Page 1

12
0
0
COL ORIMETER
.................
Instruction
MANUAL
Page 2

Page 3

TABLE OF CONTENTS
GENERAL INFORMATION
PackagingandDelivery...............................4
General Precautions ................................4
Safety Precautions .................................4
Limits of Liability ..................................4
Specifications....................................5
Parts&Accessories.................................6
EPACompliance..................................7
CEStatement....................................7
CHEMICALTESTING........................8
WaterSamplingforChemicalAnalysis.....................8-9
Filtration ......................................9
AnIntroductionToColorimetricAnalysis..................10-11
Reagent Blank ...................................11
ColorimeterTubes.................................11
SampleDilutionTechniques&VolumetricMeasurement...........12
Interferences....................................13
StrayLightInterference..............................13
GENERALOPERATINGINFORMATION.............14
Overview....................................14-15
TheKeypadandDisplay .............................16
TestingTips....................................17
CALIBRATIONPROCEDURE..................18-19
ANALYSISPROCEDURE....................20-21
AVAILABLETESTKITS ......................22
TROUBLESHOOTINGGUIDE...................23
RS232PORT ...........................24
MAINTENANCE OF THE 1200 ..................25
ReplacingtheBattery...............................25
Repairs.......................................25
MeterDisposal...................................25
WARRANTY............................26
Limitations.....................................26
Page 4
GENERAL INFORMATION
PACKAGING & DELIVERY
Experienced packaging personnel at LaMotte Company assure adequate
protection against normal hazards encountered in transportation of shipments.
After the product leaves the manufacturer, all responsibility for safe delivery is
assured by the transportation company. Damage claims must be filed immediately
with the transportation company to receive compensation for damaged goods.
Should it be necessary to return the instrument for repair or servicing, pack the
instrument carefully in a suitable container with adequate packing material. A
return authorization number must be obtained from LaMotte Company by
calling 1-800-344-3100 or faxing 1-410-778-6394. Attach a letter with the
authorization number to the shipping carton which describes the reason for the
return. This information will enable the service department to make the required
repairs more efficiently.
GENERAL PRECAUTIONS
Read the instruction manual before attempting to set up or operate this
instrument. Failure to do so could result in personal injury or damage to the
instrument.
The 1200 Colorimeter should not be stored or used in a
wet or corrosive environment. Care should be taken to
prevent water from wet colorimeter tubes from entering
the colorimeter light chamber.
NEVER PUT WET TUBES IN THE COLORIMETER.
SAFETY PRECAUTIONS
Read labels on all reagent containers. Some labels include precautionary notices
and first aid information. Certain reagents are considered hazardous and are
designated with a * in the instruction manual. To view or print a Material Safety
Data Sheet (MSDS) for these reagents see MSDS CD or our web site. To obtain a
printed copy, contact us by e-mail, phone or FAX. Additional information for all
LaMotte reagents is available in the United States, Canada, Puerto Rico, and the
US Virgin Islands from Chem-Tel by calling 1-800-255-3924. For other areas,
call 813-248-0585 collect to contact Chem-Tel’s International access number.
Each reagent can be identified by the four digit number listed on the upper left
corner of thereagent label, in the contents list and in the test procedures.
LIMITS OF LIABILITY
Under no circumstances shall LaMotte Company be liable for loss of life,
property, profits, or other damages incurred through the use or misuse of their
products.
4
Page 5

SPECIFICATIONS
Instrument Type Single wavelength, direct reading colorimeter
Measurement Wavelengths: 420 nm, 460 nm, 510 nm, 530 nm, 570 nm or
605 nm
Readable Resolution: Determined by test factor
Photometric Precision: ±0.001 Absorbance Unit
Range: 0 - 2.00 absorbance units
Display: 3 ½ digits
Response Time: 2 seconds
Warm-up Time: Not required
Lamp: LED
Detector: Silicon Photodiode
Sample: 10 mL in capped tube
Sample Chamber: Accepts 25mm diameter flat-bottomed tubes
(capped)
Power Source: Battery Operation: 9 Volt Alkaline
Line Operation: 110V/60Hz, 220V/50Hz, with available adapter
Size (L X W X H): 8.5 x 16.2 x 6.7 cm, 3.4 X 6.4 X 2.6 inches
Shipping Weight: 11 oz (312 g) meter only
Serial Interface: RS232, 8 pin mDIN, 9600b, 8, 1, n
5
Page 6

PARTS & ACCESSORIES
Included in the Model 1200 Colorimeter Kit :
1200 Colorimeter
Colorimeter tubes, set of 6
Water Sample Collecting Bottle
Reagent System
Optional Accessories
AC Adapter, 110V/60Hz Code 1726-110
AC Adapter, Universal Code 1754
Colorimeter Tubes, set of 6 Code 0290-6
6
Page 7

EPA COMPLIANCE
CE
EUROPEAN
MARK
Application of Council Directives:
Standards to which Conformity Declared:
Manufacturer's Name:
Manufacturer's Address:
Importer's Name:
Importer's Address:
Type of Equipment:
Model Number:
Year of Manufacture:
89/336/EEC
EN55022, EN50082-1, En600950
802 Washington Avenue
PO Box 329
Chestertown, MD 21620
Reagecon Diagnostics Ltd
Water Quality Meters
2020/1200
1997
13 A/D Shannon Free Zone
Shannon, Co. Clase. Ireland
LaMotte Company
I, the undersigned, hereby declare that the equipment specified above
conforms to the above Directive and Standards.
Place
Chestertown, Maryland
Date
3-19-97
Signature
Name
James K. Trumbauer
Position
V.P., Director of Research & Development
DECLARATION OF CONFORMITY
NOTE: The device complies to the product specifications for the
Low Voltage Directive when furnished with the 220V AC Adapter (Code 1774).
The 1200 Chlorine Colorimeter is an EPA Accepted instrument.
EPA Accepted means that the instrument meets
requirements for colorimeters as found in test procedures
that are approved for the National Primary Drinking
Water Regulations (NPDWR) or National Pollutant
Discharge Elimination Systems (NPDES) compliance
monitoring programs. EPA Accepted instruments may be
used with approved test procedures without additional
approval.
CE COMPLIANCE
The 1200 Colorimeter has
been independently tested
and has earned the European
CE Mark of Compliance for
electromagnetic compatibility
and safety.
7
Page 8

CHEMICAL TESTING
WATER SAMPLING FOR CHEMICAL ANALYSIS
Taking Representative Samples
The underlying factor to be considered for any type of water sampling is whether
or not the sample is truly representative of the source. To properly collect a
representative sample:
Sample as frequently as possible.
•
Collect a large sample or at least enough to conduct whatever tests are
•
necessary.
Makeacompositesampleforthesamesamplingarea.
•
Handle the sample in such a way as to prevent deterioration or contamination
•
before the analysis is performed.
Perform analysis for dissolved gases such as dissolved oxygen, carbon dioxide,
•
and hydrogen sulfide immediately at the site of sampling. These factors, as well
as samples for pH, cannot be stored for later examination.
Make a list of conditions or observations which may affect the sample. Other
•
considerations for taking representative samples are dependent upon the
source of the sample. Taking samples from surface waters involves different
considerations than taking samples from impounded and sub-surface waters.
SamplingofOpenWaterSystems
Surface waters, such as those found in streams and rivers, are usually well mixed.
The sample should be taken downstream from any tributary, industrial or sewage
pollution source. For comparison purposes samples may be taken upstream and at
the source of the pollution before mixing.
In ponds, lakes, and reservoirs with restricted flow, it is necessary to collect a
number of samples in a cross section of the body of water, and where possible
composite samples should be made to ensure representative samples.
To collect samples from surface waters, select a suitable plastic container with a
tight fitting screw cap. Rinse the container several times with the sample to be
tested, then immerse the container below the surface until it is filled to
overflowing and replace the cap. If the sample is not to be tested immediately,
pour a small part of the sample out and reseal. This will allow for any expansion.
Any condition which might affect the sample should be listed.
Sub-surface sampling is required to obtain a vertical profile of streams, lakes,
ponds, and reservoirs at specific depths. This type of sampling requires more
sophisticated sampling equipment.
8
Page 9
For dissolved oxygen studies, or for tests requiring small sample sizes, a Water
Sample Bottle (LaMotte Code 1060) will serve as a subsurface or in-depth
sampler. This weighted device is lowered to the sampling depth and allowed to
rest at this depth for a few minutes. The water percolates into the sample
chamber displacing the air which bubbles to the surface. When the bubbles cease
to rise, the device has flushed itself approximately five times and it may be raised
to the surface for examination. The inner chamber of the sampling device is
lifted out and portions of the water sample are carefully dispensed for subsequent
chemical analysis.
A Snap-Plunger Water Sampler (LaMotte Code 1077) is another “in-depth”
sampling device which is designed to collect large samples which can be used for
a multitude of tests. Basically, this collection apparatus is a hollow cylinder with a
spring loaded plunger attached to each end. The device is cocked above the
surface of the water and lowered to the desired depth. A weighted messenger is
sent down the calibrated line to trip the closing mechanism and the plungers seal
the sample from mixing with intermediate layers as it is brought to the surface. A
special drain outlet is provided to draw off samples for chemical analysis.
Sampling of Closed System
To obtain representative samples from confined water systems, such as pipe lines,
tanks, vats, filters, water softeners, evaporators and condensers, different
considerations are required because of chemical changes which occur between
the inlet and outlet water. One must have a basic understanding of the type of
chemical changes which occur for the type of equipment used. Also,
consideration should be given to the rate of passage and retaining time for the
process water.
Temperature changes play an important part in deciding exactly what test should
be performed. Process water should be allowed to come to room temperature,
20–25°C, before conducting any tests.
When drawing off samples from an outlet pipe such as a tap, allow sample to run
for several minutes, rinsing the container several times before taking the final
sample. Avoid splashing and introduction of any contaminating material.
FILTRATION
When testing natural waters that contain significant turbidity due to suspended
solids and algae, filtration is an option. Reagent systems, whether EPA, Standard
Methods, LaMotte or any others, will generally only determine dissolved
constituents. Both EPA and Standard Methods suggest filtration through a 0.45
micron filter membrane, to remove turbidity, for the determination of dissolved
constituents.** To test for total constituents, organically bound and suspended or
colloidal materials, a rigorous high temperature acid digestion is necessary.
**LaMotte offers a filtering apparatus: Syringe Assembly (Code 1050) and
Membrane Filters, 0.45 micron (Code 1103).
9
Page 10

AN INTRODUCTION TO
COLORIMETRIC ANALYSIS
Most test substances in water are colorless and undetectable to the human eye.
To test for their presence we must find a way to “see” them. The LaMotte
colorimeter can be used to measure any test substance that is itself colored or can
be reacted to produce a color. In fact a simple definition of colorimetry is “the
measurement of color” and a colorimetric method is “any technique used to
evaluate an unknown color in reference to known colors”. In a colorimetric
chemical test, the intensity of the color from the reaction must be proportional to
the concentration of the substance being tested. Some reactions have limitations
or variances inherent to them that may give misleading results. Many such
interferences are discussed with each particular test instruction. In the most basic
colorimetric method the reacted test sample is visually compared to a known
color standard. However, accurate and reproducible results are limited by the
eyesight of the analyst, inconsistencies in the light sources, and the fading of
color standards.
To avoid these sources of error, a colorimeter can be used to photoelectrically
measure the amount of colored light absorbed by a colored sample in reference to
a colorless sample (blank).
White light is made up of many different colors or wavelengths of light. A
colored sample typically absorbs only one color or one band of wavelengths from
the white light. Only a small difference would be measured between white light
before it passes through a colored sample versus after it passes through a colored
sample. The reason for this is that the one color absorbed by the sample is only a
small portion of the total amount of light passing through the sample. However,
if we could select only that one color or band of wavelengths of light to which
the test sample is most sensitive, we would see a large difference between the
light before it passes through the sample and after it passes through the sample.
A colorimeter passes a white light beam through an optical filter which transmits
only one particular color or band of wavelengths of light to the photodetector
where it is measured. The difference in the amount of colored light transmitted
by a colorless sample (blank) and the amount of colored light transmitted by a
colored sample is a measurement of the amount of colored light absorbed by the
sample. In most colorimetric tests the amount of colored light absorbed is directly
proportional to the concentration of the test factor producing the color and the
path length through the sample. However, for some tests the amount of colored
light absorbed is inversely proportional to the concentration.
The choice of the correct optical filter and therefore the correct color or
wavelength of light is important. It is interesting to note that the filter that gives
the most sensitive calibration for a test factor is the complementary color of the
test sample. For example, the Nitrate-Nitrogen test produces a pink color
proportional to the nitrate concentration in the sample (the greater the nitrate
concentration, the darker the pink color). A green filter is used since a
pinkish-red solution absorbs mostly green light.
10
Page 11
The 1200 Colorimeter has been specially calibrated to read test results directly in
parts per million (ppm) of the test factor. The sensitivity of the response has been
maximized by using a specific, complementary light source for each test.
REAGENT BLANK
Some tests will provide greater accuracy if a reagent blank is determined, to
compensate for any color or turbidity resulting from the reagents themselves. A
reagent blank is performed by running the test procedure on 10 mL of
demineralized water. Use sample water to zero the meter. Insert the reagent blank
in the colorimeter chamber and select READ. Note result of reagent blank.
Perform the tests on the unknown as described. Subtract results of
reagent blank from all subsequent test results.
COLORIMETER TUBES
The handling of the colorimeter tubes is of utmost importance. Scratches,
fingerprints and water droplets on the tube or inside the light chamber can cause
stray light interference leading to inaccurate results. It is imperative that the
tubes and light chamber be clean and dry. The glassware must be clean and
defect-free. Scratches and abrasions will permanently affect the accuracy of the
readings. Tubes can be acid washed periodically. After a tube has been filled and
capped, it should be held by the cap and the outside surface should be wiped with
a clean, lint-free absorbent cloth until it is dry and smudge-free. Handling the
tube only by the cap will avoid problems from fingerprints. Always set the clean
tube aside on a clean surface that will not contaminate the tube.
Variability in the geometry and quality of the glassware is the predominate cause
of variability in results. Orientation of the tube in the chamber will greatly affect
the test results. To obtain the most accurate results, the tubes must be positioned
so that the index line on the tube is aligned with the arrow-shaped index mark t
molded into the housing in front of the light chamber. This will ensure that the
most accurate results are obtained.
11
Page 12

SAMPLE DILUTION TECHNIQUES & VOLUMETRIC
MEASUREMENTS
If a test result using the 1200 Colorimeter gives an ER2 (over range) message, the
sample must be diluted. Then the test should be repeated to obtain a reading
which is in the concentration range for the test. (Note: This is not true for
colorimetric determination of pH.)
Example:
Measure 5 mL of the water sample into a graduated cylinder. Add demineralized
water until the cylinder is filled to the 10 mL line. The sample has been diluted
by one-half, and the dilution factor is therefore 2. Perform the test procedure,
then multiply the resulting concentration by 2 to obtain the test result.
The following table gives quick reference guidelines on dilutions of various
proportions. All dilutions are based on a 10 mL volume, so several dilutions will
require small volumes of the water sample. Graduated pipets should be used for
all dilutions.
Deionized Water to
Bring Volume to 10
Size of Sample
10 mL 0 mL 1
5mL 5mL 2
mL
Multiplication
Factor
2.5 mL 7.5 mL 4
1mL 9mL 10
0.5 mL 9.5 mL 20
If the above glassware is not available, dilutions can be made with the
colorimeter tube. Fill the colorimeter tube to the 10 mL line with the sample
then transfer it to another container. Add 10 mL volumes of demineralized water
to the container and mix. Transfer back 10 mL of the diluted sample to the
colorimeter tube and follow the test procedure. Continue diluting and testing
until a reading, which is in the concentration range for the test, is obtained. Be
sure to multiply the concentration found by the dilution factor (the number of
total 10 mL volumes used).
Example:
10 mL of sample is diluted with three 10 mL volumes of demineralized water; the
dilution factor is four.
12
Page 13

INTERFERENCES
LaMotte reagent systems are designed to minimize most common interferences.
Each individual test discusses interferences unique to that test. Be aware of
possible interferences in the water being tested.
The reagent systems also contain buffers to adjust the water sample to the ideal
pH for the reaction. It is possible that the buffer capacity of the water sample may
exceed the buffer capacity of the reagent system and the ideal pH will not be
obtained. If this is suspected, measure the pH of a reacted distilled water reagent
blank using a pH meter. This is the ideal pH for the test. Measure the pH of a
reacted water sample using the pH meter. If the pH is significantly different from
the ideal value, the pH of the sample should be adjusted before testing.
Interferences due to high concentration of the substance being tested, can be
overcome by sample dilution (see page 12).
STRAY LIGHT INTERFERENCE
Stray light interference can be minimized by always zeroing the meter and
reading a sample with the lid closed. Turbidimetric determinations (i.e. sulfate)
are most likely to exhibit a stray light interference. Colorimetric tests are less
likely to have this problem unless the samples are turbid. If sample turbidity is
causing a stray light interference, filtration may be needed.
13
Page 14
GENERAL OPERATING INFORMATION
OVERVIEW
The Model 1200 Colorimeter is a portable, microprocessor-controlled, direct
reading colorimeter. The microprocessor enables factory programmed
calibrations to optimally match non-linear curves. The auto zero feature
eliminates the need to dial in the zero manually. A sealed keypad controls the
operation. The large display presents measurements in ppm concentration and
indicates low battery warnings. The 1200 will turn off automatically after 5
minutes to prolong battery life. This will not affect the user calibration and the
meter will not have to be re-zeroed. The calibration and the last zero reading will
be stored in memory.
The 1200 is supplied with a 9 volt alkaline battery. An optional AC power
adapter is available.
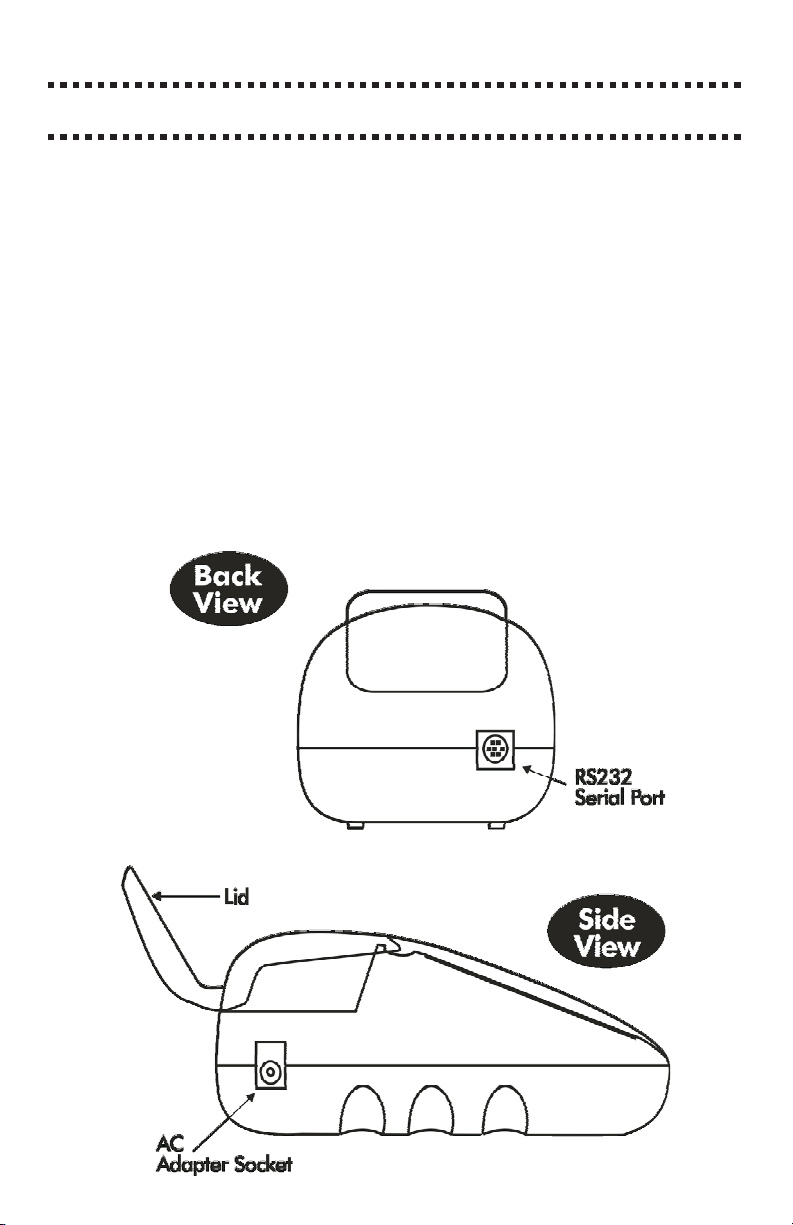
A RS-232 serial port on the back of the meter allows an interface of the
colorimeter with a computer for real time data acquisition and data storage using
the PC. This port also allows an interface with a RS-232 serial printer.
14
Page 15
READ
ZERO
Top
View
Bottom
View
2.55
Serial
Number
Battery
Compartment
I200
••••••••••••••••••
15
Page 16
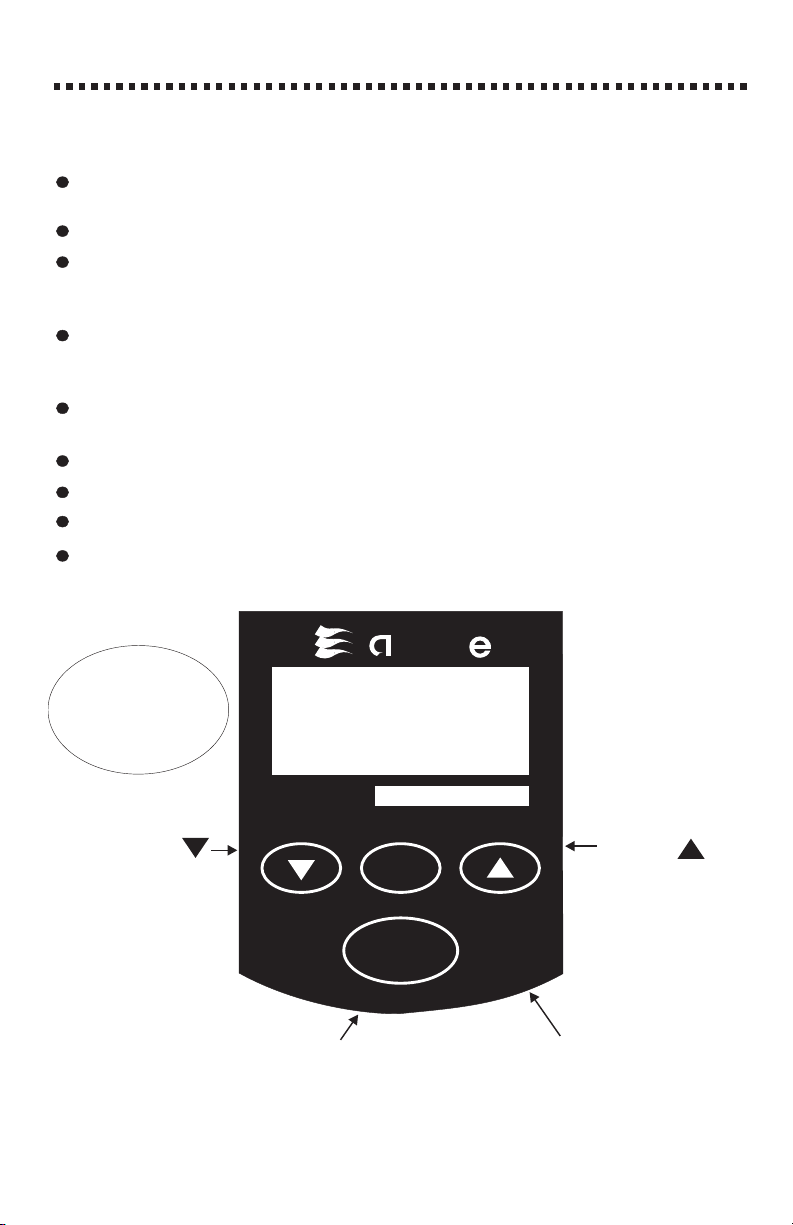
THE KEYPAD and DISPLAY
The button is used to turn the meter
ON and to take readings. Pressing the button
for 2 seconds will cause the meter to display
OFF. Releasing the button when OFF is
displayed turns the meter OFF.
The button is used
to zero the meter and for
calibration procedures.
The DOWN
ARROW will
the
numerical value
of the display.
The UP
ARROW will
the numerical
valueofthe
display.
See
TROUBLE
SHOOTING
GUIDE
page 23
The DISPLAY :
When the button is first pushed, a number will be briefly displayed that
indicates the software version number.
A walking dash ( ) will be displayed when reading is taking place.
(blank) will be displayed after the button has been pushed and held
for 2 seconds. If the button is released while is displayed, a zero or
blank reading will be taken.
(calibrate) will be displayed after the zero button has been pushed and held
for 5 seconds. If the button is released while is displayed, the
calibration mode will be entered as indicated by a flashing display.
will be displayed after the button has been held down for 2 seconds.
The meter will turn off when the button is released.
will be displayed when the battery voltage is very low.
will be displayed when concentration is over range.
will be displayed when the bulb has burned out.
will be displayed when the battery voltage is getting low. Readings are
reliable. Replace battery as soon as possible.
-
READ
ZERO
I200
••••••••••••••••••
L
Mott
DECREASE
READ
ZERO
INCREASE
READ
ZERO
ZERO
ZERO
READ
Bla
BLA
cal
CAL
OFF
Er1
Er2
Er3
BAT
16
Page 17
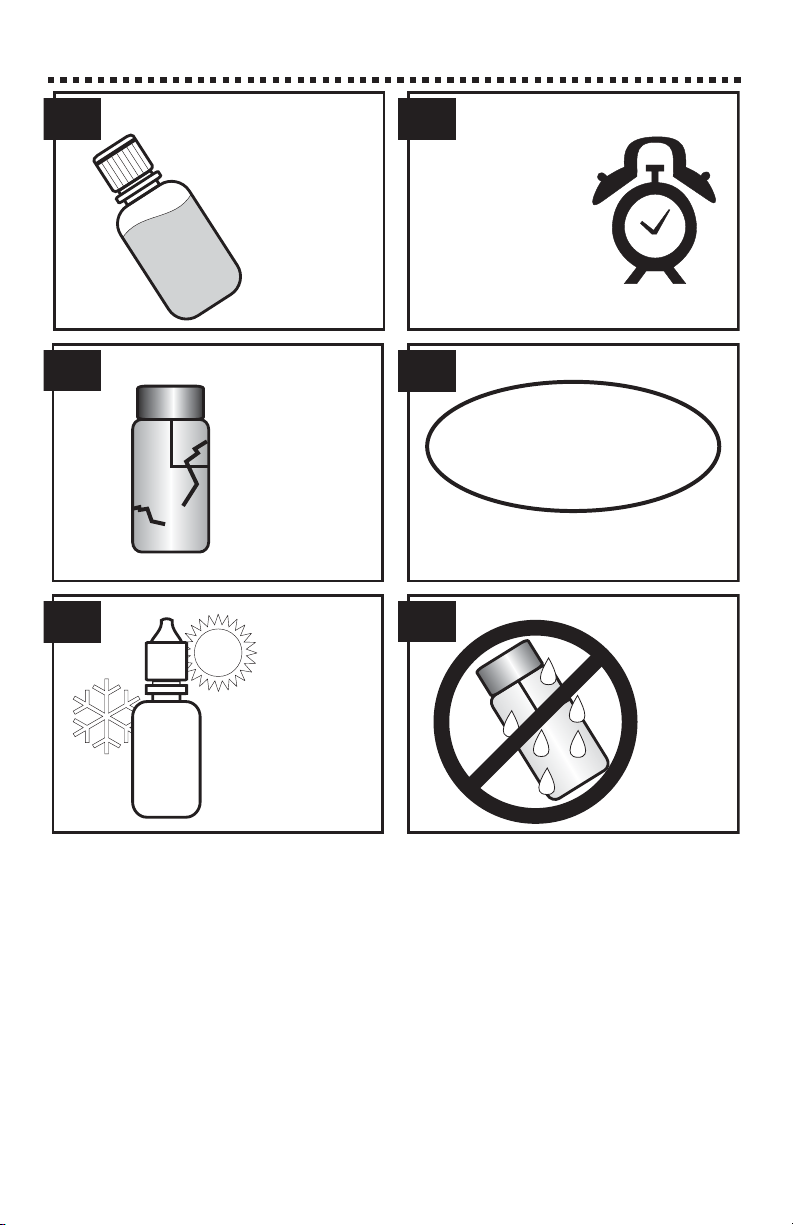
TESTING TIPS
Samples should
be collected in a
clean glass or
polyethylene
container.
Observe the shelf-life
recommendations for reagents.
Samples should be
analyzed as soon as
possible after
collection.
5.
3.
1. 2.
4.
6.
Protect reagents
and components
from extreme heat
and cold.
Never put
wet tubes in
colorimeter.
Discard tubes
that are badly
scratched.
ONE
year
SHELF
LIFE
17
Page 18
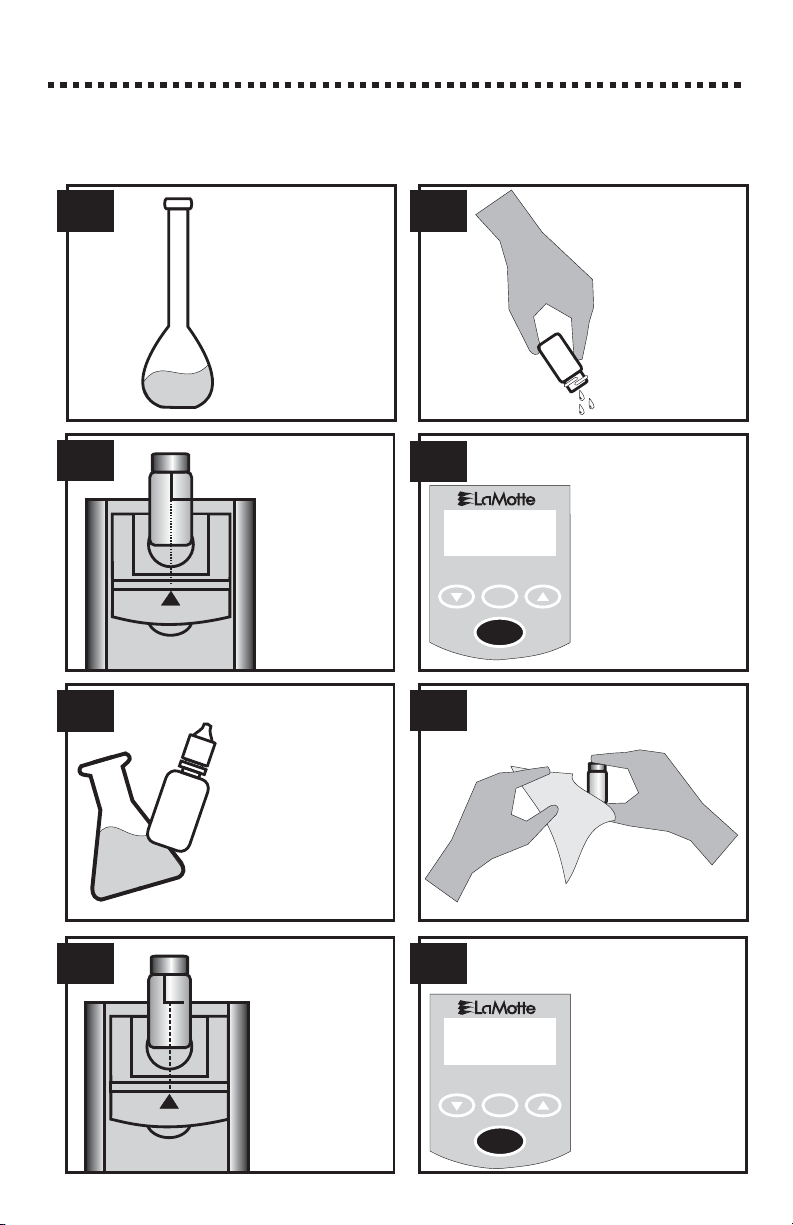
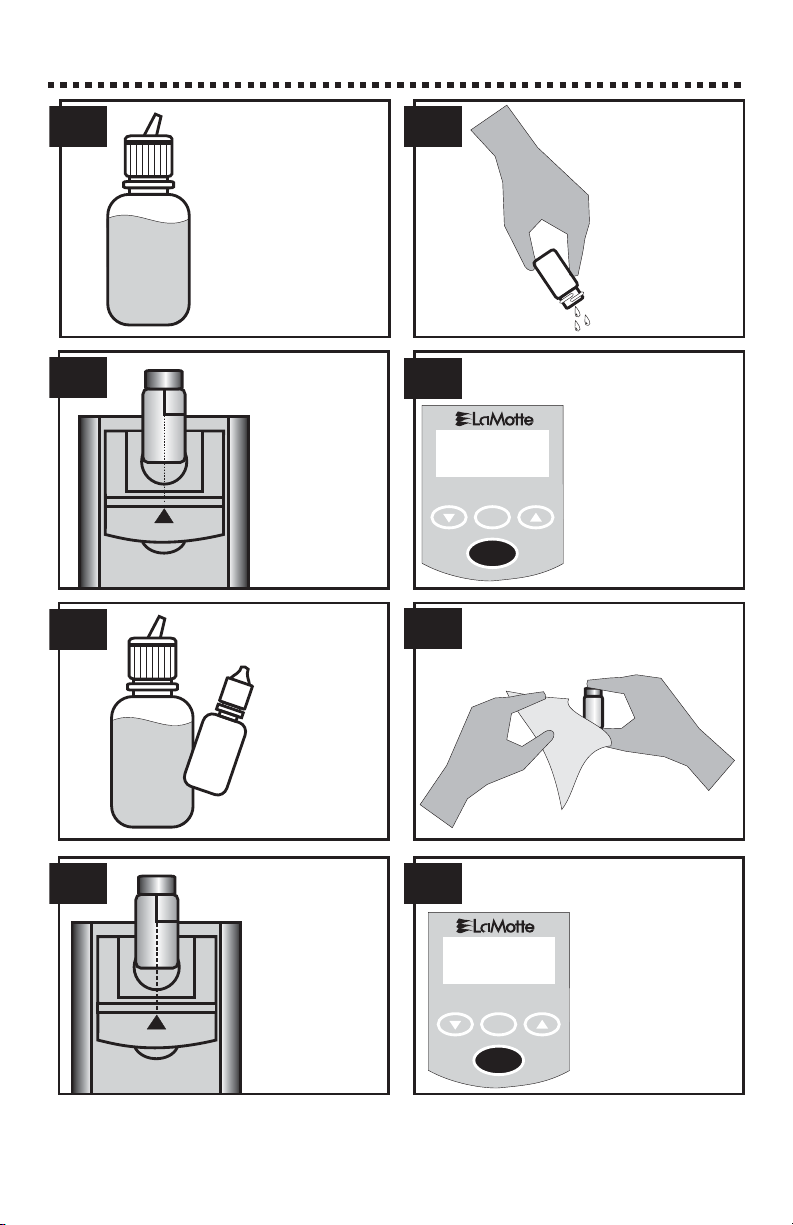
CALIBRATION PROCEDURE
Prepare standard
solutions to be
tested.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
standard
solution. Cap
and wipe dry.
7. 8.
5.
3.
1. 2.
4.
6.
React a standard
that is in the range
of the reagent
system, and similar
to the expected
range of the sample.
Follow the
individual test
procedure.
Wipe the tube clean with a
lint-free cloth.
2.50 ppm
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until
is displayed.
Release the button
to take a zero
reading (0 ppm).
Insert the
tube into the
chamber,
being sure to
align the
index line
with the
arrow on the
meter. Close
the lid.
Push the button. If the
displayed value is not the same as
thevalueofthe
reacted standard
(within the
specification
limits), continue
with the
calibration
procedure.
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
2.55
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
bla
READ
READ
ZERO
bla
The 1200 has been pre-calibrated. Recalibration of the 1200 by the user is not
required. However, a procedure to standardize the calibration (shown below)
should be performed to obtain the most accurate readings.
18
Page 19
The 1200 can be calibrated with two prepared standards. The concentrations of
Push the
button again to
memorize the
calibration. The
1200 display will
stop flashing.
Calibration is
complete.
ZERO
Turn the unit off by
holding the
button down for at
least 2 seconds, or
proceed to measure
the test samples
following the
procedure on page
20.
READ
9. 10.
11 .
Push and hold the
button for 5
seconds until is
displayed. Release
the button. The
display will flash.
Adjust the display
with the and
buttons until the
value of the standard
is displayed.
ZERO
CAL
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
“
“
“
“
2.55
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
2.50
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
the standards should be chosen from the low and high ends of the range of the
meter. The low standard must be less than 5% of over range. To calibrate the
meter with two standards, follow steps 1-11 of the calibration procedure for one
reacted sample. Repeat steps 1-11 of the calibration procedure with the second
reacted standard.
•
The calibration procedure should be followed as often as required by
regulations and laws for compliance monitoring.
•
To reset the calibration to the factory calibration: with the meter off, hold
down the t and push READ. The meter will turn on and the calibration
will be reset.
19
Page 20
ANALYSIS PROCEDURE
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Push the
button to turn the
meter on. Press the
button and
hold it for 2 seconds
until is
displayed. Release
the button to take a
zero reading (0
ppm).
READ
ZERO
bla
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry.
7. 8.
5.
3.
1. 2.
4.
6.
React a sample
following the
individual test
procedure.
Wipe the tube clean with a
lint-free cloth.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
Push the
button. The
concentration in
ppm will be
displayed within
2 seconds.
READ
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
2.55
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
bla
20
Page 21
9.
The 1200 will turn
off automatically 5
minutes after the
last button push. To
turn the meter off
manually, hold the
button for 2
seconds. Release the
button when is
displayed.
READ
OFF
If is displayed, the
concentration is over range.
The sample must be diluted
and re-tested. See page 12.
Er2
Note
I200
••••••••••••••••••
ZERO
COLORIMETER
READ
21
Page 22

AVAILABLE TEST KITS
TEST
FACTOR
Ammonia
Nitrogen
ORDER
CODE/MODEL
3680-01
DC 1200-NH
Bromine 3672-01
DC 1200-BR
Chlorine
(Free &
3670-01
DC1200-CL
Total)
Chlorine
(Free &
3670-01-LI
DC1200-CL-LI
Total)
Chlorine
Dioxide
3671-01
DC1200-CLO
Copper 3673-01
DC1200-CO
Fluoride 3674-01
DC1200-FL
Iron 3681-01
DC1200-FE
RANGE
(PPM)
DETECTION
LIMIT
TEST METHOD
(# OF REAGENTS)
0-5.00 0.05 Nessler (2)
0-7.00 0.05 DPD Tablets (1)
0-4.00 0.05 DPD Tablets (2)
0-4.00 0.05 DPD Liquid (3)
0-7.00 0.05 DPD with Glycine
Solution (2)
0-8.00 0.03 Diethyldithio-
carbamate (1)
0-2.00 0.03 Aliizarin-Zirconyl
(2)
0-4.00 0.25 1-10
Phenanthroline (2)
Manganese 3682-01
0-0.90 0.01 PAN (3)
DC1200-MN
Molybdenum 3676-01
0-50.0 0.5 Thioglycolate (3)
DC1200-MO
Nitrate
Nitrogen
Ozone 3678-01
3677-01
DC1200-NA
0-3.00 0.05 Cadmium
0-0.40 0.04 Indigo Blue (3)
DC1200-OZ
Phosphate 3679-01
0-3.00 0.07 Ascorbic Acid (2)
DC1200-PLR
Sulfate 3683-01
0-100.0 1.0 Barium Chloride
DC1200-SU
Test kit includes 1200 meter and reagent system.
22
Reduction (2)
(1)
Page 23
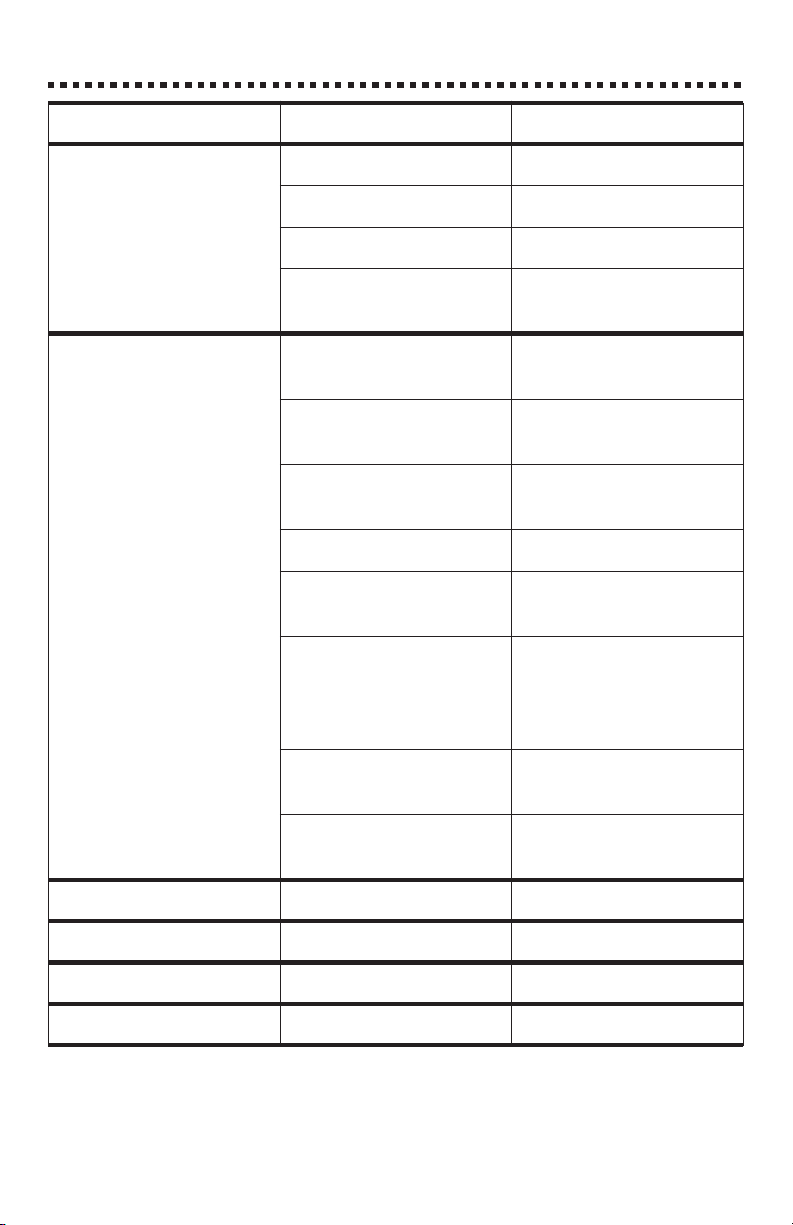
TROUBLESHOOTING
er1
er2
er3
PROBLEM CHECK ACTION
Meter won’t turn on Battery Replace
AC Adapter Plug in
AC Wall Outlet Verify power source
Contact LaMotte for
Return Authorization
Suspect Calibration Check calibration with
standards
Verify standard
concentration
Verify with another
meter
Check tube alignment Re-align tube
Check sample tubes for
dirt and scratches
Check to see if internal
meter components are
wet
Reset meter to factory
calibration
Contact LaMotte for
Return Authorization
Return to LaMotte for
repair
Use new standards
Runtestwithalternative
reagent system
Check other meter
calibrations
Check, clean and/or
replace if necessary
Always dry tubes before
inserting. Examine
chamber for visible
moisture.
With meter off, hold
down t and press READ
Return for calibration
check
Very low battery Change battery
Over range Dilute sample
Burnt out bulb Call LaMotte
BAT Low battery Change battery
23
Page 24

RS232 PORT
The 1200 Colorimeter may be interfaced with any IBM compatible computer
using an Interface cable (Code 1772). The meter may also be interfaced with an
RS-232 serial printer, using an appropriate cable and setting the printer
configuration to the output below.
Output: RS232 compatible, asynchronous serial, 9600 baud, no parity, 8 data
bits, 1 stop bit.
Computer Connection: RS232 (Code 1772) interface connection, 8 pin
mini-DIN/9 pin F D-submin.
Pin out:
5 RS-232 TxD
3 RS-232 RxD
4, 6, 8 Digital ground
24
Page 25
MAINTENANCE
REPLACING THE BATTERY
The LaMotte 1200 uses a standard 9-volt alkaline battery that is available
worldwide. The battery compartment is located on the bottom of the case. To
replace the battery:
1. Open the battery compartment lid.
2. Remove the battery and disconnect the battery from the polarized plug.
3. Carefully connect the new battery to the polarized plug and insert it into
the compartment.
4. Close the battery compartment lid.
REPAIRS
If it is necessary to return the instrument for repair, telephone LaMotte Company
at 1-800-344-3100 or fax 1-410-778-6394 for a return authorization number.
METER DISPOSAL
Waste Electrical and Electronic Equipment (WEEE)
Natural resources were used in the production of this equipment. This equipment
may contain materials that are hazardous to health and the environment. To
avoid harm to the environment and natural resources, the use of appropriate
take-back systems is recommended. The crossed out wheeled bin symbol on the
meter encourages you to use these systems when disposing of this equipment.
Take-back systems will allow the materials to be reused or recycled in a way that
will not harm the environment. For more information on approved collection,
reuse, and recycling systems contact your local or regional waste administration
or recycling service.
25
Page 26

WARRANTY
This Instrument is guaranteed to be free from defects in material and workmanship for a
period of one (1) year from the original purchase date. In the event that a defect is found
during the warranty time frame, LaMotte Company agrees that it will be repaired or
replaced without charge except for the transportation costs. This guarantee does not cover
batteries.
This product can not be returned without a return authorization number from LaMotte
Company. For warranty support or a Return Authorization Number, contact LaMotte
Company at 1-800-344-3100 or tech@lamotte.com.
Limitations
This guarantee is void under the following circumstances:
Damage due to operator negligence, misuse, accident or improper application.
•
Damage or alterations from attempted repairs by an unauthorized
•
(non-LaMotte) service.
Damage due to improper power source, AC adapter or battery.
•
Damage caused by acts of God or natural disaster.
•
Damage occurred while in transit with a shipping carrier.
•
LaMotte Company will service and repair out-of-warranty products at a nominal
charge.
26
Page 27

27
Page 28

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
626728 • 4.09
Page 29

1200 COLORIMETER
CHLORINE
DPD TABLET TEST
MODEL 1200-CL · CODE 3670-01
QUANTITY CONTENTS CODE
100 *Chlorine DPD #1 Instrument Grade Tablets *6903A-J
100 *Chlorine DPD #3 Instrument Grade Tablets *6197A-J
1 Colorimeter Tubes, w/caps,set of 6 0290-6
2 Tablet Crushers 0175
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Chlorine DPD 26728
*WARNING: Reagents marked with an * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Chlorine is added to water to kill bacteria and other disease-producing organisms,
control algae, and remove undesirable odors and colors. Chlorine added to water quickly
forms hypochlorous acid, HClO, also known as Free Available Chlorine, the active
ingredient responsible for chlorine*s sanitizing capabilities. Free Available Chlorine
combines with impurities in the water to form chloramines and other organic nitrogen
compounds. In combined form, its sanitizing capability diminishes, and higher levels of
chlorine are necessary to achieve effective sanitation.
Therefore, it is essential to chlorinate to the point of establishing a Free Available
Chlorine Residual, and then to maintain that residual at a recommended level. The
LaMotte DPD tablet test method distinguishes levels of Free Available Chlorine,
Combined Chlorine, and Total Residual Chlorine, using a single test sample.
Page 30

CHLORINE TEST PROCEDURE - DPD METHOD
7. 8.
5.
3.
1. 2.
4.
6.
I200
COLORIMETER
••••••••••••••••••
READ
READ
ZERO
CAL
••••••••••••••••••
bla
COLORIMETER
I200
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
Empty all but a few
drops of sample from
tube. Add one
*Chlorine DPD #1
IG T ablet (6903A) and
crush with tablet
crusher (0175).
NOTE: T o insure
accurate results, tablet
must be crushed before
filling tube.
Align the index line with the
arrow on the meter, insert tube
into chamber.
the lid. Push the
READ button.
Record results as
ppm Free A vailable
Chlorine. Save
sample for the T otal
Residual Chlorine
test.
Rinse and fill a
colorimeter tube
(0290) to the
10 mL line with
sample water.
Cap and wipe
dry .
Push the
READ
button to turn the
meter on. Press the
ZERO button and
hold it for 2 seconds
until is displayed.
bla
Release the button to
take a zero reading
(0.00 ppm).
Fill to 10 mL line
with sample. Cap
and mix until tablet
disintegrates. Make
readings within 30
seconds after
disintegration of the
tablet.
Add one Chlorine
DPD #3 IG T ablet
(6197A) to the sample
from Step 6. Crush
tablet.
READ
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
FREE AVAILABLE CHLORINE
Page 31

er2
TOTAL RESIDUAL CHLORINE
11.
9. 10.
12.
••••••••••••••••••
READ
2.55
ZERO
COLORIMETER
I200
Cap and mix
until tablet
disintegrates.**
Push the
READ button.
Record reading
as ppm Total
Residual
Chlorine.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
Subtract the Free Available
Chlorine reading from the
Total Residual Chlorine
reading to determine ppm
Combined Chlorine
(Monochloramine,
Dichloramine, and Nitrogen
Trichloride) present in the
water sample.
If either chlorine reading displays , repeat procedure on diluted sample, and
multiply the result by the appropriate dilution factor. See 1200 Colorimeter Instruction
Manual for procedure.
Levels of chlorine above 10 mg/L will cause a bleaching effect on the DPD indicator, and
may give a false indication that no chlorine is present. If it is possible that the chlorine
concentration is greater than 10 mg/L (e.g. after shock treatment), perform test on a
diluted sample and multiply the result by appropriate dilution factor.
CAUTION: DO NOT leave reacted DPD samples in test tubes. Discard sample and
thoroughly rinse tubes. If allowed to remain, DPD will stain tubes, significantly
impairing the operation of the 1200 Colorimeter. If necessary, acid wash, and vigorously
clean glassware with test tube brush and detergent.
**For wastewater samples, Standard Methods for the Examination of Water and Wastewater
recommends waiting 2 minutes for full color development.
Page 32

DPD CHLORINE
TEST METHOD SPECIFICATIONS
APPLICATION
Drinking water supplies and distribution systems, swimming pool and spas, sewage and
chlorinated waste waters, process waters and sanitizing solutions.
RANGE
0 to 4.0 mg/L Chlorine (may be extended by dilution)
METHOD
In the absence of Iodide, Free Available Chlorine reacts instantly with the buffered
diethyl-p-phenylenediamine indicator (DPD) to produce a red color in proportion to the
amount of chlorine present. Subsequent addition of potassium iodide produces a rapid
color response from the combined forms of chlorine (chloramines).
HANDLING & PRESERVATION
Chlorine in aqueous solutions, particularly weak solutions, is not stable. Exposure to
sunlight or agitation will accelerate the reduction of chlorine. Fill sample containers to
the top and cap tightly. Analyze samples as soon as possible after collection.
INTERFERENCES
The only interfering substance likely to be encountered is oxidized manganese. The
extent of this interference can be determined by treating a sample with sodium arsenite
to destroy the chlorine present, so that the amount of interference can be measured.
CALIBRATION
The single test colorimeter is precalibrated. In order to comply with NPDWR or NPDES
reporting regulations, the calibration should be checked periodically by using a set of
reference standards including a 0 mg/L blank and 0.3, 1.0, and 3.5 mg/L chlorine. To
prepare these standards, a LaMotte 1000 mg/L standard chlorine equivalent solution
(Code 3858) is available. Consult with your local regulatory agency to determine
standardization frequency.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
SM
63670-01 · 7/08
Page 33

1200 COLORIMETER
CHLORINE
DPD LIQUID TEST
MODEL 1200-CL-LI • CODE 3670-01-LI
QUANTITY CONTENTS CODE
30 mL DPD #1A Free Chlorine Reagent P-6740-G
30 mL *DPD #1B Free Chlorine Reagent *P-6741-G
30 mL *DPD #3 Total Chlorine Reagent *P-6743-G
1 Colorimeter Tubes, with caps 0290-6
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Chlorine DPD 26728
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view or
print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by email, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Chlorine is added to water to kill bacteria and other disease-producing organisms,
control algae, and remove undesirable odors and colors. Chlorine added to water quickly
forms hypochlorous acid, HClO, also known as Free Available Chlorine, the active
ingredient responsible for chlorine's sanitizing capabilities. Free Available Chlorine
combines with impurities in the water to form chloramines and other organic nitrogen
compounds. In combined form, its sanitizing capability diminishes, and higher levels of
chlorine are necessary to achieve effective sanitation.
Therefore, it is essential to chlorinate to the point of establishing a Free Available
Chlorine Residual, and then to maintain that residual at a recommended level. The
LaMotte DPD method distinguishes levels of Free Available Chlorine, Combined
Chlorine, and Total Residual Chlorine, using a single test sample.
Page 34

CHLORINE TEST PROCEDURE - DPD METHOD
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blank or zero.
8.
5.
3. 4.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry .
1. 2.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
7.
CAL
I200COLORIMETER
••••••••••••••••••
READ
Align the index line
with the arrow on
the meter, insert
tube into chamber.
Close the lid. Push
the button.
Record results as
ppm Free Available
Chlorine.
READ
Remove the tube and
add 5 drops DPD #1A
Free Chlorine Reagent
(6740), and 5 drops of
*DPD #1B Free
Chlorine Reagent
(6741).
6.
Cap and invert to
mix. Wipe tube dry .
Make readings
within 30 seconds.
Push the
button to turn the
meter on. Press the
button and
hold it for 2 seconds
until is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
READ
ZERO
bla
Save sample for the
Total Residual
Chlorine T est.
Proceed to Step 9.
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
FREE AVAILABLE CHLORINE
Page 35

er2
Subtract the Free Available Chlorine reading from the Total Residual
Chlorine reading to determine ppm Combined Chlorine (Monochloramine,
Dichloramine, and Nitrogen T richloride present in the water sample).
11.
9. 10.
12.
Push the
button.
Record reading
as ppm Total
Residual
Chlorine.
READ
Cap and
invert to
mix.**
Insert the tube
into the
chamber, being
sure to align
the index line
with the arrow
on the meter.
Close the lid.
I200 COLORIMETER
••••••••••••••••••
READ
2.55
ZERO
Add 5 drops *DPD
#3 T otal Chlorine
Reagent (6743) to
the sample from
Step 7.
13.
TOTAL RESIDUAL CHLORINE
If either chlorine reading displays , repeat procedure on diluted sample, and
multiply the result by the appropriate dilution factor. See 1200 Colorimeter Instruction
Manual for procedure.
Levels of chlorine above 10 mg/L will cause a bleaching effect on the DPD indicator,
and may give a false indication that no chlorine is present. If it is possible that the
chlorine concentration is greater than 10 mg/L (e.g. after shock treatment), perform test
on a diluted sample and multiply the result by appropriate dilution factor.
CAUTION: DO NOT leave reacted DPD samples in test tubes (0290). Discard sample
and thoroughly rinse tubes. If allowed to remain, DPD will stain tubes, significantly
impairing the operation of the 1200 Colorimeter. If necessary, acid wash, and vigorously
clean glassware with test tube brush and detergent.
**For wastewater samples, Standard Methods for the Examination of Water and Wastewater
recommends waiting 2 minutes for full color development.
Page 36

DPD CHLORINE TEST METHOD SPECIFICATIONS
APPLICATION
Drinking water supplies and distribution systems, swimming pool and spas, sewage and
chlorinated waste waters, process waters and sanitizing solutions.
RANGE
0 to 4.0 mg/L Chlorine (may be extended by dilution)
METHOD
In the absence of Iodide, Free Available Chlorine reacts instantly with the buffered
diethyl-p-phenylenediamine indicator (DPD) to produce a red color in proportion to the
amount of chlorine present. Subsequent addition of potassium iodide produces a rapid
color response from the combined forms of chlorine (chloramines).
HANDLING & PRESERVATION
Chlorine in aqueous solutions, particularly weak solutions, is not stable. Exposure to
sunlight or agitation will accelerate the reduction of chlorine. Fill sample containers to
the top and cap tightly. Analyze samples as soon as possible after collection.
INTERFERENCES
The only interfering substance likely to be encountered is oxidized manganese. The
extent of this interference can be determined by treating a sample with sodium arsenite
to destroy the chlorine present, so that the amount of interference can be measured.
CALIBRATION
The single test colorimeter is precalibrated. In order to comply with NPDWR or NPDES
reporting regulations, the calibration should be checked periodically by using a set of
reference standards including a 0 mg/L blank and 0.3, 1.0, and 4.0 mg/L chlorine. To
prepare these standards, a LaMotte 250 ppm standard chlorine equivalent solution
(Code 6973) is available. Consult with your local regulatory agency to determine
standardization frequency.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
3670-01-LI · 08/08
Page 37

1200 COLORIMETER
CHLORINE DIOXIDE
DPD LIQUID TEST
CODE 3671-01-LI
QUANTITY CONTENTS CODE
30 mL DPD #1A Free Chlorine Reagent P-6740-G
30 mL *DPD #1B Free Chlorine Reagent *P-6741-G
15 mL Glycine Solution 6811-E
1 Colorimeter Tubes, w/caps 0290-6
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Chlorine Dioxide 26731
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Chlorine dioxide is used as a substiture for and an adjunct to chlorine in water
treatment. It is better than chlorine in eliminating taste and odor in certain cases.
Chlorine dioxide, unlike chlorine, does not produce carcinogenic chlorinated organic
compounds when reacted with organic materials. A disadvantage is the higher cost of
producing chlorine dioxide compared to chlorine.
1
Page 38

CHLORINE DIOXIDE TEST PROCEDURE - DPD METHOD
Remove tube.
Add 5 drops
Glycine
Solution
(6811). Cap
and mix.
Cap and invert to
mix. Wipe tube dry.
Make readings
within 30 seconds.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blank or zero.
Rinse and fill a
colorimeter tube
(0290) to the
10 mL line with
sample water.
Cap and wipe
dry .
5.
7.
3.
1. 2.
4.
6.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
Add 5 drops DPD
#1A Free Chlorine
Reagent (6740),
and 5 drops of
*DPD #1B Free
Chlorine Reagent
(6741).
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
READ
ZERO
bla
8.
CAL
I200COLORIMETER
••••••••••••••••••
READ
Align the index
line with the arrow
on the meter, insert
tube into chamber.
Close the lid. Push
the
button. Record
results as ppm
Chlorine Dioxide.
READ
Read the 1200 Colorimeter Manual before proceeding.
Carefully wipe tubes dry before inserting into the colorimeter
chamber.
CHLORINE DIOXIDE
2
Page 39

NOTE: If reading displays , repeat procedure on diluted sample, and multiply the
er2
result by the appropriate dilution factor. See 1200 Colorimeter Instruction Manual for
procedure.
CAUTION: DO NOT leave reacted DPD samples in test tubes (0290). Discard sample
and thoroughly rinse tubes. If allowed to remain, DPD will stain tubes, significantly
impairing the operation of the 1200 Colorimeter. If necessary, acid wash, and vigorously
clean glassware with test tube brush and detergent.
CHLORINE DIOXIDE TEST METHOD SPECIFICATIONS
APPLICATION
Drinking and pool waters; domestic and industrial wastewater.
RANGE
0 to 7.0 ppm Chlorine Dioxide
METHOD
Chlorine dioxide reacts with DPD to form a red color in proportion to the
concentration.
HANDLING & PRESERVATION
Test as soon as possible to avoid loss of chlorine dioxide.
INTERFERENCES
Chlorine interference is eliminated by the addition of glycine to the sample before the
indicator.
3
Page 40

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 • Fax 410-778-6394
Visit us on the web at www.lamotte.com
SM
63671-01-LI• 4/08
Page 41

1200 COLORIMETER
BROMINE
DPD TABLET METHOD
MODEL 1200-BR · CODE 3672-01
QUANTITY CONTENTS CODE
100 *Chlorine DPD #1 Tablets (Instrument Grade) *6903A-J
6 Colorimeter Tubes, w/caps 0290-6
1 Tablet Crusher 0175
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Bromine DPD 26730
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Bromine is added to water to kill bacteria and other disease-producing organisms,
control algae, and remove undesirable odors and colors. Bromine added to water, forms
hypobromus acid, the sanitizing form of bromine. Bromine forms free available bromine
and combined bromine or bromamines. Combined bromine is a very active sanitizer.
Page 42

BROMINE TEST PROCEDURE - DPD METHOD
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
READ
ZERO
bla
Fill the W ater
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry .
Align the index line
with the arrow on
the meter, insert
tube into chamber .
Close the lid. Push
the
button. Record
results as ppm
Bromine.
READ
CAL
I200COLORIMETER
••••••••••••••••••
READ
7.
5.
3.
1. 2.
4.
6.
I200
COLORIMETER
••••••••••••••••••
READ
ZERO
Empty all but a few
drops of sample from
tube. Add one
*Chlorine DPD #1
Tablet (6903A) and
crush with tablet
crusher (0175).
To insure
accurate results, tablet
must be crushed before
filling tube.
NOTE:
Fill to 10 mL line
with sample. Cap
and mix until tablet
disintegrates. Make
readings within 30
seconds after
disintegration of the
tablet.
bla
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
FREE AVAILABLE BROMINE
Page 43

DPD BROMINE
TEST METHOD SPECIFICATIONS
APPLICATION
Drinking, surface, saline waters; swimming pool water; domestic and industrial waters
and wastes.
RANGE
0to7.0mg/LBromine(maybeextendedbydilution)
METHOD
In buffered sample, bromine reacts with disthyl-p-phenylene diamine (DPD) to produce
a pink-red color in proportion to the concentration of bromine present.
HANDLING & PRESERVATION
Bromine is aqueous solutions is not stable, and the bromine content of samples or
solutions, particularily weak solutions, will rapidly decrease. Exposure to sunlight or
agitation will acceslerater the reduction of bromine present in such solutions. For best
results, start analysis immediately after sampling. Samples to be analyzed for bromine
cannot be preserved or stored.
INTERFERENCES
The only interfering substance likely to be encountered in water is oxidized manganese.
The extent of this interference can be dtermined by treating a sample with sodium
arsenite to destroy the bromine present so that the degree of interference can be
estimated.
Iodine and chlorine can also interfere, but these are not normally present unless they
have been added as sanitizers.
Page 44

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
63672-01 · 10/08
Page 45

1200 COLORIMETER
COPPER
MODEL 1200-CO · CODE 3673-01
QUANTITY CONTENTS CODE
30 mL *Copper 1 *6446-G
1 Colorimeter Tubes, w/caps 0290-6
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Copper 26732
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by email, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
The copper content of drinking water generally falls below 0.03 parts per million, but
copper levels as high as 1.0 part per million will give water a bitter taste. W aters testing
as high as 1.0 part per million copper have probably been treated with a copper
compound, like those used in the control of algae, or have become contaminated from
untreated industrial wastes. The addition of copper sulfate to lakes causes an increase in
the copper content of the sediments. Acid waters and those high in free carbon dioxide
may cause the corrosion or “eating away” of copper, brass, and bronze pipes and fittings.
This corrosion results in the addition of copper into the water supply.
Page 46

COPPER TEST PROCEDURE - DIETHYLDITHIOCARBAMATE
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blank or zero.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry .
5.
3.
1. 2.
4.
6.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
7.
Align the index line with the
arrow on the meter , insert tube
CAL
I200COLORIMETER
••••••••••••••••••
READ
into chamber.
Close the lid. Push
the
button. Record
resultsasppm
Copper.
READ
Cap and invert to
mix. Wipe tube dry .
Remove tube from
colorimeter. Add 5
drops of *Copper 1
(6446).
Push the
button
to turn the meter
on. Press the
button
and hold it for 2
seconds until
is displayed.
Release the button
to take a blank
reading (0.0 ppm).
READ
ZERO
bla
L
Mott
L
Mott
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
COPPER
Page 47

NOTE: If test reading displays , repeat procedure on diluted sample, and multiply
er2
the result by the appropriate dilution factor. See 1200 Colorimeter Instruction Manual
for procedure.
COPPER TEST METHOD SPECIFICATIONS
APPLICATION
Drinking, surface, and saline waters; domestic and industrial wastes.
RANGE
0 to 5.0 ppm Copper
METHOD
Cupric ions form a yellow colored chelate with diethyldithiocarbamate around pH 9-10
in proportion to the concentration of copper in the sample.
HANDLING & PRESERVATION
Copper has a tendency to be absorbed to the surface of the sample container. Samples
should be analyzed as soon as possible after collection. If storage is necessary, 0.5 mL of
20% hydrochloric acid per 100 mL of sample will prevent “plating out.” However, a
correction must be made to bring the reaction into the optimum pH range.
INTERFERENCES
Bismuth, cobalt, mercurous, nickel and silver ions and chlorine (6 ppm or greater)
interfere and must be absent.
Page 48

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
3673-01 · 6/08
Page 49

1200 COLORIMETER
EPA
COMPLIANT
FLUORIDE
SPADNS METHOD
MODEL 1200-FL · CODE 3674-01
QUANTITY CONTENTS CODE
2 x 100 mL *Acid-Zirconyl-SPADNS Reagent *3875-J
2 x 60 mL *Sodium Arsenite Solution *4128-H
1 Pipet, 0.5 mL, plastic 0353
1 Pipet, 1.0 mL, plastic 0354
1 Colorimeter Tubes, with caps, set of 6 0290-6
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Fluoride 26733
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
NOTE: This procedure uses EPA approved SPADNS Reagent System for fluoride found
in method 4500-F-D, 18th Edfition of Standard Methods, page 1-27.
FLUORIDE INTRODUCTION
Fluoride may occur naturally in some ground waters or it may be added to public
drinking water supplies to maintain a 1.0 mg/L concentration to prevent dental cavities.
At higher concentrations, fluoride may produce an objectionable discoloration of tooth
enamel called fluorosis, though levels up to 8 mg/L have not been found to be
physiologically harmful.
Page 50

FLUORIDE TEST PROCEDURE - SPADNS METHOD
Insert the tube
into the
chamber, being
sure to align
the index line
with the arrow
on the meter.
Close the lid.
This tube is
the sample
blank.
5.
Use the 0.5 mL
pipet (0353) to
add 0.5 mL of
*Sodium Arsenite
Solution (4128).
Cap and mix.
3.
This test requires a
reagent blank.
Rinseaclean
colorimeter tube
(0290) with clear,
colorless, fluoride
free water. Fill to
the 10 mL line
with clear,
colorless, fluoride
free water.
2.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
1.
Use the 1.0 mL
pipet (0354) to
add 2 measures of
*Acid-Zirconyl
SP ADNS
Reagent (3875).
Cap and mix
thoroughly . (This
is the reagent
blank.
4.
6.
Push the
button
to turn the meter
on. Press the
button
andholditfor2
seconds until
is displayed.
Release the button
to take a blank
reading (0.0 ppm).
READ
ZERO
bla
I200 COLORIMETER
••••••••••••••••••
READ
ZERO
bla
L
Mott
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
FLUORIDE
Page 51

FLUORIDE
9.
10 .
Align the index
line with the arrow
on the meter,
insert tube into
chamber. Close
the lid. Push the
button.
Record results as
ppm Fluoride.
READ
CAL
I200COLORIMETER
••••••••••••••••••
READ
18.0
Use the 1.0 mL
pipet (0354) to add
2 measures of
*Acid-Zirconyl
SP ADNS Reagent
(3875). Cap and
mix thoroughly.
8.7.
Rinseaclean
colorimeter tube
(0290) with
sample water. Fill
to the 10 mL line
with sample
water.
Use the 0.5 mL
pipet (0353) to
add 0.5 mL of
*Sodium Arsenite
Solution (4128).
Cap and mix.
L
Mott
er2
NOTE: Zeroing the meter with sample water or an empty chamber will result in an
message. Meter must be zeroed with a reagent blank.
Page 52

FLUORIDE SPADNS TEST METHOD SPECIFICA TIONS
APPLICATION
Drinking and surface waters; domestic and industrial waters.
RANGE
0.0 to 2.0 ppm Fluoride
METHOD
Colorimetric test based upon the reaction between fluoride and zirconium dye lake. The
fluoride reacts with the dye lake, dissociating a portion of it into a colorless complex ion
and dye. As the fluoride concentration increases, the color produced becomes
progressively lighter.
HANDLING & PRESERVATION
Samples may be stored and refrigerated in plastic containers.
INTERFERENCES
The following substances produce a positive interference at the concentration given:
Chloride (Cl
Phosphate (PO
Hexametaphosphate (NaPO
-
) 7000 mg/L
-3
)16mg/L
4
1mg/L
3)6
The following substances produce a negative interference at the concentration given:
Alkalinity (CaCO
Aluminum (Al
+3
Iron (Fe
)10mg/L
Sulfate (SO
) 5000 mg/L
3
+3
)0.1mg/L
-2
) 200 mg/L
4
Color and turbidity must be removed or compensated for in the procedure. Temperature
should be maintained within 5 degrees Celcius of room temperature.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
03.08
Page 53

1200 COLORIMETER
MOL YBDENUM
MODEL 1200-MO • CODE 3676-01
QUANTITY CONTENTS CODE
60 mL *Mo Buffer *3997-H
2 x 30 mL *Molybdenum Oxidizing Reagent *6485-G
2.5 g *Molybdenum Indicator Powder *6486-S
1 Colorimeter Tubes 10 mL, w/cap, set of 6 0290-6
1 Spoon, 0.05g, plastic 0696
1 Pipets, 1.0 mL, plastic, w/cap 0372
1 Pipet, 1.0 mL 0354
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Molybdenum 26729
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
NOTE: After use, return *MO Buffer (3997) to zipper top storage bag to reduce exposure to
corrosive reagent.
INTRODUCTION
Molybdenum occurs naturally in the earth’s crust as molybdenite and wolfenite, and is
an important element in many biochemical reactions, including nitrogen fixation. In
industrial processes, such as the operation of boilers and cooling towers, molybdenum, in
the form of sodium molybdate, is used as an environmentally safe corrosion inhibitor.
1
Page 54

MOLYBDENUM PROCEDURE - Thioglycolate Method
8.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Rinse and fill a
colorimeter
tube (0290) to
the 10 mL line
with sample
water.
Use the 0.05 spoon
(0696) to add 0.05g
of *Molybdenum
Indicator Powder
(6486).
1. 2.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
sample blank.
6.
5.
Wipe the tube dry.
Use the 1.0 mL
pipet (0354) to
add1mLof
*Molybdenum
Oxidizing
Reagent
(6485). Cap
and mix.
4.
Use the 1.0 mL
pipet (0372) to
add1mLof*Mo
Buffer (3997).
Cap and mix.
3.
7.
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until is
displayed. Release
the button to take a
blank reading
(0.0 ppm).
READ
ZERO
bla
I200 COLORIMETER
••••••••••••••••••
READ
ZERO
bla
L
Mott
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber
MOLYBDENUM
2
Page 55

NOTE: If the molybdenum reading exceeds 50 ppm, repeat procedure on diluted sample,
9.
10 .
Wipe the tube dry .
Cap and mix
until the
powder
dissolves.
Solution will
turn yellow if
molybdenum is
present.
Align the index line
with the arrow on
the meter, insert
tube into chamber.
Close the lid. Push
the
button. Record
results as ppm
Molybdenum.
READ
CAL
I200COLORIMETER
••••••••••••••••••
READ
18.0
11.
L
Mott
and multiply the result by the appropriate dilution factor. See 1200 Colorimeter
Instruction Manual for procedure.
3
Page 56

MOLYBDENUM
TEST METHOD SPECIFICATIONS
APPLICATION
Boiler and cooling water
RANGE
0 to 30 ppm Molybdenum
METHOD
Calcium thioglycolate reacts with molybdenum to give a yellow color with an intensity
proportional to the amount of molybdenum present.
HANDLING & PRESERVATION
Molybdenum samples may be stored in either plastic or glass containers.
INTERFERENCES
Nickel levels less than 50 ppm do not interfere; aluminum levels less than 10 ppm do
not interfere; chromate, at higher concentrations, interferes due to the intense yellow
color. Ferrous iron levels below 50 ppm do not interfere, but low levels of ferric iron will
cause a large blank. Highly buffered samples may exceed the capacity of the system
possibly producing inaccurate results. Add an extra 1.0 mL of *Mo Buffer (3997) to
adjust the pH of the sample to approximately 4.5. Multiply the result in ppm by 1.08.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
06/08
Page 57

1200 COLORIMETER
NITRATE NITROGEN
MODEL 1200-NA · CODE 3677-01
QUANTITY CONTENTS CODE
2 x 120 mL *Mixed Acid Reagent *V-6278-J
10 g *Nitrate Reducing Reagent *V-6279-D
1 Spoon, 0.1 g, plastic 0699
1 Graduated Cylinder 0416
1 Colorimeter Tubes, w/caps 0290-6
1 Water Sample Collecting Bottle 0688
1
*WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy , contact LaMotte by email, phone or fax.
To order individual reagents or test components, use the specified code number.
NITRATE - NITROGEN INTRODUCTION
Nitrogen is essential for plant growth, but the presence of excessive amounts in water
supplies presents a major pollution problem. Nitrogen compounds may enter water as
nitrates or be converted to nitrates from agricultural fertilizers, sewage, industrial and
packing house wastes, drainage from livestock feeding areas, farm manures and legumes.
Nitrates in large amounts can cause “blue babies” (methemoglobinemia) in infants less
than six months of age. Nitrate concentration is an important factor to be considered in
livestock products, where, in addition to causing methemoglobinemia, it is responsible
for many other problems. Nitrates in conjunction with phosphate stimulate the growth
of algae with all of the related difficulties associated with excessive algae growth.
U.S. Public Health Service Drinking Water Standards state that 10 ppm nitrate nitrogen
should not be exceeded. To the sanitary and industrial engineer, concentrations of less
than 1 ppm are acceptable.
1200 Colorimeter for Nitrate Nitrogen
26734
1
Page 58

NITRATE-NITROGEN TEST PROCEDURE -
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
8.
5.
3. 4.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry .
1. 2.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
7.
Remove tube from
colorimeter and
pour off 5 mL into
graduated cylinder
or similar. Discard
the remaining
sample.
6.
Pour the 5 mL
sample from a
graduated
cylinder or
similar into the
colorimeter
tube.
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
READ
ZERO
bla
Use the graduated
cylinder or similar to
measure 5 mL of
*Mixed Acid
Reagent (V-6278)
and add to tube.
5mL
5mL
2
MINUTES
5mL
Cap and mix. Wait approximately 2
minutes before proceeding to Step 9.
L
Mott
CADMIUM REDUCTION METHOD
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
NITRATE - NITROGEN
2
Page 59

NITRATE - NITROGEN
NOTE:
11.
9. 10.
NOTE:
At the end of waiting period,
an undissolved portion of
*Nitrate Reducing Reagent
may remain in bottom of the
tube without affecting results.
Use the 0.1 g spoon
(0699) to add two
measures of *Nitrate
Reducing Reagent
(V -6279). Cap.
Hold tube by
index finger and
thumb and mix by
12.
inverting approximately
times a minute for
. Wait
for maximum
color development.
50-60
4 minutes 10
minutes
10
MINUTES
Wipe tube dry.
Align the index line with the
arrow on the meter, insert
CAL
I200COLORIMETER
••••••••••••••••••
READ
tube into chamber.
Close the lid. Push
the
button. Record
results as ppm
Nitrate Nitrogen.
READ
To convert Nitrate Nitrogen
(NO -N) results to ppm Nitrate
(NO ), multiply by 4.4.
3
3
L
Mott
3
Page 60

NITRATE-NITROGEN
TEST METHOD SPECIFICATIONS
APPLICATION
This method determines nitrate levels in drinking, surface, saline waters, domestic, and
industrial waters.
RANGE
0 - 3.0 ppm Nitrate Nitrogen (Range can be extended by dilution.)
METHOD
Powdered cadmium is used to reduce nitrate to nitrite. The nitrite that is originally
present plus reduced nitrate is determined by diazotization of sulfanilamide and nitrite
followed by coupling with N-(1 naphthyl) -ethylenediamine dihydrochloride to form a
highly colored azo dye which is measured colorimetrically.
HANDLING & PRESERVATION
Analysis should be made as soon as possible. If analysis cannot be made within 24 hours,
the sample should be preserved by refrigeration at 4°C. When samples must be stored for
more than 24 hours, they can be preserved by adding 2 mL of concentrated sulfuric acid
per liter of sample. For best results, the analysis for nitrate should be determined at
temperatures between 20°C and 25°C.
INTERFERENCES
Nitrite interferences at all levels. Strong oxidizing and reducing substances interfere.
Low results might be obtained for samples that contain high concentrations of iron and
copper.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
06.08 #63677-01
Page 61

1200 COLORIMETER
OZONE
MODEL 1200-OZ · CODE 3678-01
QUANTITY CONTENTS CODE
15 mL Chlorine Inhibitor 3990-E
250 mL *Ozone Buffer *3991-K
30 mL Indigo Blue Stock Solution 3989-G
1 Bottle, HR Reagent, amber glass 0680-J
1 Sampling Apparatus 0681
1 Pipet, transfer, 1.0 mL 2-2170
1 Pipet, 5 mL, glass volumetric 0329
1 Pipet Pump, 10 mL 2-2216
1 Graduated Cylinder, 50 mL, glass 0418
1 Colorimeter Tubes, w/caps 0290-6
1 1200 Colorimeter for Ozone 26735
*WARNING: Reagents marked with a * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents
see MSDS CD or www.lamotte.com. To obtain a printed copy, contact LaMotte by
email, phone or fax.
To order individual reagents or test kit components, use the specified code numbers.
INTRODUCTION
Ozone is sometimes used in place of, or in conjunction with, chlorine or other
halogens for disinfection of pool, spa or drinking waters. Recently, large aquatic
facilities have begun using ozone as a disinfectant in many artificial habitats.
Page 62

OZONE TEST PROCEDURE - INDIGO METHOD
Use the 1.0 mL
transfer pipet (2-
2170) and pipette
pump (2-2216) to
add 1.0 mL of HR
Reagent to each of
2 clean colorimeter
tubes (0967).
If chlorine is
present, add 3
drops Chlorine
Inhibitor (3990)
to each tube.
Cap tubes.
8.
5.
3. 4.
Use the 50 mL graduated
cylinder (0418) to carefully
add 45 mL of *Ozone Buffer
(3991) to amber glass bottle
marked HR Reagent (0680).
Use the 5 mL
volumetric pipet
(0329) and pipette
pump (2-2216) to add
5 mL of Indigo Blue
Stock Solution (3989)
to the amber glass
bottle. Cap and mix.
1. 2.
7.
Take one of the
prepared colorimeter
tubes and sampling
apparatus (0681) to
sampling site.
6.
Lower the end of the tubing
of sampling apparatus to
desired depth. Slowly
withdraw and depress
plunger several times to
purge syringe and tubing.
Slowly withdraw plunger to
fill purged syringe.
Remove plastic tubing
from syringe. Remove
Fill the second prepared
tube (0967) to the 10
mL line with ozone free
water. This is the
Reagent Blank.
HR Reagent
cap from the prepared tube.
Place tip of syringe against
inside of the prepared tube.
Slowly depress plunger and
fill to the 10 mL line and
cap. This is the Sample
Tube. NOTE: Do not shake
or invert the sample.
Read the 1200 Colorimeter Manual before proceeding.
Carefully wipe tubes dry before inserting into the
colorimeter chamber.
PROCEDURE
2
Page 63

Push the
button to turn the
meter on. Press the
button
and hold it for 2
seconds until is
displayed. Release
the button to take a
zero reading (0
ppm).
READ
ZERO
bla
13. Note:
11.
9. 10.
12.
Wipe both tubes clean with a
lint-free cloth.
Insert the
Reagent Blank
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blank or zero.
Insert the
Sample T ube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
The HR Reagent must be made
fresh each week. If reagent is
refrigerated, it may be kept up to
3 weeks.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
Push the
button. The
concentration
in ppm will be
displayed within
2 seconds.
READ
ZERO
I200
COLORIIMETER
••••••••••••••••••
READ
0.15
L
Mott
L
Mott
er2
NOTE: Zeroing the meter with sample water or an empty chamber will result in an
message when reading reacted samples. Meter must be zeroed with a reagent
blank.
3
Page 64

OZONE TEST METHOD SPECIFICATIONS
APPLICATION
Drinking, pool, and aquatic waters.
RANGE
0.0 - 0.5 ppm Ozone
METHOD
Ozone rapidly and stoichiometrically decolorizes Indigo Trisulfonate under acidic
conditions.
HANDLING & PRESERVATION
Ozone is extremely unstable in aqueous solutions. Test must be performed
immediately and the sample must not be agitated.
INTERFERENCES
Manganese at any level will interfere.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 · Chestertown · Maryland · 21620 · USA
800-344-3100 · 410-778-3100 (Outside USA) · Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
1/09
Page 65

1200 COLORIMETER
L
Mott
PHOSPHATE
MODEL 1200-PLR · CODE 3679-01
QUANTITY CONTENTS CODE
2 x 60 mL *Phosphate Acid Reagent *V-6282-H
10 g *Phosphate Reducing Reagent *V-6283-D
6 Colorimeter Tubes, 10 mL, w/cap 0290
1 Spoon, 0.1g, plastic 0699
1 Water Sample Collecting Bottle 0688
1 Pipet, 1.0 mL, plastic 0354
1 1200 Colorimeter for Phosphate 26736
WARNING: Reagents marked with a * are considered to be potential health hazards. T o view or
print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Phosphorus is an important nutrient for aquatic plants. The amount found in water is
generally not more than 0.1 ppm unless the water has become polluted from waste water
sources or excessive drainage from agricultural areas. When phosphorus is present in
excess of the concentrations required for normal aquatic plant growth, a process called
eutrophication takes place. This creates a favorable environment for an increase in algae
and weed nuisances. When algae cells die, oxygen is used in the decomposition and fish
kills often result. Rapid decomposition of dense algae scums with associated organisms
give rise to foul odors and hydrogen sulfide gas.
Page 66

PHOSPHATE PROCEDURE - ASCORBIC ACID METHOD
4.
Push the
button
to turn the meter
on. Press the
button
andholditfor2
seconds until
is displayed.
Release the button
to take a blank
reading (0.0 ppm).
READ
ZERO
bla
I200 COLORIMETER
••••••••••••••••••
READ
ZERO
bla
Insert the tube
into the
chamber, being
sure to align
the index line
with the arrow
on the meter .
Close the lid.
This tube is
the sample
blankorzero.
3.
Rinse and fill a
colorimeter tube
(0290) to the
10 mL line with
sample water. Cap
and wipe dry .
2.
Fill the W ater
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
1.
Remove tube
from colorimeter.
Use 1.0 mL pipet
(0354) to add
1.0 mL of
*Phosphate Acid
Reagent
(V -6282). Cap
and mix.
5.
6.
Use the 0.1 g spoon
(0699) to add one
measure of *Phosphate
Reducing Reagent
(V -6283).
8.
Align the index line with the
arrow on the meter, insert tube
CAL
I200COLORIMETER
••••••••••••••••••
READ
into chamber. Close
the lid. Push the
button.
Record results as
ppm
Orthophosphate.
READ
7.
Cap and shake until
powder dissolves.
Wait 5 minutes for
full color
development.
Solution will turn
blue if phosphates
are present. Wipe
tube dry.
L
Mott
L
Mott
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
PHOSPHATE
Page 67

PHOSPHATE TEST METHOD SPECIFICATIONS
APPLICATION
Drinking, surface and saline waters; domestic and industrial wastes (Method based on
reactions that are specific for orthophosphate).
RANGE
0 to 3.0 ppm Orthophosphate (Range can be extended by dilution.)
METHOD
Ammonium molybdate and antimony potassium tartrate react in a filtered acid medium
with dilute solution of PO
3-
to form an antimony-phosphomolybdate complex. This
4
complex is reduced to an intense blue colored complex by ascorbic acid. The color is
proportional to the amount of phosphate present. (Only orthophosphate forms a blue
color in this test.) Polyphosphates (and some organic phosphorus compounds) may be
converted to the orthophosphate form by sulfuric acid digestion. Organic phosphorus
compounds may be converted to the orthophosphate form by persulfate digestion.
HANDLING & PRESERVATION
If benthic deposits are present in the area being sampled, great care should be taken not
to include these deposits. If the analysis cannot be performed the same day of collection,
the sample should be preserved by the addition of 2 mL of concentrated sulfuric acid or
40 mg mercuric chloride per liter and refrigerated at 4°C.
INTERFERENCES
1. No interference from copper , iron, or silicate at concentrations many times the
concentration of sea water. However, high iron concentrations can cause
precipitation and subsequent loss of phosphorus.
2. Salt error for samples ranging from 5% to 20% salt content was found to be less
than 1%.
3. Mercuric chloride, HgCl
, when used as the preservative, interferes when the
2
chloride levels are low (less than 50mg/L). This interference is overcome by spiking
samples with a minimum of 50 mg/L of sodium chloride.
Page 68

LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
3679-01 • 10.08
Page 69

1200 COLORIMETER
AMMONIA-NITROGEN
MODEL 1200-NH · CODE 3680-01
QUANTITY CONTENTS CODE
30 mL Ammonia Nitrogen Reagent #1 V-4797-G
3 x 30 mL *Ammonia Nitrogen Reagent #2 *V-4798-G
1 Pipet, 1 mL, plastic 0354
1 Colorimeter Tubes, with caps 0290-6
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Ammonia Nitrogen 26737
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view or
print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Ammonia nitrogen is present in various concentrations in many surface and ground
water supplies. Any sudden change in the concentration of ammonia nitrogen in a water
supply is cause for suspicion. A product of microbiological activity, ammonia nitrogen is
sometimes accepted as chemical evidence of pollution when encountered in natural
waters.
Ammonia is rapidly oxidized in natural water systems by special bacterial groups that
produce nitrite and nitrate. This oxidation requires that dissolved oxygen be available in
the water. Ammonia is an additional source of nitrogen as a nutrient which may
contribute to the expanded growth of undesirable algae and other forms of plant growth
that overload the natural system and cause pollution.
1
Page 70

AMMONIA NITROGEN TEST PROCEDURE:
sure to align
the index line
with the
arrow on the
meter. Close
the lid. This
tube is the
blank or zero.
3.
Rinse and fill a
colorimeter
tube (0290) to
the 10 mL line
with sample
water. Cap and
wipe dry.
2.
Fill the W ater
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
1.
6.
Use 1.0 mL
pipet (0354) to
add 1.0 mL of
*Ammonia
Nitrogen
Reagent #2
(V -4798).
5.
Remove tube from
colorimeter.
Add 8 drops of
Ammonia Nitrogen
Reagent #1 (V-4797).
Cap and mix.
4.
the
button and hold it
for 2 seconds until
is displayed.
Release the button
to take a blank
reading (0.0 ppm).
ZERO
bla
I200
COLORIMETER
••••••••••••••••••
READ
ZERO
bla
7.
Cap and invert to mix.
Wait 5 minutes for full
color development.
Wipe tube dry.
5
MINUTES
8.
Align the index line with the
arrow on the meter, insert tube
into chamber.
Close the lid. Push
the
button. Record
results as ppm
Ammonia
Nitrogen (NH -N).
READ
3
CAL
I200
COLORIMETER
••••••••••••••••••
READ
L
Mott
L
Mott
Insert the tube into
the chamber, being
Push the button
to turn the meter on. Press
READ
NESSLER METHOD
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
AMMONIA NITROGEN
2
Page 71

NOTE: For the best possible results, carry a reagent blank through the procedure. After
scanning the blank in Step 4, perform the test procedure on clear, colorless, distilled or
deioized water. Subtract results of regent blank from all subsequent test results.
NOTE: If the reading displays
Er2, repeat procedure on diluted sample, and multiply
the result by the appropriate dilution factor. See 1200 Colorimeter Instruction Manual
for procedure.
CALCULATIONS
To express results as Unionized Ammonia (NH
Unionized Ammonia (NH
To express results as Ionized Ammonia (NH
Ionized Ammonia (NH
) = ppm Ammonia Nitrogen (NH3-N) x 1.2
3
+
) = ppm Ammonia Nitrogen (NH3-N) x 1.3
4
Ammonia in water occurs in two forms: toxic unionized ammonia (NH
relatively non-toxic ionized form, ammonium ion (NH
both forms as ammonia-nitrogen (NH
concentration in water. The actual proportion of each compound depends on
-N) to give the total ammonia-nitrogen
4
):
3
+
):
4
)andthe
+
). This test method measures
4
3
temperature, salinity, and pH. A greater concentration of unionized ammonia is present
when the pH value and salinity increase.
1. Consult the table below to find the percentage that corresponds to the temperature,
pH and salinity of the sample.
2. To express the test result as ppm Unionized Ammonia Nitrogen (NH
the total ammonia-nitrogen test result by the percentage from the table.
3. To express the test result as ppm Ionized Ammonia Nitrogen (NH
the unionized ammonia-nitrogen determined in Step 2 from the total ammonia
-N), multiply
3
+
-N), subtract
4
nitrogen.
10°C 15°C 20°C 25°C
pH FW
1
SW
2
FW SW FW SW FW SW
7.0 0.19 0.27 0.40 0.55
7.1 0.23 0.34 0.50 0.70
7.2 0.29 0.43 0.63 0.88
7.3 0.37 0.54 0.79 1.10
7.4 0.47 0.68 0.99 1.38
7.5 0.59 0.459 0.85 0.665 1.24 0.963 1.73 1.39
7.6 0.74 0.577 1.07 0.836 1.56 1.21 2.17 1.75
7.7 0.92 0.726 1.35 1.05 1.96 1.52 2.72 2.19
7.8 1.16 0.912 1.69 1.32 2.45 1.90 3.39 2.74
7.9 1.46 1.15 2.12 1.66 3.06 2.39 4.24 3.43
8.0 1.83 1.44 2.65 2.07 3.83 2.98 5.28 4.28
8.1 2.29 1.80 3.32 2.60 4.77 3.73 6.55 5.32
8.2 2.86 2.26 4.14 3.25 5.94 4.65 8.11 6.61
8.3 3.58 2.83 5.16 4.06 7.36 5.78 10.00 8.18
8.4 4.46 3.54 6.41 5.05 9.09 7.17 12.27 10.10
8.5 5.55 4.41 7.98 6.28 11.18 8.87 14.97 12.40
1
Freshwater data from Trussel (1972).
2
Seawater values from Bower and Bidwell (1978). Salinity for the Seawater values =
34% at an ionic strength of 0.701 m.
3
Page 72

FOR EXAMPLE:
A fresh water sample at 20°C has a pH of 8.5 and the test result is 1.0 ppm as total
Ammonia-Nitrogen.
1. The percentage from the table is 11.18% (or 0.1118).
2. 1 ppm total Ammonia-Nitrogen x 0.1118 = 0.1118 ppm Unionized
Ammonia-Nitrogen
3.
Total Ammonia-Nitrogen 1.0000 ppm
Unionized Ammonia-Nitrogen - 0.1118 ppm
Ionized Ammonia-Nitrogen = 0.8882 ppm
AMMONIA NITROGEN
TEST METHOD SPECIFICATIONS
APPLICATION
Drinking , surface, and saline waters; domestic and industrial wastes.
RANGE
0 to 5.0 ppm Ammonia Nitrogen
METHOD
Ammonia forms a colored complex with Nessler’s Reagent in proportion to the amount
of ammonia present in the sample. Rochelle salt is added to prevent precipitation of
calcium or magnesium in undistilled samples.
HANDLING & PRESERVATION
Preservation is accomplished by the addition of 2 mL of concentrated H
2SO4
at 4°C.
INTERFERENCES
Sample turbidity and color may interfere. Turbidity may be removed by a filtration
procedure. Color interference may be eliminated by adjusting the instrument to 100%T
withasampleblank.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
3680-01 · 6.07
Page 73

1200 COLORIMETER
IRON
MODEL 1200-FE · 3681-01
QUANTITY CONTENTS CODE
2 x 15 mL *Acid Phenanthroline Indicator *2776-E
2x5g *IronReducingReagent *2777-C
1 Colorimeter Tubes, with caps, set of 6 0290-6
1 Spoon, 0.1 g, plastic 0699
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Iron 26738
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by email, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Most natural waters contain some iron. The concentration may vary from small traces to
very large amounts in water which is contaminated by acid mine wastes. For domestic
use, the concentration should not exceed 0.2 ppm and for some industrial applications
not even a trace of iron can be tolerated. There are many means available for removing
or reducing the iron content. W a ter softening resins are effective for removing small
amounts of iron and special ion exchange materials are selective for iron removal. High
concentrations of iron can be removed by such chemical processes as oxidation and lime
or lime-soda softening. Because of the many means of removing or reducing the amount
of iron in water , the particular method employed will depend largely on the form of iron
which is present and the end use of the treated water.
1
Page 74

IRON TEST PROCEDURE - 1,10 PHENANTHROLINE METHOD
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry .
5.
3.
1. 2.
4.
6.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
Cap and invert the
tube 15 times to
mix.
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
READ
ZERO
bla
Remove tube from
colorimeter. Use the
0.1 g spoon (0699) to
add one measure of
*Iron Reducing
Reagent (2777).
8.
7.
Remove the cap and
add 6 drops of *Acid
Phenanthroline
Indicator (2776).
Cap and invert the
tube 3 times to mix
reagents.
L
Mott
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
IRON
2
Page 75

If the reading displays Er2, repeat procedure on diluted sample, and multiply the result
9. 10.
Wait 5 minutes for full
color development.
Solution will turn orange
if iron is present.
Wipe tube dry.
11.
Align the index line with the
arrow on the meter, insert
CAL
I200COLORIMETER
••••••••••••••••••
READ
tube into chamber.
Close the lid. Push
the
button. Record
results as ppm Iron.
READ
5
MINUTES
L
Mott
by the appropriate dilution factor. See 1200 Colorimeter Instruction Manual for
procedure.
3
Page 76

IRON TEST METHOD SPECIFICATIONS
APPLICATION
Drinking , surface, and saline waters; domestic and industrial wastes.
RANGE
0to4.0ppmIron
METHOD
Ferric iron is reduced to ferrous iron and subsequently forms a colored complex with
phenanthroline for a quantitative measure of total iron.
HANDLING & PRESERVATION
The sample container should be cleaned with acid and rinsed with deionized water.
Addition of acid to adjust the sample to pH 2 - 3 will prevent deposition of iron on the
container walls. Samples should be analyzed as soon as possible after collection since
ferrous iron undergoes oxidation to ferric iron.
INTERFERENCES
Strong oxidizing agents, cyanide, nitrite, phosphates, chromium, and zinc in
concentrations exceeding 10 times that of iron; cobalt and copper in excess of 5 mg/L,
and nickel in excess of 2 mg/L. Bismuth, cadmium, mercury, molybdate, and silver
precipitate phenanthroline.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
63681-01 · 4/09
Page 77

1200 COLORIMETER
MANGANESE
MODEL 1200-MN · CODE 3682-01
QUANTITY CONTENTS CODE
2 x 100 mL *Hardness Buffer Reagent *4255-J
2 x 30 mL *Manganese Indicator Reagent *3956-G
15 mL *Sodium Cyanide, 10% *6565-E
1 Pipet, 0.5 mL, plastic, w/cap 0369
1 Pipet, 1.0 mL, plastic 0354
6 Colorimeter Tubes, with caps 0290-6
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Manganese 26739
*WARNING: Reagents marked with a * are considered to be potential health hazards. To view
or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD or
www .lamotte.com. To obtain a printed copy, contact LaMotte by email, phone or fax.
To order individual reagents or test kit components, use the specified code number.
INTRODUCTION
Manganese is present in ground water in the divalent state due to the lack of oxygen. In
surface waters, manganese may be in various oxidation states as soluble complexes or as
suspended compounds. Manganese is rarely present in excess of 1 mg/L. It may cause an
objectionable taste or cause staining problems in laundry, but manganese levels normally
encountered in water seldom produce any health hazard.
Manganese is removed from water by various means including chemical precipitation,
pH adjustment, aeration, superchlorination and the use of ion exchange resins.
Page 78

MANGANESE TEST PROCEDURE - PAN METHOD
4.
Push the
button
to turn the meter
on. Press the
button
andholditfor2
seconds until
is displayed.
Release the button
to take a blank
reading (0.0 ppm).
READ
ZERO
bla
I200 COLORIMETER
••••••••••••••••••
READ
ZERO
bla
Insert the tube into
the chamber, being
3.
Rinse and fill a
colorimeter tube
(0290) to the
10 mL line with
sample water. Cap
and wipe dry.
2.
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
1.
Use the 1.0 mL
pipet (0354) to
add 2.0 mL of
*Hardness Buffer
Reagent (4255).
5.
6.
Swirl the tube to mix.
8.
7.
Add two drops of
*Sodium Cyanide,
10% (6565).
Swirl the tube to mix.
sure to align
the index line
with the arrow
on the meter.
Close the lid.
This tube is
the blank or
zero.
L
Mott
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
MANGANESE
Page 79

10.9.
Cap tube and invert
to mix.
11.
Align the index line with the
arrow on the meter, insert
tube into chamber.
Close the lid. Push
the
button. Record
results as ppm
Manganese.
READ
Note:
For the best possible results, carry a
reagent blank through the
procedure. After scanning the blank
in Step 4, perform the test procedure
on clear, colorless, distilled or
deionized water. Subtract results of
reagent blank from all subsequent
test results.
12.
Use the 0.5 mL
pipet (0369) to
add 0.5 mL of
*Manganese
Indicator Reagent
(3956).
Wipe tube dry.
CAL
I200COLORIMETER
••••••••••••••••••
READ
L
Mott
If the reading displays Er2, repeat procedure on diluted sample, and multiply the result
by the appropriate dilution factor. See 1200 Colorimeter Instruction Manual for
procedure.
Page 80

MANGANESE TEST METHOD SPECIFICATIONS
APPLICATION
Drinking and surface; domestic and industrial wastewaters.
RANGE
0.0 - 0.7 mg/L Manganese
METHOD
P AN, (1-[2-Pyridylazo]-2-Naphthol), forms a red complex with Manganese (Mn
+2
)ata
pH of 8 to 10.
HANDLING & PRESERVATION
Manganese may oxidize readily in neutral water and precipitate from solution. It may
adhere to or be absorbed by container walls, especially glass. Acidified sample can be
stored in plastic.
INTERFERENCES
None. Test is quite specific.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax: 410-778-6394
Visit us on the web at www.lamotte.com
®
63682-01 · 10.08
Page 81

1200 COLORIMETER
SULFA TE
MODEL 1200-SU · CODE 3683-01
QUANTITY CONTENTS CODE
10 g *Sulfate Reagent *V-6277-D
1 Colorimeter Tubes, with caps 0290-6
1 Spoon, 0.1 g, plastic 0699
1 Water Sample Collecting Bottle 0688
1 1200 Colorimeter for Sulfate 26740
*WARNING: Reagents marked with a * are considered hazardous substances. Material
Safety Data Sheets (MSDS) are supplied for these reagents. For your safety, read label
and accompanying MSDS before using.
To order individual reagents or test kit components, use the specified code number.
SULFA TE INTRODUCTION
The most common mineral forms of sulfur are iron sulfide, lead sulfide, zinc sulfide,
calcium sulfate and magnesium sulfate. In most fresh waters, the sulfate ion is the second
or third most abundant anion, being exceeded only by bicarbonate and, in some cases,
silicate. Sulfur, in the form of sulfate, is considered an important nutrient element.
Mineral springs are rich in sulfate and feed appreciable quantities of this compound to
the watershed. Acid mine water drainage is a form of pollution which may contribute
extremely large amounts of sulfate content to natural waters. Other sources of sulfate
include waste material from pulp mills, steel mills, food processing operations and
municipal wastes. Many bacteria obtain sulfur from sulfate for the synthesis of amino
acids. In lakes and streams low in oxygen, this process of sulfate reduction causes the
production of hydrogen sulfide, with its characteristic offensive odor. Calcium sulfate
and magnesium sulfate contribute significantly to the hardness of water. Under natural
conditions, the quantities ordinarily to be expected in lakes are between 3 and 30 parts
per million.
1
Page 82

SULFA TE TEST PROCEDURE
Fill the Water
Sample Collecting
Bottle (0688) with
sample water. This
will be used to
dispense sample
water for the tests.
Insert the tube
into the
chamber, being
sure to align the
index line with
the arrow on
the meter.
Close the lid.
This tube is the
blankorzero.
Rinse and fill a
colorimeter tube
(0290) to the 10
mL line with
sample water.
Cap and wipe
dry .
5.
3.
1. 2.
4.
6.
I200 COLORIMETER
••••••••••••••••••
READ
bla
ZERO
Cap and shake
vigorouslly for 15
seconds. A white
precipitate will
develop if sulfates
are present.
Push the
button to turn the
meter on. Press the
button
andholditfor2
seconds until is
displayed. Release
the button to take a
zero reading
(0.00 ppm).
READ
ZERO
bla
Remove tube from
colorimeter. Use the
0.1 g spoon (0699)
to add one measure
of *Sulfate Reagent
(V -6277).
8.
Invert the tube to
mix again.
5
7.
Wait 5 minutes.
MINUTES
L
Mott
- BARIUM CHLORIDE METHOD
Read the 1200 Colorimeter Manual before proceeding. Carefully
wipe tubes dry before inserting into the colorimeter chamber.
SULFATE
2
Page 83

9.
Note:
A white film is deposited on the
inside of test tubes as a result of the
sulfate test. Thoroughly clean and
rinse test tubes after each test.
Align the index line with the
arrow on the meter, insert
CAL
I200COLORIMETER
••••••••••••••••••
READ
tube into chamber.
Close the lid. Push
the
button. Record
results as ppm
Sulfate.
READ
Note:
The Sulfate test is sensitive to
temperature. Best results will
be obtained if the water sample
is 20° - 23°C.
L
Mott
3
Page 84

SULFA TE TEST METHOD SPECIFICATIONS
APPLICATION
Drinking and surface; domestic and industrial wastes.
RANGE
5 to 100 ppm Sulfate
METHOD
Sulfate ion is precipitated in an acid medium with barium chloride to form a barium
sulfate suspension in proportion to the amount of sulfate present.
HANDLING & PRESERVATION
Sulfate samples may be preserved by refrigeration at 4°C up to 7 days in glass or plastic
containers without any change in concentration.
INTERFERENCES
Suspended matter and color interference may be removed by a filtration step. Silica in
excess of 500 mg/L will interfere.
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside USA)• Fax 410-778-6394
Visit us on the web at www.lamotte.com
®
63683-01 · 11/01
 Loading...
Loading...