KORU FREEDOM60 User Manual

Instructions For Use
Introduction
Indications for Use
Caution
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
FREEDOM60® Syringe Driver Diagram
FREEDOM60® Product Line
Syringes for Use with FREEDOM60®
. . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
Testing FREEDOM60® Syringe Driver
Selected Flow Rates vs Time
Subcutaneous Infusion Instructions
Intravenous Infusion Instructions
Troubleshooting
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Care and Maintenance
FREEDOM60® Flow Prole
Technical Specications
Selected Flow Rate Combinations
Warranty Information
Denition of Symbols
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . .
. . . . . . . . . . . . . . .
2
2
3
4
4
4
5
5
6
8
11
12
14
14
15
17
19
English 1 of 20

Introduction:
The FREEDOM60® Syringe Infusion System is portable and easy to use, requiring
no batteries or electricity. There are only two operating knobs and special precision
tubing sets are used to control the ow rate.
The FREEDOM60® operates at a constant safe pressure. The constant pressure developed in the FREEDOM60® automatically decreases the ow rate if there is an increase
in resistance during the delivery. The system will nd equilibrium between the
increasing resistance and ow rate. It provides constant ow which tends to inhibit
clots, and holds full pressure after an infusion is complete to prevent blood or drug
backow. The FREEDOM60® also eliminates concerns of a bolus, overow, overdose or
runaway infusion.
Indications for Use:
The Freedom Integrated Syringe Infusion System, which includes the FREEDOM60®
and FreedomEdge® syringe drivers, is indicated for the intravenous or subcutaneous
infusion of medications and uids in the home, hospital, or ambulatory settings when
administered according to the approved biologic or drug product labeling. The
Freedom System is specically indicated for the subcutaneous infusion of the following human plasma-derived immunoglobulins when used according to the FDA
approved biologic labeling: Hizentra, Immune Globulin Subcutaneous (Human) 20%
Liquid (manufactured by CSL Behring); Gammagard Liquid, Immune Globulin Infusion
(Human) 10% (manufactured by Takeda); and Cuvitru Immune Globulin Infusion
(Human) 20% (manufactured by Takeda). The Freedom System is specically indicated
for the intravenous infusion of the following antibiotics when used according to the
FDA approved drug product labeling: meropenem, ertapenem, oxacillin, and
tobramycin.
The FREEDOM60® Syringe Infusion System is indicated for use with the BD® 50 ml
syringe (US Reference number 309653).
English 2 of 20
Caution:
• Use the FREEDOM60® Syringe Infusion System only for the patient for whom the device is
prescribed and only for its intended use.
• In order to achieve specic and repeatable ow rate performance with the Freedom Syringe
Infusion Systems’ unique constant force mechanism, use only Freedom System accessories
manufactured by KORU Medical Systems. Directly connecting extension tubing or administration
set (without the luer disc) will cause the syringe to eject from the syringe driver and eventually
cause internal damage to it. Use of any other ow control accessory may also cause over delivery
of uids or medication to the patient. For use with subcutaneous immune globulin products, use
only KORU ow control devices and HIgH-Flo needle sets, as use of generic products may result in
unknown ow rates and additional site complications such as pain, swelling and redness.
• Use only recommended BD® 50 ml syringes with the FREEDOM60®.
• Before use, carefully inspect the tubing set package. Do not use the tubing set if the package is
opened or damaged.
• Do not re-sterilize tubing sets.
• Overuse of the slide clamp or storing tubing sets with the slide clamp engaged for long periods of
time* may damage the tubing and aect the infusion rate.
• The black tab that pushes on the syringe plunger operates under high force. Do not place ngers
on the black tab or inside the syringe shield at any time. Do not attempt to interfere with the
movement of the black tab at any time.
• Carefully inspect the FREEDOM60® Syringe Driver before use. Discontinue use of a syringe driver
that has been damaged, exposed to severe impact, or which has failed to test properly.
• Do not attempt to open the syringe driver housing or remove the syringe shield. Do not operate if
the syringe shield has been removed.
• Do not attempt to remove the syringe or disconnect the tubing set without rst turning the
syringe driver OFF and fully winding the large knob clockwise until the black tab has reached the
end of its track.
• The FREEDOM60® Syringe Infusion System does not have an alarm, therefore no alarm will sound
if an interruption to ow occurs. There is no display of infusion status. The syringe driver is not
suitable for use with medication where delay or under-infusion could result in serious injury.
• Discontinue use of a syringe driver that has been submerged in uid. If any uid is allowed to
enter the syringe driver, it should be replaced immediately.
• Do not autoclave the FREEDOM60®. It will melt the ABS plastic and damage the syringe driver.
• Federal law (USA) restricts this device to sale by or on the order of a physician.
• Priming and shipping of tubing sets, packaged at temperatures below freezing, is not
recommended. This may damage the tubing.
• The FREEDOM60® Syringe Infusion System is not intended for blood transfusions.
*Generally greater than 2 hours.
English 3 of 20
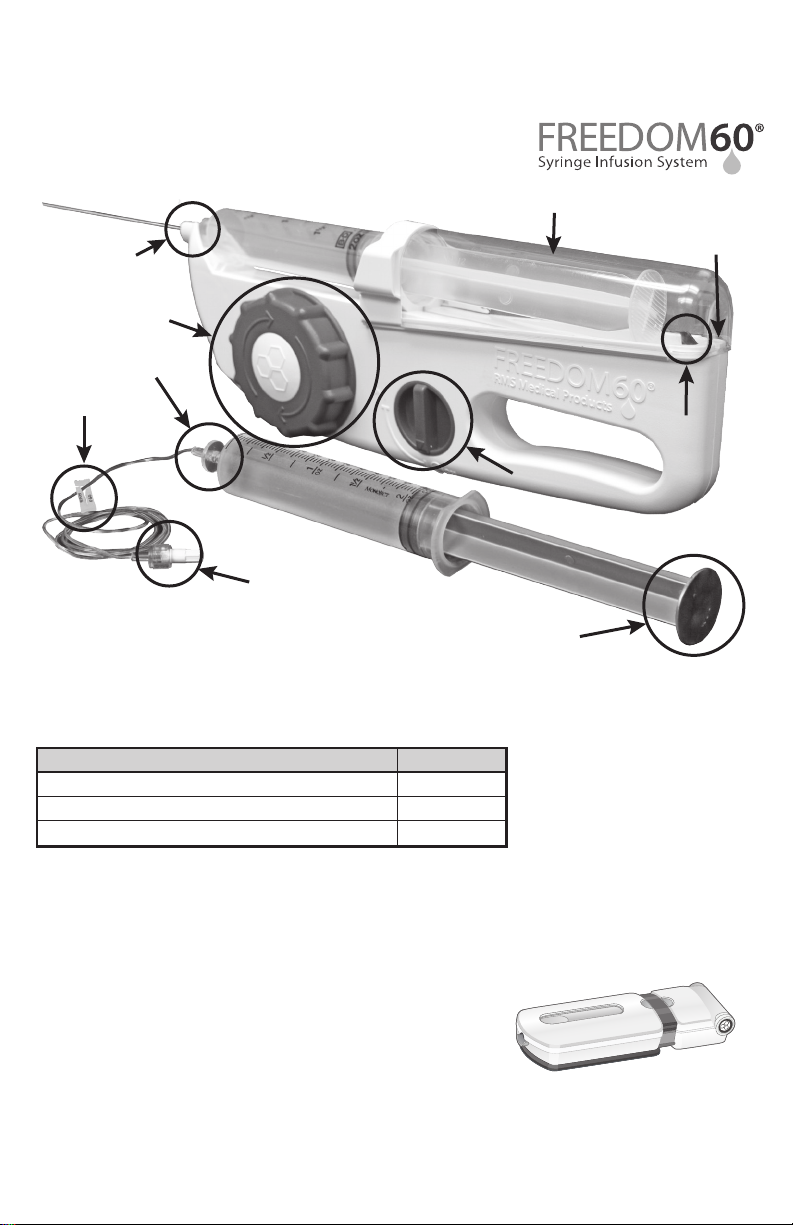
FREEDOM60® Syringe Driver Diagram:
Product
Part #
F10050
F10090
SYRINGE SHIELD
DRIVER NOSE
LARGE KNOB
(WIND UP KNOB)
LUER DISC END OF
FLOW RATE TUBING
Patent No. D398,053
END OF TRACK
(TRACK END)
SLIDE CLAMP
ON/OFF SWITCH
MALE END OF
FLOW RATE TUBING
SYRINGE
PLUNGER
BLACK TAB
FREEDOM60® Product Line:
Each FREEDOM60® Syringe Infusion System includes a travel pouch and user manual.
FREEDOM60® Syringe Driver
Replacement Travel Pouch - black
Zebra Print Travel Pouch - pattern F10080
Syringes for use with the FREEDOM60®:
• Becton Dickinson & Co. BD® Luer-Lok® 50 ml (US Reference #309653;
EU Reference #300865)
For Smaller Volume Infusions:
The FREEDOM60® is designed to accommodate BD®
50 ml syringes. For smaller volumes, the FreedomEdge®
accommodates both 20 ml and 30 ml syringes.
Part #: F10020
English 4 of 20
5
5 hrs.
2 hrs. 30 min.
10 min.
6 min. 42 sec.
5 min.
2 min. 30 sec.
10
10 hrs.
5 hrs.
20 min.
13 min. 18 sec.
10 min.
5 min.
15
15 hrs.
7 hrs. 30 min.
30 min.
20 min.
15 min.
7 min. 30 sec.
20
20 hrs.
10 hrs.
40 min.
26 min. 42 sec.
20 min.
10 min.
25
25 hrs.
12 hrs. 30 min.
50 min.
33 min. 18 sec.
25 min.
12 min. 30 sec.
30
30 hrs.
15 hrs.
60 min.
40 min.
30 min.
15 min.
35
35 hrs.
17 hrs. 30 min.
70 min.
46 min. 42 sec.
35 min.
17 min. 30 sec.
40
40 hrs.
20 hrs.
80 min.
53 min. 18 sec.
40 min.
20 min.
50
50 hrs.
25 hrs.
100 min.
66 min. 42 sec.
50 min.
25 min.
55
55 hrs.
27 hrs. 30 min.
110 min.
73 min. 18 sec.
55 min.
27 min. 30 sec.
60
60 hrs.
30 hrs.
120 min.
80 min.
60 min.
30 min.
Testing the FREEDOM60® Syringe Driver:
1. Examine the inside of the syringe shield and ensure it is free of debris or
contamination.
2. Make sure that the syringe driver’s on/o switch is in the OFF position and
that the black tab within the syringe shield is at the end of its track. If the
black tab is not at the end of its track, fully wind the large knob clockwise.
3. Turn the syringe driver ON (–) and watch that the tab moves smoothly
along the full length of its track; listen for the following sounds:
• a ‘click’ as the driver is turned ON (–)
• a ‘whirring’ sound as the black tab moves forward
• a ‘click’ as the tab reaches the end of its travel
4. Test to make sure the syringe tension tab (the one-inch long tab located at
the entry of the syringe shield) operates freely by adjusting it up and down
with your nger.
Selected Flow Rates vs Time:
Note: For assistance in determining which ow rate tubing set to use, please contact
KORU Medical Systems at 800-624-9600.
Flow Rate vs Time Chart
Syringe
Volume
1ml/hr 2ml/hr 30ml/hr 45ml/hr 60ml/hr 120ml/hr
English 5 of 20
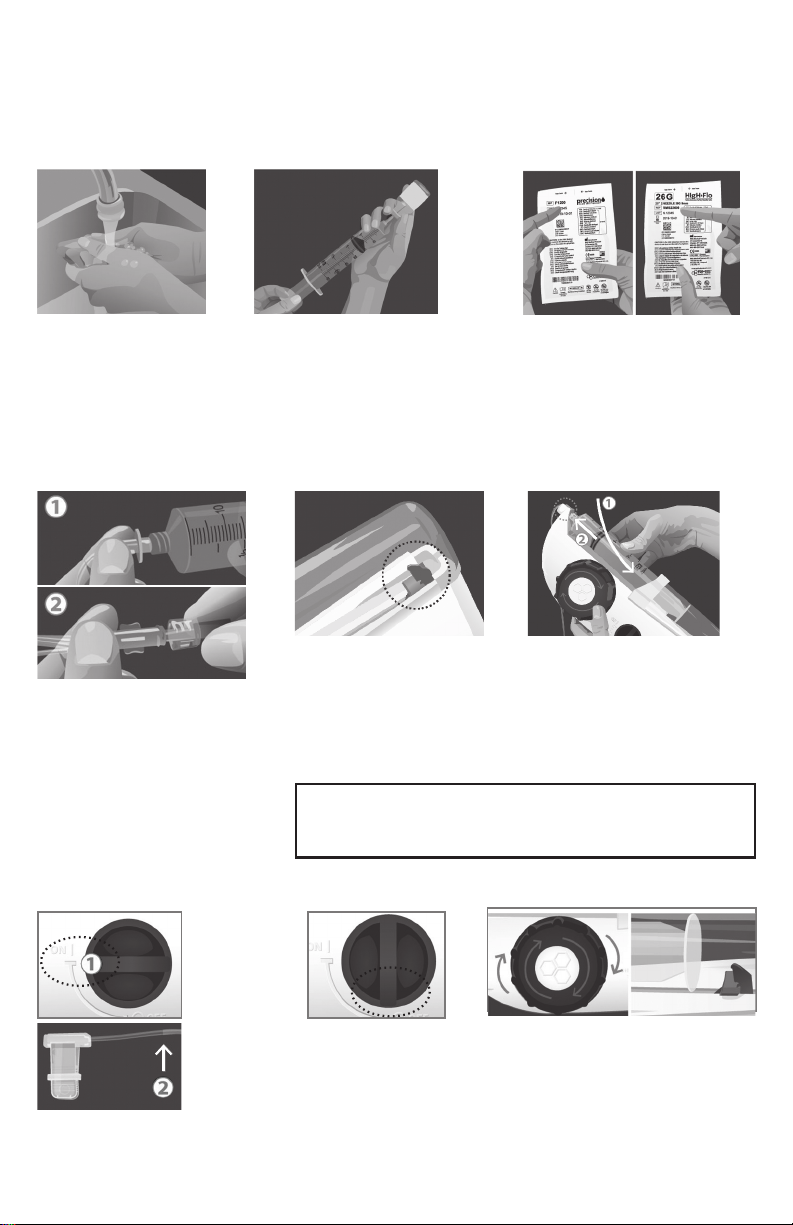
For Subcutaneous Infusions, Follow These Instructions:
Prepare the FREEDOM60® Syringe Driver:
1 Wash Hands
2 Fill Syringe 3 Verify Tubing & Needles
Gather supplies. Wash
your hands thoroughly
and, if required, put on
disposable gloves.
Be sure the product is at room
temperature before you begin
lling the BD® 50 ml syringe with
your required dose. Refer to
the manufacturer's instructions
or ask your provider for more
detailed lling instructions.
Verify that you are using the
correct tubing and needle sets
recommended by your healthcare provider and prescribed
by your doctor.
4 Attach Tubing 6 Insert Syringe5 Check Black Tab
1. Remove sterile cap from the
luer disc end of the Precision
tubing set and connect to syringe.
2. Remove sterile caps from
opposite end of tubing set and
HIgH·Flo needle set and connect,
using care not to contaminate ends.
Make sure the syringe
driver is o and the black
tab inside the clear syringe
shield is at the end of its
track. If the black tab is not
at the end of its track, wind
the large knob clockwise.
Note: You will never need to use force to load or remove
a syringe. If you’re having trouble, stop and make sure
the black tab is at the end of its track.
With syringe gradations
facing up:
1.
load the syringe and its
tubing into the syringe driver.
2.
Make sure the luer disc is
fully seated in the driver’s nose.
Prime Tubing:
7 Turn ON 8 Turn OFF 9 Wind Back
1. Turn the
syringe driver’s
ON/OFF
switch to the
ON position
to prime (ll)
the tubing.
2. Try to stop
the ow about
2” short of
the needle(s).
To stop ow to needle(s),
turn the syringe driver’s
ON/OFF switch to the
OFF position.
Wind the large knob clockwise until
the black tab is clearly not touching
the syringe. This will release pressure
on the plunger and stop the ow.
English 6 of 20
 Loading...
Loading...