LaMotte 1200-SU User Manual

1200 COLORIMETER
SULFATE
MODEL 1200-SU · CODE 3683-01
QUANTITY CONTENTS CODE
10 g *Sulfate Reagent *V-6277-D
1 Colorimeter Tubes, with caps 0290-6
1 Spoon, 0.1 g, plastic 0699
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Sulfate 26740
*WARNING: Reagents marked with a * are considered hazardous substances. Material
Safety Data Sheets (MSDS) are supplied for these reagents. For your safety, read label
and accompanying MSDS before using.
To order individual reagents or test kit components, use the specified code number.
SULFATE INTRODUCTION
The most common mineral forms of sulfur are iron sulfide, lead sulfide, zinc sulfide,
calcium sulfate and magnesium sulfate. In most fresh waters, the sulfate ion is the second
or third most abundant anion, being exceeded only by bicarbonate and, in some cases,
silicate. Sulfur, in the form of sulfate, is considered an important nutrient element.
Mineral springs are rich in sulfate and feed appreciable quantities of this compound to
the watershed. Acid mine water drainage is a form of pollution which may contribute
extremely large amounts of sulfate content to natural waters. Other sources of sulfate
include waste material from pulp mills, steel mills, food processing operations and
municipal wastes. Many bacteria obtain sulfur from sulfate for the synthesis of amino
acids. In lakes and streams low in oxygen, this process of sulfate reduction causes the
production of hydrogen sulfide, with its characteristic offensive odor. Calcium sulfate
and magnesium sulfate contribute significantly to the hardness of water. Under natural
conditions, the quantities ordinarily to be expected in lakes are between 3 and 30 parts
per million.
1
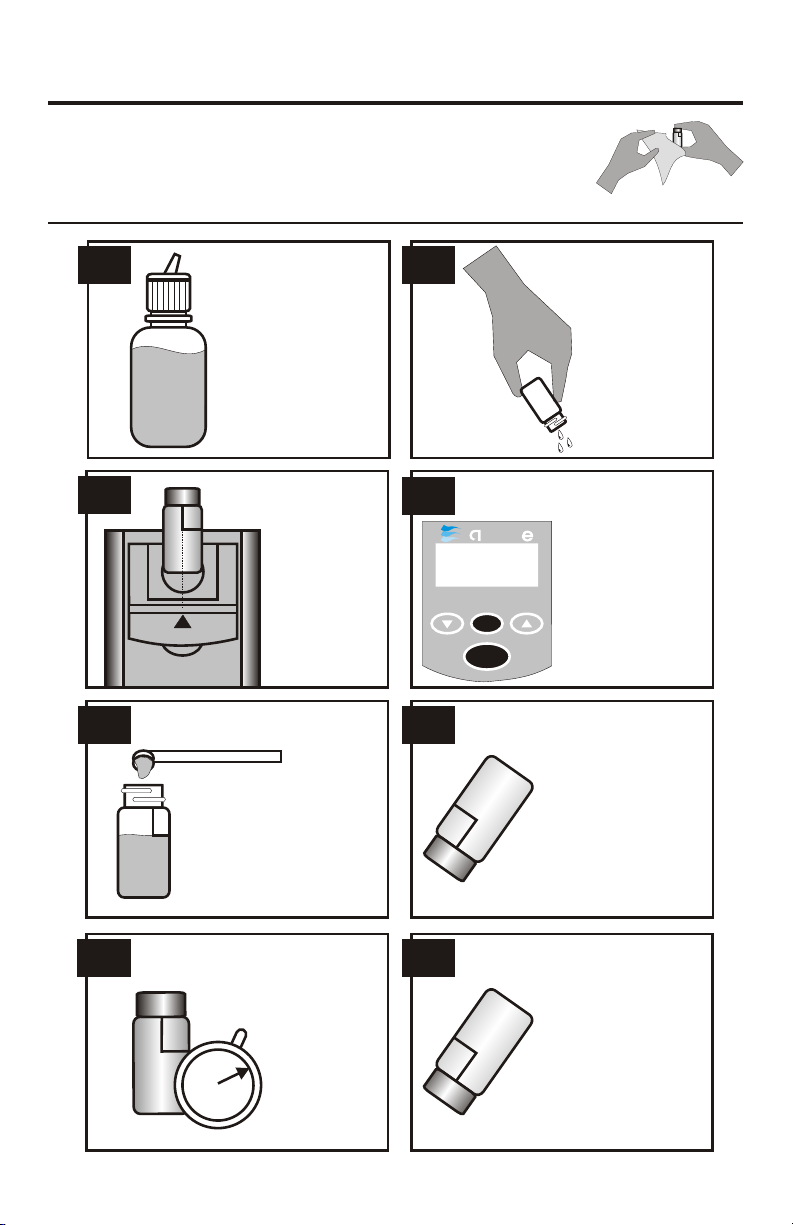
SULFATE TEST PROCEDURE
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blank or zero.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry.
5.
3.
1. 2.
4.
6.
I200 COLORIMETER
• • • • • • • • • • • • • • • • • •
READ
bla
ZERO
Cap and shake
vigorouslly for 15
seconds. A white
precipitate will
develop if sulfates
are present.
Push the READ
button to turn the
meter on. Press the
ZERO button
and hold it for 2
seconds until bla is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
Remove tube from
colorimeter. Use the
0.1 g spoon (0699)
to add one measure
of *Sulfate Reagent
(V-6277).
8.
Invert the tube to
mix again.
5
7.
Wait 5 minutes.
MI
NUT
E
S
L
Mott
- BARIUM CHLORIDE METHOD
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
SULFATE
2
 Loading...
Loading...