids, and
other plant structures, and adequate levels result in larger plants which
produce greater, more tender, yields. Since nitrogen is readily absorbed
by the plants, or leached from the soil, levels may change rapidly.
Cadmium in the *Nitrate Reducing Reagent reduces nitrate to nitrite
ions, which produce a red dyestuff through a diazotization reaction.
*Mixed Acid Reagent supplies the acid necessary for the reaction to
occur. The resulting color is matched to a color standard to determine
the nitrate nitrogen level.
QUANTITY CONTENTS CODE
500 mL *Mixed Acid Reagent *V-6278-L
2 x 10 g *Nitrate Reducing Reagent *V-6279-D
1 Test Tube, plastic, w/cap 0106
1 Spoon, 0.1 g, plastic 0699
1 Nitrate Nitrogen Octa-Slide Bar,
2.5-100ppm
*WARNING: Reagents marked with an * are considered to be potential health
hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone
or fax.
3422
16
Page 17

PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Fill a test tube (0106) to 5 mL line with soil extract.
2. Dilute to 10 mL line with *Mixed Acid Reagent (V-6278).
3. Use the 0.1 g spoon (0699) to add 2 measures of *Nitrate Reducing
Reagent (V-6279). Cap and invert 50-60 times in one minute to
mix. Wait 10 minutes.
NOTE: At the end of 10 minutes an undissolved portion of
*Nitrate Reducing Reagent may remain in the test tube. This will
not affect test results.
4. Invert the sample once to mix. Insert test tube into the Octa-Slide
Viewer (1100). Slide the Nitrate Nitrogen Octa-Slide Bar (3422)
into viewer. Match sample color to a color standard. Record as
lb/acre Nitrate Nitrogen.
NOTE: If sample color is darker than the highest color standard,
the sample must be diluted (see Sample Dilution Techniques,
page 7) and the test repeated.
17
Page 18

AMMONIA NITROGEN
Fertile soil will give low ammonia nitrogen readings, unless nitrogenous
fertilizer has recently been added. The rapid disappearance of ammonia
after fertilizer application indicates the ammonia has been transformed
to the more available nitrogen compounds, such as nitrate. In less fertile
forest soils ammonia is the most available form of nitrogen.
Nessler’s Reagent (*Ammonia Nitrogen Reagent #2) reacts in direct
proportion with the ammonia in the sample to form a reddish-brown
color. The resulting color is matched to a color standard to determine
the ammonia nitrogen concentration.
QUANTITY CONTENTS CODE
2 x 30 mL Ammonia Nitrogen Reagent #1 4797WT-G
2 x 30 mL *Ammonia Nitrogen Reagent #2 *4798PS-G
1 Test tube, plastic, w/cap 0106
1 Pipet, 0.5 mL, plastic 0353
1 Ammonia Nitrogen Octa-Slide Bar,
10-80 ppm
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Fill a test tube (0106) to 5.0 mL line with neutralized soil extract.
NOTE: See Neutralization of Soil Extract, page 15.
2. Add 6 drops of Ammonia Nitrogen Reagent #1 (4797). Cap and mix.
3. Use a 0.5 mL pipet (0353) to add 0.5 mL of *Ammonia Nitrogen
Reagent #2 (4798). Cap and mix. Wait 5 minutes.
4. Invert the sample once to mix. Insert test tube into the Octa-Slide
Viewer (1100). Slide the Ammonia Nitrogen Octa-Slide Bar (3425)
into viewer. Match sample color to a color standard. Record as
lb/acre Ammonia Nitrogen.
NOTE: If sample color is darker than the highest color standard,
the sample must be diluted (see Sample Dilution Techniques,
page 7) and the test repeated
18
3425
Page 19

NITRITE NITROGEN
Nitrites are formed as an intermediate step in the transformation of
ammonia to nitrate. This transformation is aided by well drained and
aerated by soil, so these soils often have low nitrite levels. Toxic levels of
nitrites may be found in poorly aerated soil, or in soils with high nitrate
levels, where a portion of the nitrate nitrogen decomposes to form nitrite.
Nitrite reacts with sulfanilamide in the *Color Developing Reagent
through a diazotization reaction to produce a pink azo dye. The resulting
color is matched to a color standard to determine the nitrite nitrogen
concentration.
QUANTITY CONTENTS CODE
500 mL *Mixed Acid Reagent *V-6278-L
2 x 10 g *Color Developing Reagent *V-6281-D
1 Test Tube, plastic, w/cap 0106
1 Spoon, 0.1g, plastic 0699
1 Nitrite in Soil Octa-Slide Bar,
0.5-25 lb/acre
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Fill test tube (0106) to 2.5 mL line with soil extract. Dilute to 5 mL
line with deionized water.
2. Dilute to 10 mL line with *Mixed Acid Reagent (V-6278).
3. Use the 0.1 g spoon (0699) to add 2 measures of *Color Developing
Reagent (V-6281). Cap and mix for one minute. Wait 5 minutes.
4. Invert the sample once to mix. Insert test tube into the Octa-Slide
Viewer (1100). Slide the Nitrite in Soil Octa-Slide Bar (3481) into
viewer. Match sample color to a color standard. Record as lb/acre
Nitrite Nitrogen.
NOTE: If sample color is darker than the highest color standard,
the sample must be diluted (see Sample Dilution Techniques,
page 7) and the test repeated.
19
3481
Page 20

PHOSPHORUS
Phosphorus is an important element for both plants and animals. It is
contained in the nucleus of the plant cell, which controls cell division
and growth, and has an important role in energy storage and chemical
transfer within the plant. Phosphorus is also important to fruiting and
seed production.
Phosphorus reacts with molybdate in *VM Phosphate Reagent to form a
phospho-molybdate compound. Reduction with stannous chloride in the
*Reducing Reagent produces a molybdenum blue color. The resulting
color is matched to a color standard to determine the phosphorus
concentration.
QUANTITY CONTENTS CODE
2 x 60 mL *VM Phosphate Reagent *4410-H
5 mL *Reducing Reagent *6405-C
2 x 60 mL *Special NF Phosphorus Extracting Solution *6362-H
60 mL Charcoal Suspension 5638-H
1 Test Tube, 5-10-15 mL, plastic, w/cap 0701
100 Filter Papers 0465
1 Funnel 0459
1 Test tube, plastic, w/cap 0106
1 Pipet, 1.0 mL, plastic 0354
1 Pipet, 0.5 mL, plastic 0353
1 Pipet, plain 0352
1 Spoon, 1 g, plastic 0697
1 Phosphorus Octa-Slide Bar, 15-150 lb/acre 3423
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
20
Page 21

PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Use a 1.0 mL pipet (0354) to add 1 mL of soil extract to a test tube
(0106). Dilute to 5 mL line with deionized water.
2. Use a 0.5 mL pipet (0353) to add 0.5 mL of *VM Phosphate (4410).
Cap and invert several times to mix. Wait 5 minutes.
3. Use the plain pipet (0352) to add 2 drops of *Reducing Reagent
(6405). Cap and mix. Solution should turn blue in 10 seconds.
4. Invert the sample once to mix. Insert test tube into the Octa-Slide
Viewer (1100). Slide the Phosphorus Octa-Slide (3423) into
viewer. Match sample color to a color standard. Record as lb/acre
Phosphorus.
NOTE: If sample color is darker than the highest color standard,
the sample must be diluted (see Sample Dilution Techniques,
page 7) and the test repeated.
PHOSPHORUS IN ALKALINE SOILS
A special extraction procedure is used for determining the available
phosphorus content of soils where the pH value is above 7.0.
1. Use the 1.0 mL pipet (0354) to add 1 mL of *Special NF
Phosphorus Extracting Solution (6362) to the test tube (0701).
Dilute to the 15 mL line with deionized water.
2. Use the 1 g spoon (0697) to add 3 measures of the soil sample. Add
0.5 mL of Charcoal Suspension (5638). Cap and shake for 5
minutes.
3. Use filter paper (0465) and funnel (0459) to filter solution (see
How to Use a Funnel and Filter Paper, page 10). Collect the filtrate.
Follow the Phosphorus procedure above.
21
Page 22

POT ASSIUM
Potassium is found in great supply as a component of common minerals,
but slow solubility limits availability to plants. Although it is not part of
the actual plant structure, potassium is important in many biochemical
functions, including cell division and resistance to disease.
Potassium present in an alkaline solution combines with sodium
tetraphenylboron in *Potassium TPB Solution, to form a potassium
tetraphenylboron precipitate. The resulting turbidity is used to
determine the potassium level.
QUANTITY CONTENTS CODE
250 mL *Potassium TPB Solution *3825-K
1 Double Tube, Potassium 0796
2 Pipets, 1.0 mL, plastic 0354
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
PROCEDURE
1. Use a 1.0 mL pipet (0354) to add 2 mL of soil extract to the round
tube (0796).
2. Use a second 1.0 mL pipet (0354) to add 2 mL of *Potassium TPB
Solution (3825). Wait 5 minutes.
3. Dilute to top line with deionized water. Cap and shake to mix.
4. Remove the cap and slowly insert the square tube with the collar.
The square tube will slide up and down through the collar and fill
with liquid.
5. Viewing from above, lower the square tube into the solution until
the black dot on the base can no longer be seen. Hold the round
tube at the top to avoid blocking the light.
6. Read the level of the liquid level in the square tube. Record as
lb/acre Potassium.
NOTE: To convert to potash, multiply result by 1.2.
22
Page 23

IRON
Only small quantities of iron are used by plants, but it is essential to
growth as an activator in numerous enzyme systems. Since iron is more
soluble in acidic solutions, it will be more available in soils with a low
pH.
A bipyridal indicator, *Iron Reagent #2 Powder, reacts with iron at the
proper pH to produce a pink color. The resulting color is matched to a
color standard to determine the iron concentration.
QUANTITY CONTENTS CODE
30 mL *Iron Reagent #1 *4450-G
10 g *Iron Reagent #2 Powder *V-4451-D
1 Test Tube, plastic, w/cap 0106
1 Spoon, 0.05 g, plastic 0696
1 Iron Octa-Slide Bar, 2.5-50 ppm 3479
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Fill test tube (0106) to 5 mL line with neutralized soil extract.
NOTE: See Neutralization of Soil Extract, page 15.
2. Add 5 drops of *Iron Reagent #1 (4450).
3. Use the 0.05 g spoon (0696) to add 1 measure of *Iron Reagent #2
(V-4451). Cap and mix until the powder has dissolved. Wait 5
minutes.
4. Invert the sample once to mix. Insert test tube into the Octa-Slide
Viewer (1100). Slide the Iron Octa-Slide Bar (3479) into viewer.
Match sample color to a color standard. Record as ppm Iron.
NOTE: If sample color is darker than the highest color standard,
the sample must be diluted (see Sample Dilution Techniques,
page 7) and the test repeated.
23
Page 24

SULFUR
Sulfur is essential to the formation of protein, and affects various aspects
of plant metabolism. Plants which are deficient in sulfur can be
distinguished by the pale green color and thin, reedy stems. The major
sources of sulfur are fertilizers containing sulfate compounds, and
atmospheric sulfur dioxide carried into the soil by precipitation.
*Sulfate Reagent contains barium chloride, which reacts with sulfur to
form a barium sulfate precipitate. The resulting turbidity is matched to a
standard to determine the sulfur concentration.
QUANTITY CONTENTS CODE
60 mL Charcoal Suspension 5638-H
10 g *Sulfate Reagent *V-6277-D
1 Test Tube, 5-10-15 mL, w/cap 0701
1 Spoon, 1.0 g, plastic 0697
100 Filter Papers 0465
1 Funnel 0459
1 Test tube, sulfur, turbidity, plastic, w/cap 0106-WL
1 Sulfur Octa-Slide Bar, 0-100 ppm 3480
*WARNING: Reagents marked with an * are considered to be potential health
hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone
or fax.
24
Page 25

PROCEDURE
Read Use of the Octa-Slide Viewer on page 5 before proceeding.
1. Fill the test tube (0701) to the 15 mL line with deionized water.
2. Use the 1.0 g spoon (0697) to add 3 measures of soil.
3. Add 0.5 mL of Charcoal Suspension (5638). Cap and shake for 5
minutes.
4. Use the filter paper (0465) and funnel (0459) to filter sample(see
page 10, How to Use a Funnel and Filter Paper). Collect liquid. Use
this liquid as the extract for the sulfur test.
5. Fill the tube (0106-WL) to the 5 mL line with soil extract. Dilute to
10 mL line with deionized water.
6. Use the 0.1 g spoon (0699) to add 1 measure of *Sulfate Reagent
(V-6277). Cap and shake until the powder is dissolved. A white
precipitate will form if sulfur is present. Wait 5 minutes.
7. Invert the sample once to mix. Insert test tube into the Octa-Slide
Viewer (1100). Place tube in Viewer with printing facing away from
operator. Slide the Sulfur Octa-Slide Bar (3480) into viewer. Match
sample color to a color standard. Record as ppm Sulfur.
NOTE: If sample turbidity is greater than the highest standard, the
sample must be diluted (see Sample Dilution Techniques,
page 7) and the test repeated.
25
Page 26

COPPER
Copper acts as a catalyst in enzyme systems. In acidic soils, aluminum
may compete with copper, resulting in decreased uptake by plants. The
balance of copper with iron and molybdenum may be more important
than the actual amounts present in the plant.
A Standard Color Solution is added to an untreated sample until it
matches the color of the sample in which the copper has reacted with
sodium diethyeldithiocarbamate in the Copper Reagent.
QUANTITY CONTENTS CODE
2 x 15 mL *Copper 1 Reagent *6446-E
2 x 15 mL Copper 2 Reagent 6613-E
2 Test tubes, plastic, w/cap 0106
1 Sheet, white, plastic 32961
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
PROCEDURE
1. Fill two test tubes (0106) to the 10 mL line with neutralized soil
extract.
NOTE: See Neutralization of Soil Extract, page 15.
2. Add 5 drops of *Copper Reagent (6446) to one test tube. Cap and
mix. Remove cap.
3. Hold both test tubes one-half inch above the white plastic sheet.
The extract with the reagent will appear yellow if copper is present.
4. Add Copper 2 Reagent (6613) to the second, untreated sample, one
drop at a time, with mixing, until the color of the two samples is the
same. Count the number of drops added. Hold bottle vertically.
5. Multiply number of drops of Copper 2 Reagent used in Step 4 by
0.25. Record as ppm Copper.
1 ppm = 4 drops
2 ppm = 8 drops
3 ppm = 12 drops
26
Page 27

CALCIUM & MAGNESIUM
Calcium is found in rapidly growing root tips, indicating that it is a
necessary ingredient for cell division. It also tends to make cells more
selective in their absorption of nutrients.
Magnesium is an ingredient in chlorophyll which makes plants green. It
also aids in the formation of fats and oils, as well as phosphorus uptake.
The Schwarzenbach EDTA titration, used to determine calcium and
magnesium levels, involves two separate titrations. The first titration
determines the combined calcium and magnesium level, and the second
titration indicates the calcium level only. Magnesium is determined by
calculation.
QUANTITY CONTENTS CODE
30 mL *Sodium Hydroxide Solution, 15% *7886WT-G
30 mL Calcium-Magnesium Inhibitor Reagent 3922-G
30 mL *Calcium & Magnesium Buffer *5126-G
2 x 15 mL *CM Indicator Reagent *6522-E
250 mL Standard EDTA Reagent 5254-K
15 mL *Inhibitor Solution *9258-E
15 mL *TEA Reagent * 3921-E
30 mL *Sodium Hydroxide Reagent
w/Metal Inhibitors
100 Calcium Hardness Indicator Tablets T-5250-J
1 pH Hydrion Test papers, 4.5-7.5 2953
1 Graduated Cylinder, glass, 25 mL 0417
1 Beaker, plastic, 50 mL 0944
1 Test Tube, 5-10-15 mL, glass, w/cap 0778
1 Direct Reading Titrator, 0-1000 Range 0384
*WARNING: Reagents marked with an * are considered to be potential health
hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone
or fax.
*4259-G
27
Page 28

PROCEDURE
Read the Direct Reading Titrator instructions on page 6 before
proceeding.
DILUTION OF SOIL EXTRACT
1. Use the graduated cylinder (0417) to transfer 10 mL of soil extract
to a 50 mL beaker (0944).
2. Add10mLofdeionizedwater.Mixandneutralize.
NOTE: See Neutralization of Soil Extract, page 15.
TITRATION A: CALCIUM & MAGNESIUM
1. Fill test tube (0778) to 5 mL line with diluted soil extract. Dilute to
10 mL line with deionized water.
2. Add 5 drops of Calcium-Magnesium Inhibitor Reagent (3922).
Swirl to mix. Wait 5 minutes.
3. Add 5 drops of *Calcium & Magnesium Buffer (5126). Swirl to mix.
4. Add 10 drops of *CM Indicator Reagent (6522). Swirl to mix.
Solution will turn red.
5. Fill the Direct Reading Titrator (0384) with Standard EDTA
Reagent (5254). Insert Titrator tip into the center hole of the test
tube cap.
6. While swirling the tube, slowly press the plunger to titrate sample
until color changes from red to blue.
7. Read the result where the plunger tip meets the scale. Multiply by
5.16. Record as Value A.
28
Page 29

TITRATION B: CALCIUM
1. Fill test tube (0778) to 5 mL line with diluted soil extract. Dilute to
10 mL line with deionized water.
2. Add 2 drops of *Inhibitor Solution (9258). Swirl to mix.
3. Add 2 drops of *TEA Reagent (3921). Swirl to mix.
4. Add 8 drops of *Sodium Hydroxide Reagent w/Metal Inhibitors
(4259). Swirl to mix.
5. Add one Calcium Hardness Indicator Tablet (T-5250). Cap and
swirl until tablet disintegrates. Solution will turn red.
6. Fill the Direct Reading Titrator (0384) with Standard EDTA
Reagent (5254). Insert Titrator tip into the center hole of the test
tube cap.
7. While swirling the tube, slowly press the plunger to titrate sample
until color changes from red to blue, and does not revert to red for at
least one minute.
8. Read the result where the plunger tip meets the scale. Multiply by
5.16. Record as Value B.
FINAL RESULTS
Calcium:
Value B x 0.4 = ppm Ca
Magnesium:
0.24 (Value A - Value B) = ppm Mg
NOTE: To obtain results in lb/acre, multiply results by 2.
29
Page 30

CHLORIDE
No natural soil deficiencies of chloride are known to exist. Chloridecontaining fertilizers may lead to excessive or even toxic levels. A high
test reading, particularly where stunted growth has been observed, may
indicate poisoning due to high levels.
This test is valuable on saline soils or when contamination from sea
water or sea spray is suspected. Normal soils of humid regions rarely give
readable test results, except when liberal amounts of chloride-containing
fertilizers were recently applied.
Chloride is titrated with silver nitrate in *Chlorine Reagent 2S, after
potassium dichromate in *Chloride Reagent #1 has been added as an
indicator. The final result is read directly from the Titrator.
QUANTITY CONTENTS CODE
60 mL Charcoal Suspension 5638-H
15 mL *Chloride Reagent #1 *4504-E
2 x 60 mL *Chloride Reagent 2S *7624DR-H
1 Test Tube, 5-10-15 mL, w/cap 0701
1 Spoon, 1 g, plastic 0697
100 Filter papers 0465
1 Funnel 0459
1 Test Tube, 5-10-15 mL, w/cap 0778
1 Direct Reading Titrator, 0-1000 Range 0384
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
30
Page 31

PROCEDURE
Read the Direct Reading Titrator instructions on page 6 before
proceeding. The Titrator is calibrated in ppm chloride. Each minor
division on the Titrator scale equals 20 ppm.
1. Fill a clean test tube (0701) to 15 mL line with deionized water.
2. Use the 1 g spoon (0697) to add 3 measures of soil sample. Add 0.5
mL of Charcoal Suspension (5638). Cap and shake for 5 minutes.
3. Use funnel (0459) and filter paper (0465) to filter soil (see How to
Use a Funnel and Filter Paper, page 10). Collect the soil filtrate. It
will be used as the extract for the chloride test.
NOTE: The extract may be slightly turbid. This will not interfere
with the test result.
4. Fill the test tube (0778) to 10 mL line with soil extract.
5. Add 3 drops of *Chloride Reagent #1 (4504). Cap and mix.
Solution should turn yellow.
6. Fill the Titrator (0384) with *Chloride Reagent 2S (7624). Insert
Titrator into center hole of test tube cap.
7. While gently swirling the tube, slowly press plunger to titrate with
*Chloride Reagent 2S until yellow color changes permanently to
brick-red. Read result where plunger meets the scale. Record as ppm
Chloride.
8. If the Titrator reaches the bottom mark on the scale before the color
change occurs, refill and continue titration. Include original amount
(1000 ppm) in final result.
31
Page 32

ALUMINUM
All soils contain significant concentrations of aluminum, in the form of
inorganic colloidal material and undecomposed minerals. In neutral,
slightly alkaline, or slightly acid soils the aluminum is in inert
combinations that do not affect plant growth. In more acidic soils,
aluminum can form potentially toxic salts. A high test result indicates an
undesirable acid soil. Plants which normally thrive on acid soils may fail
in a soil with a high active aluminum test reading. A medium test result
is tolerable to many plants, including grasses, corn, oats, potatoes, and
tobacco; a low or negative aluminum result is preferable.
Aluminum reacts with hematein in the *Aluminum Test Solution to
form a colored solution. The resulting color is matched to a color chart
to determine the aluminum concentration.
QUANTITY CONTENTS CODE
30 mL *Aluminum Test Solution *5101-G
1 Pipet, transfer 0364
1 Spot Plate 0159
1 Pipet, transfer, plastic 0364
1 Stirring rod, plastic 0519
1 Aluminum in Soil Color Chart 1301
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
32
Page 33

PROCEDURE
1. Use pipet (0364) to add 2 drops of soil extract to the large
depression on a spot plate (0159).
2. Add 2 drops of deionized water.
3. Use a clean pipet (0364) to add 1 drop of *Aluminum Test Solution
(5101). Use a stirring rod (0519) to mix. Wait one minute.
4. Match sample color to a color standard on the Aluminum in Soil
Color Chart (1301). Record result. Use chart below to convert
reading to an approximate concentration. Record as ppm
Aluminum.
Very Low 5 ppm
Low 10 ppm
Medium 30 ppm
High 80 ppm
Very High 125 ppm
33
Page 34

MANGANESE
An essential element in the enzyme system of plants, manganese plays a
role in metabolic reactions affecting germination, photosynthesis, and
other vital aspects of plant development. Yellowing and stunted growth
may indicate manganese deficiency.
Some insoluble manganese is present in all soils, and its solubility or
availability is related to the pH. Calcareous soils, or soils which have
been heavily limed, may be deficient in manganese, which can be
corrected by applying manganese sulfate or another soluble manganese
salt. Highly acid soils may have extremely high, even toxic, levels of
manganese, which can be lowered by applying lime.
Since available manganese may be leached from the soil, or may be
altered to less available forms by oxidation, tests should be performed
just before planting and during plant growth. A positive test reading,
even a very low reading, generally indicates sufficient available
manganese to meet plant requirements. A high test reading is
undesirable and indicates the need for lime.
Periodate oxidizes soluable manganous compounds to form
permanganate.
QUANTITY CONTENTS CODE
10 g Manganese Buffer Reagent 6310-D
30 mL *Manganese Periodate Reagent *6311-E
1 Spot Plate 0159
1 Pipet, transfer, plastic 0364
2 Spoons, 0.05 g 0696
1 Manganese in Soil Color Chart 1307-01
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
34
Page 35

PROCEDURE
1. Use a transfer pipet (0364) to add 10 drops of soil extract to the
large depression on a spot plate.
2. Use the 0.05 g spoon (0696) to add one measure of Manganese
Buffer Reagent (6310). Mix with a clean stirring rod (0519) until
the powder dissolves.
3. Use the other 0.05 g spoon (0696) to add one measure of
*Manganese Periodate Reagent (6311). Mix with a clean stirring
rod for 20 seconds.
NOTE: Immediately clean the spot plate to prevent staining.
4. Match the color in the spot plate to a color standard on the
Manganese in Soil Color Chart (1307-01). Record as ppm
Manganese.
Low 5 ppm
Medium 12 ppm
High 25 ppm
Very High 40 ppm
35
Page 36

HUMUS
Humus consists of the complex remains of fresh plant and animal residue
after extensive chemical and biological breakdown. It accounts for 60 to
70% of the total organic carbon in soil. It can modify the physical
properties of soil, affecting the chemical and biological properties.
*Humus Screening Reagent Powder is EDTA, which extracts the humus
from the soil. The resulting color is matched to a color standard to
determine the humus concentration.
QUANTITY CONTENTS CODE
50 g *Humus Screening Reagent Powder *5119-H
2 x 60 mL Soil Flocculating Reagent 5643WT-H
1 Extraction tube 0704
2 Spoon, 0.5 g, plastic 0698
100 F ilter Papers 0465
1 Funnel 0459
1 Humus Color Chart 1384
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these
reagents go to www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
36
Page 37

PROCEDURE
1. Use the 0.5 spoon (0698) to add 8 level measures of soil to an
extraction tube (0704).
2. Add deionized or tap water to 14 mL line. Cap and shake to mix.
3. Use the 0.5 g spoon (0698) to add 2 measures *Humus Screening
Reagent Powder (5119). If necessary, add more water to bring level
to 14 mL mark. Cap and mix vigorously for one minute.
4. Add 15 drops of Soil Flocculating Reagent (5643WT). Cap and mix
gently. Allow the soil to settle for several minutes.
5. Use filter paper (0465) and funnel (0459) to filter mixture (see How
to Use a Funnel and Filter Paper, page 10). Collect filtrate in a
second extraction tube.
6. Match sample color of the filtrate to a color standard on the Humus
Color Chart (1384). Record result. Use chart to convert result to a
value.
HumusorOrganicMatterinSoil
HumusReading 12345
Agricultural Soils
Garden Greenhouse Soils
Organic Soils
Low Medium High
Low Medium High
Low Medium High
37
Page 38

GREEN PLANT TISSUE TESTS
Nutrient deficiencies during plant growth can be verified by using an
extract prepared from fresh plant tissue. A procedure for testing nitrate
nitrogen, phosphorus, and potassium in plant tissue is described below.
Additional information on plant tissue testing is discussed in the
LaMotte Soil Handbook (Code 1504).
These tests are meant to be used in a comparative manner. When testing
plant tissue, it is important to compare tissue from healthy plants to
tissue from the problem plants. Plants of the same species, the same age
and grown in the same environment should be compared. Since test
reactions vary from species to species, and even from environment to
environment within a species, it is not possible to accurately quantify
results. Relative values from very deficient to abundant have been
assigned to the range of possible test reactions under each factor below.
PREPARATION OF TISSUE EXTRACT
1. Select a small lot of the leaf petioles or succulent portions of the
stem. When testing problem plants, collect tissues from those areas
where the abnormality is most visible.
2. Use a clean, sharp knife or razor blade to cut the material into small
pieces, not more than 1/8" to 1/16" in length and thickness.
3. Fill a extraction vial (0701) half way with plant material.
NOTE: Do not pack material into vial.
4. Use a 1 mL pipet (0354) to add 1 mL of *Acid Extracting Solution
(6361).
5. Dilute to line with deionized water. Cap and shake for 5 minutes.
6. Use filter paper (0465) and a funnel (0459) to filter sample (see
How to Use a Funnel and Filter Paper, page 10). Collect filtrate in a
second vial. Use tissue extract in test procedures instead of soil
extract.
38
Page 39

PLANT TISSUE PROCEDURE
Follow the test procedures for nitrate nitrogen, phosphorus and
potassium, using tissue extract instead of soil extract. Results should be
used in a comparative manner, which can be used with the lists below to
determine relative concentrations. The most meaningful test results will
be obtained by comparing healthy plants to problem plants.
Guidelines for Interpreting Plant Tissue Tests
Relative Amount Of
Test F a c tor Test Re a c tion
Nutrient In Plant Tissue
Nitrate Nitrogen Dark Pink Color Abundant
Light Pink Color Adequate
Colorless No Reserve/Probably Deficient
Phosphorus Deep Blue Color Adequate
Light Blue Color Adequate
Yellow to Colorless Low to Deficient
Potassium Heavy Precipitate Adequate to Abundant
Medium Precipitate Low to Deficient
Trace Precipitate Deficient
No Precipitate Very Deficient
39
Page 40

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-6394(Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
36071-7/11
 Loading...
Loading...