Page 1

1200 COLORIMETER
NITRATE NITROGEN
MODEL 1200-NA · CODE 3677-01
QUANTITY CONTENTS CODE
2 x 120 mL *Mixed Acid Reagent *V-6278-J
10 g *Nitrate Reducing Reagent *V-6279-D
1 Spoon, 0.1 g, plastic 0699
1 Graduated Cylinder 0416
1 Colorimeter Tubes, w/caps 0290-6
1 Water Sample Collecting Bottle 0688
1
*WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email, phone or fax.
To order individual reagents or test components, use the specified code number.
NITRATE - NITROGEN INTRODUCTION
Nitrogen is essential for plant growth, but the presence of excessive amounts in water
supplies presents a major pollution problem. Nitrogen compounds may enter water as
nitrates or be converted to nitrates from agricultural fertilizers, sewage, industrial and
packing house wastes, drainage from livestock feeding areas, farm manures and legumes.
Nitrates in large amounts can cause “blue babies” (methemoglobinemia) in infants less
than six months of age. Nitrate concentration is an important factor to be considered in
livestock products, where, in addition to causing methemoglobinemia, it is responsible
for many other problems. Nitrates in conjunction with phosphate stimulate the growth
of algae with all of the related difficulties associated with excessive algae growth.
U.S. Public Health Service Drinking Water Standards state that 10 ppm nitrate nitrogen
should not be exceeded. To the sanitary and industrial engineer, concentrations of less
than 1 ppm are acceptable.
1200 Colorimeter for Nitrate Nitrogen
26734
1
Page 2
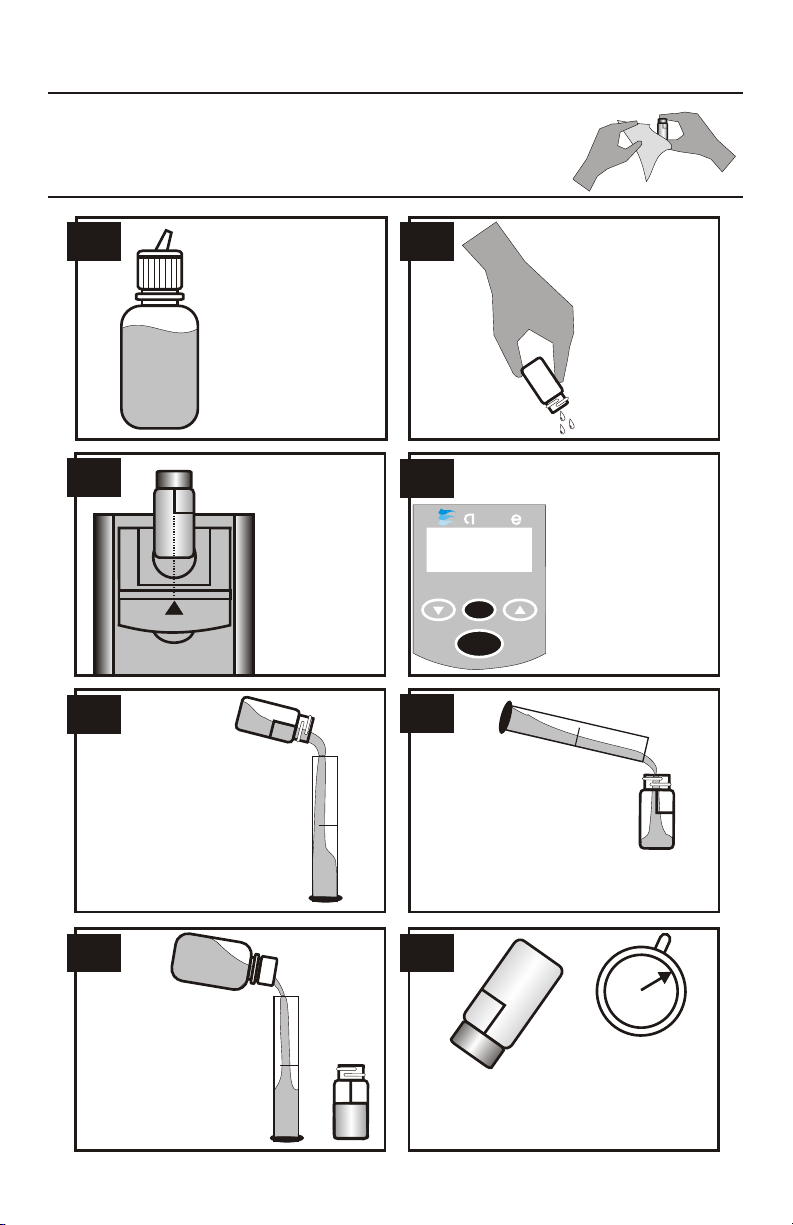
NITRATE-NITROGEN TEST PROCEDURE -
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blank or zero.
8.
5.
3. 4.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry.
1. 2.
I200 COLORIMETER
• • • • • • • • • • • • • • • • • •
READ
bla
ZERO
7.
Remove tube from
colorimeter and
pour off 5 mL into
graduated cylinder
or similar. Discard
the remaining
sample.
6.
Pour the 5 mL
sample from a
graduated
cylinder or
similar into the
colorimeter
tube.
Push the READ
button to turn the
meter on. Press the
ZERO button
and hold it for 2
seconds until bla is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
Use the graduated
cylinder or similar to
measure 5 mL of
*Mixed Acid
Reagent (V-6278)
and add to tube.
5 mL
5
m
L
2
M
I
N
U
T
E
S
5 mL
Cap and mix. Wait approximately 2
minutes before proceeding to Step 9.
L
Mott
CADMIUM REDUCTION METHOD
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
NITRATE - NITROGEN
2
Page 3

NITRATE - NITROGEN
NOTE:
11.
9. 10.
NOTE:
At the end of waiting period,
an undissolved portion of
*Nitrate Reducing Reagent
may remain in bottom of the
tube without affecting results.
Use the 0.1 g spoon
(0699) to add two
measures of *Nitrate
Reducing Reagent
(V-6279). Cap.
Hold tube by
index finger and
thumb and mix by
12.
inverting approximately
50-60 times a minute for
4 minutes. Wait 10
minutes for maximum
color development.
10
M
I
N
U
T
E
S
Wipe tube dry.
Align the index line with the
arrow on the meter, insert
CAL
I200COLORIMETER
• • • • • • • • • • • • • • • • • •
READ
tube into chamber.
Close the lid. Push
the READ
button. Record
results as ppm
Nitrate Nitrogen.
To convert Nitrate Nitrogen
(NO -N) results to ppm Nitrate
3
(NO ), multiply by 4.4.
3
L
Mott
3
Page 4

NITRATE-NITROGEN
TEST METHOD SPECIFICATIONS
APPLICATION
This method determines nitrate levels in drinking, surface, saline waters, domestic, and
industrial waters.
RANGE
0 - 3.0 ppm Nitrate Nitrogen (Range can be extended by dilution.)
METHOD
Powdered cadmium is used to reduce nitrate to nitrite. The nitrite that is originally
present plus reduced nitrate is determined by diazotization of sulfanilamide and nitrite
followed by coupling with N-(1 naphthyl) -ethylenediamine dihydrochloride to form a
highly colored azo dye which is measured colorimetrically.
HANDLING & PRESERVATION
Analysis should be made as soon as possible. If analysis cannot be made within 24 hours,
the sample should be preserved by refrigeration at 4°C. When samples must be stored for
more than 24 hours, they can be preserved by adding 2 mL of concentrated sulfuric acid
per liter of sample. For best results, the analysis for nitrate should be determined at
temperatures between 20°C and 25°C.
INTERFERENCES
Nitrite interferences at all levels. Strong oxidizing and reducing substances interfere.
Low results might be obtained for samples that contain high concentrations of iron and
copper.
LaMOTTE COMPANY
Helping Peo ple Solve An a lyt i cal Chal lenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
06.09 #63677-01
 Loading...
Loading...