Page 1

KERN & Sohn GmbH
Ziegelei 1
D-72336 Balingen
E-Mail: info@kern-sohn.com
Tel: +49-[0]7433- 9933-0
Fax: +49-[0]7433-9933-149
Internet: www.kern-sohn.com
Operating instructions
Step-On Personal scale
KERN MPD_M
Version 1.1
10/2012
GB
MPD_M-BA-e-1211
Page 2

2 MPD_M-BA-e-1211
GB
KERN MPD 250K100M
Version 1.1 10/2012
Operating instructions
Step-On Personal scale
Contents
1 Technical data ............................................................................................ 3
2 Declaration of conformity .......................................................................... 4
2.1 Explanation of the graphic symbols ........................................................................ 4
3 Appliance overview .................................................................................... 6
3.1 Overview of display ................................................................................................ 6
4 Basic Information (General ) ...................................................................... 7
4.1 Specific function ..................................................................................................... 7
4.2 Proper use ............................................................................................................. 7
4.3 Improper Use ......................................................................................................... 8
4.4 Warranty ................................................................................................................ 8
4.5 Monitoring of Test Resources ................................................................................ 8
5 Basic Safety Precauti on s .......................................................................... 9
5.1 Pay attention to the instructions in the Operation Manual ....................................... 9
5.2 Personnel training .................................................................................................. 9
5.3 Preventing contamination ....................................................................................... 9
6 Transport and storage ............................................................................. 15
6.1 Testing upon acceptance ......................................................................................15
6.2 Packaging / return transport ..................................................................................15
7 Unpacking, Setup and Commissioning .................................................. 16
7.1 Installation Site, Location of Use ...........................................................................16
7.2 Unpacking .............................................................................................................16
7.3 Scope of delivery ..................................................................................................16
7.4 Placing ..................................................................................................................17
7.5 Mains connection ..................................................................................................17
7.6 Rechargeable battery operation (optional) ............................................................18
7.7 Initial Commissioning ............................................................................................18
8 Operation .................................................................................................. 19
9 Error messages ........................................................................................ 19
10 Service, maintenance, disposal .............................................................. 20
10.1 Cleaning ...............................................................................................................20
10.2 Cleaning / disinfecting ...........................................................................................20
10.3 Service, maintenance ...........................................................................................20
10.4 Disposal ................................................................................................................20
11 Instant help ............................................................................................... 21
12 Verification ................................................................................................ 22
12.1 Verification validity period (current status in G) .....................................................23
13 Adjustment ............................................................................................... 24
Page 3

MPD_M-BA-e-1211 3
1 Technical data
KERN MPD 250K100M
Display 6-digit
Weighing range (max) 250 kg
Minimum load (Min) 2 kg
Verification value (e) 100 g
Reproducibility 0.1 kg
Linearity ± 0.1 kg
Display LCD with 25mm high digits
Recommended adjustment
weight, (Class)
200 kg
(M1)
Stabilization time (typical) 2 sec.
Warm-up time 10 min
Operating temperature 0° C …. + 40° C
Humidity of air max. 80 % (not condensing)
Electric Supply Input voltage 220V-240V AC, 50 Hz
Balance
(W x D x H) mm
365 x 490 x 120
Weighing plate mm 365 x 360 x 80
Weight kg (net) 10
Verified in accordance with
90/384/EEC
Medical grade III
Medical product in
accordance with 93/42/EEC
Category I with measuring function
Rechargeable battery
operation
optional
Page 4

4 MPD_M-BA-e-1211
2 Declaration of conformity
Declaration of conformity: see separate document showing serial number of device
CE marking:
0297
93/42/EEC
2009 / 23 / EG
Non-automatic Weighing Instruments Directive
2.1 Explanation of the graphic symbols
This EC verification mark indicates that these scales are in
conformity with EU Directive 2009/23/EG for Non-Automatic
Weighing Instruments. Weighing instruments bearing this m ar k
are approved for medical purposes within the European Union.
WF 130012
Designation of the serial number of every device, applied at the
device and on the packaging
Number here as example
2012-10
Identification of the manufacturing date of the medical product.
Year and month here as example
“Please note the accompanying documents“
or “Please note operating instructions”
“Please note operating instructions”
“Please note operating instructions”
M
year
0103
Page 5

MPD_M-BA-e-1211 5
Kern & Sohn GmbH
D–72336 Baligen,German y
www.kern-sohn.com
Identification of manufacturer of medical product including
address
“Electro-medical appliance“
with attachment for type B
Device protection category II
Dispose of old appliances separately from your household
waste!
Instead, take them to communal collection points.
Temperature limit indicating the upper and the lower limit
(storage temperature on packaging)
(Temperature serving as an example)
12 V DC / 500 mA
Display of supply voltage for scales with polarity display.
+70°C
-30°C
Page 6

6 MPD_M-BA-e-1211
3 Appliance overview
1 Display unit
2 Weighing platform
(anti-slip surface)
3 Foot switch
Underside
4 Mains port
5 Rechargeable battery
compartment
6 Rubber feet
(adjustable height)
7 Transportation Securing
3.1 Overview of display
Display Description Description
STABLE
Stability display Scales are in a steady state
ZERO
Zeroing display Weighing scale shows „0.0“.
GROSS
Gross weight display Illuminated when gross weight is displayed
Page 7

MPD_M-BA-e-1211 7
4 Basic Information (General)
Weighing instruments have to be verified for the purposes stated
below in accordance with Directive 2009/23/EC.
Article 1,
paragraph 4. “Determination of mass in the practice of medicine
that is, weighing patients
for reasons of medical supervision
during medical surveillance, examination and treatment.”
4.1 Specific function
Indication
Determining the body weight in the medical practice area.
Use as „non-standalone weighing scale“, that is, a person
steps carefully onto the weighing platform‘s centre. Once a
steady display value is shown, you can read the weight value.
Contra-
indication
No contraindication known
4.2 Proper use
This weighing scale is designed for determining the weight of a person whilst
standing, such as in doctor’s surgeries. The balance is suitable for recognising,
preventing and controlling illnesses.
Scales fitted with a serial interface may only be connected to
appliances in compliance with Directive EN60601-1.
On personal weighing scales, the person should step onto the centre of the weighing
platform and remain standing without moving.
As soon as a stable weighing value is reached the weighing value can be read.
The weighing scale is designed for continuous duty.
The weighing platform may only be stepped on by persons
capable of standing on both feet on the weighing platform.
The weighing platforms are fitted with an anti-slip surface that must not be covered
during weighing a person.
The balance should be checked for correct condition prior to each utilisation by a
person familiar with proper operation of the balance.
Page 8

8 MPD_M-BA-e-1211
4.3 Improper Use
Do not use these scales for dynamic weighing processes.
Do not leave permanent load on the weighing pan. This may damage the measuring
system.
Impacts and overloading exceeding the stated maximum load (max) of the weighing
plate, minus a possibly existing tare load, must be strictly avoided. This could cause
damage to the balance.
Never operate balance in explosive environment. The serial version is not explosion
protected. It should be noted that a flammable mixture of anaesthetics and oxygen or
laughing gas may occur.
The structure of the balance may not be modified. This may lead to incorrect
weighing results, safety-related faul ts and dest ruc ti on o f the bala nce.
The balance may only be used according to the described conditions. Other areas of
use must be released by KERN in writing.
4.4 Warranty
Warranty claims shall be voided in case
• Our conditions in the operation manual are ignored
• The appliance is used outside the described uses
• The appliance is modified or opened
• mechanical damage and damage caused by media, liquids,
• natural wear and tear
• The appliance is improperly set up or incorrectly electrically connected
• The measuring system is overloaded
• Dropping the balance
4.5 Monitoring of Test Resources
In the framework of quality assurance the measuring-related weighing properties of
the balance and, if applicable, the testing weight, must be checked regularly. The
responsible user must define a suitable interval as well as type and scope of this test.
Information is available on KERN’s home page (www.kern-sohn.com with regard to
the monitoring of balance test substances and the test weights required for this. In
KERN’s accredited DKD calibration laboratory test weights and balances may be
calibrated (return to the national standard) fast and at moderate cost.
Page 9
MPD_M-BA-e-1211 9
5 Basic Safety Precautions
5.1 Pay attention to the instructions in the Operation Manual
Carefully read this operati on ma nual be for e
setup and commissioning, even if you are
already familiar with KERN balances.
All language versions contain a non-binding
translation. The original German is binding.
5.2 Personnel training
The medical staff must apply and follow the operating instructions for proper use and
care of the product.
5.3 Preventing contamination
The prevention of cross-contamination (fungal skin infections,……) requires regular
cleaning of the weighing platform. Recommendation: after a weighing procedure that
could potentially result in contamination (e. g. after weighing that involves direct skin
contact).
Page 10

10 MPD_M-BA-e-1211
6 Electromagnetic compatibility (EMC)
6.1 General hints
The installation and use of this electrical medical device requires
special precautionary measures as outlined in the EMC information
below.
This device complies with the limits set for medical electrical devices of group 1,
class B (as per EN 60601-1-2).
Electromagnetic compatibility (EMC) describes a device’s ability to perform reliably
within an electromagnetic environment without causing inadmissible electromagnetic
interference at the same time. Amongst other things, such disturbances may be
emitted by connecting cables or the air.
Inadmissible disturbances from the environment may result in incorrect displays,
inaccurate measured values or incorrect behaviour of the medical device. By the
same token the medical device may in some cases cause such disturbances in other
devices. To eliminate problems of that kind, we recommend you to take one or
several of the measures listed below:
• Change the alignment or distance of the device to the source of EMI.
• Install or use the personal scale MPD-M at a different location.
• Connect the personal scale MPD-M to a different power source.
• For further questions please contact our customer services.
Disturbances may be caused by improper modification or add-ons to the device or
not recommended accessories (such as power units or connecting cables). The
manufacturer will not be responsible for these. Modifications may also result in a loss
of authorisation relating to the use of the device.
Devices emitting high frequency signals (mobile telephones, radio
transmitters, radio receivers) may cause interference in the medical
device. For that reason do not use the m near the me di c al device.
Chapter 6.4 contains details about recommended minimum dist anc e s .
Page 11
MPD_M-BA-e-1211 11
6.2 Electromagnetic interferences
Guidelines and manufacturer’s declaration – electromagnetic interferences
The personal scale MPD-M is designed for use in an electromagnetic environment that meets the
requirements stated below. The customer or user of the medical electrical device must ensure that
operation takes place in such an environment.
Emitted interference
measurements
Conformity
Electromagnetic environment guideline
HF emissions
as per CISPR 11 / EN 55011
Group 1
The personal scale MPD-M uses HF
energy merely for its internal working.
Its HF emission therefore is very low
and it is highly unlike to interfere with
adjacent electronic devices.
HF emissions
as per CISPR 11 / EN 55011
Class B
The personal scale MPD-M is
designed for use in all equipment
including those in living areas and
those connected directly to the public
power grid that also supplies buildings
used for living purposes.
Emission of harmonics
as per IEC 61000-3-2
Class A
Emission of voltage fluctuations /
flicker
as per IEC 61000-3-3
Conforms with
Do not put the personal scale MPD-M directly next to other devices and do not stack
it with other devices. If this type of operation is necessary, observe the personal
scale MPD-M to ensure normal operation in such an arrangement.
Page 12

12 MPD_M-BA-e-1211
6.3 Electromagnetic noise immunity
Guidelines and manufacturer’s declaration - electromagnetic noise immunity
The personal scale MPD-M is designed for use in an electromagnetic environment that meets the
requirements stated below. The customer or user of the medical electrical device must ensure that
operation takes place in such an environment.
Noise immunity tests
IEC 60601 test level
Conformity
Electromagnetic
environment - guideline
Discharge static
electricity (DSE)
as per IEC 61000-4-2
± 6 kV contact discharge
± 8 kV air discharge
± 6 kV
± 8 kV
Floors should be made of
wood or concrete or tiled with
ceramic tiles. If floors are
covered with synthetic
material, relative air humidity
must be at least 30%.
Fast transient electrical
disturbances / bursts
as per IEC 61000-4-4
± 2 kV for power lines
+ 1 kV for input and
output lines
± 2 kV
+ 1 kV
The quality of the supply
voltage should match that of
the typical business or hospital
environment.
Impulse voltages /
surges
as per IEC 61000-4-5
± 1 kV voltage
Live wire - live wire
± 2 kV voltage
Live wire - earth
± 1 kV
Inapplicable
The quality of the supply
voltage should match that of
the typical business or hospital
environment.
Voltage dips, short-term
disruptions and
fluctuations in supply
voltage
as per IEC 61000-4-11
< 5 %
(> 95 % dip of )
for ½ period
40 %
(> 60 % dip of )
for 5 periods
70 %
(> 30 % dip of )
for 25 periods
< 5 %
(> 95 % dip of )
for 5 s
Compliance
with
requirements
under all
postulated
conditions
Controlled
switch off
Return to
undisturbed
situation after
user
intervention.
The quality of the supply
voltage should match that of
the typical business or hospital
environment. Where the user
of the personal scale MPD-M
demands continuous operation
even during disruptions to the
power supply, we recommend
powering the personal scale
MPD-M by no-break power
supply or battery.
Magnetic field for supply
frequency
(50/60 Hz)
as per IEC 61000-4-8
3 A/m
3 A/m
50/60 Hz
Magnetic fields for the supply
frequency should match the
typical values found in the
particular business or hospital
environment.
NOTE equals AC line voltage prior to application of test level.
Page 13

MPD_M-BA-e-1211 13
Guidelines and manufacturer’s declaration - electromagnetic noise immunity
The personal scale MPD-M is designed for use in an electromagnetic environment that meets the
requirements stated below. The customer or user of the medical electrical device must ensure that
operation takes place in such an environment.
Noise immunity tests
IEC 60601 test
level
Conformity
Electromagnetic environment guideline
Conducted HF
disturbance variables
as per IEC 61000-4-6
3
150 kHz to 80 MHz
3 V
Do not use portable or mobile radio
sets nearer to the personal scale MPDM or its wires than the distance
recommended as safety distance
which is calculated according to the
equation relevant for its transmission
frequency.
Recommended safety distance:
for 80 MHz to 800 MHz
for 800 MHz to 2.5 GHz
Use P as rated capacity of radio
transmitter in Watt (W) as per details
given by the radio transmitter
manufacturer and d as recommended
safety distance in metres (m).
The field intensity of stationary radio
transmitters should for all frequencies
be lower according to an in situ
a
examination than the conformity level.
b
Interference may occur near devices
bearing the symbol below.
Emitted HF disturbance
variables
as per IEC 61000-4-3
3
80 MHz to 2.5 GHz
3 V/m
NOTE 1 Higher frequency range applies to 80 MHz and 800 MHz.
NOTE 2 These guidelines may not be applicable in all cases.
The spread of electromagnetic variables is influenced by absorption and reflections in
buildings, objects and humans.
a
The field intensity of stationary radio transmitters such as base stations of wireless telephones and
mobile radio sets, amateur radio stations, AM and FM radio and television stations cannot be reliably
predicted in advance. To determine the electromagnetic environment in respect of stationary
transmitters, you should consider a study of electromagnetic phenomena at the location. If the
measured field intensity at the location where the personal scale MPD-M is to be used exceeds the
conformity level above, you should observe the personal scale MPD-M in order to ensure normal
operation. If you observe unusual features of performance you may have to take additional measures
such as a change in alignment or a different location for the personal scale MPD-M.
b
For a frequency range of 150 kHz to 80 MHz field intensity should be less than 3 V/m.
Page 14

14 MPD_M-BA-e-1211
6.3.1 Crucial features of performance
Note:
The personal scale MPD-M does not have any crucial features of
performance as per IEC 60601-1. The system may be subject to
interference by other devices even if these devices conform to current
emission requirements as per CISPR.
6.4 Minimum distances
Recommended safety distances between portable and mobile HF
telecommunication devices and the medical device
The medical device is designed for use in an electromagnetic environment in which HF disturbance
variables are controlled. The customer or user of the medical electrical device can help avoiding
electromagnetic disturbances by keeping the minimum distance between portable and mobile HF
telecommunication devices (transmitters) and the medical device – depending on the output
performance of the communication device, as stated below.
Rated capacity of
transmitter
W
The safety distance depends on the tra n smission frequency
m
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2.5 GHz
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.20
1.20
2.30
10
3.80
3.80
7.30
100
12.00
12.00
23.00
For transmitters with a maximum rated capacity not stated in the table above you can calculate the
recommended safety distance in metres (m) yourself by using the equation belonging to each column,
whereby P equals the maximum rated capacity of the transmitter in Watt (W) as per details provided
by the transmitter manufacturer.
NOTE 1 Higher frequency range applies to 80 MHz and 800 MHz.
NOTE 2 These guidelines may not be applicable in all cases.
The spread of electromagnetic variables is influenced by absorption and reflections in
buildings, objects and humans.
Page 15
MPD_M-BA-e-1211 15
7 T ransport and storage
7.1 Testing upon acceptance
When receiving the appliance, please check packaging immediately, and the
appliance itself when unpacking for possible visible damage.
7.2 Packaging / return transport
Keep all parts of the original packaging for a possibly required
return.
Only use original packaging for returning.
Prior to dispatch disconnect all cables and remove loose/mobile
parts.
Reattach possibly supplied transport securing devices.
Secure all parts such as the weighing platform, power unit etc.
against shifting and damage.
Page 16
16 MPD_M-BA-e-1211
8 Unpacking, Setup and Commis si oning
8.1 Installation Site, Location of Use
The balances are designed in a way that reliable weighing results are achieved in
common conditions of use.
You will work accurately and fast, if you select the right location for your balance.
On the installation site observe the following:
• Place scales on a stable, even surface;
• Avoid extreme heat as well as temperature fluctuation caused by installing
next to a radiator or in the direct sunlight;
• Protect the balance against direct draughts due to open windows and doors;
• Avoid jarring during weighing;
• Protect the balance against high humidity, vapours and dust;
• Do not expose the device to extreme dampness for longer periods of time.
Non-permitted condensation (co nd ens a ti on o f air humidi ty on the appliance)
may occur if a cold appliance is taken to a considerably warmer environment.
In this case, acclimatize the disconnected appliance for ca. 2 hours at room
temperature.
• Avoid static charge of the balance and of the person to be weighed.
• Avoid contact with water.
Major display deviations (incorrect weighing results) may be experienced should
electromagnetic fields (e.g. due to mobile phones or radio equipment), static
electricity accumulations or instable power supply occur. Change location or remove
source of interference.
8.2 Unpacking
Remove the individual components of the balance or the complete balance from the
packaging with care and install at the intended location. When using the pow er pack,
ensure that the power cable does not produce a risk of stumbling.
8.3 Scope of delivery
Serial accessories:
• Balance
• Power pack unit (EN 60601-1 attestation of conformity)
• Operating instructions
Page 17
MPD_M-BA-e-1211 17
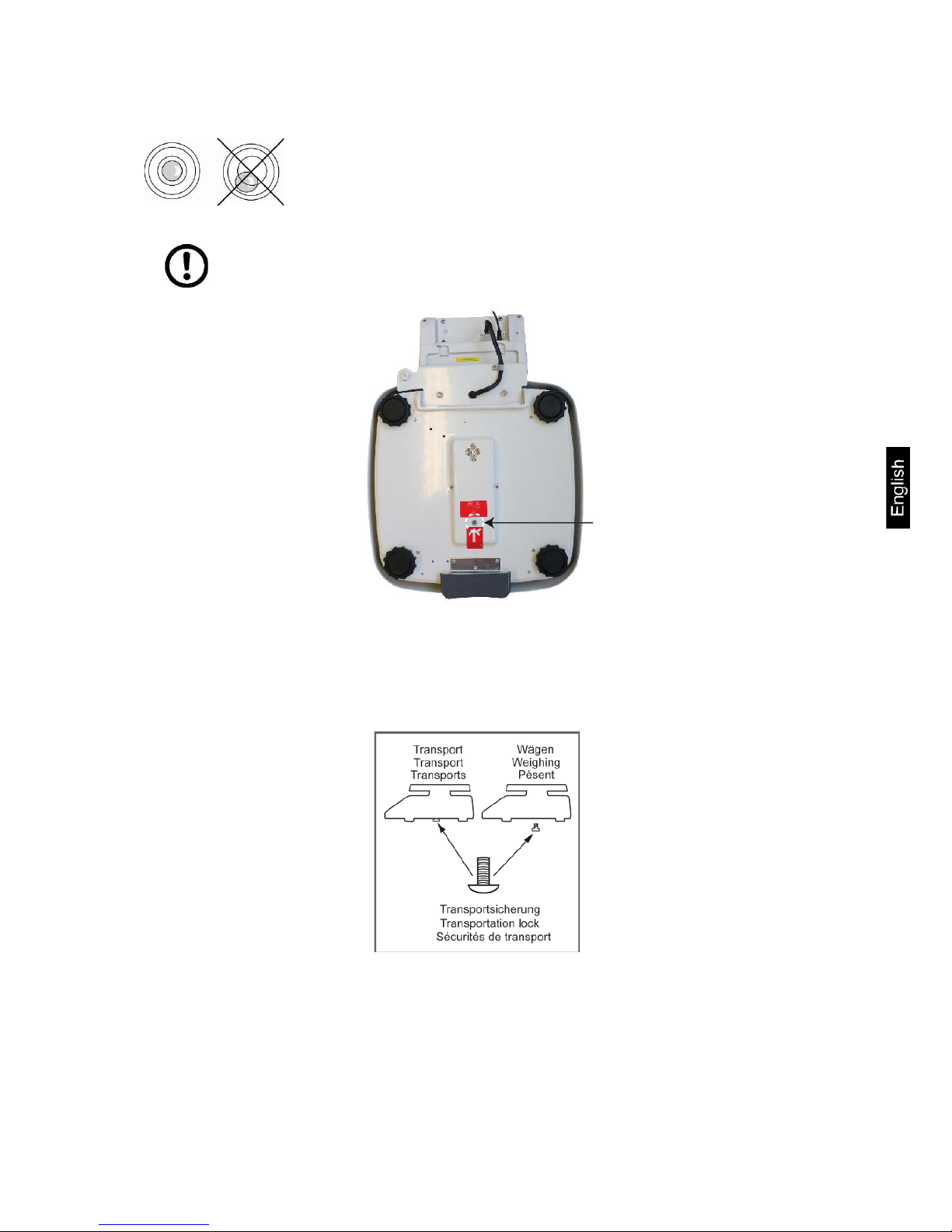
8.4 Placing
Level balance with foot screws until the air bubble of the
water balance is in the prescribed circle.
Check levelling regularly.
Make sure that all transport locking devices are removed
To loosen the transport guard screw out transport screw [1] anticlockwise.
For transportation carefully screw-in transport screw clockwise till to the stopper
and then fix it using locknut.
8.5 Mains connection
Power is supplied by the external power unit which also serves to isolate the mains
supply from the scale. The stated voltage value must be the same as the local
voltage.
Always use genuine approved KERN power pack units as per EN 60601-1 directive.
Page 18

18 MPD_M-BA-e-1211
The small sticker attached to the side of the display unit indicates the power port:
The LED remains illuminated as long as the weighing scale remains connected to the
mains. The LED display informs you during loading about the loading status of the
rechargeable battery.
green: Rechargeable battery completely reloaded
blue: Charging storage battery
8.6 Rechargeable battery operation (optional)
Open battery compartment cover (1) at the base of the display unit and insert battery.
Charge the battery for at least 12 hours before initial use.
The appearance of the symbol in the weight display indicates that the battery
is almost exhausted. The weighing scale will remain ready for operation for a few
more minutes before switching off in order to save battery. Load rechargeable
battery.
Voltage has dropped below prescribed minimum.
Rechargeable battery very low.
Rechargeable battery completely reloaded
If the balance is not used for a longer time, take out the rechargeable battery and
store it separately. Leaking liquid could damage the balance.
8.7 Initial Commissioning
In order to obtain exact results with the electronic balances, your bala nce must have
reached the operating temperature (see warming up time chap. 1). During this
warming up time the balance must be connected to the power supply (mains,
accumulator or battery) and be switched on.
The accuracy of the balance depends on the local acceleration of gravity.
The value of gravity acceleration is shown on the type plate.
Page 19

MPD_M-BA-e-1211 19
9 Operation
Turn on weighing scale by foot switch (chap. 3).
The balance will carry out a self-test
The scales are ready for operation as soon as the weight
display for “0.0 kg“ has appeared.
Have person stand in the centre of the scales. Wait unt il the
standstill display „STABLE“ appears, then read the weighing
result.
10 Error messages
Display
Description
OL or------ Weighing range has been exceeded (excess load)
------or Null Below weighing range (underload)
Zero range exceeded
(on start-up or when pressing the key)
• Load on weighing pan
• Excess load, during zero setting of weighing scale
• Incorrect adjusting process
• Fault on load cell
Value outside the A/D converter range
• Damaged weighing cell
• Damaged electronics
Should other error messages occur, switch balance off and then on again. If the error
message remains inform manufacturer.
Page 20
20 MPD_M-BA-e-1211
11 Service, maintenance, disposal
11.1 Cleaning
Before any maintenance, cleaning and repair work disconnect the
appliance from the oper ati ng vol tage.
11.2 Cleaning / disinfecting
Clean weighing platform (such as seat) as well as casing with household detergents
or commercially available disinfectants. Please follow manufacturer’s instructions.
Do not use abrasive or aggressive cleaners such as spirits or alcohol or similar as
they might damage the high-quality surface.
The prevention of cross-contamination (fungal skin infections,……) requires regular
cleaning of the weighing platform. Recommendation: after a weighing procedure that
could potentially result in contamination (e. g. after weighing that involves direct skin
contact).
Do not spray disinfectants onto appliance.
Make sure that disinfectant does not penetrate the interior of
the appliance.
Remove dirt immediately.
11.3 Service, maintenance
The appliance may only be opened by trained service technicians who are authorized
by KERN.
Disconnect the scales before openi ng .
11.4 Disposal
Disposal of packaging and appliance must be carried out by operator according to
valid national or regional law of the location where the appliance is used.
Page 21
MPD_M-BA-e-1211 21
12 Instant help
In case of an error in the program process, briefly turn off the balance and disconnect
from power supply. The weighing process must then be restarted from the beginning.
Fault Possible cause
The displayed weight does
not glow.
• The balance is not switched on.
•
The mains supply connection has been interrupted
(mains cable not plugged in/faulty).
• Power supply interrupted.
• Rechargeable battery inserted incorrectly or empty
• No rechargeable battery inserted
The displayed weight is
permanently changing
• Draught/air movement
• Table/floor vibrations
• The weighing plate is in contact with foreign
bodies or is not correctly positioned.
• Electromagnetic fields / static charging (choose
different location/switch off interfering device if
possible)
The weighing result is
obviously incorrect
• The display of the balance is not at zero
• Adjustment is no longer correct.
• Great fluctuations in temperature.
• Warm-up time was ignored.
• Electromagnetic fields / static charging (choose
different location/switch off interfering device if
possible)
Should other error messages occur, switch balance off and then on again. If the error
message remains inform manufacturer.
Page 22
22 MPD_M-BA-e-1211
13 Verification
General introduction:
According to EU directive 2009/23/EC balances must be officially verified if they are
used as follows (legally controlled area):
a) For commercial transactions if the price of goods is determined by weighing.
b) For the production of medicines in pharmacies as well as for analyses in the
medical and pharmaceutical laboratory.
c) For official purposes
d) For manufacturing final packages
In cases of doubt, please contact your local trade in standard.
Verification notes:
An EU type approval exists for balances described in their technical data as
verifiable. If a balance is used where obligation to verify exists as described above, it
must be verified and re-verified at regular intervals.
Re-verification of a balance is carried out according to the respective national
regulations. For verification validity period, s. chap. 11.1.
The legal regulation of the country where the balance is used must be observed!
Verification of the balance is invalid without the seal.
The seal marks attached on balances with type approval point out that the
balance may only be opened and serviced by trained and authorised
specialist staff. If the seal mark is destroyed, verification looses its validity.
Please observe all national laws and legal regulations. In Germany a
re-verification will be necessary.
Balances with obligation to verify must be taken out of operation if:
•
The weighing result of the balance is outside the error limit. Therefore, in
regular intervals load balance with known test weight (ca. 1/3 of the max.
load) and compare with displayed value.
•
The reverification deadline has been exceeded.
Page 23
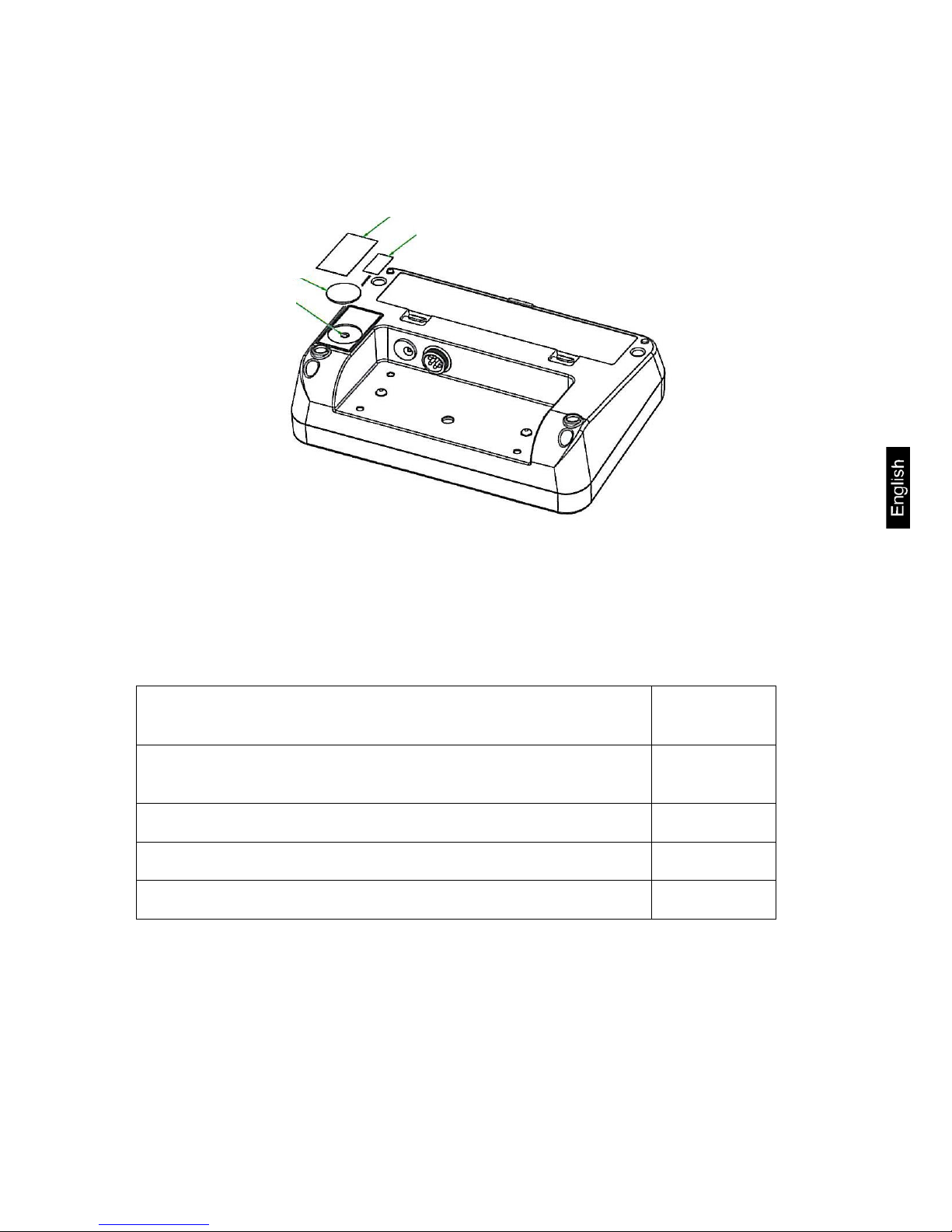
MPD_M-BA-e-1211 23
Position adjustment switch and seals:
1. Self-destroying seal mark
2. Cover
3. Adjustment switch
13.1 Verification validity period (current status 2012 in G)
Personal scales (including chair and wheelchair scales) in
hospitals
4 year
Personal scales, when not located in hospitals (for example,
doctor's offices and nurs ing homes)
unlimited
Baby weighing scales and mechanical birth weight scales 4 year
Bed scales 2 year
Scales in dialysis stations unlimited
Rehab clinics and health authorities are treated as hospitals.
(4 years of verification validity)
Not treated as hospitals (verification validity not limited) are dialysis stations, nursing
homes and doctor’s surgeries.
(Details derived from: „Information by the verification authority, weighing scales
applied in medical use“)
1 1 2
3
Page 24
24 MPD_M-BA-e-1211
14 Adjustment
As the acceleration value due to gravity is not the same at every location on earth,
each display unit with connected weighing plate must be coordinated - in compliance
with the underlying physical weighing principle - to the existing acceleration due to
gravity at its place of location (only if the weighing system has not already been
adjusted to the location in the factory). This adjustment process must be carried out
for the first commissioning, after each change of location as well as in case of
fluctuating environment temperature. To receive accurate measuring values it is also
recommended to adjust the display unit periodically in weighing operation.
• Prepare the required adjustment weight. The adjustment weight to be
applied depends on the capacity of a weighing scale, see chap. 1.
Carry out adjustment as closely as possible to admissible maximum
load of weighing scale. Information abo ut test weights you will find in
the internet under http://www.kern-sohn.com
• Observe stable environmental conditions. For warm-up time required
for stabilisation see chap. 1.
The adjustment function is locked for verified balances.
To disable the access lock, destroy the seal and actuate the adjustment
switch. Position of the adjustment switch see chap. 11.
Attention:
After destruction of the seal the weighing system must be re-veri fied by an
authorised agency and a new verification wire/seal mark fitted before it can
be reused for applications subject to verification.
Page 25
MPD_M-BA-e-1211 25
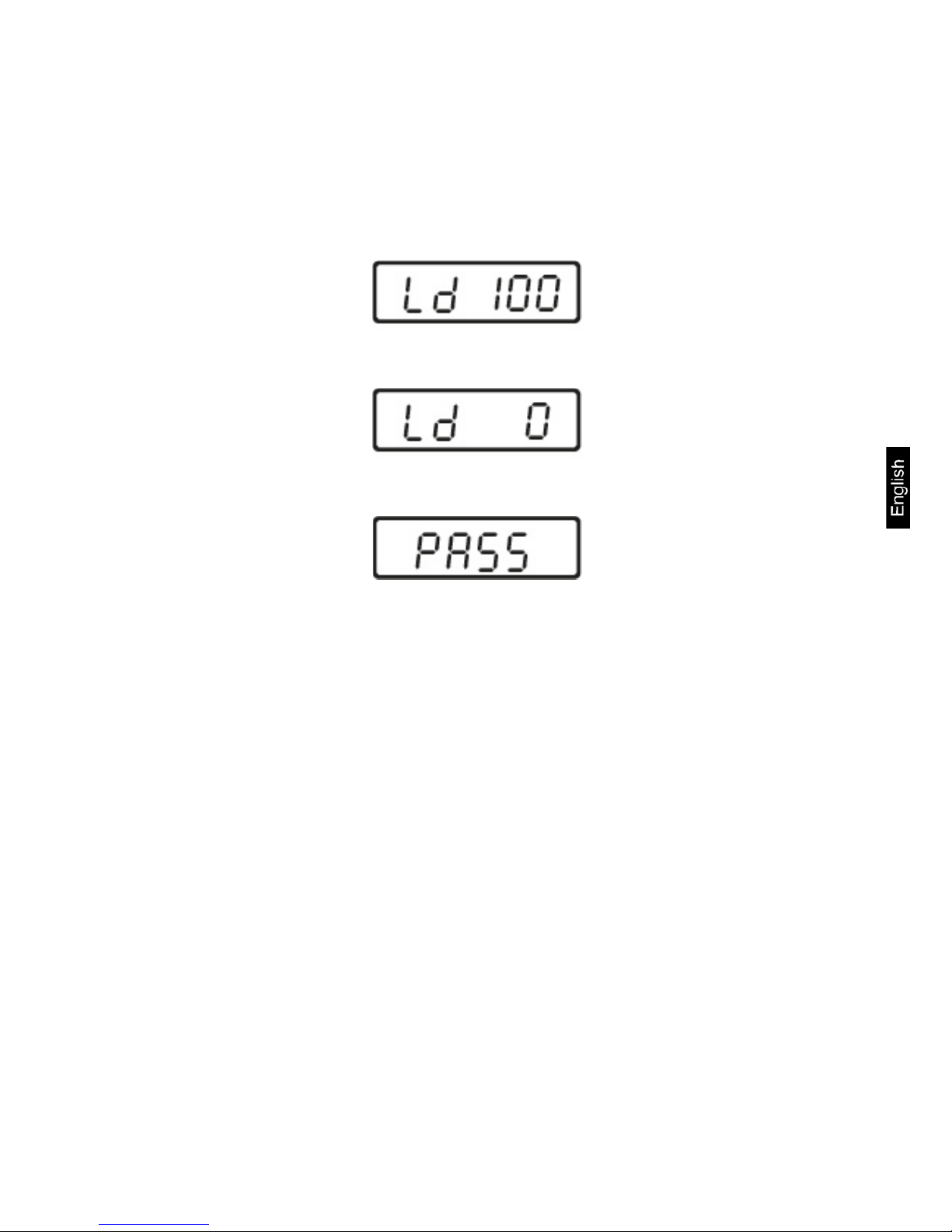
Procedure:
Switch off the balance.
Press adjustment switch and turn on weighing scale by operating foot switch
(chap. 3)..
Wait until the size of the adjustment weight (see chap. 1) required is di spl ayed.
Place adjustment weight in the centre of the weighing platform. Wait unti l „Ld 0“
is shown.
Take away adjustment weight. Ensure that there are no other objects on the
weighing pan.
Wait for a few seconds until „PASS“ is displayed.
After successful adjustment the balance automatically returns to weighing
mode.
 Loading...
Loading...