Page 1
3 IN 1 COMBO STIMULATOR
INSTRUCTION MANUAL
MA LT3011A REF.LTK545 EN 01 B_02-2015
Manufacturer:
Name: Shenzhen Dongdixin Technology Co., LTD.
Add: No.3 Building XiliBaimang Xusheng Industrial
Estate, Nanshan Shenzhen, China 518108
Tel: 0086-755-27652316
Authorized EC-representative:
Shanghai International Holding Corp. GmbH (Europe)
Eiffestraße 80, 20537 Hamburg Germany
Tel: 0049-40-2513175 Fax: 0049-40-255726
Copyright 2013 by Shenzhen Dongdixin
Technology Co., LTD.
Edition: V1.2
Distributed by:
Via Bruxelles, 3 - Meleto
52022 Cavriglia (Arezzo)
Tel. +39 055 96 21 11
Fax. +39 055 96 21 200
www.morettispa.com
info@morettispa.com
Page 2

This manual is valid for the LT3011A REF.LTK545
This user manual is published by Shenzhen Dongdixin
Technology Co., LTD.
Shenzhen Dongdixin Technology Co., LTD. does not
guarantee its contents and reserves the right to improve and
amend it at any time without prior notice. Amendments will
however be published in a new edition of this manual.
All Rights Reserved
LT3011A REF. LTK545 Stimulator Rev.V1.2 2013
Dongdixin
Declaration of conformity:
Shenzhen Dongdixin Technology Co., LTD. declares that
the LT3011A REF.LTK545 complies with following
normative documents:
Table of Contents
1. Foreword.......................................................................... 4
1.1 Introduction
1.2 Medical background
2. Safety information.............................................................7
2.1 Indication for use
2.2 Contraindication
2.3 Warning
2.4 Precautions
2.5 Adverse reactions
3. Presentation....................................................................12
3.1 Accessories
3.2 Presentation of the device
3.3 LCD display
3.4 Technical information
4. Operation instrucions......................................................16
4.1 Check batteries
4.2 Connect electrodes to lead wires
4.3 Connect lead wires to device
4.4 Place electrodes on skin
4.5 Turn on
4.6 Select the therapy mode and treatment part
4.7 Select the therapy program
4.8 Adjust the intensity and start to treatment
4.9 User program setting
4.10 Checking the memory
4.11 Turn the device off
4.12 Replace batteries
4.13 Low battery indicator
-1-
-2-
EN/IEC60601-1, EN/IEC60601-1-2, EN/IEC60601-2-10,
EN/IEC60601-1-11, ISO10993-5, ISO10993-10, ISO 10993-1
Complies with MDD 93/42/EEC and
amended by directive 2007/47/EC requirements
Page 3

5. Therapy program............................................................24
6. Cleaning and maintenance.............................................29
6.1 Cleaning the device
6.2 Cleaning the electrodes
6.3 Cleaning the Electrodes cords
6.4 Maintenance
7. Troubleshooting..............................................................32
8. Storage...........................................................................34
9. Disposal..........................................................................34
10. Normalized symbols.....................................................35
11. Warranty.......................................................................36
Appendix:Stimulation position.............................................37
1.1 Introduction
The LT3011A Ref.LTK545 is a battery operated pulse
generator that sends electrical impulses to the body and
reach the nerves and underlying muscle group,this is a unit
to be used for pain relief,muscle stimulation and
massage.The device provides two controllable output
channels,each independent of each other,a pair of
electrodes can be connected to each output channel.The
parameters of units are controlled by button.Its intensity
level is adjustable according to the needs of patients.
1.2 Medical background
What is TENS?
TENS (Transcutaneous Electrical Nerve Stimulation) gives
good results in acute and chronic pain conditions of many
kinds. It is clinically proven and used daily by
physiotherapists, other caregivers and top athletes around
the world.
High-frequency TENS activates the pain-inhibiting
mechanisms of the nervous system. Electrical impulses
from electrodes, placed on the skin over or near the painful
area, stimulate the nerves to block the pain signals to the
brain, and the pain is not perceived. Low-frequency TENS
stimulates the release of endorphins, the body's natural
painkillers. TENS is a safe treatment method and has, in
contrast to drugs and other pain relief methods, no side
effects. It may be sufficient as the only treatment form, but it
is also a valuable complement to other pharmacological
-3- -4-
1. Foreword
Page 4

and/or physical treatments. TENS does not always treat the
cause of pain. Consult your doctor if pain persists.
How Does TENS Control Pain?
The device provides pain relief in two ways. The first is the
gate control method. When the body is injured, both pain
and non-pain impulses are sent to the brain from the ervous
system. These pulses travel through the cutaneous nerves
to the deeper, afferent nerves, and then to the spinal cord
and brain. Along the path are many areas referred to as
“gates,” which determine which impulses are allowed to
continue on to the brain. The gates prevent the brain from
receiving too much information too quickly. Since the same
nerve cannot carry a pain and a non-pain impulse at the
same time, the stronger, non-pain impulse from the device
“controls the gate.” The second method of pain control is the
endorphin release method. The device can be set to trigger
the body's natural pain killers, called endorphins. These
chemicals interact with receptors, blocking the perception of
pain. This is similar to the way the pharmaceutical drug
morphine works, but without the side effect associated with
morphine. No matter which pain control method is
employed, the Device has been proven useful in pain
management. By reading this manual and carefully following
the treatment instructions provided by your clinician, you
can attain maximum benefit from your device.
What is EMS?
EMS (Electrical muscle stimulation) is achieved by sending
small electrical impulses through the skin to the underlying
motor units (nerves and muscles) to create an involuntary
muscle contraction. Neuromuscular stimulation has many
uses beyond its traditional application to prevent disuse
atrophy.
How does EMS work?
Because the transdermal stimulation of nerves and muscles
may be accomplished by electrical pulses, this modality can
help prevent disuse atrophy. Accordingly, incapacitated
patients can receive therapeutic treatment to create
involuntary muscle contractions thereby improving and
maintaining muscle tone without actual physical activity.
The goal of electrical muscle stimulation is to achieve
contractions or vibrations in the muscles. Normal muscular
activity is controlled by the central and peripheral nervous
systems, which transmit electrical signals to the muscles.
EMS works similarly but uses an external source (the
stimulator) with electrodes attached to the skin for
transmitting electrical impulses into the body. The impulses
stimulate the nerves to send signals to a specifically
targeted muscle, which reacts by contracting, just as it does
with normal muscular activity.
-5- -6-
Page 5

2.1 Indications for use
This device is used in following instance:
1) Symptomatic relief of chronic intractable pain, acute
post traumatic pain or acute post surgical pain.
2) Increase of blood flow in the treatment area.
3) Relaxation of muscle spasm.
4) Immediate post-surgical stimulation of muscles to
prevent venous thrombosis.
5) Prevention or retardation of disuse atrophy.
6) Muscle re-education
7) Maintaining or increasing range of motion.
2.2 Contraindications
1) This device should not be used when cancerous
lesions are present in the treatment area.
2) Stimulation should not be applied over swollen, infected,
inflamed areas or skin eruptions
(e.g. phlebitis, thrombophlebitis, varicose veins, etc.).
3) Safety has not been established for the use of
therapeutic electrical stimulation during pregnancy.
4) Demand type implanted pacemaker or defibrillator.
5) Epilepsy
6) Serious arterial circulatory problems in the lower limbs
7) Abdominal or inguinal hernia
8) No suitable for use during pregnancy or labor women
9) Do not use this device when pain syndromes are
undiagnosed. Use only after origin of pain has been
diagnosed.
-7-
2.3 Warning
1)
2)
3)
4)
5)
6)
7)
8)
9)
10)
The long-term effects of chronic electrical stimulation
are unknown.
For external use only.
This device should be used only under the continued
supervision of a licensed medical practitioner.
Stimulation should not be applied over the carotid sinus
nerves, particularly in patients witha known sensitivity
to the carotid sinus reflex.
Do not apply stimulation over thyroid or carotid sinus
region of the neck or mouth, or from electrodes placed
on the chest and the upper back, because this could
severe muscle spasms resulting in closure of the
airway, difficulty in breathing, or adverse effects on
heart rhythm or blood pressure.
Stimulation should not be applied transthoracically in
that the introduction of electrical current into the heart
may cause cardiac arrhythmias.
Stimulation should not take place while the user is
connected to high-frequency surgical equipment, it may
cause burn injuries on the skin under the electrodes, as
well as problems with the stimulator.
Do not use the stimulator in the vicinity of shortwave or
microwave therapy equipment, since this may affect the
output power of the stimulator.
Never use in environments with high humidity such as
in the bathroom or when having a bath or shower.
Never use near the heat. Stimulation electrodes should
never be placed anywhere on the front of the thorax
(marked by ribs and breastbone),but above all not on
the two large pectoral muscles. Here it can increase the
-8-
2. Safety information
Page 6

-9- -10-
2.4 Precautions
1)
2)
3)
4)
5)
6)
7)
8)
9)
10)
11)
12)
13)
14)
The stimulator is suitable for private use.
Caution should be used for patients with suspected or
diagnosed heart problems.
Some patients may experience skin irritation or
hypersensitivity due to the electrical stimulation or gel. If
rash develops or pain persists, discontinue use and
consult a doctor.
Electrode placement and stimulation settings should be
based on the guidance of prescribing practitioner.
Effectiveness is highly dependent upon patient selection
by a person qualified in the management of pain
afflicted patients.
Isolated cases of skin irritation may occur at the site of
the electrode placement following long-term application.
The electrodes are only to be placed on healthy skin.
Avoid skin irritation by ensuring that good contact is
achieved between electrodes and skin.
If the stimulation levels are uncomfortable or become
uncomfortable, reduce the stimulation amplitude to a
comfortable level and contact your physician if problems
persist.
The device may not be used whilst driving a car or whilst
operating and controlling machinery.
Never use the device in rooms where aerosols(sprays)
are used or pure oxygen is being administered.
Do not use it near any highly flammable substances,
gases or explosives.
Do not use this device at the same time as other
equipment which sends electrical pulses to your body.
Do not confuse the electrode cables and contacts with
your headphones or other devices, and do not connect
the electrodes to other devices.
Do not use sharp objects such as pencil point or
ballpoint pen to operate the buttons on the control panel.
risk of ventricular fibrillation and lead to cardiac arrest.
Never use on the eye area, or applied across or through
the head.
Never use near the genitals.
Never use on the areas of the skin which lack normal
sensation
This stimulator is never use by patients who have
noncompliant, emotionally disturbed, dementia, or low
IQ.
Apply the electrodes to clean, dry, and unbroken skin
only.
Keep electrodes separate during treatment, electrodes
in contact with other could result in improper stimulation
or skin burns.
Keep the stimulator out of reach of children.
Consult your doctor if you are in any doubt whatsoever.
11)
12)
13)
14)
15)
16)
17)
18)
Page 7
3.1 Reception of equipment and accessories
Your stimulator is supplied with case containing:
A. 1pc the stimulator
B. 2pcs electrode cable
C. 4pcs Electrode pad (40x40mm)
D. 1pc user manual
E. 4pcs AAA battery
3.2 Presentation of the device
Inspect Applicator cables and associated connectors
before each use.
Electrical stimulators should be used only with the leads
and electrodes recommended for use by the
manufacturer.
Possible skin irritation or electrode burn under the
electrodes may occur.
Possible allergic skin reaction to tape or gel may occur.
-11-
15)
16)
2.5 Adverse Reactions
1)
2)
-12-
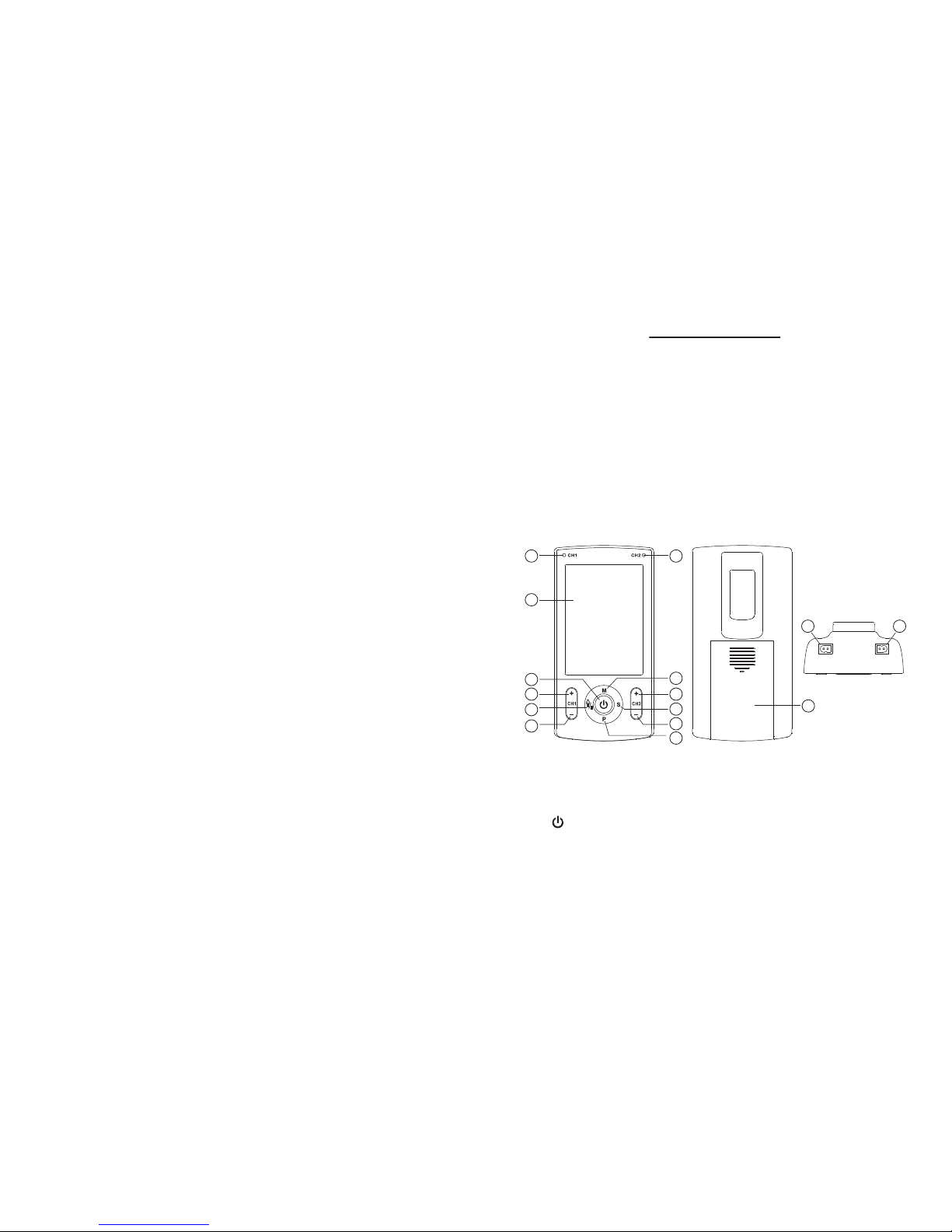
1
3
4
5
6
7
11
12
10
9
8
2
15 16
14
3. Presentation
(1) LED for channel 1
(2) LED for channel 2
(3) LCD
(4) [ ] key:
◆ Turn on or turn off the device;
◆ In working mode:Stop the treatment;
◆ In memory mode:Exit from the memory state.
◆ In setting mode: Exit from the setting state
Page 8

-13-
(5) [CH1+] key:
◆ Increasing the output intensity of channel 1.
(6) [ ] key:
◆ In user select mode: To select the user (U1 or U2);
◆ In memory mode: To check the memory data and
confirm whether remove the memory data;
◆ In working mode: Pause treatment and resume
treatment
(7) [ ] key:
◆ Decreasing the output intensity of channel 1.
◆ In setting mode: To select the next parameter .
(8) [ ] key:
◆ In waiting mode: To select the therapy program.
Press and hold to enter setting mode.
(9) [ ] key:
◆ Decreasing the output intensity of channel 1.
◆ In setting mode: Adjust the user program parameter.
(10) [ ] key:
◆ In user select mode: Confirm to select U1 or U2;
◆ In waiting mode: To select the treatment part circularly;
◆ In waiting mode: Press and hold on 3 seconds to enter
memory mode;
◆ In memory mode: Press and hold on 3 seconds to ask
whether remove the memory data.
(11) [ ] key:
◆ Increasing the output intensity of channel 1.
◆ In setting mode: Adjust the user program parameter.
(12) [ ] key:
◆ In waiting mode: To select the therapy mode: “TENS”,
“MASS”, “EMS”.
-14-
3.3 LCD display
1) Therapy mode: TENS, MASS, EMS
2) Therapy part: SHOULDER, NECK, BACK,
ELBOW, HIP, ANKLE, FOOT, WRIST, KNEE
3) Pause indicator
4) Setting mode indicator
5) Pulse width and Output intensity of channel 1
6) Treatment time
7) Pulse rate and Output intensity of channel 2
8) Low battery indicator
9) Intensity lock indicator
8
9
7
6
5
4
3
2
1
(14) Battery cover
(15) Socket for channel 1
(16) Socket for channel 2
Page 9

4 .1 Check Battery
Insert a fresh 4xAAA batteries into the battery compartment.
Make sure you are installing the battery properly.The battery
is inserted in the casing on the back of the stimulator.
Be sure to match the positive and negative ends of the
battery to the markings in the battery compartment of
unit.
To remove the battery cover, press and pull down following
the direction of on the battery cover.
Remove the batteries if the device is not in use for long
periods of time.
Do not mix old and new batteries or difference type of
batteries.
Warning: If batteries leak and come into contact with the
skin or eyes, wash immediately with copious amounts of
water.
Batteries must be handled by an adult. Keep batteries
out of the reach of children.
Only batteries of the same or equivalent type are
recommended.
Remove exhausted batteries from the unit.
Dispose of batteries safety according to local regulation.
1.
2.
3.
4.
5.
6.
7.
-16-
-15-
3.4 Technical information
Material:-------------------------------ABS
Channel:-------------------------------Two channel
Power supply:----------------------- 4x AAA batteries
Waveform:----------------------------Biphase square-wave pulse
Pulse duration:---------------------- 30-350μs
Pulse frequency:--------------------1-290Hz
Treatment time:--------------------- 5 to 90min
Intensity:------------------------------ Adjustable from 0 to 90mA
(at 1000 ohm )
Operating conditions:--------------5°C to 40°C with a relative
humidity of 30%-85%,
Atmospheric pressure from
700 hpa to 1060 hpa
Storage conditions:---------------- -10°C to 50°C with a relative
humidity of 10%~90%,
atmospheric pressure from
700 hpa to 1060 hpa
Dimensions:--------------------------117x60x25mm
Weight:--------------------------------110g (without batteries)
140g (with batteries)
Service life of the device:---------3 years
Service life of the battery:---------With new super heavy duty
batteries, approx. 20 days
when used for 30 minutes a
day in TENS NECK P01
program at 40mA intensity.
4. Instructions for use
Caution:
Page 10

4.2 Connect electrodes to lead wires
Insert the lead wire connector into
electrodes connector. Make sure no
bare metal of the pins is exposed.
Caution:
Always use the electrodes with
CE mark, or which are legally
marketed in the US under 510(K) procedure.
4.3 Connect lead wires to the device
LT3011A Ref.LTK545 has two output channels,
the user can use one channel with
a pairs of electrode pads or use
both channels with two pairs of
electrode pads. Before Connect
lead wires to the device, please
make sure the device is completely
turned off. Hold the cable plug and insert into the socket on
the top of the device.
Caution:
For the safety, please always use lead wires which supplied
by manufacturer or distributor.
4.4 Place electrodes on skin
Apply electrodes to the exact site indicated by your
physician (Recommend stimulation position please refers to
Appendix: Stimulation position). Before applying electrodes,
be sure the skin surface over which electrodes are placed is
thoroughly cleaned and dried. Make sure the electrodes are
Before applying the self-adhesive electrodes, it is
recommended to wash and degrease the skin, and then
dry it.
Do not switch the device on when the self-adhesive
electrodes is not positioned on the body.
Never remove the self - adhesive electrodes from the
skin while the device is still switched on.
It is recommended that, at minimum,4cm x 4cm self adhering based, square electrodes are used at the
treatment area
1.
2.
3.
4.
-17-
placed firmly to the skin and make good contact between
the skin and the electrodes. Place the electrodes over the
skin; attach them properly, firmly, and evenly.
Caution:
4.5 Turn on
Before using the device for the first time, you are strongly
advised to take careful note of the contraindications and
safety information of this manual. As this powerful
equipment is neither a toy nor a gadget!
In order to turn on the device, press [ ] button and the
device will enter into user select mode, LCD displays like
figure I:
Figure I
-18-
Page 11

You can select U1 or U2 by press [ ] button, the device will
enter into waiting mode after press [ S ] button.
Caution:
1)The device will enter into user select mode too, after you
insert batteries.
2) The device will enter into waiting mode too if no any
button to press in 5 seconds.
4.6 Select the therapy mode and treatment part
There are three therapy modes in this device, Press [ M ]
button to select therapy mode: “TENS”, “MASSAGE” or
“EMS”. The therapy mode which you selected will flash like
figure II.
There are 9 treatment parts display on LCD, press [ S ]
button to select therapy part circularly. The therapy part will
flash like figure II when you selected it.
Figure II
-19-
4.7 Select the therapy program
There are 3 therapy modes and 9 treatment parts in this
device, each treatment part has 3 or 4 programs in one
therapy mode. So, the total therapy program is 78, among
them, TENS program and EMS program each is 27 and
MASSAGE program is 24. The details please refer to
“Therapy program” in page 24.
Press [P] button to select the therapy program for the
treatment part which you selected. The LCD will display
program number like figure II.
4.8 Adjust the intensity and start to treatment
Press [CH1+] or [CH2+] button to increase the output
intensity of channel 1 or channel 2, and the device start to
work. Press [CH1-] or [CH2-] button to decrease the output
intensity of channel 1 or channel 2. The LCD will display the
current output intensity like Figure III, the remaining working
time also display on LCD.
Figure III
-20-
Page 12

Caution:
4.9 User program setting
Each treatment body part has one user program-U1 in
TENS and EMS mode. Turn on the device and select the
user program by press [P] button, and then press and hold
[P] button to enter into the setting mode. In this mode, you
can set the pulse width from 50 to 350µs, pulse rate from 1
to 150Hz and treatment time from 5 to 90min.
-21-
1) Select the parameter
Press [CH1-] button to switch the parameters- pulse rate,
pulse width, and treatment time.
2) Adjust the parameter
Press [CH2+] or [CH2-] button to adjust the parameter.
Press [ ] button to confirm the parameters and the device
enter into the waiting mode.
Note:
Press and hold [CH2-] button until you install all batteries,
the parameter will come back to factory default.
4.10 Checking the memory
The device will record the treatment parameter (e.g.
recording number, program, treatment part, the average
intensity and treatment time) after you finished treatment.
The maximum memory number is 30, the front of record will
be deleted when the memory number over 30.
If you want to check the memory data, please press and
hold [S] button for 3 seconds, the device will enter into
memory mode. Press [ ] button to read the memory
parameter, and press [CH1] button to read the next record
when the LCD display memory number.
The strength/intensity of stimulation may be adjusted
depending upon the individual requirement of the user.
The maximum intensity level is 90, 1level/step.
Before removing the electrode pads please make sure
the device turned off first.
The safety lock feature automatically activates after there
is no press any button in 20 seconds. You cannot
increase the output intensity when the indicator " "
display on LCD. You can press [CH1-] or [CH2-] button
to unlock the device.
When the remaining working time returns to zero, the
output intensity will stop automatically.
If there is any emergency happen, Please press [ ]
button to stop treatment, the device will enter into waiting
mode, or press [ ] button to pause treatment and press
again to continue the treatment. If the device is locked,
please press [CH1-] or [CH2-] button to unlock the
device first.
The amplitude level will be reset to 0mA when the
amplitude level is 10mA or greater and an open circuit at
either channel is detected.
1.
2.
3.
4.
5.
6.
7.
-22-
Page 13

Caution:
4.11 Turn the device off
You can Press and hold [ ] button to turn the device off
Note:
Except for working mode, the device will automatically turn
off after there is no press any button in 3min.
4.12 Replace batteries
To replace the batteries, open the lid cover and extract the
battery. Replace it with new AAA batteries. Make sure you
insert the battery correctly.
4.13 Low battery indicator
When the low power indicator flashes, the batteries should
be replaced with new batteries as soon as possible.
However, the stimulator will continue to operate for several
more hours.
-24--23-
◆ Decreasing the output intensity of channel 1.
◆ In setting mode: Adjust the user program parameter.
(10) [ ] key:
◆ In user select mode: Confirm to select U1 or U2;
◆ In waiting mode: To select the treatment part circularly;
◆ In waiting mode: Press and hold on 3 seconds to enter
memory mode;
◆ In memory mode: Press and hold on 3 seconds to ask
whether remove the memory data.
(11) [ ] key:
◆ Increasing the output intensity of channel 1.
◆ In setting mode: Adjust the user program parameter.
(12) [ ] key:
◆ In waiting mode: To select the therapy mode: “TENS”,
“MASS”, “EMS”.
1) Therapy mode: TENS, MASS, EMS
2) Therapy part: SHOULDER, NECK, BACK,
ELBOW, HIP, ANKLE, FOOT, WRIST, KNEE
3) Pause indicator
4) Setting mode indicator
5) Pulse width and Output intensity of channel 1
6) Treatment time
7) Pulse rate and Output intensity of channel 2
8) Low battery indicator
9) Intensity lock indicator
The device will return to waiting mode when you
press [ ] button or 30 seconds later.
Press and hold [S] button for 3 seconds, the total
recording number will flash on LCD when the device in
memory mode. If you want to delete the memory, please
press [ ] button to delete them; if you want to keep
them, you should press [ ] button and the device return
to waiting mode.
1.
2.
There are 3 therapy modes (TENS, EMS and MASSAGE)
and 9 treatment parts (SHOULDER, NECK, BACK, ELBOW,
HIP, ANKLE, FOOT, WRIST and KNEE) in this device, each
treatment part has 3 or 4 programs in one therapy mode. So,
the total therapy program is 78, among them, TENS program
and EMS program each is 27 and MASSAGE program is 24.
The factory default details please refer to following:
5.1 TENS program:
5. Therapy program
Page 14
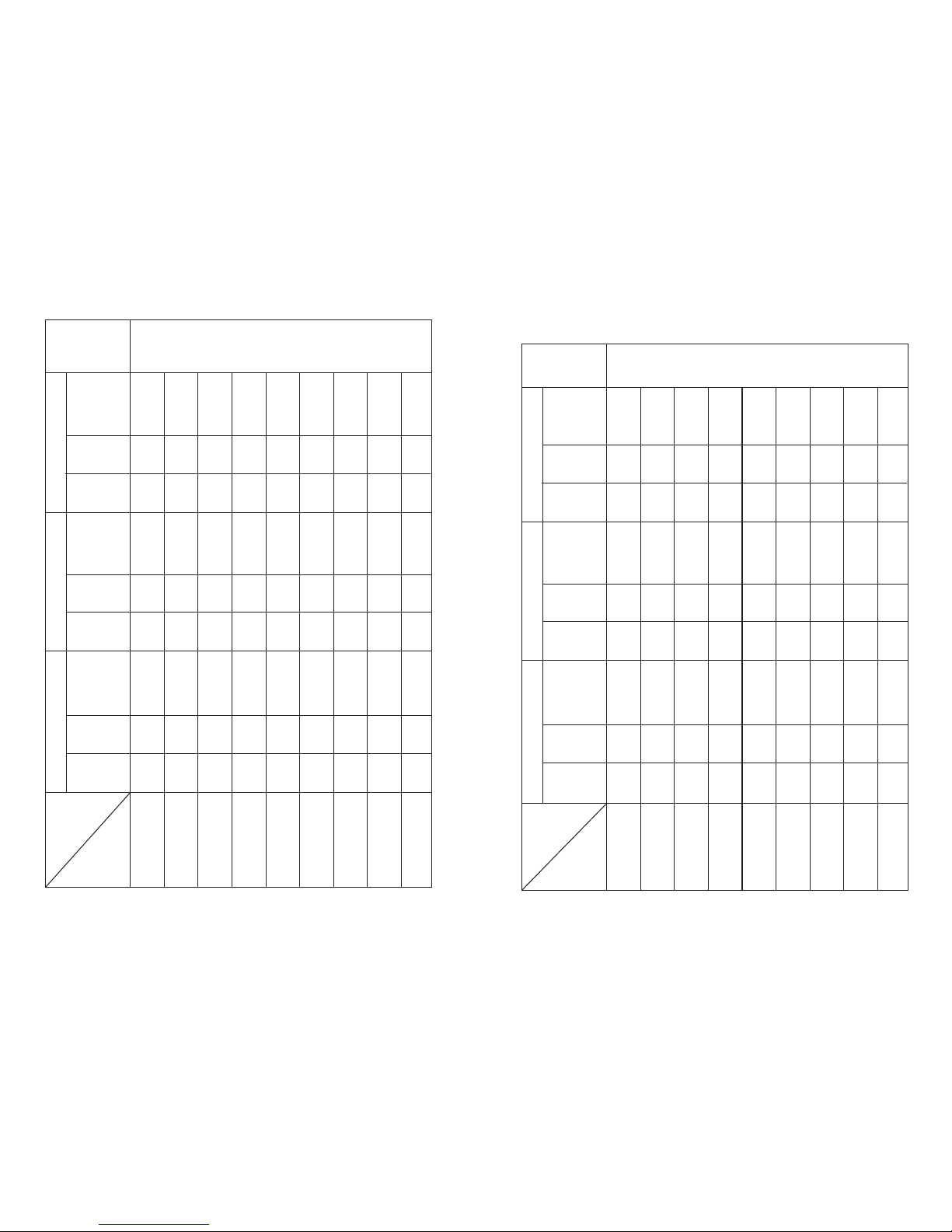
Therapy
part
program
P1
Treat-
ment
time
(min)
P2 U1(Default)
SHOULDER
NECK
BACK
ELBOW
HIP
ANKLE
FOOT
WRIST
KNEE
Waveform Waveform Waveform
Hans Hans
Hans
Pulse
rate
(Hz)
2~
125
80
1002~125
80
2~40
2~
100
2~80
80
Pulse
width
(μs)
100~
200
180
330/
200
100~
200
330/
200
100~
200
100~
200
100~
200
200
30
Pulse
rate
(Hz)
50
80
80
80
125
2~8
80
65
2
Pulse
width
(μs)
200
70~
180
70/
180
70~
180
330/
200
300
70/
180
200
180
Pulse
rate
(Hz)
2~80
2
80/2
2
100
2~60
80
50
50
Pulse
width
(μs)
100~
200
180
180
180
330/
200
100~
200
70~
180
200
350
Continuous Continuous
Continuous
Continuous
Amplitude
modulated
Amplitude
modulated
Amplitude
modulated
Amplitude
modulated
Asynchron-
ous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Synchronous
Pulse width
modulated
Pulse width
modulated
Pulse width
modulated
Pulse rate
modulated
Simple
modulated
pulse
Simple
modulated
pulse
Simple
modulated
pulse
Simple
modulated
pulse
Simple
modulated
pulse
Simple
modulated
pulse
Simple
modulated
pulse
Alternate
Ramped
Burst
Therapy
part
program
P1
Treat-
ment
time
(min)
P2 U1(Default)
SHOULDER
NECK
BACK
ELBOW
HIP
ANKLE
FOOT
WRIST
KNEE
Waveform Waveform Waveform
Pulse
rate
(Hz)
40
2~8
80
50
50
50
65
50
40
Pulse
width
(μs)
200
300
150
350
350
200
200
350
350
30
Pulse
rate
(Hz)
50
2~60
65
50
40
65
50
50/8
50
Pulse
width
(μs)
200
100~
200
350
200
200
200
200
200
350
Pulse
rate
(Hz)
80
1
50
50
65
50
50
50
50
Pulse
width
(μs)
150
50
350
150
350
200
200
200
350
Continuous
Pulse rate
modulated
Asynchronous
Asynchronous
Asynchronous
Simple
modulated
pulse
5.2 EMS program:
-25- -26-
Page 15

-27- -28-
5.3 MASSAGE program:
P1
P2
P3
SHOULDER
NECK
BACK
ELBOW
HIP
ANKLE
FOOT
WRIST
KNEE
P1,P2, P3,P4
P2,P3,P4
P1,P2,P3
P2,P3
P1,P2,P3,P4
P3,P4
P3,P4
P2,P3
P1,P2
Therapy part program
Work
time
(s)
Rest
time
(s)
Treatment
Time(min)
PhaseProgram
1
1
2
3
4
5
6
7
8
9
10
11
12
13
1
2
3
4
5
Pulse width
(µs)
100
30~220
30~220
30~220
30~220
200
200
200
200
200
30~220
30~220
30~220
30~220
50~220
50~220
50~220
50~220
50~220
Pulse
rate
(Hz)
8
25
25
33
43
53
69
79
69
53
43
33
25
25
83
100
111
118
132
4
3.5
2.5
1.9
1.3
0.9
0.7
0.5
0.7
0.7
1.3
1.9
2.5
3.5
4
3.8
3.1
2.6
2.3
/
1.0
0.9
0.9
0.8
0.7
0.6
0.5
0.6
0.6
0.8
0.9
0.9
1
1
0.8
0.72
0.6
0.6
30
P3
P4
Work
time
(s)
Rest
time
(s)
Treatment
Time(min)
PhaseProgram
6
7
8
1
2
3
4
5
6
7
8
9
10
11
Pulse width
(µs)
50~220
50~220
50~220
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
30~220~150
Pulse
rate
(Hz)
118
111
100
147
169
196
237
285
290
238
197
191
168
150
2.6
2.8
3.3
12.0
10.3
8.5
6.8
5.1
5.7
6.3
8
8.5
9.1
10.8
0.6
0.7
0.8
1.0
0.9
0.6
0.6
0.4
0.5
0.5
0.6
0.7
0.8
0.9
30
Page 16

-29- -30-
Remove the batteries from the device every time
before cleaning.
Clean the device with a soft,slight moistened cloth. In
case of more extreme soiling you can also moisten the
cloth with mild soapy water.
Do not use any chemical cleaners or abrasive agents for
cleaning.
1.
2.
3.
Use the device only with the leads and electrodes
provided by the manufacturer. Use only the electrode
placements and stimulation settings prescribed by your
practitioner.
It is recommended that, at minimum 4cm * 4cm selfadhering based, square electrodes are used at the
treatment area.
Inspect your electrodes before every use. Replace
electrodes as needed.Reusable electrodes may cause
slight skin irritation, lose adhesion and deliver less
stimulation if over used.
1.
2.
3.
Attach the electrode to the lead wire.
Remove the protective backing from the electrode
surface. Do not throw away the protective backing
because it is reused after the treatment session has
been completed.
Place the tacky surface to the prescribed skin area by
pressing the electrode firmly against the skin.
1.
2.
3.
Lift the corner of the electrode and gently remove it from
the skin.
Apply the protective backing to the tacky side of the
electrode.Place the electrode on the side of the
protective backing that is labeled with the word, on.
Store the electrodes in the resalable pouch or a plastic
bag.
1.
2.
3.
Do not pull on the electrode wire. Doing so may damage
the and electrode.
Always use the electrodes with CE mark, or are legally
marketed in the US under 510(K) procedure.
1.
2.
To use these electrodes:
To remove your electrodes:
Caution:
6.3 Cleaning the Electrodes cords
Clean the electrode cords by wiping them with damp cloth.
Coating them lightly with talcum powder will reduce tangles
and prolong the life.
6. Cleaning and maintenance
6.1 Cleaning the device
6.2 Electrodes
Page 17

-31- -32-
Maintenance and all repairs should only be carried out
by an authorized agency. The manufacturer will not be
held responsible for the results of maintenance or repairs
by unauthorized persons.
The user may not attempt any repairs to the device or any
of its accessories. Please contact the retailer for repair.
Opening of the equipment by unauthorized agencies is
not allowed and will terminate any claim to warranty.
Check the device before each use for signs of wear
and/or damage. Replace wear items as required.
1.
2.
3.
4.
7.Trouble-shooting
6.4 Maintenance
If your device does not seem to be operating correctly, refer
to the chart below to determine what may be wrong. Should
none of these measures correct the problem, the device
should be serviced.
Page 18

-33- -34-
For a prolonged pause in treatment, store the device in a
dry room and protect it against heat, sunshine and
moisture.
Store the device in a cool, well-ventilated place
Never place any heavy objects on the device.
1.
2.
3.
8. STORAGE
9. DISPOSAL
Used fully discharged batteries must be disposed of in a
specially labeled collection container, at toxic waste collection
points or through an electrical retailer. You are under legal
obligation to dispose of batteries correctly.
Please dispose of the device in accordance with
the directive 2002/96/EC WEEE (Waste Electrical
and Electronic Equipment). Contact your local
distributor for information regarding disposal of
the unit and accessories.
Page 19

The following is excluded under the warranty:
Liability for direct or indirect consequential losses caused
by the unit are excluded even if the damage to the unit
is accepted as a warranty claim.
All damage which has arisen due to improper
treatment, e.g. nonobservance of the user instruction.
All damage which is due to repairs or tampering by
the customer or unauthorized third parities.
Damage which has arisen during transport from the
manufacturer to the consumer or during transport to
the service centre.
Accessories which are subject to normal wear and
tear.
a.
b.
c.
d.
4.
5.
-35-
LOT
SN
-36-
The warranty period for LT3011A Ref.LTK545 products
is one year from date of purchase. In case of a warranty
claim, the date of purchase has to be proven by means
of the sales receipt or invoice.
Defects in material or workmanship will be removed free
of change within the warranty period.
Repairs under warranty do not extend the warranty
period either for the unit or for the replacement parts.
1.
2.
3.
Please contact your dealer or the device centre in case of a
claim under the warranty. If you have to send in the unit,
enclose a copy of your receipt and state what the defect
is.The following warranty terms apply:
10. Normalized symbols
11. Warranty
Applied part of type BF
Disposal in accordance with Directive
2002/96/EC (WEEE)
Batch code
Serial number
Refer to instruction manual because of the
higher levels of output
Keep dry
Complies with the European Medical Device
Directive (93/42/EEC) and amended by
directive 2007/47/EC requirements. Notified
body TÜV Rheinland (CE0197)
The name and the address of the manufacturer
Page 20

-37- -38-
Appendix: Stimulation position
Muscular
groups
Plantar
arch
muscles
Calf
muscles
Tibialis
anterior
Take up a seated
position with your
feet placed under
a piece of
furniture, to avoid
any ankle
bending
Contract your
tibialis anterior
muscles while
vigorously trying to
raise the tip of your
foot against an
object that resists
and prevents this
movement
Vigorously contract
your calf muscles
while strongly trying
to push the tip of
your foot against an
object that r
esistsand prevents
this movement
Take up a seated
position with your
back and feet firmly
placed against
supports This
position is easy to
adopt by sitting,
for example,in a
doorframe
Peroneous
muscles
Placement of
electrodes
Stimulation
positions
Take up a seated
position with your
feet resting on
the floor
Take up a seated
position with your
feet resting on
the floor
Contract your
peroneous muscles
by exercising a
vigorous pressure
on the floor with
your big toe, while
also trying to raise
the outer toes from
the floor
Vigorously contract
the muscles of your
plantar arch,trying
to dig your toes into
the floor
Voluntary start of the
contraction phase
1
2
3
4
1
2
Contract vigorously
the muscles on the
back of the thigh
(hamstrings) while
trying to bend your
knees
Lie flat on your
stomach with
your ankles fixed
in a convenient
way
Take up a seated
position and
place a rigid(but
comfortable)
object between
your knees
Take up a seated
position The work
can be done in two
ways:statically, if you
have taken the
necessary measures
to block the
movement of your
knees dynamically,
if you want to
emphasise work with
movement, against
an object offering
resistance created by
using heavy weights
Lie down on your
stomach or stand up
Special
recommendations:
For the buttocks, the
mode requires very
good muscular
qualities and is not
compatible with
certain morphological
configurations In the
event of repeated
failures with the mode,
we recommend work
on the buttocks in the
“classic” mode
Strongly contract
your quadriceps,
while trying to
extend your legs
Vigorously contract
your buttock
muscles, while
strongly trying to
close your buttocks
and trying to bring
your thighs behind
your trunk
Buttocks
Strongly contract
your adductors,
while vigorously
trying to bring your
knees together
Hamstrings
Adductors
Quadriceps
Muscular
groups
Placement of
electrodes
Stimulation
positions
Voluntary start of the
contraction phase
5
1
2
6
1 2
7
1
2
8
1
2
Page 21

Lie down stretched out
on your back, which
can be slightly raised.
The work can be done
in two ways: statically,
if you are simply trying
to voluntarily start the
muscular contraction
phase exercise with
the movement that
dynamically, if you
want to associate an
consists of bringing the
trunk towards the
thighs;in this case,take
care not to accentuate
the lumbar region arch
(lordosis); to do this, it
is essential to work
always with your knees
well bent
Take up a seated position.
Special recommendations:
Because of the anatomical
and morphological specificity
of the low back muscles
region, it is necessary to have
muscles with particularly good
performance to work in the
mode.
In the event of repeated
failures with this work mode,
we recommend work in the
“classic”mode or to use the
recommended placement
for combined stimulation of
the low back muscles and
erector spinalis; in this case,
always take care to position
the system at the level of the
dorsal region muscles, as
shown in the picture.
The application “Preparation
for a sportsman who wishes
to improve the efficiency of
the muscular reinforcement
of his abdomen” of the
Sportcategory gives advice
for work on the abdominal
and the low back muscles
with the greatest
effectiveness
Strongly contract
your abdominal
muscles, while
strongly trying to
raise your head and
shoulders from their
support
Vigorously contract
the low back
muscles, while
making an effort to
sit as tall as
possible
Abdominal
muscles
Low back
muscles
Muscular
groups
Placement of
electrodes
Stimulation
positions
Voluntary start of the
contraction phase
1
2
10
9
1
2
2
1
11
Erector
spinalis
Cervical
muscles
Trapezius
Deltoids
Latissimus
dorsi
Take up a seated
position
Take up a seated
position
Take up a seated
position
Take up a seated
position, with
your elbows
placed inside
armrests to create
resistance of the
arms to their
movement away
from the body
Take up a seated
position, with your
elbows placed
outside armrests
to create resistance
of the arms to their
movement
towards the body
Vigorously contract
the dorsal region
muscles, while
making an effort to
sit as tall as possible.
Vigorously contract
the dorsal region
muscles, while
making an effort to
sit as tall as possible
Strongly contract
your trapezius
muscles, while
vigorously trying to
shrug your shoulders
Vigorously contract
your deltoids, while
strongly trying to
move your elbows
away from your body
Vigorously contract
your latissimus dorsi,
while strongly trying
to move your elbows
towards your body
Muscular
groups
Placement of
electrodes
Stimulation
positions
Voluntary start of the
contraction phase
1
2
12
1
2
13
14
1
2
15
1
2
16
1
2
-40--39-
Page 22

-42-
Pectoralis
Triceps
Biceps
Hand
extensors
Take up a seated
position, with the
palms of your hands
in contact with each
other Current
international
standards require
that a warning be
given concerning the
application of to the
thorax: increased risk
of cardiac fibrillati
Vigorously contract
your pectoral muscles,
while trying to press
strongly the palms of
your hands against
each other
electrodeson
Vigorously contract
your triceps, while
strongly trying to dig
the palms of your
hands into the
armrests
Vigorously contract
your biceps, while
trying strongly to
move the palms of
your hands towards
your shoulders
Vigorously contract
your hand extensors
muscles, while trying
to raise yourhands
Take up a seated
position, with your
forearms and
hands resting on
armrests
Take up a seated
position, with your
forearms resting
on armrests and
the palms of your
hands imperatively
facing upwards.
Use a fixing system
to avoid any
movement of your
elbows during
stimulation
Take up a seated
position, with your
forearms and the
palms of your
hands resting on
armrests Fix your
hands solidly to
the armrests
Muscular
groups
Placement of
electrodes
Stimulation
positions
Voluntary start of the
contraction phase
18
1
2
17
19
20
-41-
Hand
flexors
Strongly contract
your hand flexors
muscles, while
strongly trying to grip
the object that you
were holding in your
hands
Take up a seated
position, with your
forearms resting
on armrests. Hold
a crush-proof
object in your
hands so that your
fingers are slightly
bent
Muscular
groups
Placement of
electrodes
Stimulation
positions
Voluntary start of the
contraction phase
21
 Loading...
Loading...