Page 1

Instructions for use
ARCUSevo
Always be on the safe side.
Page 2

Sales:
KaVo Dental GmbH
Bismarckring 39
D-88400 Biberach
Tel. +49 7351 56-0
Fax +49 7351 56-1488
Manufacturer:
Kaltenbach & Voigt GmbH
Bismarckring 39
D-88400 Biberach
www.kavo.com
Page 3

Instructions for use ARCUSevo
Contents
Contents
Contents ...........................................................................................................................................................
1
1 User instructions ............................................................................................................................................ 2
1.1 User guide ...............................................................................................................................................2
1.1.1 Abbreviations .................................................................................................................................. 2
1.1.2 Symbols .......................................................................................................................................... 2
1.1.3 Target group ...................................................................................................................................2
1.2 Service ....................................................................................................................................................3
1.3 Warranty provisions ................................................................................................................................. 4
1.4 Transportation and storage .....................................................................................................................5
1.4.1 Packaging ordinance of August 28,1998 ........................................................................................ 5
1.4.2 Transportation damage ..................................................................................................................5
1.4.3 Storage ...........................................................................................................................................7
2 Safety ............................................................................................................................................................8
2.1 Description of safety instructions ............................................................................................................. 8
2.1.1 Warning symbol .............................................................................................................................. 8
2.1.2 Structure .........................................................................................................................................8
2.1.3 Description of hazardous steps ......................................................................................................8
2.2 Proper use ...............................................................................................................................................9
2.2.1 General information ........................................................................................................................9
2.2.2 Product-specific ............................................................................................................................10
2.3 Safety ....................................................................................................................................................11
2.3.1 General information ......................................................................................................................11
3 Product description ...................................................................................................................................... 12
3.1 ARCUSevo ............................................................................................................................................12
3.2 Technical data .......................................................................................................................................13
3.3 Scope of delivery ...................................................................................................................................14
4 Operation ..................................................................................................................................................... 15
4.1 Adapting the facial bow .........................................................................................................................15
4.2 Mount the facial bow .............................................................................................................................16
4.3 Removing the facial bow .......................................................................................................................18
5 Preparation methods DIN EN ISO 17664 .................................................................................................... 19
5.1 Cleaning ................................................................................................................................................19
5.1.1 Manual cleaning ...........................................................................................................................19
5.1.2 Machine cleaning .........................................................................................................................19
5.2 Disinfection ............................................................................................................................................20
5.2.1 Manual disinfection ....................................................................................................................... 20
5.2.2 Automated disinfection .................................................................................................................20
5.3 Sterilisation ............................................................................................................................................21
6 Accessories .................................................................................................................................................22
7 Spare parts sheet ........................................................................................................................................23
1/24
Page 4
Instructions for use ARCUSevo
1 User instructions | 1.1 User guide
1 User instructions
1.1 User guide
Requirement
Read these instructions before the initial startup to prevent misuse and damage.
1.1.1 Abbreviations
Short
form
Explanation
GA Instructions for use
PA Care instructions
MA Assembly instructions
TA Technician's instructions
STK Safety check
IEC International Electrotechnical Commission
RA Repair instructions
EMC Electromagnetic compatibility
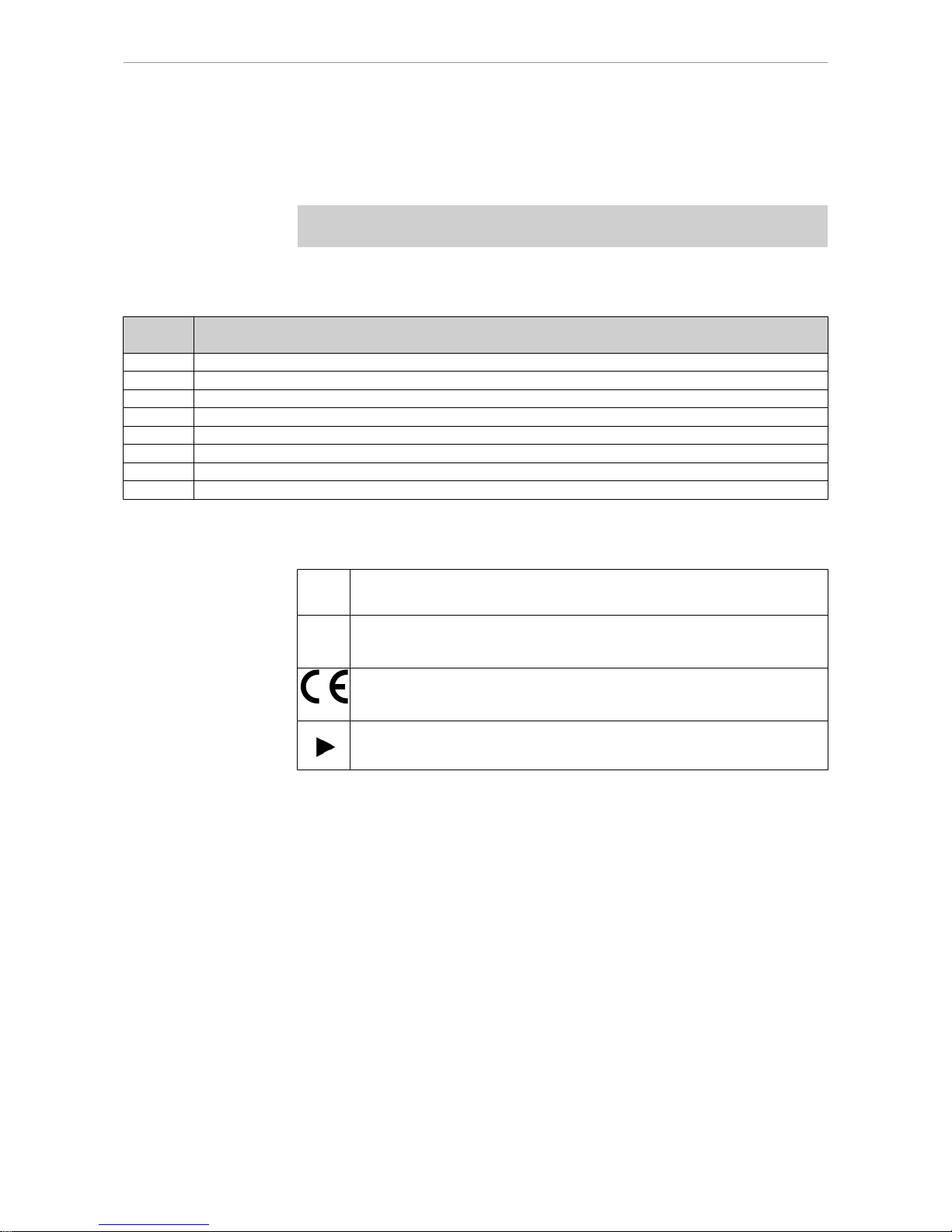
1.1.2 Symbols
See the section Safety/Warning Symbols
Important information for users and technicians
CE mark (Communauté Européenne). A product with this mark meets the
requirements
of the corresponding European directive, i.e., the applicable
European standard.
Action required
1.1.3 Target group
This document is for dentists and office personnel.
2/24
Page 5

Instructions for use ARCUSevo
1 User instructions | 1.2 Service
1.2 Service
Service hotline:
+49 7351 56-1600
Service.Zahntechnik@kavo.com
Please indicate the product serial number in all requests.
Additional information can be obtained at: www.kavo.com
3/24
Page 6

Instructions for use ARCUSevo
1 User instructions | 1.3 Warranty provisions
1.3 Warranty provisions
The following warranty conditions apply to this KaVo medical device:
KaVo provides the end customer with a warranty of proper function and guarantees
zero defects in respect of material and processing for a period of 12 months from
data of invoice, subject to the following conditions:
In case of justified complaints, KaVo will honour its warranty with a repair or free
replacement. Other claims of any nature whatsoever, in particular with respect to
compensation,
are excluded. In the event of default, gross negligence or intent, this
shall only apply in the absence of mandatory legal regulations to the contrary.
KaVo cannot be held liable for defects and their consequences that are or may be
due to natural wear, improper handling, cleaning or maintenance, non-compliance
with operating or connection instructions, calcination or corrosion, contaminated air
or water supplies or chemical or electrical factors deemed abnormal or impermis‐
sible in accordance with KaVo's instructions for use or other manufacturer specifi‐
cations. The warranty does not usually cover lamps, light conductors made of glass
and glass fibres, glassware, rubber parts and the colourfastness of plastic parts.
Defects or their consequences that can be attributed to interventions on or changes
made to the product by the customer or a third party not authorised by KaVo are
excluded from the warranty.
Service warranty claims will only be accepted if the product is submitted along with
proof of purchase in the form of a copy of the invoice/delivery note. The dealer,
purchase date, unit number or type and serial number must be clearly visible on this
document.
4/24
Page 7

Instructions for use ARCUSevo
1 User instructions | 1.4 Transportation and storage
1.4 Transportation and storage
1.4.1 Packaging ordinance of August 28,1998
Note
Only applicable for the Federal Republic of Germany.
KaVo transport packaging must be disposed of and recycled by local disposal ser‐
vice providers and recycling companies in accordance with Dual System require‐
ments.
For more information about disposal and recycling, and an up-to-date list of local
disposal service providers and recycling companies, please visit the following In‐
ternet sites:
http://www.umweltdatenbank.de
http://www.quality.de
KaVo will bring
KaVo transport packaging returned by the customer at the custo‐
mer's own cost to the appropriate recycling companies without reimbursement..
1.4.2 Transportation damage
In Germany
If external damage to the packaging is visible upon delivery, follow the procedure
below:
1. The recipient must record the loss or damage in the notice of delivery. The re‐
cipient and employee of the transportation firm must sign the notice of delivery.
2. Leave the product and packaging unchanged.
3. Do not use the product.
4. Report damage to the shipping company.
5. Report damage to KaVo.
6. A damaged product cannot be returned before talking with KaVo.
7. Send the signed notice of delivery to KaVo.
If the product is damaged and there is no discernable damage to the packaging
upon delivery, proceed as follows:
1. Report damage immediately or at least 7 days after the delivery to the delivery
company. .
2. Report damage to KaVo.
3. Leave the product and packaging unchanged.
4. Do not use a damaged product.
Note
If the recipient does not follow one of the above instructions, the damage will be
held to have occurred after the delivery
(according to ADSp. Art. 28)..
5/24
Page 8
Instructions for use ARCUSevo
1 User instructions | 1.4 Transportation and storage
Outside of Germany
Note
KaVo is not liable for damage arising from transportation.
Immediately inspect the delivery after receipt!
If external damage to the packaging is visible upon delivery, follow the procedure
below:
1.
The recipient must record the loss or damage in the notice of delivery. The re‐
cipient and employee of the transportation firm must sign the notice of delivery.
The recipient can only assert damages against the transportation company ba‐
sed on these records.
2. Leave the product and packaging unchanged.
3. Do not use the product.
If the product is damaged and there is no discernable damage to the packaging
upon delivery, proceed as follows:
1. Report the damage immediately or at least 7 days after the delivery to the deli‐
very company .
2. Leave the product and packaging unchanged.
3. Do not use a damaged product.
Note
If the recipient does not follow one of the above instructions, the damage will be
held to have occurred after the delivery
(according to . CMR law , section 5, Art.
30).
6/24
Page 9
Instructions for use ARCUSevo
1 User instructions | 1.4 Transportation and storage
1.4.3 Storage
Note
Keep the packaging for returning the product for service or repairs
.
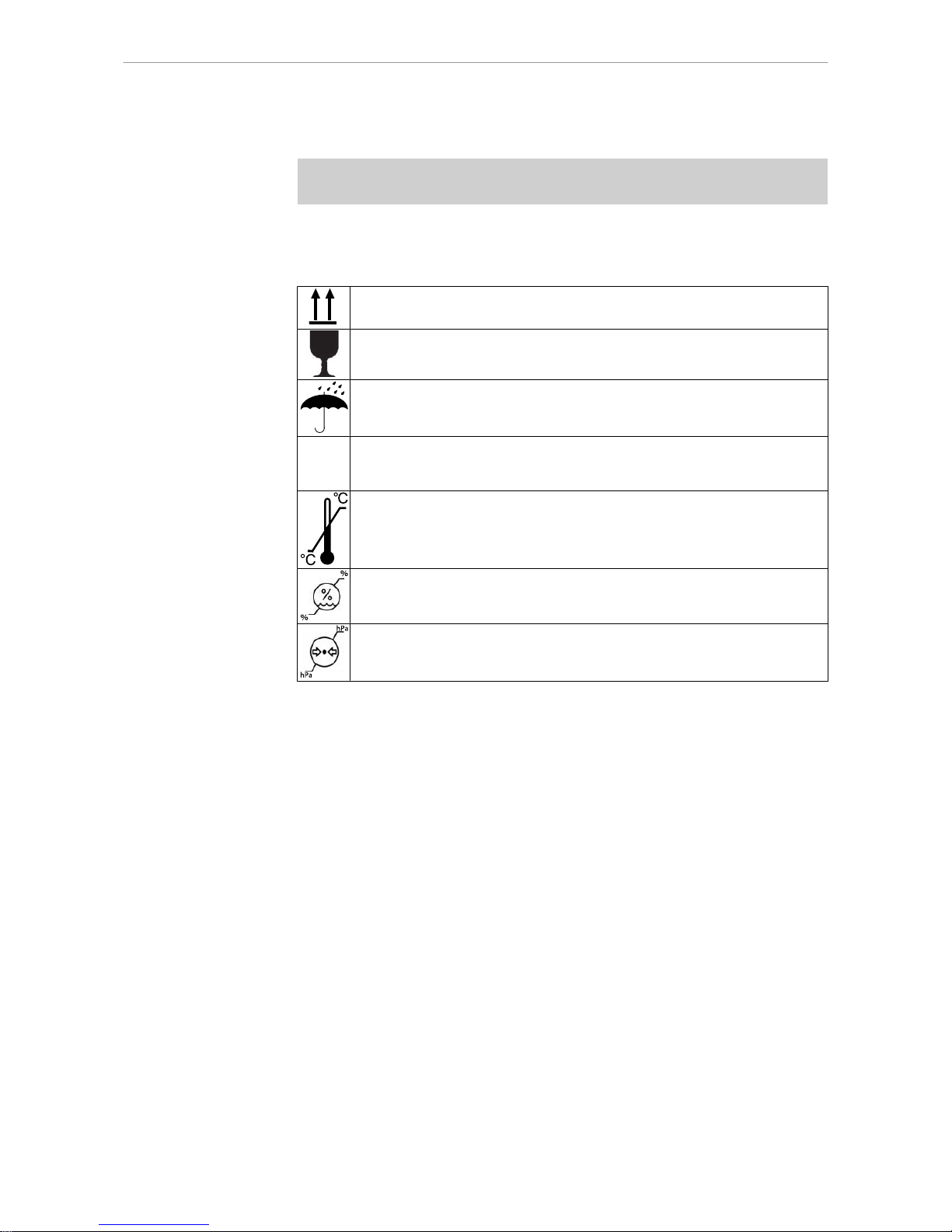
The symbols printed on the outside are for transportation and storage, and have the
following meaning:
Transport upright with the arrows pointing upwards
Fragile - protect against knocks
Keep dry
Maximum permitted stacking load
Temperature range
Humidity
Air pressure
7/24
Page 10

Instructions for use ARCUSevo
2 Safety | 2.1 Description of safety instructions
2 Safety
2.1 Description of safety instructions
2.1.1 Warning symbol
Warning symbol
2.1.2 Structure
DANGER
The introduction describes the type and source of the hazard.
This section describes the potential consequences of non-observance.
▶
The optional step contains necessary measures for avoiding hazards.
2.1.3 Description of hazardous steps
Safety instructions with three hazard levels are used in this document for avoiding
personal and property damage.
CAUTION
CAUTION
indicates a hazardous situation that can lead to property damage or minor to mo‐
derate injury.
WARNING
WARNING
indicates a hazardous situation that can lead to serious injury or death.
DANGER
DANGER
indicates a maximum hazardous situation that can directly cause serious injury or
death.
8/24
Page 11

Instructions for use ARCUSevo
2 Safety | 2.2 Proper use
2.2 Proper use
2.2.1 General information
The user must ensure that that the device works properly and is in a satisfactory
condition before each use.
This KaVo product is intended only for use in the field of dentistry. It is impermissible
to use the product for a purpose for which it was not intended.
"Proper use" includes following all the instructions for use and ensuring that all in‐
spections and service tasks are performed.
Apply and meet the overarching guidelines and/or national laws, national regulati‐
ons and the rules of technology for medical devices applicable for startup and use
of the KaVo product for the intended purpose.
Responsibility is accepted for the safety, reliability and performance of the compo‐
nents supplied by KaVo provided:
▪ installation,
upgrades, adjustments, changes or repairs are carried out by tech‐
nicians trained by KaVo or third parties authorised by KaVo, or by the personnel
of authorised distributors.
▪ The unit is operated in accordance with the instructions for use, care and instal‐
lation.
▪ The IT components supplied by the operator meet the technical requirements in
these instruction for use for hardware and software, and they are installed and
set up according to the descriptions of these components.
▪ If it is repaired, the requirements of VDE 0751-1 "Repeat tests and tests before
start-up of electrical items of medical equipment and systems - general regula‐
tions" must be met in full.
The user must observe the following:
▪ - only use properly operating equipment.
▪ protect himself or herself and third parties from danger.
▪ avoid contamination from the product.
During use, national legal regulations must be observed, in particular:
▪ the applicable health and safety regulations.
▪ the applicable accident prevention regulations.
Authorised to repair and service the KaVo product:
▪ The technicians of KaVo branches.
▪ Technicians of authorised dealers specially trained by KaVo.
In Germany, the operator, person responsible for the device and user must operate
their devices in accordance with the provisions of the Medical Device Law.
9/24
Page 12

Instructions for use ARCUSevo
2 Safety | 2.2 Proper use
These service tasks include all testing tasks that are stipulated in the Operator Or‐
dinance (MPBetreiber V) § 6.
Note
The product must be cleaned and serviced according to instructions if it is not to
be used for a long period.
Note
Only those accessories may be used that are approved for the device.
Disposal
Note
The
waste that arises must be recycled or disposed of in a manner safe for humans
and the environment. Observe the applicable national regulations.
Please direct all questions regarding the proper disposal of KaVo products to the
nearest KaVo branch.
2.2.2 Product-specific
The ARCUSevo facial bow records the position of the patient's maxilla. When crea‐
ting a prosthesis, a working or tooth model can be correctly positioned in an
articulator.
The
facial bow is suitable for recording the Frankfurter horizontals and the Camper's
plane.
10/24
Page 13

Instructions for use ARCUSevo
2 Safety | 2.3 Safety
2.3 Safety
2.3.1 General information
CAUTION
Premature weary and malfunctions from improper servicing and care.
Reduced production time.
▶
Perform regular proper care and maintenance.
WARNING
Injury or damage from damaged functional parts.
When functional parts are damaged, it can cause additional damage or personal
injury.
▶
When operating parts are damaged: Stop working and eliminatethe damage,
or notify a service technician.
▶ Check the electrode lines and accessories for damage to the insulation.
CAUTION
The reference pointer can contact patients.
Hitting/scaring the patient.
▶
Move the reference pointer carefully toward the patient.
11/24
Page 14

Instructions for use ARCUSevo
3 Product description | 3.1 ARCUSevo
3 Product description
3.1 ARCUSevo
① Bow ⑦ Fastening nut of the bite fork support
② Fastening nut for the earbud ⑧ Adjustment wheel for adjusting the
facial width
③ Earbud ⑨ Bite fork support
④ Nose support ⑩ Knurled screw of the bite fork support
⑤ Lock lever for the nose support ⑪ Bite fork
⑥ Reference pointer for the Frankfurter
horizontal and Camper's plane
12/24
Page 15

Instructions for use ARCUSevo
3 Product description | 3.2 Technical data
3.2 Technical data
Dimensions and weight
Max. width 345 mm
Depth 300 mm
Height 100 mm
Weight 250 g
Weight of bite fork/bite fork support 100 g
Information on the planes and reference points
Frankfurter horizontal ② (FH):
Connection between the infraorbital point ① and porion ③
Camper's plane ⑤ (CP):
Connection between the subnasal point ④ and Targus medialis ⑥
The angle difference between the FH and CP is 15°.
13/24
Page 16

Instructions for use ARCUSevo
3 Product description | 3.3 Scope of delivery
3.3 Scope of delivery
Figure Designation
ARCUSevo facial bow incl.
Earbuds
Nose support
Reference pointer
Bite fork support
Bite fork
Instructions for use
14/24
Page 17

Instructions for use ARCUSevo
4 Operation | 4.1 Adapting the facial bow
4 Operation
4.1 Adapting the facial bow
▶ Turn the adjustment wheel ③.
The earbuds ② move further apart. The bow ① can be placed on the patient.
15/24
Page 18

Instructions for use ARCUSevo
4 Operation | 4.2 Mount the facial bow
4.2 Mount the facial bow
The facial bow is mounted with reference to the Frankfurter horizontal and the Cam‐
per's plane.
CAUTION
Unapproved registration materials
Endangerment of the patient
▶
Only use approved registration materials according to 93/42 EEC!
CAUTION
The reference pointer can contact patients.
Hitting/scaring the patient.
▶
Move the reference pointer carefully toward the patient.
▶ Place the prepared bite fork in the patient's mouth.
▶ Adapt the bow ⑥ to the width of the patient's face.
See also: 4.1 Adapting the facial bow, Page
15
▶ Insert the earbuds ⑦ into the outer auditory channel of the patient.
▶ Align the facial bow with the desired reference point (infraorbital point for the
Frankfurter horizontal or the subnasal point for the Camper's plane).
▶ Affix the nose support ① with the the lock lever ②.
Note
If
the Frankfurter horizontal is used, the reference pointer must be inserted with the
pointer guide at position ④ as in the picture.
If the Camper plane is used, the reference pointer must be moved with the pointer
guide below the adjustment wheel at position ⑤.
16/24
Page 19

Instructions for use ARCUSevo
4 Operation | 4.2 Mount the facial bow
▶ Remove the knurled screw ②.
▶ Shove the bite fork support ③ onto the bite fork ④.
▶ Suspend
the bite fork support ③ on the facial bow and tighten it with the fastening
nut ①. Make sure that the guide pins of the bite fork support ③ engage in the
guide groove of the facial bow.
See also: following picture.
▶ Tighten the knurled screw ② to affix the bite fork ④.
① Guide pins of the bite fork support ② Guide groove of the facial bow
17/24
Page 20

Instructions for use ARCUSevo
4 Operation | 4.3 Removing the facial bow
4.3 Removing the facial bow
▶ Open the lock lever ④ of the nose support ③, push back the nose support ③
and fix.
▶ Turn
the setting wheel ⑤ to remove the earbuds ② of the facial bow ① from the
ears.
▶ Completely remove the facial bow from the patient with bite fork ⑥ fixed in the
correct position.
Transfer to the articulator:
See also: Instructions for use of the articulator
18/24
Page 21

Instructions for use ARCUSevo
5 Preparation methods DIN EN ISO 17664 | 5.1 Cleaning
5 Preparation methods DIN EN ISO 17664
Note
All of the components that contact the patient's mucous membrane must be steri‐
lized after use.
The following components must be sterilized:
▪
Normal bite fork (Mat. no. 0.622.0911)
5.1 Cleaning
5.1.1 Manual cleaning
▶ Before sterilization, clean the bite fork under flowing water (tap water quality,
30°C ± 5°C, flow rate: 2 litre/min) 30 seconds with a medium-hard toothbrush.
▶ Sterilise directly before cleaning.
5.1.2 Machine cleaning
Not applicable.
19/24
Page 22

Instructions for use ARCUSevo
5 Preparation methods DIN EN ISO 17664 | 5.2 Disinfection
5.2 Disinfection
5.2.1 Manual disinfection
Allowed disinfectants
▪
Microcide liquid (Schülke & Mayr)
▶ Wipe-disinfect all components.
5.2.2 Automated disinfection
Not applicable.
20/24
Page 23

Instructions for use ARCUSevo
5 Preparation methods DIN EN ISO 17664 | 5.3 Sterilisation
5.3 Sterilisation
▶ Sterilise the bite fork in a fractionated initial vacuum at 134°C ± 1°C, 3.04 bar
for 4 minutes (sterilisable up to max. 138°C).
21/24
Page 24

Instructions for use ARCUSevo
6 Accessories
6 Accessories
Designation Material number
1 pair of adjustable axial pins 1.004.7640
22/24
Page 25

Instructions for use ARCUSevo
7 Spare parts sheet
7 Spare parts sheet
2/2
Verk.-Nr. Gesichtsbogen
ARCUSevo
11/2007
09.30
1.004.7623
1.004.7637
0.258.1442
0.622.2852
0.258.6030
A
A
0.622.2862
0.252.4020
0.622.2872
0.622.2192
0.622.2182
0.622.2752
0.240.2240
0.622.2772
0.252.3016
0.622.0931
0.622.2762
1.004.7640
1.001.4046
0.622.0911
Zubehör
23/24
Page 26

Instructions for use ARCUSevo
7 Spare parts sheet
24/24
Page 27

Page 28

1.006.1320 · Fk · 20071217-01 · en
 Loading...
Loading...