Page 1
FreeZone
Models
User’s Manual
®
Benchtop Shell Freezers
7949020
7949030
7949040
To receive important product updates,
complete your product registration card
online at register.labconco.com
Labconco Corporation
8811 Prospect Avenue
Kansas City, MO 64132-2696
800-821-5525, 816-333-8811
FAX 816-363-0130
E-MAIL labconco@labconco.com
HOME PAGE www.labconco.com
Please read the User’s Manual before operating the equipment.
Page 2
Copyright © 2004, 2007 Labconco Corporation. All rights reserved.
The information contained in this manual and the accompanying products are copyrighted and all rights
reserved by Labconco Corporation. Labconco Corporation reserves the right to make periodic design
changes without obligation to notify any person or entity of such change.
Warranty
Labconco provides a warranty on all parts and factory workmanship. The warranty includes areas
of defective material and workmanship, provided such defect results from normal and proper use of
the equipment.
The warranty for all Labconco products will expire one year from date of installation or two years
from date of shipment from Labconco, whichever is sooner, except the following;
• Purifier® Delta® Series Biological Safety Cabinets and PuriCare® Lab Animal Research
Stations carry a three-year warranty from date of installation or four years from date of
shipment from Labconco, whichever is sooner.
• SteamScrubber® & FlaskScrubber® Glassware Washers carry a two-year warranty from
date of installation or three years from date of shipment from Labconco, whichever is
sooner.
• Blood Drawing Chairs carry a ten year warranty.
• Carts carry a lifetime warranty.
• Glassware is not warranted from breakage when dropped or mishandled.
This limited warranty covers parts and labor, but not transportation and insurance charges. In the
event of a warranty claim, contact Labconco Corporation or the dealer who sold you the product. If
the cause is determined to be a manufacturing fault, the dealer or Labconco Corporation will repair
or replace all defective parts to restore the unit to operation. Under no circumstances shall
Labconco Corporation be liable for indirect, consequential, or special damages of any kind. This
statement may be altered by a specific published amendment. No individual has authorization to
alter the provisions of this warranty policy or its amendments. Lamps and filters are not covered by
this warranty. Damage due to corrosion or accidental breakage is not covered.
Returned or Damaged Goods
Do not return goods without the prior authorization from Labconco. Unauthorized returns will not be
accepted. If your shipment was damaged in transit, you must file a claim directly with the freight carrier.
Labconco Corporation and its dealers are not responsible for shipping damages.
The United States Interstate Commerce Commission rules require that claims be filed with the delivery
carrier within fifteen (15) days of delivery.
Limitation of Liability
The disposal and/or emission of substances used in connection with this equipment may be governed by
various federal, state, or local regulations. All users of this equipment are required to become familiar with
any regulations that apply in the user’s area concerning the dumping of waste materials in or upon water,
land, or air and to comply with such regulations. Labconco Corporation is held harmless with respect to
user’s compliance with such regulations.
Contacting Labconco Corporation
If you have questions that are not addressed in this manual, or if you need technical assistance, contact
Labconco’s Customer Service Department or Labconco’s Product Service Department at 1-800-821-5525
or 1-816-333-8811, between the hours of 7:00 a.m. and 6:00 p.m., Central Standard Time.
Part #7392209, Rev. A
ECO E218
Page 3

T
AABBLLEE
T
CHAPTER 1: INTRODUCTION 1
Freeze Dry Process 1
Freeze Dry Rates 2
Freeze Dry Capacity 3
Samples Containing Volatile Substances 4
Sample Surface Area and Thickness of the Sample 4
About This Manual 5
Typographical Conventions 7
CHAPTER 2: PREREQUISITES 8
Electrical Requirements 8
Location Requirements 9
CHAPTER 3: GETTING STARTED 10
Unpacking Your Benchtop Shell Freezer 10
Benchtop Shell Freezer Components 11
Installing Your Benchtop Shell Freezer 12
Electrical Connection 12
Solvent Safety Precautions 12
CHAPTER 4: USING YOUR BENCHTOP SHELL FREEZER 14
Benchtop Shell Freezer Controls 15
Operating the Benchtop Shell Freezer 15
CHAPTER 5: MAINTAINING YOUR BENCHTOP
APPENDIX A: BENCHTOP SHELL FREEZER COMPONENTS 19
APPENDIX B: BENCHTOP SHELL FREEZER DIMENSIONS 21
APPENDIX C: BENCHTOP SHELL FREEZER
Electrical Specifications 22
Environmental Conditions 22
Wiring Diagram (115V) 24
Wiring Diagram (230V) 25
DECLARATION OF CONFORMITY 26
O
O
SHELL FREEZER 17
SPECIFICATIONS 22
FF
C
C
OONNTTEENNTTSS
Page 4

C
HHAAPPTTEERR
C
I
NNTTRROODDUUCCTTIIOONN
I
Congratulations on your purchase of a Labconco FreeZone®
Benchtop Shell Freezer, which is designed to prepare samples for
laboratory lyophilization procedures. The unit is easy to install
and maintain. Proper care and maintenance of this product will
result in many years of dependable service.
1
1
Freeze Dry Process
Freeze drying is an important process in sample preparation and
for the preservation and storage of biologicals, pharmaceuticals
and foods. Of the various methods of dehydration, freeze drying
(lyophilization) is especially suited for substances that are heat
sensitive. Other than food processing (e.g., coffee, whole dinners),
freeze drying has been extensively used in the development of
pharmaceuticals (e.g., antibiotics) and preservation of biologicals
(e.g., proteins, plasma, viruses and cell lines). The nondestructive
nature of this process has been demonstrated by the retention of
viability in freeze dried viruses and microorganisms.
Freeze drying is a process whereby water or other solvent is
removed from frozen material by converting the frozen water
directly into vapor without the intermediate formation of liquid
water. The basis for this sublimation process involves the
absorption of heat by the frozen sample in order to vaporize the
ice; the use of a vacuum pump to enhance the removal of water
vapor from the surface of the sample; the transfer of water vapor to
a collector; and the removal of heat by the collector in order to
condense the water vapor. In essence, the freeze dry process is a
balance between the heat absorbed by the sample to vaporize the
ice and the heat removed from the collector to convert the water
vapor into ice.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
1
Page 5

Chapter 1: Introduction
Freeze Dry Rates
The efficiency of the freeze drying process is dependent upon the
surface area and the thickness of the sample, the collector
temperature and vacuum obtained, the eutectic point and solute
concentration of the sample. It is important to remember these
factors when trying to obtain efficient utilization of your freeze dry
system. A listing of selected materials and their approximate
drying times are shown in Table 1 for your reference.
SAFE TEMPERATURE AND DRYING TIMES
FOR SELECTED MATERIALS
Material
10mm Thick
Milk -5 -40 10
Urea -7 -40 10
Blood Plasma -10 to -25 -40 16
Serum -25 -40 18
Vaccinia -30 to -40 -50 22
Influenza Vaccine -30 -50 24
Human Tissue -30 to -40 -50 48
Vegetable Tissue -50 -80 60
*Total sample quantities are contingent on various freeze dryer capacities.
Up to the point of overloading the system, the greater the surface
area of the sample, the faster the rate of freeze drying. By contrast,
for a given surface area, the thicker the sample the slower the rate
of freeze drying. This is based on the fact that the heat of
sublimation is usually absorbed on one side of the frozen sample
and must travel through the frozen layer to vaporize water at the
other surface. In addition, as the sample is freeze dried, the water
vapor must travel through the layer of dried material. The thicker
the sample, the greater the chance that the dried layer may collapse
which would cause an additional decrease in the rate of freeze
drying.
The surface area and thickness of the sample can usually be
ignored when each sample contains only a few milliliters.
However, for larger volumes, the samples should be shell frozen to
maximize the surface area and minimize the thickness of the
sample. The volume of the freeze dry flask should be two to three
times the volume of the sample.
Safe
Temperature
°C
Collector
Temperature
°C
Table 1
Hours
(Approx.)
2
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 6

Chapter 1: Introduction
In order for lyophilization to occur, ice must be removed from the
frozen sample via sublimation. This is accomplished by the
collector and the vacuum pump. The collector, which should be at
least 15 to 20°C colder than the eutectic temperature (melting
temperature) of the sample, traps vapor as ice. Since the vapor
pressure at the collector is lower than that of the sample, the flow
of water vapor is from the sample to the collector. Since this vapor
diffusion process occurs very slowly under normal atmospheric
conditions, a good vacuum is essential to maintain an efficient rate.
In many applications, the maintenance of a vacuum of 0.133 mBar
or less is recommended.
The rate of freeze drying is directly proportional to the vapor
pressure and the vapor pressure is dependent upon both eutectic
temperature and solute concentration of the sample. For example,
a solution of sodium chloride and water would freeze dry at a
slower rate than pure water. The eutectic temperature of a sodium
chloride solution is about –21°C and at this temperature the vapor
pressure is about 1/16 that of water at 0°C. Although the eutectic
temperature is not dependent upon the concentration of sodium
chloride, the vapor pressure of the water would decrease as the
concentration of sodium chloride increased. This is due to the fact
that as the solute concentration increases, less of the surface area of
the frozen sample is occupied by water. In general, most solutions
or biological samples will have a eutectic temperature of –10° to
–25°C. However, if the sample contains a simple sugar such as
glucose or if the sample is animal or plant tissue, the eutectic
temperature may be as low as –30° to –50°C.
Freeze Dry Capacity
The volume of a sample that can be freeze dried at one time is
related to factors discussed previously and the size and design of
the freeze dry system. With any given instrument, the capacity is
based on the surface area of the sample, the eutectic temperature
and concentration of the sample and the rate and amount of heat
transferred to the frozen sample. Of these factors, the eutectic
temperature is the most important factor in determining the amount
of sample that can be freeze dried at one time, particularly when
flasks are used. This is because as the eutectic temperature
decreases, the vapor pressure decreases but the rate of heat
absorption by the sample does not change. This tends to promote
melting of the sample, which leads to a marked increase in vapor
pressure and ultimately overloads the collector and vacuum pump.
Samples that have eutectic temperatures of –20°C or lower should
be placed on the freeze dry system one flask at a time so that the
Product Service: Domestic 1-800-522-7658, International 816-333-8811
3
Page 7

Chapter 1: Introduction
vacuum in the system may recover before adding another sample
to the system. If the vacuum does not recover, the capacity of the
freeze dry system has been exceeded and the sample should be
removed.
If there is a problem with a particular type of sample melting when
placed on the freeze dry system, dilution of the sample with more
water or providing some insulation around the flask to decrease the
rate of heat absorption by the sample may help. If the eutectic
temperature of the sample is –40 to –60°C, the freeze dry system
selected for use must be equipped with cascade type refrigeration
so that the collector temperature can be cooled to below –75°C, or
a dry ice/solvent trap may be used between the collector and the
vacuum pump.
Samples Containing Volatile
Substances
In certain cases the solvent in a sample to be freeze dried may
contain volatile components such as acetonitrile, methanol, acetic
acid, formic acid or pyridine. In addition to these substances
having an effect on the eutectic temperature, they may increase the
vapor pressure at the surface of the sample. Also, compared to
water, they will require the absorption of less heat for sublimation
to occur. Hence, samples that contain volatile substances will have
a greater tendency to melt, particularly when placed in flasks or
exposed to room temperature. If a sample containing a volatile
substance tends to melt when placed on a freeze dry system,
dilution of the sample with more water will help keep the sample
frozen. For example, a 0.2M solution of acetic acid is much easier
to freeze dry than a 0.5M solution.
Sample Surface Area and
Thickness of the Sample
The volume and configuration of the suspension to be freeze dried
often determines how the material will be freeze dried. For
example, the greater the ratio of the surface area to the volume of
the suspension, the faster drying will occur. This is because a
greater area for the water molecules to leave the product exists
compared to the distance they have to travel to reach the surface of
the frozen matrix. Drying occurs from the top of the product and
initially the removal of water molecules is efficient. However, as
the drying front moves down through the product, drying becomes
more and more difficult. The water molecules must now travel
4
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 8
Chapter 1: Introduction
through the dried portions of the product, which impedes their
progress. As the drying front moves further and further down the
matrix, the application of heat to the product becomes more
important.
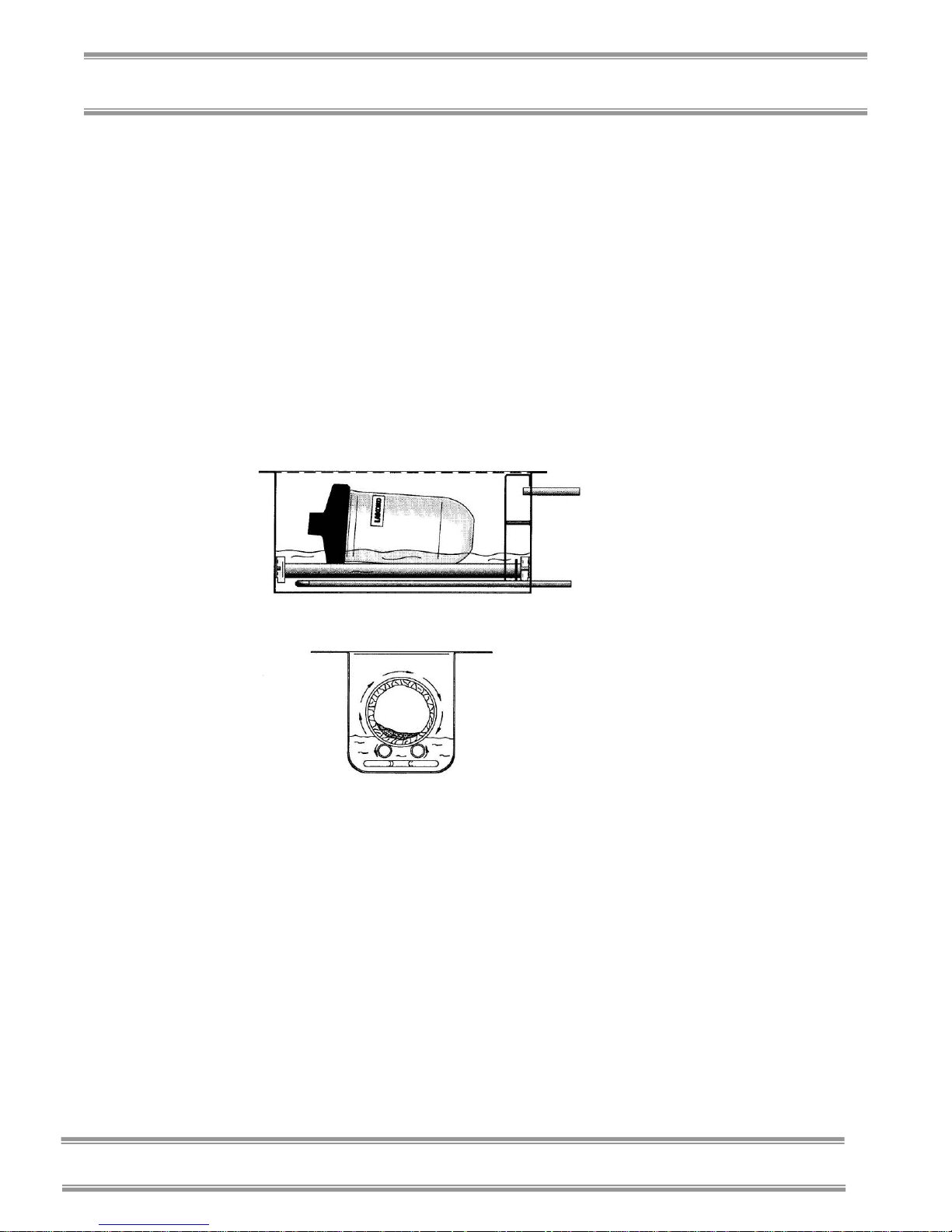
Shell freezing as a method of prefreezing the product can increase
the surface area-to-volume ratio by spreading out the frozen
product inside the vessel. Shell freezing is accomplished by
rotating the vessel in a low temperature bath causing the product to
freeze in a thin layer on the inside surface of the vessel. The
thickness of the frozen suspension depends on the volume of the
product in comparison to the size of the vessel. The diagram
below shows how a Shell Freezer operates.
About This Manual
This manual is designed to help you learn how to install, use, and
maintain your Benchtop Shell Freezer. Instructions for performing
routine maintenance and making minor modifications to your
Benchtop Shell Freezer are also included.
Chapter 1: Introduction provides a brief overview of the freeze dry
process, explains the organization of the manual, and defines the
typographical conventions used in the manual.
Chapter 2: Prerequisites explains what you need to do to prepare
your site before you install your Benchtop Shell Freezer.
Electrical requirements are discussed.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
5
Page 9

Chapter 1: Introduction
Chapter 3: Getting Started contains the information you need to
properly unpack, inspect and install your Benchtop Shell Freezer.
Chapter 4: Using Your Benchtop Shell Freezer discusses the basic
operation and information on how to load samples.
Chapter 5: Maintaining Your Benchtop Shell Freezer explains how
to perform routine maintenance.
Chapter 6: Troubleshooting contains information about problems
you may encounter while using your Benchtop Shell Freezer,
including the probable causes of the problems, and suggested
corrective actions.
Appendix A: Benchtop Shell Freezer Components contains labeled
diagrams of the key components of the Benchtop Shell Freezer.
Appendix B: Benchtop Shell Freezer Dimensions contains
diagrams showing the dimensions for the Benchtop Shell Freezer.
Appendix C: Benchtop Shell Freezer Specifications contains
product specifications and wiring diagrams.
6
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 10

!
)
Chapter 1: Introduction
Typographical Conventions
Recognizing the following typographical conventions will help
you understand and use this manual:
• Book, chapter, and section titles are shown in italic type (e.g.,
Chapter 3: Getting Started).
• Steps required to perform a task are presented in a numbered
format.
• Comments located in the margins provide suggestions,
reminders, and references.
• Critical information is presented in boldface type in paragraphs
that are preceded by the exclamation icon. Failure to comply
with the information following an exclamation icon may result
in injury to the user or permanent damage to your Shell
Freezer.
• Important information is presented in capitalized type in
paragraphs that are preceded by the pointer icon. It is
imperative that the information contained in these paragraphs
be thoroughly read and understood by the user.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
7
Page 11

C
HHAAPPTTEERR
C
P
RREERREEQQUUIISSIITTEESS
P
Before you install your Benchtop Shell Freezer, you need to
prepare your site for installation. The Benchtop Shell Freezer may
be mounted on top of a benchtop work surface. Carefully examine
the location where you intend to install your Benchtop Shell
Freezer. You must be certain that the area is level and of solid
construction. An electrical source must be located near the
installation site.
Carefully read this chapter to learn:
• the electrical supply requirements.
• location requirements.
Refer to Appendix C: Benchtop Shell Freezer Specifications for
complete Benchtop Shell Freezer electrical and environmental
conditions, specifications and requirements.
2
2
Electrical Requirements
The Benchtop Shell Freezer requires a dedicated electrical outlet.
This outlet requires a 15 Amp circuit breaker or fuse for models
rated at 115V (60 Hz). An outlet equipped with a 8 Amp circuit
breaker or fuse is required for models rated at 230V (50/60 Hz).
The power cord on 115V models is equipped with a 15 Amp
NEMA 5-15P plug. The power cord on 230V models is equipped
with a NEMA 6-15P plug. If this does not match with the
available receptacle, remove this plug and replace it with an
approved plug of the suitable style.
8
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 12

Chapter 2: Prerequisites
Location Requirements
The Benchtop Shell Freezer should be located in an area that
provides an unobstructed flow of air around the cabinet. This air
cools the refrigeration system. A minimum of 3" must be allowed
between the back and both sides of the Benchtop Shell Freezer and
adjacent wall surfaces. Restriction of airflow during operation
could adversely affect performance.
Refer to Appendix B: Benchtop Shell Freezer Dimensions for
dimensional drawings of the Benchtop Shell Freezer.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
9
Page 13

C
HHAAPPTTEERR
C
G
EETTTTIINNGG
G
Now that the site for your Benchtop Shell Freezer is properly
prepared, you are ready to unpack, inspect, install and test your
Benchtop Shell Freezer. Read this chapter to learn how to:
• unpack and move your Benchtop Shell Freezer.
• set up your Benchtop Shell Freezer.
• connect the electrical supply source to your Benchtop Shell
Freezer.
• safely use solvents with your Benchtop Shell Freezer.
The Benchtop Shell Freezer weighs over 90 lbs.
!
(41 Kg). The carton allows for lifting with a
mechanical lift truck or hand truck. If you must
lift the Benchtop Shell Freezer manually, use at
least two (2) persons and follow safe lifting
guidelines.
S
3
3
TTAARRTTEEDD
S
Unpacking Your Benchtop Shell
Freezer
The United States
Interstate Commerce
Commission rules
require that claims be
filed with the delivery
carrier within fifteen (15)
days of delivery.
10
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Carefully unpack your Benchtop Shell Freezer and inspect it for
damage that may have occurred in transit. If your Benchtop Shell
Freezer is damaged, notify the delivery carrier immediately and
retain the entire shipment intact for inspection by the carrier.
Page 14

)
)
)
Chapter 3: Getting Started
DO NOT RETURN GOODS WITHOUT THE
PRIOR AUTHORIZATION OF LABCONCO.
UNAUTHORIZED RETURNS WILL NOT BE
ACCEPTED.
IF YOUR BENCHTOP SHELL FREEZER WAS
DAMAGED IN TRANSIT, YOU MUST FILE A
CLAIM DIRECTLY WITH THE FREIGHT
CARRIER. LABCONCO CORPORATION AND
ITS DEALERS ARE NOT RESPONSIBLE FOR
SHIPPING DAMAGE.
DO NOT DISCARD THE CARTON OR
PACKING MATERIALS UNTIL YOU HAVE
CHECKED ALL OF THE COMPONENTS AND
INSTALLED AND TESTED THE BENCHTOP
SHELL FREEZER.
Benchtop Shell Freezer
Components
Locate the model of Benchtop Shell Freezer you received in the
following table. Verify that the components listed are present and
undamaged.
Catalog # Product Description
7949020 Benchtop Shell Freezer - 115V, 60 Hz
7949030 Benchtop Shell Freezer - 230V, 50 Hz
7949040 Benchtop Shell Freezer - 230V, 60 Hz
Plus the following:
Part # Qty. Component Description
7392209 1 User’s Manual
1334500 1 Power Cord – 115V
Or
1338000 1 Power Cord – 230V
If you did not receive one or more of the components listed for
your Benchtop Shell Freezer, or if any of the components are
damaged, contact Labconco Corporation immediately for further
instructions.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
11
Page 15

Chapter 3: Getting Started
Installing Your Benchtop Shell
Freezer
After you verify receipt of the proper components, move your
Benchtop Shell Freezer to the location where you want to install it.
Then, follow the steps listed below.
1. Check that the solvent bath drain plug is securely installed
in the drain hose.
2. Remove bath compartment cover and add solvent to bath
compartment. Commonly used solvents are methanol or
ethanol. Recommended solvent depth is 1/4" to 1/2" above
the top of the rollers (approximately 2 liters).
If flammable solvents are used in the shell
!
freezer bath, be sure to keep away from open
flame.
Electrical Connection
Plug the power cord into the receptacle on the back
of the Benchtop Shell Freezer and plug the other
end into a suitable power receptacle.
DO NOT ATTEMPT TO PLUG THE BENCHTOP
)
SHELL FREEZER INTO A FREEZONE FREEZE
DRY SYSTEM.
Solvent Safety
!
Precautions
Solvents used in the Benchtop Shell Freezer may
be flammable or hazardous to your health. Use
extreme caution and keep sources of ignition
away from the solvents.
Hazardous materials such as strong acids or
bases, radioactive substances and volatile
organics must be handled carefully and
promptly cleaned up if spilled. If a sample is
spilled, it must immediately be cleaned up.
12
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 16

Chapter 3: Getting Started
WARNING: The disposal of substances used in
connection with this equipment may be governed
by various Federal, State or local regulations.
All users of this equipment are urged to become
familiar with any regulations that apply in the
user’s area concerning the dumping of waste
materials in or upon water, land or air and to
comply with such regulations.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
13
Page 17

C
HHAAPPTTEERR
C
U
SSIINNG
U
S
HHEELLL
S
After your Benchtop Shell Freezer has been installed as detailed in
Chapter 3: Getting Started, you are ready to begin using your
Benchtop Shell Freezer. Read this chapter to learn how to:
• operate the controls.
• understand the display.
• load samples.
!
Y
G
Y
F
L
F
Do not use the Benchtop Shell Freezer in a
manner not specified by the manufacturer (refer
to Appendix C: Benchtop Shell Freezer
Specifications). The electrical protection
properties of the Benchtop Shell Freezer may be
impaired if the Benchtop Shell Freezer is used
inappropriately.
4
4
B
OOUURR
B
EENNCCHHTTOOP
RREEEEZZEERR
P
14
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 18

Chapter 4: Using Your Benchtop Shell Freezer
Benchtop Shell Freezer Controls
The control panel for the Benchtop Shell Freezer is shown below
with a description about its function.
1. Main Power Switch- (Not shown - Located on right side of
cabinet) turns the unit on or off.
2. Bath Temperature Graph Display – This display indicates
the temperature of the solvent in the Benchtop Shell
Freezer bath.
The top LED turns on when the main power switch is
turned on.
The second LED turns on when the bath temperature
reaches approximately –20°C.
The third LED turns on when the bath temperature reaches
approximately –30°C.
The fourth LED turns on at approximately –35°C.
The fifth LED turns on at approximately –38°C.
The sixth LED turns on at approximately –40°C.
The bottom LED turns on at approximately –42°C.
Operating the Benchtop Shell
Freezer
The Benchtop Shell Freezer is designed for shell freezing
samples in flasks in preparation for freeze drying. It can
process the following Labconco flasks
Quantity Flask Size
1 1200 ml Flask
1 900 ml Flask
1 750 ml Flask
1 600 ml Flask
1 300 ml Flask
2 150 ml Flask
2 120 ml Flask
2 80 ml Flask
2 40 ml Flask
Product Service: Domestic 1-800-522-7658, International 816-333-8811
15
Page 19

Chapter 4: Using Your Benchtop Shell Freezer
Follow the steps below each time you use the shell freezer to
obtain optimum performance:
1. Check that the solvent bath drain plug is securely installed
in the drain hose.
2. Remove the bath compartment cover and add solvent to the
bath compartment. Commonly used solvents are methanol
or ethanol. Recommended solvent depth is 1/4" to 1/2"
above the top of the rollers (approximately 2 liters).
If flammable solvents are used in the shell
freezer bath, be sure to keep away from open
flame.
!
!
3. Press the main power switch. The top LED will illuminate.
The shell freezer refrigeration module will start and the
bath rollers will begin rotating.
4. Fill a freeze dry container no more than ½ full with sample
and stopper the container top. Lay the container on the
rollers in a horizontal position. Replace the bath
compartment cover during shell freezing.
5. When the sample is completely frozen in the container,
remove the container from the bath compartment and freeze
dry the sample or place it in a suitable storage freezer for
future freeze drying.
6. Press the main power switch to turn shell freezer off.
7. If the Benchtop Shell Freezer is to be used for cold bath
operation, additional solvent may be added to submerge
small flasks. Do not fill pan more than 2 inches below the
top of the stainless steel pan with vessels submerged.
Allow additional time for larger volumes of solvent to
reach lower temperatures.
8. Always drain flammable solvents from the bath when
the shell freezer is not in use and store the solvent in a
suitable container. This is accomplished by first pulling
the drain hose out of the left side panel of the unit and
then removing the drain plug. Place the hose in a
suitable container to collect the solvent.
16
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 20

C
HHAAPPTTEERR
C
M
B
F
Under normal operation, the Benchtop Shell Freezer requires little
maintenance. The following maintenance schedule is
recommended:
As needed:
1. If the heat transfer liquid in the bath becomes contaminated,
2. Clean up all spills.
3. Clean the lid and gasket using a soft cloth, sponge or chamois
Monthly:
1. Check the drain hose and lid gasket and replace if they show
2. Using a soft cloth, sponge or chamois and a mild, non-abrasive
3. Using a soft cloth, sponge, or chamois and a mild, non-abrasive
AAIINNTTAAIINNIINNG
M
EENNCCHHTTOOP
B
RREEEEZZEERR
F
drain and replace. It is the user’s responsibility to dispose of it
in accordance with all applicable regulations.
and a mild, non-abrasive soap or detergent.
signs of hardening, permanent set or deterioration.
soap or detergent, clean the bath lid.
soap or detergent, clean the exterior surfaces of the unit.
Liquid spray cleaners and polishes may be used on the exterior
P
5
5
S
G
Y
S
HHEELLL
Y
OOUURR
L
Product Service Domestic 1-800-522-7658, International 816-333-8811
17
Page 21

Chapter 5: Maintaining Your Benchtop Shell Freezer
surfaces. Do not use solvents to remove stains from the exterior
surfaces as they may damage the finish.
Annually:
1. Every 12 months, or more often if the Benchtop Shell Freezer
is operated in a dusty environment, the refrigeration system
condenser should be cleaned. Using a vacuum cleaner and
brush attachment, clean the condenser to ensure proper airflow
for peak performance. It can be accessed by removing the
front lower panel.
18
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 22

A
PPPPEENNDDIIXX
A
B
EENNCCHHTTOOP
B
F
RREEEEZZEERR
F
C
OOMMPPOONNEENNTTSS
C
The following pages list components that are available for your
Benchtop Shell Freezer. The parts shown are the most common
replacement parts. If other parts are required, contact Product
Service.
P
A
A
S
S
HHEELLL
L
Product Service Domestic 1-800-522-7658, International 816-333-8811
19
Page 23

Appendix A: Benchtop Shell Freezer Components
Item Qty Part No. Description
1 1 7403100 Lid
2 1 7408400 Gasket – Lid
3 1 1305200 Switch
4 1 7394400 Printed Circuit Board
5 1 7392208 Label
6 1 7624100 Hose – Drain
7 1 7728000 Plug – Drain
8 1 7519100 Drive Motor 115V
7519101 Drive Motor 230V
9 1 7515300 Temperature Sensor
1
2
4
7
5
6
9
8
5
3
20
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 24

A
PPPPEENNDDIIXX
A
B
EENNCCHHTTOOP
B
F
RREEEEZZEER
F
R
B
B
P
S
D
D
S
HHEELLL
IIMMEENNSSIIOONNSS
L
Product Service Domestic 1-800-522-7658, International 816-333-8811
21
Page 25

A
PPPPEENNDDIIXX
A
B
EENNCCHHTTOOP
B
F
RREEEEZZEERR
F
S
PPEECCIIFFIICCAATTIIOONNSS
S
This Appendix contains technical information about the Freeze
Dryer including electrical specifications, environmental operating
conditions and wiring diagrams.
Electrical Specifications
Catalog
Number
7949020 115 103-127 60 1 6
100 90-110 50 1 6
7949030 230 198-254 50 1 2.5
7949040 230 187-253 60 1 2.5
Environmental Conditions
• Indoor use only.
• Maximum altitude: 6562 feet (2000 meters).
• Ambient temperature range: 41° to 104°F (5° to 40°C).
• Maximum relative humidity: 80% for temperatures up to
88°F (31°C), decreasing linearly to 50% relative humidity
at 104°F (40°C).
• Main supply voltage fluctuations not to exceed ±10% of the
nominal voltage.
• Transient over voltages according to Installation Categories
II (Over voltage Categories per IEC 1010). Temporary
voltage spikes on the AC input line that may be as high as
1500V for 115V models and 2500V for 230V models are
allowed.
• Used in an environment of Pollution degrees 2 (i.e., where
normally only non-conductive atmospheres are present).
Nominal
C
C
P
E
E
Operating
S
S
HHEELLL
L
LLEECCTTRRIICCAALL
Range
Frequency Phase Amperage
22
Product Service Domestic 1-800-522-7658, International 816-333-8811
Page 26

Appendix C: Benchtop Shell Freezer Specifications
Occasionally, however, a temporary conductivity caused by
condensation must be expected, in accordance with IEC
664.
Product Service: Domestic 1-800-522-7658, International 816-333-8811
23
Page 27

Appendix C: Benchtop Shell Freezer Specifications
Wiring Diagram (115V, 60 Hz Model)
24
Product Service: Domestic 1-800-522-7658, International 816-333-8811
Page 28

Appendix C: Benchtop Shell Freezer Specifications
Wiring Diagram (230V, 60 or 50 Hz Models)
Product Service: Domestic 1-800-522-7658, International 816-333-8811
25
Page 29

DECLARATION OF CONFORMITY
Application Council Directive(s): 73/23/EEC, 89/336/EEC
Standard(s) to which conformity is declared: EN61010, EN55022, EN50082-1
Manufacturer’s Name: Labconco Corporation
Manufacturer’s Address: 8811 Prospect Avenue
Kansas City, MO 64132 USA
Importer’s Name: See Shipping/Customs Documents*
Importer’s Address: See Shipping/Customs Documents for your equipment
Type of Equipment: Laboratory Equipment – Benchtop Shell Freezer
Model No.: 7949030
Serial No.: Various – See Individual Declaration
Year of Manufacture: 2004 and Subsequent
I, the undersigned, hereby declare that the equipment specified above conforms to the
above Directive(s) and Standard(s).
See individual Declaration of Conformity which
will be signed by the importer for your country.
Place: _______________________________________
(Signature)
Date: _______________________________________
(Full Name)
_______________________________________
(Position)
*An individual version of this declaration is included with your shipping/customs
documentation.
Labconco P/N 36960-40, Rev. A, ECO C729
26
Product Service Domestic 1-800-522-7658, International 816-333-8811
 Loading...
Loading...