Page 1

Page 2

WARNING! This set contains chemicals
that may be harmful if misused. Read
cautions on individual containers
carefully. Not to be used by children
except under adult supervision
Page 3

Index of Tests
Page
AlkalinityTest ...............6
FieldTestMethod.................7
CalculationofAlkalinityRelationships ......... 8
Overhead Projection Demonstration .......... 9
AmmoniaNitrogenTest............ 10
FieldTestMethod ................ 10
Overhead Projection Demonstration .......... 11
Calcium,Magnesium,&TotalHardnessTests..... 12
FieldTestMethod-TotalHardness.......... 13
FieldTestMethod-CalciumHardness......... 14
Overhead Projection Demonstration .......... 15
FreeCarbonDioxideTest........... 17
FieldTestMethod ................ 17
Overhead Projection Demonstration .......... 18
ChlorideTest............... 19
FieldTestMethod ................ 20
Overhead Projection Demonstration .......... 20
ChlorineTest............... 21
FieldTestMethod ................ 21
Overhead Projection Demonstration .......... 22
Chromium(Chromate)Test .......... 23
FieldTestMethod ................ 23
Overhead Projection Demonstration .......... 24
CopperTest ............... 25
FieldTestMethod ................ 25
Overhead Projection Demonstration .......... 26
CyanideTest............... 27
FieldTestMethod ................ 28
Overhead Projection Demonstration .......... 29
IronTest................. 31
FieldTestMethod ................ 31
Overhead Projection Demonstration .......... 32
3
Page 4

Page
NitrateTest................ 33
FieldTestMethod ................ 34
Overhead Projection Demonstration .......... 35
pHTest................. 36
FieldTestMethod ................ 36
Overhead Projection Demonstration .......... 37
Phosphorus(Phosphate)Test.......... 38
FieldTestMethod ................ 39
Overhead Projection Demonstration .......... 40
SalinityTest................ 41
FieldTestMethods................ 42
Overhead Projection Demonstration .......... 42
SulfideTest................ 43
FieldTestMethods................ 44
Overhead Projection Demonstration .......... 45
TotalDissolvedSolidsTest........... 46
FieldTestMethods................ 47
CareofResinColumn............... 48
Overhead Projection Demonstration .......... 49
Care of Resi n Column 50
4
Page 5

Introduction
This kit employs two typical quantitative chemical test methods:
colorimetric comparison with standards of known value or titration of the
sample with solutions of known value. The reagent systems employed in
these testing sets can also be used to make simple qualitative tests where the
presence or absence (not the amount) of the factor being investigated is of
concern to the investigator. These qualitative tests can be conducted in the
field or in the classroom where the overhead projector can be used to project
the colorful reactions.
The colorimetric comparison outfits provide color standards of known
values. If the color of the test sample does not match the color of one of the
standards, but is between two color standards, the value assigned to the test
sample is the midpoint between the two standards that bracket the color of
the sample. For example, if the color of the test sample is between the colors
of 0.2 and 0.6 ppm, the result is read as 0.4 ppm. In the pH test, if the color
of the sample is between pH 7.6 and 7.8, the result is read as pH 7.7. When
the color of the test sample (other than pH) is greater than the standard of
the highest value, the test is repeated on a portion of the test sample that has
been diluted on a one-to-one ratio with distilled water . The values of the
color standards are multiplied by a factor of 2 to compensate for the dilution.
Dilutions of higher ratio can be made; however, it must be remembered that
the values of the standards must be multiplied by the ratio of the dilution. A
dilution procedure cannot be used when measuring pH.
The titration procedures are performed using a Direct Reading Titrator
which accurately measures the amount of titration reagent used. Carefully
read the enclosed instruction manual on the Direct Reading Titrator before
performing any of the titrations.
5
Page 6
Alkalinity Test
The normal conditi ons of the alkalini ty of natural waters are associated with
the carbon dioxide, bicarbonate, carbonate and hydroxide components.
These factors are characteristic of the source of water and the natural
processes taking place at any given time. For particular industrial and
domestic use, it is often desirable to change these characteristics by
treatments such as aeration, neutralization, softening, etc. The particular
treatment and the extent to which it is employed will depend upon the end
use of the water .
Alkalinity of a water is determined by titration with a standard acid to
successive indicator endpoints, thus permitting the calculation of the various
forms of alkalinity.
Field T est Method
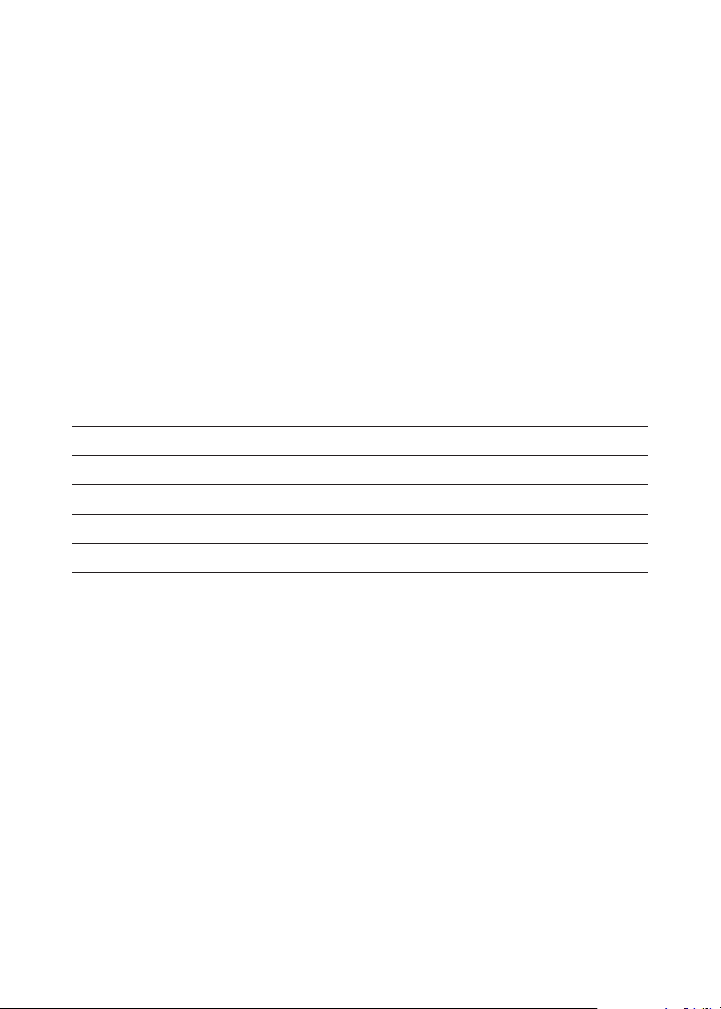
Quantity Contents Code
15 mL Total Alkalinity Indicator 2786-E
100 Phenolphthalein Tablets T-2246-J
30 mL *Alkalinity Titration Reagent B *4493-G
1 Test Tube, 5-10-15 mL, glass, w/cap 0778
1 Direct Reading Titrator, 0-200 Range 0382
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
The alkalinity titration tube is calibrated so that the result can be read
directly from the scale on the titrator in ppm calcium carbonate (CaCO
The result can be translated to grains per gallon by multiplying the reading
by the factor 0.0585.
Read the LaMotte Direct Reading Titrator Manual before prceeding.
).
3
6
Page 7

Procedure
1. Fill the test tube (0778) to the 5.0 mL line with the sample water.
2. Add one Phenolphthalein Table (T -2246). Cap and mix until the tablet
is disintegrated. If no red color develops, the “P” Alkalinity is zero. If the
“P” Alkalinity is zero, go to Step 5.
3. Fill the Direct Reading Titrator (0382) with *Alkalinity Titration
Reagent B (4493).
4. Insert the titrator tip into the test tube cap. Slowly add *Alkalinity
T itration Reagent B (4493) while swirling to mix, until the red color
disappears. Read the test result directly from the scale where the large
ring on the Titrator meets the T itrator barrel. This is the “P” or
Phenolphthalein Alkalinity. (Do not refill the Titrator for Step 6.)
5. Remove the cap and add 3 drops of Total Alkalinity Indicator (2786) to
the test sample. Replace the cap and swirl the tube to mix the indicator
with the sample.
6. Continue to add the *Alkalinity Titration Reagent B (4493) with
mixing until the color of the sample changes from greenish blue to a
definite pink color. This is the “T” or Total Alkalinity reading, also know
as the “M” Alkalinity.
7
Page 8

Calculation of Alkalinity Relationships
The results obtained from the phenolphthalein and total alkalinity
determination offer a means for the stoichiometric classification of the three
principal forms of alkalinity present in many water supplies. The
classification ascribes the entire alkalinity to bicarbonate, carbonate, and
hydroxide; and assumes the absence of other weak acids of inorganic or
organic composition, such as silicic, phosphoric, and boric. This
classification system further presupposes the incompatibility of hydroxide
and bicarbonate alkalinities in the same sample. Since the calculations are
on a stoichiometric basis, ion concentrations in the strictest sense are not
represented in the results.
Carbonate alkalinity is present when the phenolphthalein alkalinity is not
zero but is less than the total alkalinity.
Hydroxide alkalinity is present if the phenolphthalein alkalinity is more than
one-half the total alkalinity .
Bicarbonate alkalinity is present if the phenolphthalein alkalinity is less than
one-half the total alkalinity .
The mathematical conversion of the results is shown in the following table:
Relationships Between Phenolphthalein Alkalinity, Total Alkalinity ,
Carbonate Alkalinity, And Hydroxide Alkalinity:
Result of
Titration
P=0 0 0 T
P<½T 0 2P T-2P
P=½T 0 2P 0
P>½T 2P-T 2(T-P) 0
P=T T 0 0T
Hydroxide
Alkalinity
as CaCO
Carbonate
Alkalinity as
3
CaCO
8
3
Bicarbonate
Alkalinity
as CaCO
3
Page 9
Overhead Projection Demonstration
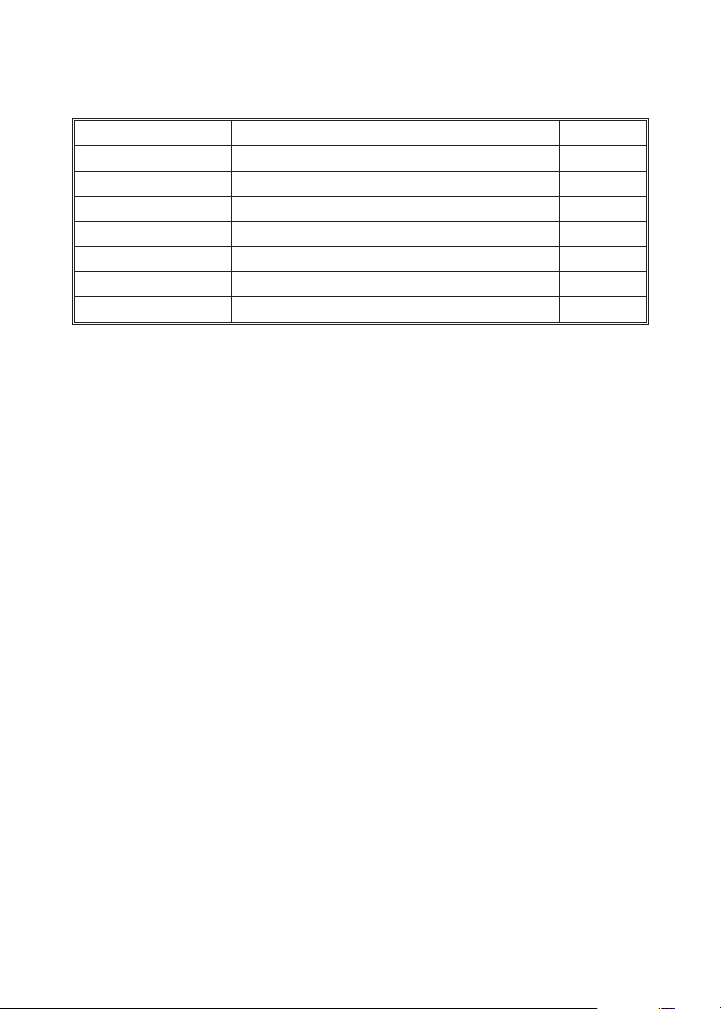
Quantity Contents Code
100 Phenolphthalein Tablets T-2246-J
15 mL Total Alkalinity Indicator 2786-E
30 mL *Alkalinity Titration Reagent B *4493
1 Test Tube, 5-10-15 mL, glass, w/cap 0778
1 Direct Reading Titrator, 0-100 Range 0381
1 Demonstration Stage, six cell 1038
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill the test tube (0778) to the 10 mL line with sample water and transfer
to a cell on the demonstration stage (1038). (A second cell can be filled
with an identical amount of water sample to be used as a “before” color
standard. Add the indicator in Step 3, but do not titrate.)
3. Add three drops of Total Alkalinity Indicator (2786) and gently stir the
contents of the cell with spatula (0691). If there is any alkalinity present,
a blue-green color will appear.
4. Fill the Direct Reading Titrator (0381) with *Alkalinity Titration
Reagent B (4493).
5. The titrator is held by hand over the cell. Discharge one drop of the
reagent at a time. Stir the mixture after each addition of the titration
solution.
6. When the color of the liquid in the cell changes permanently to pink,
read the test result directly from the scale where the large ring on the
T i trator meets the Titrator barrel. Each minor division equals 2 ppm
CaCO
“P” Alkalinity, use the procedure described in the field test method on
Alkalinity . This value is the “T” Alkalinity . To determine the
3
page 6.
9
Page 10
Ammonia Nitrogen Test
Ammonia nitrogen is present in variable concentrations in many surface and
ground waters, however, any sudden change in the analysis of a supply which
has been rather constant composition is cause for suspicion. A product of
microbiological activity, ammonia nitrogen is sometimes accepted as
chemical evidence of sanitary pollution when encountered in raw surface
waters.
Ammonia in water is detected by means of *Nessler’s Reagent (4798) which
reacts with ammonia to form a yellow color. The amount of color developed
is directly proportional to the amount of ammonia present.
Field T est Method
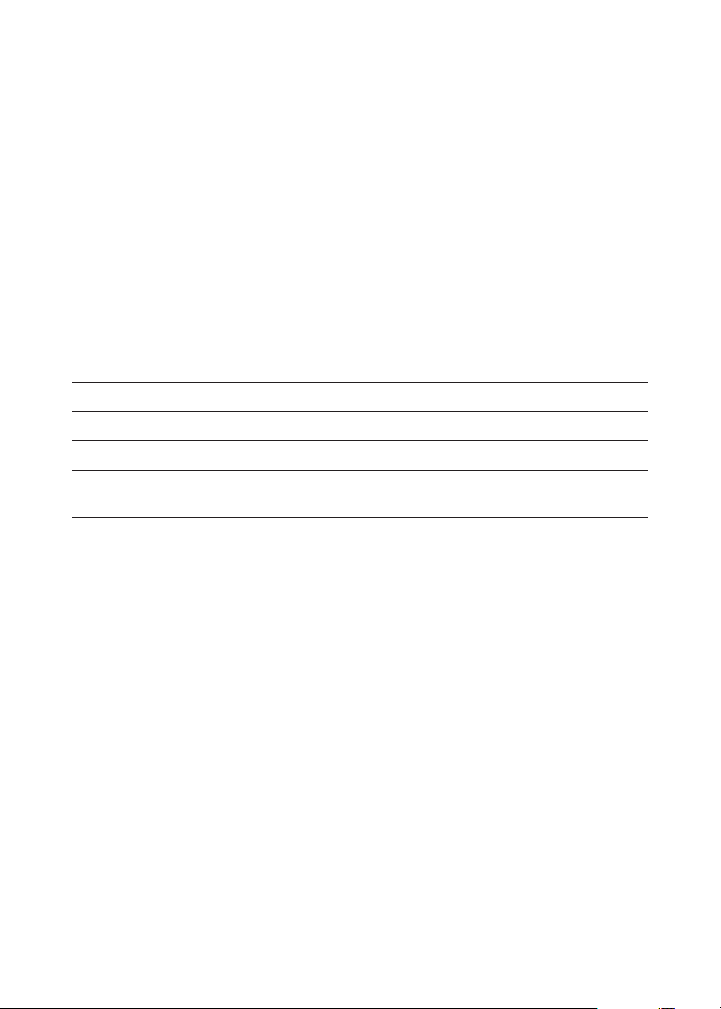
Quantity Contents Code
30 mL Ammonia Nitrogen Reagent #1 4797WT-G
30 mL *Ammonia Nitrogen Reagent #2 *4798WT-G
1 Test Tube, 5.0 mL, w/cap 0230
1 Ammonia Nitrogen Comparator,
1.0 and 5.0 ppm
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill the test tube (0230) to the 5.0 mL line with sample water .
2. Add 4 drops of Ammonia Nitrogen Reagent #1 (4797). Cap and mix.
3. Add 8 drops of *Ammonia Nitrogen Reagent #2 (4798). Cap and mix.
4. Insert test tube into the Ammonia Nitrogen Comparator (7471). Match
sample color to a color standard. Record as ppm Ammonia Nitrogen.
7471
10
Page 11

Overhead Projection Demonstration
Quantity Contents Code
30 mL Ammonia Nitrogen Reagent #1 4797WT-G
30 mL *Ammonia Nitrogen Reagent #2 *4798WT-G
1 Test Tube, 5.0 mL, w/cap 0230
1 Demonstration Stage, six cell 1038-P
1 Spatula 6091
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill test tube (0230) to the 5 mL line with sample water and transfer to a
cell on the demonstration stage (1038). (As a control, measure 5.0 mL of
sample water and add this to a second cell on the stage. Do not add any
reagents to the control sample.)
3. Add two drops of Ammonia Nitrogen Reagent #1 (4797) and gently stir
with a clean spatula (6091).
4. Add eight drops of *Ammonia Nitrogen Reagent #2 (4798). Mix by
stirring with the spatula.
5. If ammonia is present, a yellow color will form. High concentrations of
ammonia will produce a full yellow color. Lower concentrations will
produce varying shades of yellow and a faint yellow tint will indicate the
presence of a trace quantity of ammonia.
11
Page 12

Calcium, Magnesium, & Total Hardness Test
Calcium, magnesium and total hardness factors of a water should be
considered as a group since the total hardness of a water generally represents
the total concentration of calcium and magnesium ions expressed as calcium
carbonate. Other ions may contribute to the hardness of water, but in natural
waters all but calcium and magnesium are present in insignificant quantities.
When the hardness of a water is greater than the sum of the carbonate and
bicarbonate alkalinity, the amount in excess is called “noncarbonate
hardness” and such waters may contain considerable amounts of chloride
and sulfate ions. This is an important factor to consider when treating
potable water by ion exchange methods. The hardness of water may range
from zero to hundreds of milligrams per liter, (or parts per million),
depending on the source or the treatment to which it has been subjected.
A knowledge of the hardness of water is of great importance in the industrial
uses since it is the chief source of scale in heat exchange equipment, boilers,
pipe lines, etc. From the domestic standpoint, hard water consumes
excessive quantities of soap, forming curds and depositing a film on hair,
fabrics and glassware.
Total Hardness of water is determined by titration with a EDTA solution,
using Calmagite as the endpoint indicator. The total hardness minus the
calcium hardness equals the magnesium hardness. Calcium is determined by
EDT A titration in a manner similar to the total hardness determination.
Drinking water quality standards, as determined by the US Public Health
Service, set limits of calcium hardness at 200 ppm and magnesium hardness
at 150 ppm. Waters with a total hardness in the range of 0-60 ppm are
termed soft; from 60-120 ppm medium hard, from 120-180 ppm hard and
above 180 ppm very hard.
12
Page 13

Field Test Method - Total Hardness
Quantity Contents Code
100 Hardness Reagent #6 Tablets 4484-J
15 mL *Hardness Reagent #5 *4483-E
15 mL *Sodium Hydroxide Reagent w/Metal Inhibitor *4259-E
50 Calcium Hardness Indicator Tablets T-5250-H
30 mL Hardness Reagent #7 4487DR-G
1 Test Tube, 12.9 mL, w/cap 0608
1 Direct Reading Titrator, 0-200 Range 0382
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
The Hardness DR Titration Tube (0608) is calibrated so that the hardness
can be read directly from the scale on the Direct Reading Titrator in ppm.
Each minor division on this scale is equal to 4 ppm. Divide this direct
reading by the factor 17.1 to obtain hardness in grains per gallon (gpg).
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Fill the test tube (0608) to the 12.9 mL line with sample water .
2. Add 5 drops of *Hardness Reagent #5 (4483) and mix. Add 1 Hardness
Reagent #6 Tablet (4484) and swirl to disintegrate the tablet. A red color
will develop.
3. Fill the Direct Reading Titrator (0382) with the Hardness Reagent #7
(4487).
4. Add Hardness Reagent #7 (4487) one drop at a time, swirling to mix
after each drop, until the red color changes to clear blue.
5. Read the test result directly from the scale where the large ring on the
T i trator meets the Titrator barrel. Record as Total Hardness as ppm
Calcium Carbonate.
13
Page 14

Field T est Method—Calcium Hardness
Procedure
1. Fill the test tube (0769) to the 12.9 ml line with the sample water.
2. Add six drops of *Sodium Hydroxide Reagent with Metal Inhibitors
(4259).
3. Add one Calcium Hardness Indicator Tablet (5250). Cap and mix until
tablet is disintegrated. A red color will appear if calcium is present.
4. Hardness Reagent #7 (4487) is added as described in the Field Test
Method for Total Hardness, until the red color changes to blue. The
results are read as Calcium Hardness in ppm CaCO
5. The Magnesium Hardness level is determined by subtracting the
.
3
Calcium Hardness level from the Total Hardness level.
14
Page 15

Overhead Projection Demonstration
Quantity Contents Code
100 Hardness Reagent #6 Tablets 4484-J
15 mL *Hardness Reagent #5 *4483-E
15 mL *Sodium Hydroxide Reagent
w/Metal Inhibitors
50 Calcium Indicator Tablets T-5250-H
30 mL Hardness Reagent #7 4487DR-G
1 Test Tube, 12.9 mL, w/cap 0608
1 Direct Reading Titrator, 0-200 Range 0382
1 Demonstration Stage, six cell 1038-P
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
*4259-E
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill the test tube (0608) to the 12.9 mL line with sample water and
transfer to a cell on the demonstration stage (1038). Repeat this
operation so that four cells of the demonstration stage are filled. One cell
will be used for the Total Hardness test, one cell will be used for the
Calcium Hardness test and the other two cells will be used as controls.
Number the cells 1, 2, 3 and 4.
3. T o cells 1 and 2, add 5 drops of *Hardness Reagent #5 (4483) and mix.
Add 1 Hardness Reagent #6 Tablet (4484) and swirl until tablet is
disintegrated. A red color will develop.
4. Fill the Direct Reading Titrator (0382) with the Hardness Reagent #7
(4487). Add one drop at a time to cell 2 until the sample color changes
from red to clear blue. Stir the sample after the addition of each drop.
Read the test result directly from the scale where the large ring on the
T i trator meets the Titrator barrel. Record the result as ppm Total
Hardness.
15
Page 16

5. T o cells 3 and 4, add six drops of *Sodium Hydroxide Reagent with
Metal Inhibitors (4259) and stir the contents of the cells with the
spatula.
6. T o cells 3 and 4, add one Calcium Hardness Indicator Tablet (5250) to
each and stir until tablets are disintegrated or until the liquid has
developed a full red color. Cell 3 will be used as the “before” color change
standard.
7. Refill the Direct Reading Titrator with Hardness Reagent #7 and add
dropwise to cell 4, stirring the solution after each drop. Continue until
the sample color changes from red to blue. Read the test result directly
from the scale where the large ring on the Titrator meets the Titrator
barrel. Record the result as ppm Calcium Hardness.
8. Subtract the Calcium Hardness level from the Total Hardness level to
determine the Magnesium Hardness level.
16
Page 17

Free Carbon Dioxide T est
Surface waters normally contain less than 10 ppm free carbon dioxide while
some ground waters may easily exceed that concentration. Corrosion is the
principal difficulty caused by high concentrations of carbon dioxide due to
lowering of pH when carbon dioxide dissol ves in water to form carbonic
acid.
Free carbon dioxide is determined by a titration procedure using a base
solution with phenolphthalein as the endpoint indicator.
Field T est Method
Quantity Contents Code
15 mL *Phenolphthalein Indicator, 1% *2246-E
30 mL *Carbon Dioxide Reagent B *4253-G
1 Test Tube, 5-10-12.9-15-20-25 mL,
glass, w/cap
1 Direct Reading Titrator, 0-50 Range 0380
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Fill test tube (0608) to the 20 mL line with sample water. For best results
the test should be made on a freshly obtained sample, preferably a
sample obtained with a minimum of contact with the air (avoid
splashing, etc.)
2. Add 2 drops *Phenolphthalein Indicator, 1% (2246). If the test sample
turns red, no free carbon dioxide is present.
3. If the solution is colorless, titrate with *Carbon Dioxide Reagent B
(4253) until a faint but permanent pink color is produced and persists for
at least 30 seconds. Swirl the sample gently during the titration. Read the
result directly from the scale where the large ring on the Titrator meets
the Titrator barrel. Record the result in ppm Carbon Dioxide.
0608
17
Page 18

Overhead Projection Demonstration
Quantity Contents Code
15 mL *Phenolphthalein Indicator, 1% *2246-E
30 mL *Carbon Dioxide Reagent B 4253-G
1 Test Tube, 5-10-12.9-15-20-25 mL,
glass, w/cap
1 Direct Reading Titrator, 0-50 Range 0380
1 Demonstration Stage, three cell 1039
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
0608
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Place the demonstration stage (1039) on the overhead projector and
turn on the projector light.
2. Fill the test tube (0608) to the 20 mL line with sample water and transfer
this liquid to a cell on the demonstration stage (1039).
3. Add two drops *Phenolphthalein Indicator, 1% (2246) and gently stir
the contents of the cell with spatula (0691).
4. If the solution turns red, no free carbon dioxide is present. If the solution
is colorless, it is titrated to determine the amount of carbon dioxide
present.
5. Fill the Direct Reading Titrator (0382) with *Carbon Dioxide Reagent B
(4253).
6. The Titrator is held by hand over the cell. Discharge one drop of the
reagent at a time from the titrator. Stir the mixture after the addition of
each drop. Add the titration reagent until a permanent pink color is
produced and persists for at least 30 seconds. Read the test result directly
from the scale where the large ring on the Titrator meets the Titrator
barrel. Record the result as ppm Carbon Dioxide.
NOTE: The accuracy of the test method for carbon dioxide is reduced with
the overhead projection demonstration because of the increased
exposure of the sample to the air.
18
Page 19

Chloride Test (also see Salinity Test)
Chloride is one of the major anions to be found in water and sewage. Its
presence in large amounts may be due to natural processes such as the
passage of water through natural salt formations in the earth or it may be an
indicator of pollution from sea water or industrial and domestic wastes. Any
change from the normal chloride content of a natural water should be reason
for suspecting polluti on from one of these sources. US Public Health Service
Drinking Water Standards recommend a maximum chloride content of 250
ppm as chloride.
Chloride is determined by titrating the sample with a silver nitrate solution
using potassium chromate as the endpoint indicator.
The same test reagents are used in both the Chloride and the Salinity
determinations. The chloride test is run on an undiluted sample. The salinity
test is run on a sample that has been diluted with chloride-free water.
19
Page 20

Field T est Method
Quantity Contents Code
15 mL *Chloride Reagent #1 *4504-E
60 mL *Chloride Reagent #2 *4505DR-H†
1 Test Tube, 5-10-15 mL, glass, w/cap 0778
1 Direct Reading Titrator, 0-200 Range 0382
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
† Chloride Reagent #2 (4505DR)contains Silver Nitrate, which will cause a dark
stain where it contacts the skin. Care should be taken to avoid spilling.
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Fill the test tube (0778) to 15 mL line so that the result may be read directly
from the scale on the Direct Reading Titrator in ppm Chloride. Each minor
division on the scale is equal to 4 ppm chloride. The chloride content in
grains per gallon (gpg) may be obtainedbydividingthetitratorreadingbya
factor of 17.1.
Procedure
1. Fill the test tube (0778) to the 15 mL line with sample water .
2. Add three drops of *Chloride Reagent #1 (4504). Cap and swirl to mix.
A yellow color will result.
3. Fill the Direct Reading Titrator (0382) with *Chloride Reagent #2
(4504). Insert the titrator in the center hole of the cap.
4. Add the *Chloride Reagent #2 (4505) until the yellow color is
permanently changed to pinkish-brown.
5. Read the test result directly from the scale where the large ring on the
T i trator meets the Titrator barrel. Read the result as ppm Chloride.
Overhead Projection Demonstration
The reaction of the Chloride-Salinity test procedure does not work
satisfactorily on the overhead projector. A precipitate is formed in this
reaction. This precipitate blocks out the passage of light through the cell and
a blank circle is projected.
20
Page 21

Chlorine Test
Chlorine in the form of chlorine gas, hypochlorite, chloramines and organic
chlorine compounds is widely used for sterilization and disinfection.
Chlorine is not present in natural waters and is found only as a result of
chlorination of a water supply. The presence of any amount more than
would normally be used to sterilize water could be considered evidence of
pollution from chlorine treated effluents from a process in which high
concentrations of chlorine are used.
The most widely used method for detecting chlorine is based on its reaction
with the DPD indicator to produce a pink color.
Field T est Method
Quantity Contents Code
50 *Chlorine DPD #4R Tablets *6899A-H
1 Test Tube, plastic, w/cap 0106
1 Chlorine DPD Comparator, 0.1 and 0.4 ppm 4551
*WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill a 10 mL Test Tube (0106) to the 5 mL line with the sample water.
2. Add 1 *Chlorine DPD #4R Tablet (6899A). Cap tube and invert to
disintegrate tablet.
3. Chlorine is present if a pink color develops. Immediately insert test tube
into Chlorine DPD Comparator (4551). Match sample color to a color
standard. Record as ppm Chlorine.
21
Page 22

Overhead Projection Demonstration
Quantity Contents Code
50 *Chlorine DPD #4R Tablets *6899A-H
1 Test Tube, plastic, w/cap 0106
1 Chlorine DPD Comparator, 0.1 and 0.4 ppm 4551
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax
Procedure
1. Place the Overhead Projection Stage (1038) on the overhead projector
and turn on the projector light.
2. Fill test tube (0106) to the 5 mL line with sample water twice, and
transfer two tubes full (10 mL) to a cell on the demonstration stage.
Repeat this operation, filling a second cell with 10 mL of water to be
tested. The first cell will be used as a control (nothing more is added to
this cell) and the second cell will be used for the test.
3. Add one *Chlorine DPD #4R Tablet (6899A). Stir the mixture with the
spatula (0691) until the tablet disintegrates.
4. If the chlorine is present in the water sample, a color will develop. The
color may range from very light pink (trace) to a deep pink color that
indicates a high chlorine content.
22
Page 23

Chromium (Chromate) Test
Chromium may be present in water containing waste from industry such as
the plating industry. It is considered to be a toxic chemical, and if present in
an amount of over 0.5 ppm it is evidence that contamination is from
untreated or incompletely treated waste. This calls for more careful waste
disposal control by the offending plant. It is determined colorimetrically by a
reaction with diphenylcarbohydrazide in acid solution to produce a pink or
red color.
Field T est Method
Quantity Contents Code
30g *Chromate Indicator Powder *4431-G
1 Spoon, 0.5 g 0698
1 Test Tube, 5.0 mL 0230
1 Chromate Comparator, 0.5 and 2.0 ppm 7484
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill test tube (0230) to the 5 mL line with the sample water .
2. Use the 0.5g spoon (0698) to add one level measure of the Chromate
Indicator Powder (4431) to the water sample. Mix until the powder is
dissolved. Wait two minutes for color development.
3. Insert the test tube in the Chromate Comparator (7484) and compare
the color of the test sample with the colors of the color standards.
The readings on the comparator are in terms of Sodium Chromate. To
convert Sodium Chromate reading to Hexavalent Chromium, multiply the
comparator value by 0.32.
23
Page 24

Overhead Projection Demonstration
Quantity Contents Code
30 g *Chromate Indicator Powder *4431-G
1 Spoon, 0.5 g 0698
1 Test Tube, 5.0 mL 0230
1 Demonstration Stage, six cell 1038
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill test tube (0230) to the 5.0 mL line with sample wa ter , and transfer to
a cell on the demonstration stage (1038). Repeat this operation so that
two cells are filled with 5.0 mL of the sample water. One cell will act as a
control and no chromate indicator is added to it.
3. Use the 0.5g spoon (0698), to add one level spoonful of Chromate
Indicator (4431) to the cell to be used for measuring the test sample. Stir
the mixture with the spatula (0691) until all the powder is dissolved. If a
trace of chromium or chromate is present in the water sample, a slight
pink color will develop. Greater amounts of chromium or chromate in
the water sample will cause a deep red or purple color to form.
24
Page 25

Copper Test
The copper content of drinking water generally falls below 0.03 ppm and a
copper content as low as 1.0 ppm can impart a bitter taste to water. Waters
testing as high as 1.0 ppm copper have probably been treated with a copper
compound, as used in the control of algae, or have become contaminated
from untreated industrial wastes. Acid waters and those high in free carbon
dioxide may cause the corrosion of copper, brass and bronze pipe and
fittings. This results in the introduction of copper into the water supply .
Presence of copper in water in amounts as small as 0.05 ppm can be detected
by a chemical reaction with an indicator which forms a color in proportion
to the amount of copper present.
Field T est Method
Quantity Contents Code
15 mL *Copper 1 *6446-E
2 x 15 mL Copper 2 6613-E
2 Test Tubes, “A & B” 0804
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill a test tube (0804) to the upper line marked “A” with the sample
water.
2. Add 5 drops of *Copper 1 (6446) and mix. A yellow color indicates the
presence of copper.
3. Fill the second test tube (0804) to the lower line marked “B” with
distilled water.
4. Add Copper 2 (6613) to the second tube of distilled water, one drop at a
time, counting the drops and mixing after each addition. Hold the two
tubes about one-half inch above a plain white surface and look down
through the tubes to compare the colors. Continue adding the color
reagent to the second tube until the color matches the reaction in the
first tube.
5. The test result is calculated as:
Copper (ppm) = 0.025 x No. Drops Copper 2
25
Page 26

Overhead Projection Demonstration
Quantity Contents Code
15 mL *Copper 1 *6446-E
2 x 15 mL Copper 2 6613-E
2 Test Tubes, “A & B” 0804
1 Demonstration Stage, three cell 1039
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill a test tube (0804) to the upper line marked “A” with the sample
water.
2. Add 5 drops of *Copper 1 (6446) and mix. A yellow color indicates the
presence of copper. Pour this into one of the cells of the demonstration
stage (1039). Mark this cell “Test Sample.”
3. Fill the second test tube (0804) to the lower line marked “B” with
distilled water. Pour this into a second cell of the demonstration stage
(1039). Mark this cell “Reference Sample.”
4. Add Copper 2 (6613) to the Reference Sample in the second cell, one
drop at a time, stirring the solution with the Spatula (0691) after each
drop. Count the drops and continue the addition until the Reference
Sample color matches the T est Sample color.
5. The test result is calculated as:
Copper (ppm) = 0.025 x No. Drops Copper 2
26
Page 27

Cyanide Test
Cyanide may be present in water containing waste from a metal finishing
plant. It is very toxic and cannot be tolerated, even at the lowe st levels. A
positive test is evidence of untreated or incompletely treated waste which
calls for more careful waste disposal control by the offending plant. Presence
of cyanide in amounts as small as 0.05 ppm can be detected by a chemical
reaction with an indicator which forms a color in proportion to the amount
of cyanide present.
Field T est Method
Quantity Contents Code
25 mL *Cyanide Reagent #1 *7388-G
25 mL *Cyanide Reagent #2 *7389-G
25 mL *Cyanide Reagent #3 *7390-G
2 Test Tubes, “A & B” 0804
1 Funnel, plastic 0459
1 Filter Paper, 9 cm 0465
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
27
Page 28

Procedure
WA R N I N G : This cyanide test is a field test designed to screen samples for
the presence of cyanide. Oxidizing agents, as well as certain metal complexes
formed with iron, copper , manganese and platinum, may produce a false
positive result. All positive tests for cyanide should be verified or confirmed
by an independent laboratory using the appropriate procedures given in the
most recent edition of Standard Methods for the examination of Water and
Wastewater.
1. Fill test tube (0804) to the upper line marked “A” with the sample water.
2. Add two drops of *Cyanide Reagent #1 (7388). Cap and mix. Add two
drops of *Cyanide Reagent 2 (7389). Cap and mix. If a precipitate forms,
filter the solution into the second test tube (0804) until the tube is filled
to the line marked “B.” A pink color indicates the presence of Cyanide.
3. Rinse the first test tube carefully and then fill it to the line marked “B”
with deionized or cyanide-free tap water .
4. Add two drops of *Cyanide Reagent #2 (7389) to the deionized or
cyanide-free tap water sample and mix the contents.
5. Add *Cyanide Reagent #3 (7390) to the distilled water sample test tube
(from step 4) one drop at a time until the color of the liquid matches the
color of the test sample (from step 2). Ma tch the colors by looking down
through the test tubes held about a half inch above a plain white surface.
Count the number of drops of *Cyanide Reagent #3 (7390) that were
required to match the color of the test sample. Each drop of *Cyanide
Reagent #3 (7390) added is equal to 0.05 ppm Cyanide.
28
Page 29

Overhead Projection Demonstration
Quantity Contents Code
30 mL *Cyanide Reagent #1 *7388-G
30 mL *Cyanide Reagent #2 *7389-G
30 mL *Cyanide Reagent #3 *7390-G
2 Test Tubes, “A & B” 0804
1 Funnel, plastic 0459
1 Filter Paper, 9 cm 0465
1 Funnel Holder 0694
1 Demonstration Stage, three cell 1039
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill a test tube (0804) to the upper line marked “A” with the sample
water.
2. Add two drops of *Cyanide Reagent #1 (7388) and mix; then add 2
drops of *Cyanide Reagent #2 (7389) and mix.
3. Place the demonstration stage (1039) on the overhead projector and
turn on the projector light.
4. Place the Funnel (0459) in the Funnel Holder (0694) and place the
funnel holder inside one of the cells of the stage. Insert a piece of Filter
Paper (0465) in the funnel. The contents of the test tube from Step 2 are
filtered directly into the cell of the demonstration stage. It is not
necessary to collect the last few drops that may remain on the filter
paper. A pink color in the filtrate indicates the presence of Cyanide.
Mark this as the “T est Sample.”
29
Page 30

5. Fill a second test tube (0804) to the lower line marked “B” with
deionized or cyanide free tap water and add this amount to a second cell
on the demonstration stage. Mark this as the “Reference Sample.”
Compare the colors of the liquid in the two cells.
6. Add two drops of *Cyanide Reagent #2 (7389) to the Reference Sample
and mix by stirring with the Spatula (0691).
7. Add *Cyanide Reagent #3 (7390) one drop at a time to the Reference
Sample until the color matches the color of the Test Sample. Each drop
of *Cyanide Reagent #3 (7390) added is equal to 0.05 ppm Cyanide.
NOTE: If a strong oxidizing agent, such as chlorine, bromine, hydrogen
peroxide, or permanganate, is present in the sample to be tested,
the cyanide test may give a false positive indication that cyanide is
present. It is strongly recommended that positive cyanide test results
be verified by a distillation procedure as given in “Standard
Methods,” APHA,15th Edition.
30
Page 31

Iron Test
Most natural waters contain some iron. Its presence may vary from the
smallest trace to very large amounts in water which is contaminated by acid
mine wastes. For domestic use, the concentration should not exceed 0.2 ppm
and for some industrial applications, not even a trace of iron can be
tolerated. There are many means available for removing or reducing the iron
content of waters. Wa ter softening resins are effective for removing small
amounts of iron and special ion exchange materials are selective for iron
removal. High concentrations of iron can be removed by such chemical
processes as oxidation and lime or lime-soda softening. Because of the many
means of removing or reducing the amount of iron in water, the particular
method employed will depend largely on the form of iron which is present
and the end use of the finished water. The chemical test for iron is based on a
very sensitive chemical reaction with bipyridal to produce a pink to deep red
color, depending upon the amount of iron in the water.
Field T est Method
Quantity Contents Code
15 mL *Iron Reagent #1 *4450-E
4.5g *Iron Reagent #2 *4451-S
1 Test Tube, 5.0 mL 0230
1 Spoon, 0.05 g 0696
1 Iron Comparator 7474
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill test tube (0230) to the 5 mL line with sample water.
2. Add 5 drops of *Iron Reagent #1 (4450). Cap and mix.
3. Use the 0.05g spoon (0696) to add one level measure of *Iron Reagent
#2 Powder (4451). Mix until the powder dissolves. Wait 3 minutes.
4. If Iron is present in the water sample, a pink or red color will develop.
Insert the test tube into the Iron Comparator (7474). Match sample
color to a color standard.
31
Page 32

Overhead Projection Demonstration
Quantity Contents Code
15 mL *Iron Reagent #1 *4450-E
4.5g *Iron Reagent #2 *4451-S
1 Test Tube, 5.0 mL 0230
1 Spoon, 0.05g 0696
1 Demonstration Stage, six cell 1038
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill test tube (0230) to the 5 mL line with sample water and transfer to a
cell on a demonstration stage (1038).
3. Add five drops of *Iron Reagent #1 (4450). Stir the mixture with the
spatula (0691).
4. Use the 0.05g spoon (0696) to add one level measure of *Iron Reagent
#2 (4451). Stir the contents of the cell until the powder has completely
dissolved.
5. If iron is present in the water sample, a pink or red color will develop. A
trace of iron will cause a faint pink color to appear. Greater
concentration will produce a full pink color and very high
concentrations will produce a deep red color.
Magnesium Test
See Calcium, Magnesium & T otal Hardness (p. 12).
32
Page 33

Nitrate Test
Nitrogen is essential for plant growth but the presence of excessive amounts
in water suppli es presents a major pollution problem. Nitrogen compounds
that may enter water as nitrates, or be converted to nitrates, can originate
from agricultural fertilizers, sewage, industrial and packing house wastes,
drainage from livestock feeding areas, farm manures and legumes. Nitrates in
large amounts can cause “blue babies” (methemoglobinemia) in infants less
than six months of age and is an important factor to be considered in
livestock production, where, in addition to causing methemoglobinemia, it is
responsible for many other symptoms arising from the intake of nitrates in
water supplies. Nitrates, in conjunction with phosphates, stimulate the
growth of algae with all of the related difficulties associated with excessive
algae growth.
US Public Health Service Drinking Water Standards state that 10 ppm
Nitrate Nitrogen is a limit which should not be exceeded. However, to the
sanitary and industrial engineer, the concentration which is of concern is less
than 1 ppm.
In the chemical test for nitrates, a red dye is formed by the coupling of two
chemical intermediates through the action of nitrates derived from the
reduction of the nitrate ion.33
33
Page 34

Field T est Method
Quantity Contents Code
60 mL *Mixed Acid Reagent *V-6278-H
5g *Nitrate Reducing Reagent *V-6279-C
1 Spoon, 0.1g 0699
2 Test Tube, 2.5 and 5 mL 0820
1 Nitrate-N Comparator, 2 and 10 ppm 7493
1 Pipet 0352
1 Dispenser Cap 0692
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
NOTE: Place dispenser cap (0692) on *Mixed Acid Reagent (V-6278-H). Save this cap
for refill reagents.
Procedure
1. Fill a test tube (0820) to the 2.5 mL line with the sample water.
2. Add *Mixed Acid (V -6278) until the tube is filled to the 5.0 mL line.
Capandmix.Waittwominutes.
3. Use the 0.1g spoon (0699) to add one level measure of *Nitrate
Reducing Reagent (V-6279) to the mixture in the test tube. Invert the
test tube 50-60 times in one minute. Wait 10 minutes.
4. A very light pink color indicates a trace of Nitrate Nitrogen present in
the sample. High concentrations of Nitrates will produce a deep magenta
color. Insert the tube into the Nitrate-N Comparator (7493). Match
sample to a color standard.
34
Page 35

Overhead Projection Demonstration
Quantity Contents Code
60 mL *Mixed Acid Reagent *V-6278-H
5g *Nitrate Reducing Reagent *V-6279-C
1 Spoon, 0.1g 0699
1 Test Tube, 2.5 and 5 mL 0820
1 Demonstration Stage, six cell 1038
1 Spatula 0691
1 Pipet 0352
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Place the demonstration stage (1038) on an overhead projector and turn
on the projector light.
2. Fill test tube (0820) to the 5 mL line with sample water and transfer this
liquid to a cell on the demonstration stage (1038).
3. Because the invert/shaking of this test is critical, the sample must be
reacted in the tube. Follow instructions from the Field Test Method and
pour over into second cell on demonstration stage.
4. A faint pink color will indicate that a trace of Nitrate is present. High
concentrations of Nitrates will produce a deep magenta color .
35
Page 36

pH Test
Most natural waters will have pH values from pH 5.0-8.5. Acidic, freshly
fallen rain water may have a pH value of pH 5.5-6.0. If it reacts with soils
and minerals containing weak alkaline materials, the hydroxyl ions will
increase and the hydrogen ions decrease; the water may become slightly
alkaline with a pH of pH 8.0-8.5. Sea water wi ll have a pH value close to pH
8.0.
Waters more acidic than pH 5.0 and more alkaline than pH 8.5-9.0 should
be viewed with suspicion. Mine drainage and acid industrial wastes are the
principal factors in increasing the acidity of water, and alkaline industrial
wastes are the cause of high pH values.
The pH test, which is one of the most important tests for detecting industrial
pollution, is also one of the simplest to perform. *Range Finding Indicator
Solution (2220) contains several indicators. A specific color forms at each
pH as a result of the reaction between the water sample and the indicators
Field T est Method
Quantity Contents Code
60 mL *Range Finding Indicator Solution *2220-H
1 Test Tube, 5.0 ml 0230
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill test tube (0230) to the 5.0 mL line with the sample water .
2. Add ten drops of the *Range Finding pH Indicator Solution (2220).
3. The color that results from the mixture will indicate the approximate pH
value of the sample. Check the color of the sample with the table below:
pH 3.0 Red pH 8.0 Green
pH 4.0 Red-Orange pH 9.0 Blue-Green
pH 5.0 Orange pH 10.0 Blue
pH 6.0 Yellow pH 11.0 Purple
pH 7.0 Yellow-Green
36
Page 37

Overhead projection demonstration
Quantity Contents Code
60 mL *Range Finding Indicator Solution *2220-H
1 Demonstration Stage, six cell 1038
1 Test Tube, 5.0 mL 0230
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
NOTE: Buffer solutions of various pH values are available separately that may be used to
develop reference color standards at their pH values.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill test tube (0230) to the 5 mL line with sample water and transfer this
liquid to a cell on the demonstration stage (1038).
3. Add ten drops of the *Range Finding pH Indicator Solution (2220), stir
the mixture. The characteristic color will appear immediately. Check the
color of the solution with the table of colors listed in Step 3. (If buffer
solutions are used, add 5.0 mL of the buffer solution to a cell on the stage,
add ten drops of the pH indicator and mix. Note the pH value of the
buffer solution on the side of the stage near the cell.)
37
Page 38

Phosphorus (Phosphate) Test
Phosphorus is an important nutrient for aquatic plants. The amount found
in water is generally not more than 0.1 ppm unless the water has become
polluted from waste water sources or excessive drainage from agricultural
areas. When phosphorus is present in excess of the concentrations required
for normal aquatic plant growth, a process called eutrophication takes place.
This creates a favorable environment for the increase in algae and weed
nuisances that produce scums and odors. When algae cells die, oxygen is
used in the decomposition and fish kills often result. Rapid decomposition of
dense algae scums with associated organisms give rise to foul odors and
hydrogen sulfide gas. Inorganic phosphate, which is largely the form of
phosphorus required for plant growth, is determined by its reaction with a
molybdate solution to form a phosphomolybdate which, when reduced,
forms a blue color which is the basis for a very sensitive test for phosphorus.
The production of more than a faint blue color in this test is cause for
suspicion of phosphate pollution, and when the other factors such as
available nitrogen, iron, trace metals, etc. are present, will cause the
conditions described above.
38
Page 39

Field T est Method
Quantity Contents Code
60 mL *VM Phosphate Reagent *4410-H
5 mL Reducing Reagent 6405-C
1 Test Tube, 5.0 mL 0230
1 Pipet, 1.0 mL 0354
1 Pipet, unmarked 0352
1 VM Phosphate Comparator, 1.0 and 5.0 ppm 7482
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill test tube (0230) to the 5 mL line with the sample water .
2. Use the 1.0 mL pipet (0354) to add 1.0 mL of *VM Phosphate Reagent
(4410) to the test sample. Cap and mix. Wait 5 minutes. A light yellow
color may appear at this point.
3. Use the pipet (0352) to add 3 drops of Reducing Reagent (6405) to the
mixture. Invert to mix the contents.
4.
If Phosphate is present, a blue color will form immediately. Insert the test
tube in the VM Phosphate Comparator (7482). Match sample color to a
color standard.
39
Page 40

Overhead Projection Demonstration
Quantity Contents Code
60 mL *VM Phosphate Reagent *4410-H
5 mL Reducing Reagent 6405-C
1 Test Tube, 5.0 mL 0230
1 Pipet, 1.0 mL 0354
1 Pipet, unmarked 0352
1 Demonstration Stage, six cell 1038
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill test tube (0230) to the 5 mL line with sample water and transfer to a
cell on the demonstration stage (1038).
3. Use the 1.0 mL pipet (0354) to add 1.0 mL of the *VM Phosphate
Reagent (4410). Stir the contents of the cell with the spatula (0691).
Wait five minutes. A light yellow color may appear.
4. Use the pipet (0352) to add 3 drops of the Reducing Reagent (6405).
Stir the contents of the cell.
5. If Phosphate is present, a blue color will form. High concentrations of
Phosphates will produce a deep blue color . Lower concentration will
produce varying shades of light blue and a faint blue tint will indicate the
presence of trace quantities.
40
Page 41

Salinity Test (also see Chlorides)
The extent of contamination of a fresh water supply in areas adjacent to salt
water sources can be easily detected by a determination of its salinity .
Salinity , in this case, is a term used to describe the total solids content of sea
water and has a different meaning than the term as used to describe the
solids content of fresh water used for agricultural purposes. Because of the
relatively constant chemical balance of sea water , its salinity can be
determined by a measure of its total halide content, which is principally in
the form of chlorides.
The salinity of a water can be completely removed by distillation or
demineralization by ion exchange resins. Also, practical use has been made
of special ion exchange membrane systems for reducing the salt content to
within limits permissible for potable purposes.
The chemical test for salinity involves titration of the test sample with silver
nitrate using potassium chromate as the indicator .
The same test reagents are used in both the chloride and the salinity
determinations. The chloride test is run on an undiluted sample. The salinity
test is run on a sample that has been diluted with chloride-free water.
41
Page 42

Field T est Method
Quantity Contents Code
60 mL *Chloride Reagent #1 *4504-E
15 mL *Chloride Reagent #2 *4505DR-H†
1 Test Tube, 5-10-15 mL, glass, w/cap 0778
1 Direct Reading Titrator, 0-20 Range 0378
1 Pipet, plain 0352
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
† Chloride Reagent #2 (4505DR) contains Silver Nitrate, which will cause a dark
stain where it contacts the skin. Care should be taken to avoid spillage
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Use the pipet (0352) to add three drops of the sample water to the test
tube (0778).
2. Carefully add chloride-free water until the tube is filled to the 15 mL
line. (This is a one part to one hundred part (1:100) dilution.)
3. Add three drops of the *Chloride Reagent #1 (4504). Cap and mix. A
yellow color will result.
4. Fill the Direct Reading Titrator (0378) with *Chlroide Reagent #2
(4505). Insert the titrator in the center hole of the cap.
5. Add *Chloride Reagent #2 (4505) until the color of the solution
changes from yellow to pinkish-brown. Swirling to mix after each
addition.
6. Read the test result directly from the scale where the large ring on the
T i trator meets the Titrator barrel. Record the result as ppt Salinity. Each
minor division equals 0.4 ppt.
Overhead Projection Demonstration
The reaction of the Chloride-Salinity test procedure does not work
satisfactorily on the overhead projector. A precipitate is formed in this
reaction. This precipitate blocks out the passage of light through the cell and
a blank circle is projected.
42
Page 43

Sulfide Test
Sulfide occurs in many well water supplies and sometimes is formed in lakes
or surface waters. In distribution systems it may be formed as a result of
bacterial action on organic matter under anaerobic conditions. It may also be
found in waters receiving sewage or industrial wastes. Concentrations of a
few hundredths of a milligram per liter cause a noticeable odor. Removal of
sulfide odor is accomplished by aeration or chlorination. Hydrogen sulfide is
a toxic substance acting as a respiratory depressant in both humans and fish.
Hydrogen sulfide or soluble sulfides are detected by treating the sample with
para-aminodimethylaniline and ferric chloride in acid solution to form the
well known dye Methylene Blue. The reaction is sensitive to very small
traces of sulfide and can be applied to the determination of hydrogen sulfide
in air.
NOTE: The sample should be collected with a minimum of aeration and
should be analyzed promptly. *Sulfide Reagent A (4458) is a strong
acid solution and should be handled with great care.
43
Page 44

Field T est Method
Quantity Contents Code
15 mL *Sulfide Reagent A *4458-E
15 mL Sulfide Reagent B 4459-E
30 mL Sulfide Reagent C 4460-G
1 Test Tube, 5.0 mL 0230
1 Pipet, 1.0 mL 0354
1 Sulfide Comparator, 0.2 and 2.0 ppm 7477
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Fill test tube (0230) to the 5 mL line with sample water.
2. Add 15 drops of *Sulfide Reagent A (4458). Cap and mix. Remember
that the test sample now has a high acid content.
3. Add three drops of Sulfide Reagent B (4459). Cap and mix. W ait one
minute.
4. Use the 1.0 mL pipet (0354) to add 1.0 mL of Sulfide Reagent C (4460).
Cap and mix.
5. If sulfide is present, a blue color will develop. Insert the test tube into the
Sulfide Comparator (7477). Match sample color to a color standard.
44
Page 45

Overhead Projection Demonstration
Quantity Contents Code
15 mL *Sulfide Reagent A *4458-E
15 mL Sulfide Reagent B 4459-E
30 mL Sulfide Reagent C 4460-G
1 Test Tube, 5.0 mL 0230
1 Pipet, 1.0 mL 0354
1 Demonstration Stage, six cell 1038
1 Spatula 0691
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Procedure
1. Place the demonstration stage (1038) on the overhead projector and
turn on the projector light.
2. Fill test tube (0230) to the 5 mL line with sample water and transfer this
liquid to a cell on the demonstration stage (1038).
3. Add 15 drops of *Sulfide Reagent A (4458) to the sample in the cell.
Stir with the spatula (0691). Remember that this is a strongly acidic
solution.
4. Add 3 drops of Sulfide Reagent B (4459) and stir. Wait one minute.
5. Add 1.0 mL of Sulfide Reagent C (4460) and stir the contents of the
cell.
6. If sulfide is present, a blue color will develop. Traces of sulfide will
produce faint blue colors and high concentrations will produce deep blue
colors.
45
Page 46

Total Dissolved Solids Test
Dissolved solids in a natural water are usually composed of the sulfate,
bicarbonate and chlorides of calcium, magnesium and sodium. The US
Public Health Service recommends that the total solids of a potable water be
limited to 500 ppm, but if such a water is not available a total solids content
of up to 1000 ppm may be permitted. From the standpoint of irri gation of
agricultural crops. total solids of 185 ppm or less would be considered low;
between 175 and 500 medium; 500 to 1500 high; and above 1500 ppm very
high. The term salinity is also used to describe the solids content of irrigation
water. In addition to potable and irrigation uses, a high solids content is
undesirable in most industrial process waters. While sodium-hydrogen zeolite
softening and lime-soda softening may affect a reduction in dissolved solids,
for complete removal, however , it is necessary to employ demineralization or
distillation.
Dissolved solids are determined by electrical conductivity methods; by
weighing the residue after evaporation and by ion exchange methods. A
combination of ion exchange and direct titration is used here to estimate the
solids content of a water.
46
Page 47

Field T est Method
The Direct Reading Titrators used in this procedure are calibrated so that the
test result is read directly from the scale on the T itrator in ppm Total
Dissolved Solids.
Quantity Contents Code
60 mL *TDS Reagent A *4802-H
60 mL *TDS Reagent B *4803-H
2 Resin Column 1079
15 mL Methyl Orange Indicator with Halidex 2299-E
2 Test Tube, 5-10-15 mL, glass, w/cap 0778
60 mL Deionized Water 5115PT-H
2 Direct Reading Titrators, 0-1000 Range 0384
1 Pipet, 1.0 mL 0354
*WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Fill test tube (0778) to the 10 mL line with the sample water .
2. Add 3 drops of Methyl Orange Indicator with Halidex (2299). Cap and
mix.
3. Fill a Direct Reading Titrator (0384) with *TDS Reagent A (4802).
Insert the Titrator in the center hole of the test tube cap.
4. Add the *TDS Reagent A (4802) until the yellow color changes to pink,
swirling to mix after each addition. Read the test result directly from the
scale where the large ring on the Titrator meets the Titrator barrel. This
is result “A.” Discard this portion of the test sample and wash the test
tube.
The second part of the TDS test involves passing the sample through an ion
exchange column which exchanges the various cations (Na, Ca, Mg, etc.)
for hydrogen ions which are then titrated with *TDS Reagent B (4803)
(Standard Sodium Hydroxide Solution).
47
Page 48

The LaMotte Total Dissolved Solids Outfit is furnished with two
ready-to-use resin columns. Each resin column can be used for twenty water
samples, after which it should be discarded. Keep a record of the number of
times the resin column is used.
5. Suspend the resin column (1079) in the second test tube (0778).
6. Use 1 mL pipet (0354) to add 3-4 mL of deionized water (5115) to the
resin column.
7. Use the 1 mL pipet (0354) to add 5 mL of sample water. Discard all of
the solution that has passed through the column then continue adding
the water sample until at least 10 mL has been collected.
8. Water that has passed through the resin column is now poured into the
first test tube (0778) and the volume adjusted to exactly 10 mL.
9. Add 3 drops of Methyl Orange Indicator with Halidex (2299). Cap and
mix.
10. The second T itrator (0384) is filled with *TDS Reagent B (4803). The
*TDS Reagent B (4803) is added until the red color changes to yellow,
swirling to mix after each addition. Read the test result directly from the
scale where the large ring on the Titrator meets the Titrator barrel. This
is result “B.”
11. Add result “A” to result “B.” The sum is equal to the total dissolved
solids in parts per million expressed as calcium carbonate.
Care of Resin Column
At the conclusion of any test, the resin column should be treated with
Distilled Water (5115) as in step 6, then stoppered and capped until used
again.
48
Page 49

Overhead Projection Demonstration
Quantity Contents Code
60 mL *TDS Reagent A 4802-H
60 mL *TDS Reagent B 4803-H
2 Resin Column 1079
15 mL Methyl Orange Indicator with Halidex 2299-E
1 Test Tube, 5-10-15 mL, glass, w/cap 0778
60 mL Deionized Water 5115PT-H
2 Direct Reading Titrators, 0-1000 Range 0384
1 Pipet, 1.0 mL 0354
1 Demonstration Stage, three cell 1039
1 Spatula 0691
*WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email , phone or fax.
Read the LaMotte Direct Reading Titrator Manual before proceeding.
Procedure
1. Place the demonstration stage (0139) on the overhead projector and
turn the projector light on.
2. Fill the test tube (0778) to the 10 mL line with sample water and transfer
to a cell on the demonstration stage (0139).
3. Add three drops of Me thyl Orange Indicator with Halidex (2299) to the
cell and stir with the clean spatula.
4. Fill a Direct Reading Titrator (0384) with *TDS Reagent A (4802), add
one drop at a time, while stirring until the yellow color changes to pink.
Read the test result directly from the scale where the large ring on the
T i trator meets the Titrator barrel. This is result “A.” Rinse the titraton
tube.
5. Suspend the resin column (1079) in the second test tube (0778).
49
Page 50

6. Use the 1 mL pipet (0354) to add 3-4 mL of deionized water (5115) to
the resin column.
7. Use the 1 mL pipet (0354) to add 5 mL of sample water. Discard all of
the solution that has passed through the column then continue adding
the water sample until at least 10 mL has been collected.
8. Water that has passed through the resin column is now poured into the
first test tube and the volume adjusted to exactl y 10 mL.
9. T ransfer to a second cell on the demonstration stage.
10. Add three drops of Methyl Orange Indicator with Halidex (2299) to the
second cell and mix by stirring with the spatula (0691).
11. Use the other Titrator (0384) to add TDS Reagent B (4803) to the
second cell until the red color changes to yellow. Read the test result
directly from the scale where the large ring on the T itrator meets the
T itrator barrel. This result is “B.”
12. Add result “A” to result “B.” The sum is equal to the total dissolved
solids content of the sample given in parts per million expressed as
Calcium Carbonate.
Care of Resin Column
At the conclusion of any test, the resin column should be treated with
Deionized Water (5115) as in step 6 then stoppered and capped until used
again.
50
Page 51

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-6394(Outside USA) • F ax 410-778-6394
Visit us on the web at www.lamotte.com
®
36071 • 07/07
 Loading...
Loading...