Page 1
ACID RAIN
Study Guide
L
M
o
tt
LaMOTTE COM PANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Mary land • 21620 • USA
800-344-3100 • 410-778-3100 (Out side U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
SM
63604 · 07/07
Page 2

ACID RAIN STUDY OUT FIT
WARNING! This set contains chemicals
that may be harmful if misused. Read
cautions on individual containers
carefully. Not to be used by children
except under adult supervision
MODEL ARO · CODE 3604
QUAN TITY CON TENTS CODE
1 Rain Gauge 1047
1 Wide Range Com para tor, pH 3.0 - 6.5 2193
1 Wide Range Com para tor, pH 7.0 - 10.5 2196
2 x 30ml *Wide Range In di ca tor *2218-G
2 Test Tubes, 5 mL, w/caps 0230
1 Acid Rain Study Guide 63604
*WARNING: Reagents marked with a * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents
see MSDS CD or www.lamotte.com. To obtain a printed copy, contact LaMotte by
e-mail, phone or fax.
To or der in di vid ual re agents or test kit com po nents, use the spec i fied code num ber.
TA BLE OF CON TENTS
pH ................................................................................................................................3
Rain..............................................................................................................................4
Ef fects of Acid Pre cip i ta tion........................................................................................6
Mea suring Rain fall with the Rain Gauge ....................................................................8
Mea suring pH ...............................................................................................................9
Testing the pH of Other So lu tion..............................................................................10
Sug ges tions for the Use of the Chem i cal Testing Equip ment....................................12
Notes to the Teacher..................................................................................................12
Glos sary of Terms .......................................................................................................13
2
Page 3
Leaf Margin - The border or edge on a leaf. A leaf margin may take many shapes or
14
13
12
11
10
9
8
7
6
5
4
3
2
1
0
BASIC
NEUTRAL
ACIDIC
Household Lye
Bleach
Ammonia
Milk of Magnesia
Borax
Baking Soda
Blood
Distilled Water
Milk
Boric Acid
Orange Juice
Vinegar
Battery Acid
Fig. 1 The approximate pH values of some common substances.
forms such as smooth, saw-like, or tooth-like.
Litmus Paper - Paper which is soaked in a coloring matter obtained from primitive
plants called lichens. In alkaline solution litmus turns blue; in an acidic solution
litmus turns red.
Mineral Deficiency Disease - A disease in plants which is caused by the lack of one
or more of the important plant nutrients.
Neutral - A substance that is neither acidic nor basic but having a pH of 7.0 on the
pH scale.
Nitrogen Oxides - Gaseous compounds of oxygen and nitrogen which can
contribute to air pollution.
Nutrient, Plant - Any element taken in by a plant which is essential to its growth.
Nutrients are used by the plant to produce food and tissue.
Organic Matter - Animal and plant materials that are decomposed through the
action of micro-organisms.
Oxidation - The process by which oxygen combines with other compounds to
change their chemical state.
pH - The concentration of hydrogen ions in a substance. A pH scale is used to
indicate whether a substance is acidic, neutral, or basic.
Plankton - Very small plants and animals that live in water.
Pollution - The presence of matter or energy whose nature, location, or quantity
produces undesired environmental effects.
Root Hairs - Very small roots of plants which take up nutrients from the soil
solution.
Run-off - The portion of precipitation or snow melt that runs off the land into
streams or other surface water.
Solution - One or more substances dissolved in a liquid.
Sediments - Soil, sand, and minerals that wash from the land into water and settle
to the bottom of streams and lakes.
Smelter - A facility that melts or fuses ore to separate metals.
Sulfur Dioxide (SO2) - A heavy, pungent, colorless and gaseous air pollutant.
Surface Water - All water naturally open to the atmosphere (rivers, lakes, reservoirs,
streams, impoundments, seas, estuaries, etc.); also springs and wells which are
directly influenced by surface water.
Weathering - The disintegrating action of the elements (wind, rain, sleet, snow,
freezing, and thawing) on rocks and soils.
pH
The pH of a liquid, soil or other substance indicates whether a substance is acidic,
neutral, or basic. The pH measurement is based on the number of hydrogen ions
(H+) or hydroxyl ions (OH-) there are in a solution of the substance. If the solution
has more hydrogen ions than hydroxyl ions, the solution is acidic. On the other
hand, if the hydroxyl ions outnumber the hydrogen ions, the solution is basic. When
both the hydrogen ions and hydroxyl ions are present in equal numbers, the solution
is neutral.
THE pH SCALE
The pH scale ranges from 0
(very acid) to 14 (very
alkaline or basic). On this
scale a neutral substance is
7 (the mid-point of the
scale), an acid substance is
lower than 7 on the scale,
and a basic or alkaline
substance is higher than 7
on the scale. (Fig. 1 )
When an acid and a base
react, neutralization occurs.
The result is a solution that
is not as acidic or as basic as
the original substances. It is
important to know that the
pH scale is logarithmic.
Every one-unit change in
pH represents a ten-fold
change in acidity or
alkalinity. In other words,
pH 6 is ten times more
acidic than pH 7; pH 5 is
one hundred times more
acidic than pH 7. Pure
distilled water has a pH of 7
but quickly becomes slightly
acidic when exposed to air.
This is also the case with
rain water in the
atmosphere.
14
3
Page 4

RAIN
GLOS SARY OF TERMS
Normal rain may have a pH as low as 5.6 due to the absorption of small amounts of
carbon dioxide (CO2) in the atmosphere. The carbon dioxide reacts with rainwater
to form carbonic acid (H2CO3). The pH of the rain is lowered because a small
portion (about 10% or less) of the carbonic acid dissociates into bicarbonate
-
(HCO
) and hydrogen ions (H+):
3
H2O + CO2 <=> H2CO
H2CO3 <=> HCO
-
+ H
3
3
+
Carbonic acid is a weak acid which can be easily neutralized by the buffering
substances found in streams, lakes, and soil.
ACID RAIN
Precipitation having a pH less than 5.6 is considered to be abnormally acidic. A pH
of less than 5 indicates the presence of strong acids in addition to carbonic acid.
CAUSES OF ACID RAIN
Acid rain is formed from air pollutants, sulfur dioxide and nitrogen oxides, which are
released as gases into the atmosphere during the refining of metal ores and the
burning of fossil fuels such as coal, heating oil and gasoline. Power plants, smelters,
automobiles, and even volcanic activity can contribute to this problem. These gases
may travel miles from their sources. Eventually, they combine with moisture in the
atmosphere to form sulfuric acid and nitric acid solutions which fall to earth in rain,
snow, dew, fog, frost, and mist.
SUL FUR DI OX IDE (SO2)
When fuel or coal that contains sulfur is burned, the sulfur compounds react with
oxygen during combustion to form sulfur dioxide. Sulfur dioxide is also produced
when sulfite ores such as sphalerite (ZnS), pyrite (FeS2), and chalcocite (Cu2S) are
roasted or heated in air during the production of the metals zinc, iron, and copper.
Sulfur dioxide also occurs in volcanic gases. The “rotten egg” odor of burning sulfur
is due to sulfur dioxide.
Absorption - The addition of one substance through the surface of another.
Acid - A compound that donates a hydrogen ion (H+).
Acid Deposition - The depositing of acidic material from the atmosphere as gases,
particles, rain, snow, or fog.
Acidic - The degree or level of the acid content of a substance. An acidic substance
is below 7.0 on the pH scale.
Algae - Simple rootless plants that grow in sunlit waters in proportion to the
amounts of nutrients available. Algae have chlorophyll which is used to convert
solar energy (sunlight) to chemical energy. They are food for fish and small aquatic
animals.
Akalinity - A measure of the capacity of water to neutralize acids.
Aquatic - Living or growing in a water environment.
Bacteria - Microscopic living organisms which help break down complex substances,
such as dead animal and plant matter, by decay, and convert these substances to
simpler forms.
Base - A compound that accepts a hydrogen ion (H+).
Basic - A basic substance is above 7.0 on the pH scale.
Buffer - A substance that resists pH changes when small amounts of acid or base are
added.
Carbon Dioxide (CO2) - A colorless, odorless, non-poisonous gas, which is a normal
part of the ambient air.
Corrosion - The deterioration of metal parts slowly eaten away by acid solutions or
acid water supplies.
Detritus - Dead plant and animal material.
Fungus - A primitive groups of organisms that lack chlorophyll; includes the
mushrooms, molds, mildews, yeasts, rusts and smuts which live primarily on dead or
living organic matter.
Groundwater - Water that has soaked into the ground; often used to supply wells.
Hydroxyl Ion (OH-) - A negatively charged particle containing an oxygen atom
and a hydrogen atom. See pH.
Hydrogen Ion (H+) - A positively charged particle containing only a hydrogen
atom. See pH.
Indicator Solution - A liquid containing a chemical compound added to a test
sample to bring about a color reaction to show the presence or absence of a
particular substance.
Leaching - The removal of dissolved chemical compounds by the passage of water
through soil.
4
13
Page 5

SUG GES TIONS ON THE USE
OF THE CHEM I CAL TEST EQUIP MENT
A. Follow all of the instructions carefully. Read to the end of each procedure before
starting the actual work. Measure reagents and water samples accurately.
B. Handle the reagents with great care. Avoid contact between the reagents and
the skin and eyes. Some of the reagents are capable of causing minor skin
irritations if they are not washed off immediately after contact. None of the
reagents should be taken internally.
C. Keep all reagent containers tightly capped. Replace the cap immediately after
use. This prevents contamination and eliminates the possibility of loss of the
reagent due to leakage or spilling. Do not interchange caps.
D. The test tubes should be rinsed thoroughly in clean tap water and allowed to dry
before putting them back into the package. No soap or detergents are required
unless there are stubborn stains which will not come out in clean tap water. If
soaps or detergents are used, be sure to rinse the test tubes several times before
allowing them to dry.
E. Avoid storing the equipment where it will be exposed to extreme heat or cold.
Do not leave the reagents exposed to direct sunlight for a prolonged period of
time. Store the equipment and reagents out of the reach of very young children.
NOTES TO THE TEACHER
The materials and manual in this kit are provided as the tools needed to conduct
many important investigations. It is hoped the students’ and teachers’ interest will
not be limited to the experiments or procedures as they are outlined in this manual.
They provide the basic information needed to conduct many fascinating
investigations and activities. A number of suggested activities are provided in the
form of additional, open-ended experiments. They should spur the student’s
imagination to investigate other problems which are related to the subject. The
inquiring student may devise other experiments using the materials provided in this
unit or they may make modifications to the methods and incorporate additional
materials and test kits.
NI TRO GEN OX IDES (NOx)
Internal combustion engines in automobiles, trucks, and other vehicles produce
gaseous nitrogen oxides (NOx) because nitrogen and oxygen from the air combine
during combustion. Lightning during thunderstorms also forms nitric oxides by the
direct union of nitrogen and oxygen in the air.
Sulfur dioxide and nitrogen oxides dissolve in atmospheric moisture to form nitric
acid and sulfuric acid. Nitric acid and sulfuric acid are strong acids. They dissociate
completely as carbonic acid in rain water to form hydrogen ions, making the pH of
rain water more acidic.
HNO
nitric
acid
H2SO
sulfuric
acid
-
3
4
H2O
+
+
rain
H2O
rain
=>
=>
NO
3
Nitrate
ion
HSO
4
hydrogen
sulfate
+
-
+
+
H
hydrogen
ion
+
H
hydrogen
ion
+
+
H2O
rain
H2O
rain
12
5
Page 6

EF FECTS OF ACID PRE CIP I TA TION
EF FECTS ON MA TE RIALS
Acid precipitation can affect many of the materials we depend on every day. The
rusting of metals is an oxidation reaction that is accelerated by the presence of
acidic rain, fog and dew. Corrosion of steel, bronze and copper is increased, affecting
the condition and maintenance costs of buildings, bridges, and vehicles. Acid rain
hastens the natural weathering of marble, limestone and mortar. These substances
are composed of calcium carbonate (CaCO3) which reacts with sulfuric acid in acid
rain to become water, carbon dioxide, and powdery gypsum. The durability of paints
and textiles are also affected. In many areas, rain water is used for drinking purposes.
The pH of water must be carefully adjusted to make it non-corrosive before it is
circulated in plumbing systems that contain metal components so that metals will
not enter drinking water.
EF FECTS ON STREAMS, PONDS AND LAKES
A healthy, productive freshwater lake has a pH of about 8, slightly basic. The pH
level is maintained at a steady level by the presence of “buffering” chemicals in the
water, primarily carbonate and bicarbonate ions. The buffering chemicals in a
stream, pond or lake are an indication of the types of soils, minerals and rocks in the
area. In some areas, borate (BO
silicates contribute to the buffer system. The amount of basic buffering materials in
the water is termed the “alkalinity” of the water. The “alkalinity” of water does not
refer to pH but rather the ability to resist pH changes (buffering capacity).
The presence of these buffering materials helps to neutralize acids as they are added
to or created in the water ecosystem. If a body of water has an abundance of these
buffering materials (high alkalinity), it is more resistant to changes in pH. If a body
of water has very little buffering material (low alkalinity), it is very susceptible to
changes in pH.
As increasing amounts of acids are added to ponds and lakes, their buffering capacity
is consumed. If additional buffering material can be obtained from surrounding soils
and rocks, the alkalinity level may eventually be restored. However, a temporary loss
of buffering capacity can permit pH levels to drop to levels harmful to life in the
water.
An entire season of acid precipitation can be stored up in the form of snow and ice.
Areas which receive a lot of snowmelt each spring are especially susceptible to the
seasonal loss of buffering capacity.
_
3
), phosphates (PO
3
-
, HPO
4
-
, and H2PO
4
-
), and
4
TESTING THE pH OF HOUSE HOLD PROD UCTS
Test the pH of a number of household products that are readily available from your
home or school. For solutions which are not highly colored, the pH test can be
made without any preparation of the sample. Substitute a sample of the colorless
material for the water sample in the procedure above.
Solutions which are cloudy or colored may be filtered or diluted with distilled water
before the pH test is performed. (Distilled water is unbuffered and will not
significantly affect the pH of a strong sample.)
Use the chart in the preceding section to identify the pH of the solution.
6
11
Page 7

TESTING THE pH OF OTHER SO LU TIONS
The *Wide Range Indicator (2218) included in this kit can be used to measure the
pH of solutions ranging from 3.0 to 10.0 pH. Surface water, tap water, foods and
household products can be tested.
TESTING THE pH OF STREAMS, LAKES, AND PONDS
1. Select a sampling location where the water sample is typical of the water source
and does not represent a localized condition. For example, a water sample taken
next to a discharge pipe may not contain the same types and amounts of
substances that would be found in a sample collected away from the discharge
pipe.
2. Record the date and time of day, weather and other observations such as water
color, the presence of aquatic plants, algae, insects, or fish.
3. Keep the water sample free of foreign matter such as aquatic plants or sediment
from the bottom.
4. Use a clean, plastic or glass water sample container that has a suitable cap. The
container should hold enough water to conduct all of the tests.
5. Unless the sample is going to be tested immediately, the water sample container
should be filled until it overflows and then capped. Avoid air bubbles in the
sample that can cause chemical changes in the water. Water samples should be
tested as soon as possible.
6. Pour water sample into a clean test tube (0230) to the 5mL line.
7. Holding the bottle in a vertical position, add 10 drops of *Wide Range Indicator
(2218) to the sample (figure 3). Cap and mix.
8. Place the test tube in the Octet Comparator (2193) and record the pH value
from the color standard in the comparator that most closely matches the sample
tube color. (figure 4).
9. When the color observed in your sample is between two colors on the
comparator, the value may be reported to the nearest 0.25 unit. If the color
produced by your sample is not in the range of the color standards in the
comparator, use the following chart to estimate the pH of the sample:
pH 7 apple green
pH 8 green
pH 9 blue-green
pH 10 blue
pH 11 purple
EF FECTS ON AQUATIC LIFE
The pH of natural waters does not fall evenly as acid contamination proceeds. The
natural buffering materials in water slow the decline of pH to around 6.0. This
gradual decline is followed by a rapid pH drop as the bicarbonate buffering capacity
is used up. At pH 5.5, the buffering capacity is very low and pH drops further with
additional acid. Sensitive species and immature animals are affected first. As food
species disappear, even larger, resistant animals are affected.
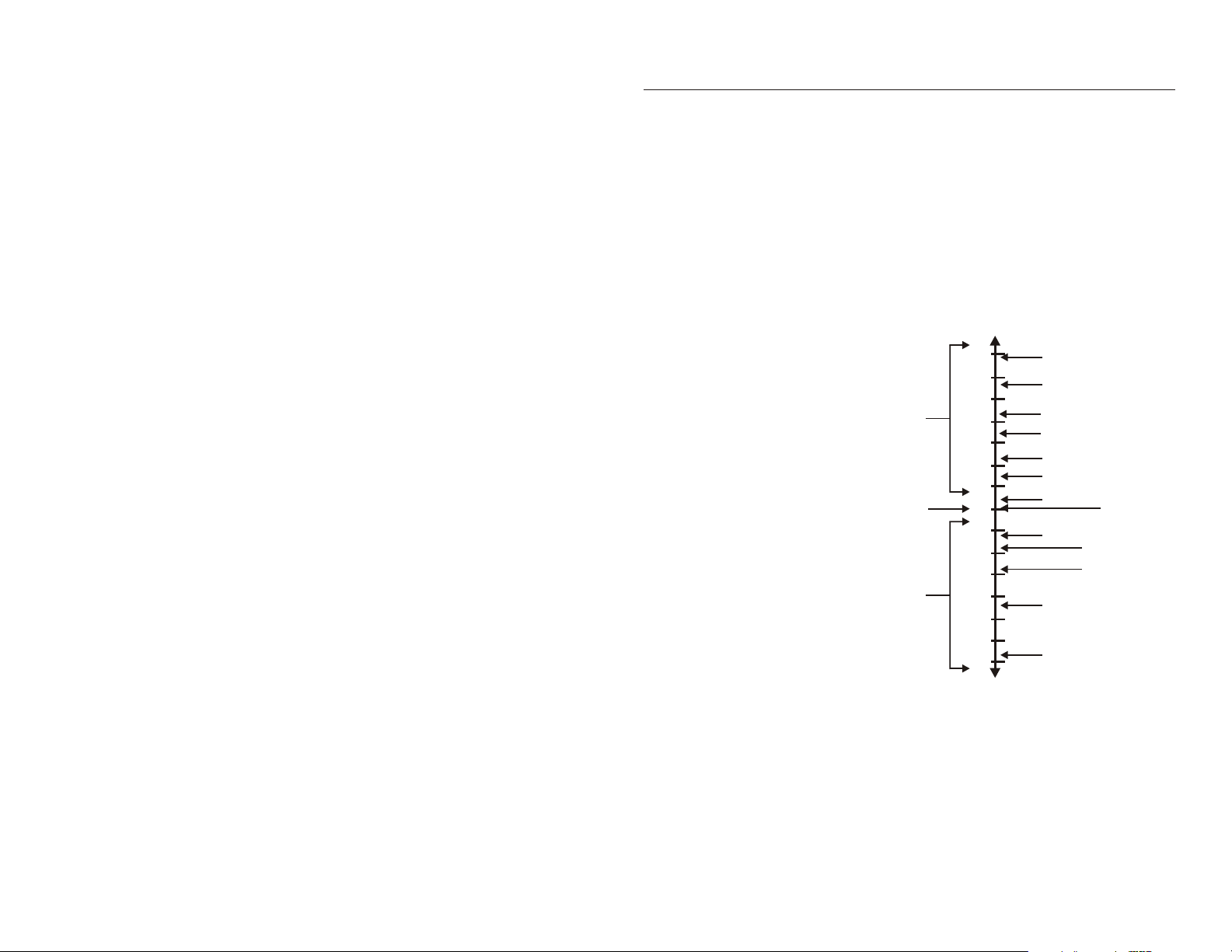
pH EFFECT ON AQUATIC LIFE
6.0 Freshwater shrimp absent
5.5 Bottom-dwelling bacteria (decomposers) begin to die
Leaf litter and detritus begin to accumulate, locking up essential
nutrients and interrupting chemical cycling
Plankton begin to disappear
Snails and clams absent
Mats of fungi begin to replace bacteria in the substrate
Metals (aluminum, lead) normally trapped in sediments are
released into the acidified water in forms toxic to aquatic life
5.0 Mayfly and many other insect eggs will not hatch
Most fish will not hatch
4.5 All fish, most frogs, insects absent
Sphagnum moss may invade, covering the substrate and inhibiting
nutrient cycling
EF FECTS ON PLANTS AND SOILS
The leaves of living plants are covered with a protective waxy cuticle. Acid
deposition (dust, rain, fog, dew) damages this coating, permitting desiccation of leaf
tissues and leaching important nutrients directly from the leaves. Damaged plants
become more vulnerable to drought and disease.
The pH of soil affects the availability of nutrients to plants growing in the soil.
When acid precipitation infiltrates forest soils, important cations such as potassium
(K+) and calcium (Ca++) may be displaced by the hydrogen ions of the acid. The
cations can then be “leached” away by groundwater and surface runoff. In areas
where the soil contains limestone or calcite, much of the acidity can be neutralized.
However, in areas with thin soils overlying granite rocks, the runoff of acid
precipitation to lakes and streams will be acidic and may contain ions leached from
the soil, such as aluminum, which is toxic to fish at levels less than 1 part per
million.
Soil acidification also inhibits helpful soil bacteria, limiting nitrogen fixation and
nitrification in the soil and slowing the decomposition of organic matter.
10
7
Page 8

MEA SURING RAIN FALL WITH THE RAIN GAUGE
L
Mott
45°45°
MEA SURING pH
Place the rain gauge in an open area far enough from buildings, trees, overhead wires
and other obstructions that may cause air turbulence or contamination. Rain falling
on rooftops or trees collects chemicals which will affect the pH. There should be no
obstruction above a 45 degree angle from the top of the rain gauge. In other words,
locate the rain gauge at least 20 feet away from a 20 foot tall obstruction (Fig. 2).
Fig. 2
A plastic spike serves as the base for the rain gauge. Select a location for the rain
gauge and push the spike straight into the ground so that the top of the rain gauge is
parallel to the ground. The rain gauge can also be mounted on a post by using the
screws included to fasten the base unit in a vertical position.
Record the amount of rainfall and empty the rain gauge after each rain event before
evaporation occurs. The rain gauge can collect up to 5" of rain. During a very heavy
storm, the rain gauge may be recorded, emptied, and reset. Record the partial
readings and add them to figure the total rainfall for the storm event.
Do not leave the rain gauge outdoors in freezing weather.
A pH test indicates whether a substance is acidic, basic, or neutral. Scientists take
pH measurements for water, soil, food, and many other substances. The pH of a
substance can be measured by adding pH indicator solutions to the substance. The
pH indicators are dyes that change color according to the pH of the solution. These
colors are then compared to color standards of a known pH value.
RAIN pH TEST
When a rain storm is expected, carefully clean and thoroughly rinse the rain gauge.
(Dust and other airborne residue inside the rain gauge will affect the pH of the
collected rain.) Rinse the rain gauge and pH kit test tubes with distilled or deionized
water and hang upside-down to dry. Place the rain gauge outdoors in its holder
immediately before the rain begins.
Perform the pH test as soon as possible after the rain has fallen.
PRO CE DURE:
1. Fill a clean test tube (0230) to the 5 mL line with rain water from
the rain gauge.
2. Holding the bottle in a vertical position (Fig. 3), add 10 drops of
*Wide Range Indicator (2218). Cap and mix.
Fig. 3
3. Place the test tube in the Wide Range
pH Comparator (2193 or 2196). Match the
color of the sample to the color
standards (Fig. 4). Record the pH value.
4. If the color observed in your sample is between two colors on the comparator,
Fig. 4
the value may be reported to the nearest 0.25 pH unit. If the color produced by
your sample is not in the range of the color standards in the comparator, use the
following chart to estimate the pH of the sample:
pH 7 apple-green
pH 8 green
pH 9 blue-green
pH 10 blue
pH 11 purple
8
9
 Loading...
Loading...