Page 1
AM MO NIA NI TRO GEN TEST KIT
L
Mott
WARNING! This set contains chemicals
that may be harmful if misused. Read
cautions on individual containers
carefully. Not to be used by children
except under adult supervision
OCTA-SLIDE METHOD
MODEL SL-NH • CODE 3351-01
QUANTITY CONTENTS CODE
30 mL Ammonia Nitrogen Reagent #1 4797WT-G
30 mL *Ammonia Nitrogen Reagent #2 *4798WT-G
2 Test Tubes, 2.5-5.0-10.0 mL, plastic, w/caps 0106
1 Ammonia Nitrogen Octa-Slide Bar,
0.2-3.0 ppm
1 Octa-Slide Viewer 1100
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
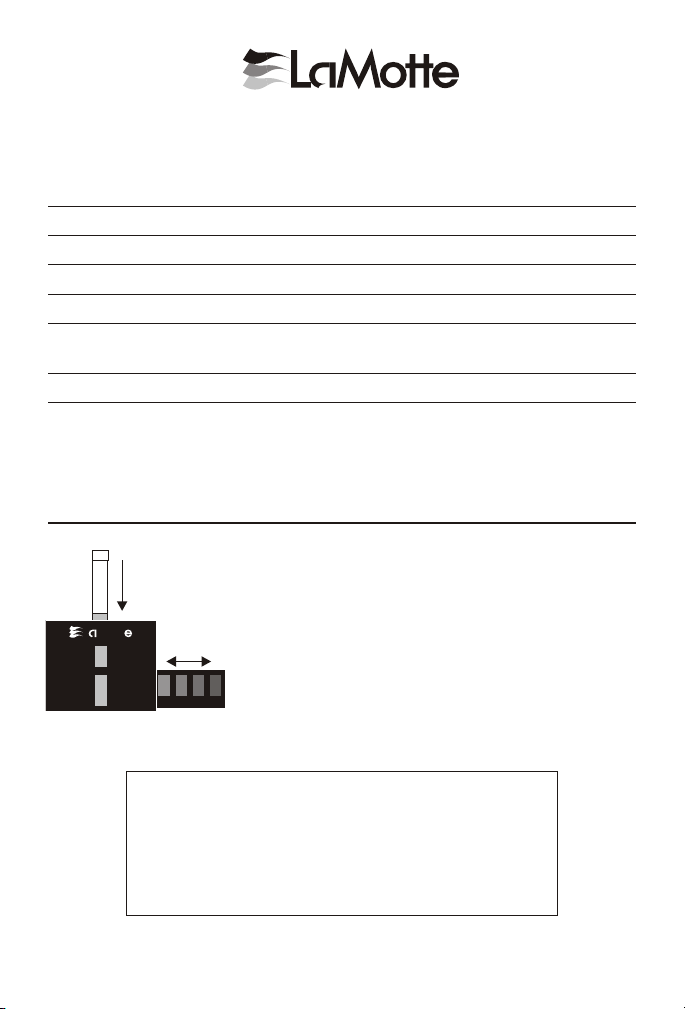
USE OF THE OCTA-SLIDE VIEWER
The Octa-Slide Viewer should be held so non-direct
light enters through the back of the viewer. With
sample tube inserted at top, slide the Octa-Slide bar
through the viewer and match with color standard.
3438
Page 2

PRO CE DURE
1. Fill test tube (0106) to the 5 mL line with sample water.
2. Add 4 drops of Ammonia Nitrogen Reagent #1 (4797WT). Cap and
mix. Wait 1 minute.
NOTE: When testing salt (sea) water, increase the amount of
Ammonia Nitrogen Reagent #1 to 8 drops.
3. Add 12 drops of *Ammonia Nitrogen Reagent #2 (4798WT). Cap
and mix. Wait five minutes.
NOTE: When testing salt water, the reading should be taken after
1 minute to prevent precipitation.
4. Insert the Ammonia Nitrogen Octa-Slide (3438) into the Octa-Slide
Viewer (1100). Insert test tube into the top of the viewer. Match
sample color to a color standard. Record as ppm Ammonia Nitrogen
(NH3-N).
To express results as Unionized Ammonia (NH3):
ppm Unionized Ammonia (NH3) =
ppm Ammonia Nitrogen (NH3-N) x 1.2
To express results as Ionized Ammonia (NH
ppm Ionized Ammonia (NH
+
):
4
+
) =
4
ppm Ammonia Nitrogen (NH3–N) x 1.3
Ammonia in water occurs in two forms: toxic unionized ammonia (NH3) and
the relatively non-toxic ionized form, ammonium ion (NH
+
). This test
4
method measures both forms as ammonia nitrogen (NH3–N) to give the total
ammonia-nitrogen concentration in water. The actual proportion of each
compound depends on temperature, salinity, and pH. A greater concentration
of unionized ammonia is present when the pH value and salinity increase.
1. Consult the table to find the percentage that corresponds to the
temperature, pH, and salinity of the sample.
2. To express the test result as ppm Unionized Ammonia Nitrogen
(NH3–N), multiply the total ammonia nitrogen test result by the
percentage from the table.
3. To express the test result as ppm Ionized Ammonia Nitrogen
+
(NH
–N), subtract the unionized ammonia-nitrogen determined in
4
step 2 from the total ammonia nitrogen.
Page 3

10°C 15°C 20°C 25°C
pH FW1SW
2
FW SW FW SW FW SW
7.0 0.19 — 0.27 — 0.40 — 0.55 —
7.1 0.23 — 0.34 — 0.50 — 0.70 —
7.2 0.29 — 0.43 — 0.63 — 0.88 —
7.3 0.37 — 0.54 — 0.79 — 1.10 —
7.4 0.47 — 0.68 — 0.99 — 1.38 —
7.5 0.59 0.459 0.85 0.665 1.24 0.963 1.73 1.39
7.6 0.74 0.577 1.07 0.836 1.56 1.21 2.17 1.75
7.7 0.92 0.726 1.35 1.05 1.96 1.52 2.72 2.19
7.8 1.16 0.912 1.69 1.32 2.45 1.90 3.39 2.74
7.9 1.46 1.15 2.12 1.66 3.06 2.39 4.24 3.43
8.0 1.83 1.44 2.65 2.07 3.83 2.98 5.28 4.28
8.1 2.29 1.80 3.32 2.60 4.77 3.73 6.55 5.32
8.2 2.86 2.26 4.14 3.25 5.94 4.65 8.11 6.61
8.3 3.58 2.83 5.16 4.06 7.36 5.78 10.00 8.18
8.4 4.46 3.54 6.41 5.05 9.09 7.17 12.27 10.10
8.5 5.55 4.41 7.98 6.28 11.18 8.87 14.97 12.40
1
Freshwater data from Trussel (1972).
2
Seawater values from Bower & Bidwell (1978). Salinity for Seawater values = 34% at
an ionic strength of 0.701 m.
FOR EXAMPLE:
A fresh water sample at 20°C has a pH of 8.5 and the test result is 1.0 ppm as
total Ammonia-Nitrogen.
1. The percentage from the table is 11.18% (or 0.1118).
2. 1 ppm total Ammonia Nitrogen x 0.1118 = 0.1118 ppm Unionized
Ammonia-Nitrogen
3.
Total Ammonia-Nitrogen 1.0000 ppm
Unionized Ammonia-Nitrogen
0.1118 ppm
-
Ionized Ammonia-Nitrogen = 0.8882 ppm
Page 4

LaMOTTE COM PANY
Helping Peo ple Solve An a lyt i cal Chal lenges
PO Box 329 • Chestertown • Mary land • 21620 • USA
800-344-3100 • 410-778-3100 (Out side U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
SM
8/11
 Loading...
Loading...