Kinetik Wellbeing JPD500E Instruction manual
Pulse Oximeter JPD-500E
Instructions to User
Thank you for purchasing the Pulse Oximeter.
This Manual is written and compiled in accordance with the council directive
MDD 93/42/EEC for medical devices and harmonized standards. In case of
modifications and software upgrades, the information contained in this
document is subject to change without notice.
Please read the User Manual carefully before using this product.
WARNING:
■ Discomfort or pain may occur if using the device for long periods of time,
especially for microcirculation barrier patients. It is recommended that the
sensor is not applied to the same finger for over 2 hours.
■ The light (infrared is invisible) emitted from the device is harmful to the
eyes, so the user and all maintenance should not stare at the light.
■ Enamel or acrylic nail polish or other nail applications may distort and/or
produce inaccurate readings.
■ Please refer to the correlative literature about any clinical restrictions or
cautions.
■ This device is not intended for treatment.
1 Safety
1.1 Instructions for Safe Operation
■ Check the main unit and all accessories periodically to make sure that
there is no visible damage that may affect patient’s safety and monitoring
performance about cable and transducers. It is recommended that the
device is inspected once a week at least. When there is obvious damage,
stop using the monitor.
■ Necessary maintenance must be performed by qualified service
engineers ONLY. Users are not permitted to maintain it by themselves.
■ The oximeter cannot be used together with devices not specified in the
User Manual. Only accessories appointed or recommended by the
manufacture can be used with this device.
■ This product is calibrated before leaving factory.
1.2 Warnings
■ Explosive hazard: DO NOT use the oximeter in an environment with
inflammable gas such as ignitable anaesthetic agents.
■ DO NOT use the oximeter if the user is being measured by an MRI or
CT scan.
■ DO NOT use the device if allergic to rubber.
■
The disposal of scrap material, the device’s accessories and packaging
(including batteries, plastic, foam and paper) should follow th
and regulations.
■ Please check the pack’s contents before use to make sure the device
and accessories are in accordance with the packing list, otherwise the
device may work abnormally.
1.3 Attentions
● Keep the oximeter away from dust, vibration, corrosive substances,
explosive materials, high temperature and moisture.
● If the oximeter gets wet, stop operation.
● If the device is moved from a cold environment to a warm or humid
environment, please do not use it immediately.
● High temperature or high pressure steam disinfection of the oximeter is
not permitted. Refer to section 7 of the User Manual for instructions on
cleaning and disinfection.
● Do not immerge the oximeter in liquid. To clean, wipe its surface with
alcoholic wipes. Do not spray any liquid on the device directly.
● Do not use the device on small children and babies.
● The product is suitable for 4+ years old and for those who weigh
between 15kg – 110kg.
e local laws
● The device may not work for all patients. If you are unable to achieve stable
readings, discontinue use.
● The update period of data is less than 5 seconds, which may change
according to the individual’s pulse rate.
● The device has a normal lifespan of five years since the first electrified use.
● The device shows low-voltage but does not have a low-voltage alarm
function. Please change the battery when the battery dies.
● Batteries must be removed if the device is going to be stored for more than a
month in case batteries leak.
1.4 Indication for use
The Finger Pulse Oximeter is a non-invasive device intended for the spot-check
of oxygen saturation of arterial haemoglobin (SpO2) and the pulse rate of adult
and paediatric patients in home and hospital environments (including clinical
use in internist/surgery, anaesthesia, intensive care etc.) This device is not
intended for continuous monitoring.
2 Overview
The pulse oxygen saturation is the percentage of HbO2 in the total Hb in the
blood, also known as the O2 concentration in the blood. It is an important
bio-parameter for respiration. For the purpose of measuring the SpO2 more
easily and accurately, our company developed the Finger Pulse Oximeter. The
device can also measure pulse rate simultaneously.
The Pulse Oximeter features a convenient operation, low power consumption,
small size and portability.
small volume, low power consumption, convenient operation and portability. A
patient needs to put just one finger onto the photoelectric sensor for diagnosis,
and a display screen will directly show the measured value of Haemoglobin
Saturation.
2.1 Classification:
Class II b (MDD 93/42/EEC IX Rule 10)
2.2 Features
■ Simple and convenient operation
■
Small size, lightweight and portable (total weight is 50g est. including
batteries)
■ Low power consumption
■ The product will automatically turn off when out of use for over 16 seconds
2.3 Major Applications and Scope of Application
The Pulse Oximeter can be used to measure Haemoglobin Saturation
pulse rate through the finger, and indicate the pulse intensity by the bar-display.
The product is suitable for use at home, in hospital, oxygen bar, social medical
organisations and to measure oxygen saturation and pulse rate.
The product is not suitable for continuous supervision of patients.
The product is not suitable for use in continuous supervision for patients.
2.4 Environment Requirements
Operation Temperature: 5ºC-40ºC
Storage Temperature: -10ºC-50ºC
Ambient Humidity: 15%-80% RH, no condensation in operation
Atmospheric Pressure: 70 kPa to 106 kPa, in operation
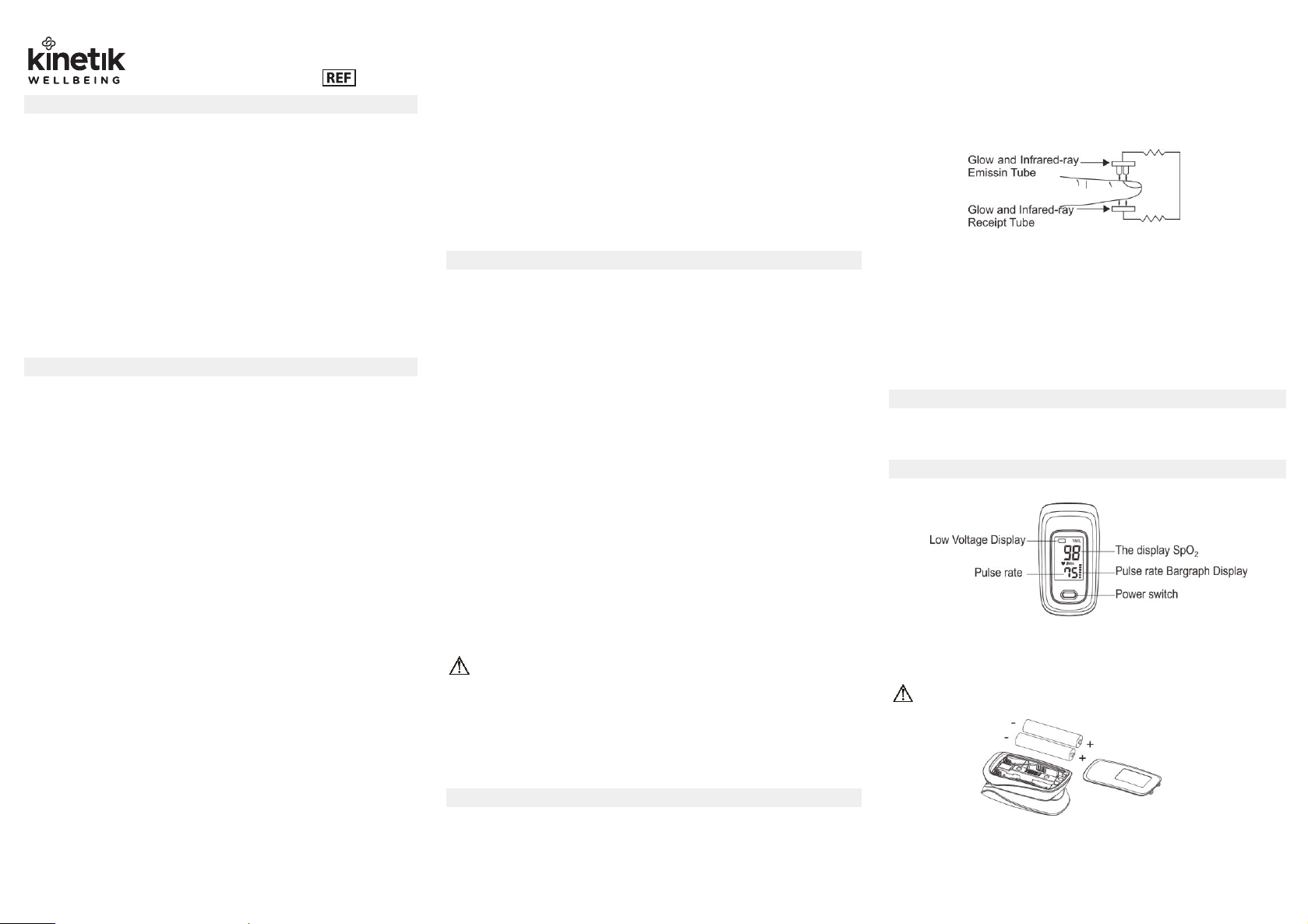
3 Principle and Caution
3.1 Principle of Measurement
Principle of Oximeter: An experienced formula of data processing is established
using Lambert Beer Law according to Spectrum Absorption Characteristics of
Reductive Haemoglobin (Hb) and Oxyhaemoglobin (HbO2) in glow &
near-infrared zones.
10%-93% RH, no condensation in storage
50kPa–106 kPa, in storage
and
Operation of Oximeter: Photoelectric Oxyhaemoglobin inspection
Technology is adopted in accordance with Capacity Pulse Scanning &
Recording Technology, so that two beams of different wavelength of lights
can be focused onto the fingertip through a perspective clamp finger-type
sensor. The measured signal can be obtained by a photosensitive element,
information acquired through which will be shown on screen through
treatment in electronic circuits and a microprocessor.
Figure 1 Operating principle
3.2 Caution
1. Place the finger properly (see Figure 5) to avoid inaccurate
measurement.
2. The SpO2 sensor and photoelectric receiving tube should be arranged
with the subject’s arteriole in between.
3. Excessive ambient light may affect the measuring result. This includes
fluorescent lamps, dual ruby lights, infrared heaters direct sunlight and etc.
4. Strenuous action or extreme electrosurgical interference may also affect
the accuracy.
4 Accessories
■ 1 x Lanyard
■ 2 x AAA Batteries
■ 1 x User Manual.
5 Installation
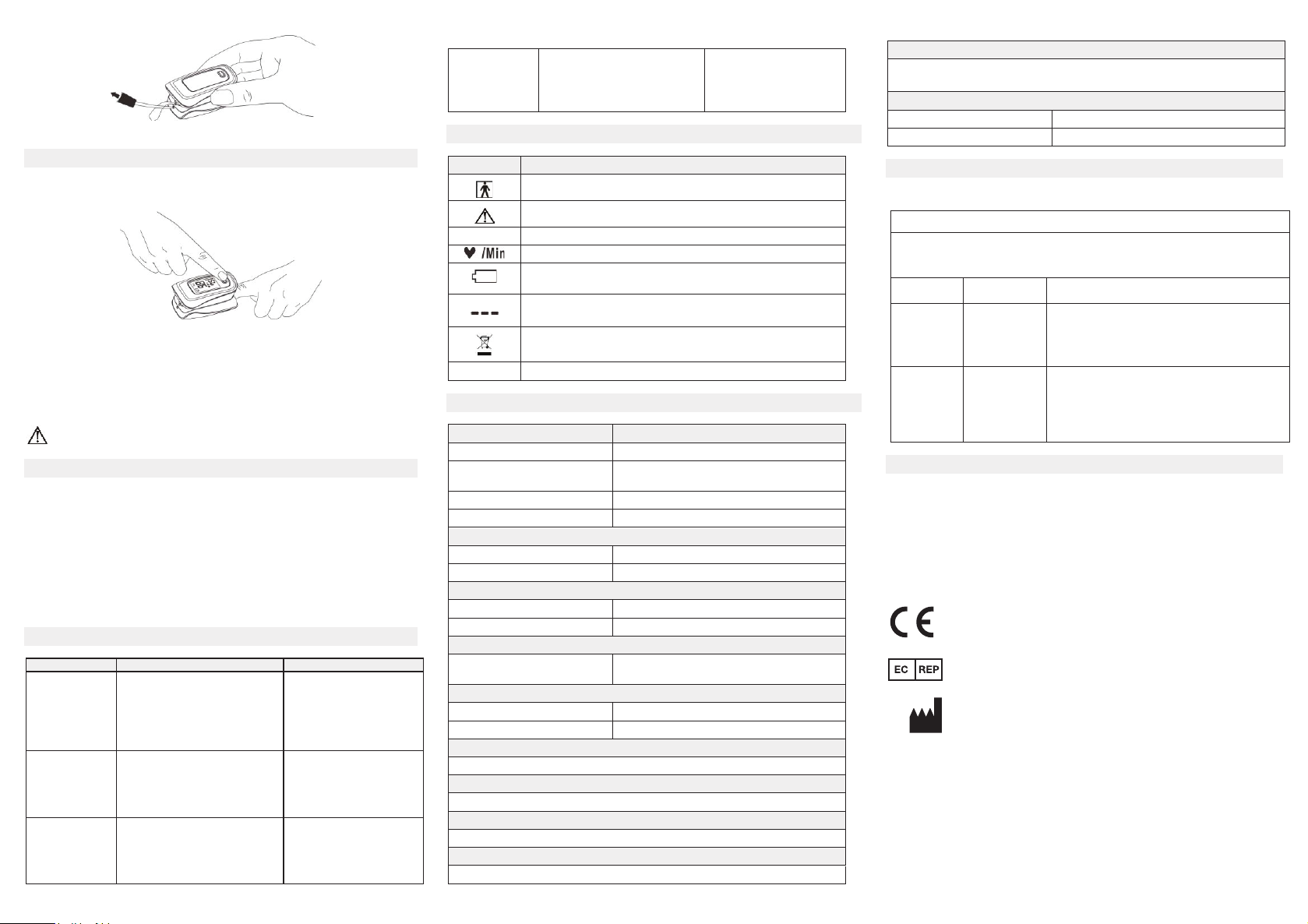
5.1 View of the Front panel
Figu
5.2 Battery
Step 1. Refer to Figure 3. and insert the two AAA in the correct direction.
Step 2. Replace the cover.
Please take care when you insert the batteries as improper
insertion may damage the device.
Figure 3. Batteries Installation
5.3 Attaching the Lanyard
Step 1. Put one end of the lanyard through the hole.
Step 2. Put the other end of the lanyard through the first one and tighten.
re 2. Front View
The display is
1. The device will power off
drained.
1. Normal.
Description
%SpO2
The pulse oxygen saturation (%)
Pulse rate (bpm)
The battery voltage indication is deficient (change the
battery in time avoiding the inexact measure)
1. No finger inserted
2. An indicator of signal inadequacy
IP22
Ingress of liquids rank
Display Mode
Display Format
LED display
The Pulse Oxygen
Digital
Pulse Intensity (bar-graph)
Digital bar-graph display
SpO2 Parameter Specification
Measuring range
35%-100% (the resolution is 1%).
Accuracy
70%-100%;±2%, Below 70% unspecified.
Pulse Parameter Specification
Measuring range
25bpm-250bpm (the resolution is 1 bpm)
Accuracy
±2bpm
Pulse Intensity
Range
Continuous bar-graph display, the higher
Alert conditions
SpO2
Less than 94%
PR
Less than 50bpm or more than 130bpm
Battery Requirement
2 X 1.5V (AAA size) alkaline battery
Power Consumption
Smaller than 35 mA.
Battery Useful Life
Two batteries can work continually for 24 hours
Power off
The oximeter will switch off if there is no activity for 16 seconds.
Optical Sensor
Red light (wavelength is 660nm)
Dimensions and Weight
Dimensions
62 (L)X37 (W)X32(H) mm
Weight
About 50g (with the batteries)
Guidance and manufacture’s declaration – electromagnetic emission
The JPD-500E is intended for use in the electromagnetic environment
Emission
test
Compliance
Electromagnetic environment –
guidance
The JPD-500E uses RF energy only for its
equipment.
The JPD-500E is suitable for use in all
for domestic purposes.
Display Information
Symbol
Figure 4. Attaching the Lanyard
6 Operating Guide
6.1 Insert the two batteries properly to the direction, and the
cover.
6.2 Open the clip as shown in Figure 5.
n replace the
off suddenly
9 Key of Symbols
Type BF applied part
Warning, see User manual.
automatically when there is
no signal within 16 seconds.
2. The batteries are almost
2. Change batteries.
Infrared (wavelength is 905nm)
11 Appendix: Electromagnetism Compatibility
Guidance and manufacture’s declaration – electromagnetic
emissions-for all EQUIPMENT and SYSTEMS
Figure 5. Put finger in position
6.3 Place finger into the clip with the fingertip over the sensor, and then clip
the finger.
6.4 Press the button once on the front panel.
6.5 Do not shake the finger and remain still during the process.
6.6 Read the information on the screen display.
6.7 In boot-strap state press the button and the device will reset.
Fingernails and the luminescent tube should be on the same side.
7 Repairing and Maintenance & cleaning and disinfection
■ Change the batteries when the low-voltage displays on the screen.
■ Clean the surface of the device with a medical alcoholic wipe before
using. Allow it to air dry in air or dry with a clean fabric first.
■ Use a medical alcoholic wipe to disinfect the product after use to
prevent cross infection in future use.
■ Please take out the batteries if the oximeter is not in use for a long time.
W
arning: High-pressure sterilization cannot be used on the de
Warning: Do not immerse the device in liquid.
Warning: It is recommended that the device should be kept in a dry
environment. Humidity may reduce the device’s lifespan, or even damage it.
8 Troubleshooting
Trouble Possible Reason Solution
The SpO2 and
Pulse Rate
can not be
displayed
normally
The SpO2 and
Pulse Rate
are not
displayed
stably
The device
can not be
turned on
1. The finger is not properly
positioned.
2. The patient’s SpO2 is too
low to be detected.
1. The finger is not placed
inside deep enough.
2. The finger is shaking or
the patient is moving.
1. Low battery or no battery.
2. The batteries are not
inserted properly.
3. The malfunction of the
device.
1. Place the finger
properly and try again.
2. Try again; Go to a
hospital for a diagnosis
if you are sure the
device works all right.
1. Place the finger
properly and try again.
2. Let the patient keep
calm
1. Change batteries.
2. Reinstall batteries.
3. Please contact the
local service center.
vice.
When end users abandon this product, they must send the
product to the collection place for recycling.
10 Technical Specification
Saturation (SpO2)
Pulse Rate (PR) Digital
display indicates the stronger pulse.
specified below. The customer of the user of the JPD-500E should assure
that it is used in such and environment.
RF
emissions
CISPR 11
RF
emission
CISPR 11
Warranty
Your product is warranted to be free of defects in materials and workmanship
for one year from the original purchase date. The device was built to
exacting standards and carefully inspected prior to shipment. In the event of
a defect covered by this warranty there is the option to repair or replace the
device. This warranty does not cover device failure due to owner misuse or
negligence, or normal wear and tear. If you have questions about your
device or the warranty, ple
Group 1
Class B
internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
establishments, including domestic
establishments and those directly
connected to the public low-voltage power
supply network that supplies buildings used
ase contact the local distributor.
0482
WellKang Ltd
Suite B, 29 Harley Street, London W1G 9QR, UK
Shenzhen Jumper Medical Equipment Co., Ltd
Address: Building D, No. 71, Xintian Road, Fuyong Street,
Baoan, Shenzhen, Guangdong, P. R. China, 518103
KINETIK JPD-500E UK IB 20181231
 Loading...
Loading...