Johnson & Johnson Sterrad 100NX User Manual

STERRAD
®
STERRAD®100NX
STERRAD® 100NX® Sterilization System
User’s Guide
Ref 99994
99994_05
January 2017

STERRAD® 100NX® Sterilization System
User’s Guide
AT
BE
1-888-STERRAD ASP U.S.A. Professional Services
ASP International 949-581-5799
Please visit www.aspjj.com
For warranty information, please visit our website or contact ASP Professional Services.
ASP International Customer Support; call your local ASP Representative
©. 2008-2016 Division of Ethicon. All rights reserved. STERRAD®, CYCLESURE®, SEALSURE®, APTIMAX® and 100NX®
are registered trademarks of Advanced Sterilization Products (ASP). Tefl on
E.I. du Pont de Nemours and Company. Radel
Kraton Polymers LLC. Santoprene™ is a trademark of ExxonMobil Corporation. Ultem
da Vinci
their respective owners. Please note: the screen displays shown in this guide are for reference only. The actual displays on your
system may be slightly different depending on your system’s confi guration and software revision. Reproduction, adaptation, or
®
is a registered trademark of Intuitive Surgical, Inc. Other products mentioned in this publication are trademarked by
translation of this publication without prior written permission is prohibited. Printed in the U.S.A.
®
is a registered trademark of Solvay SA. Kraton® is a registered trademark of
®
, Delrin®, and Tyvek® are registered trademarks of
®
is a registered trademark of SABIC.

Contents
Chapter 1. Introduction .....................................................................................5
How to Use This Guide ............................................................................................5
Intended Use ............................................................................................................5
The STERRAD
If You Have Questions .............................................................................................6
Chapter 2. Safety Information ............................................................................ 7
Personal Safety and First Aid ...................................................................................7
Personal Protective Equipment ................................................................................9
Device Safety ...........................................................................................................9
Warnings, Cautions, and Notes ..............................................................................13
Symbols..................................................................................................................14
Chapter 3. Load Preparation ............................................................................ 15
®
Sterilization Process .....................................................................6
Load Weight Requirements ....................................................................................15
Cycles and Materials Processing ...........................................................................18
Recommended Materials .......................................................................................22
Thermoplastics ..................................................................................................22
Thermoplastic Elastomers .................................................................................22
Thermosetting Elastomers .................................................................................23
Glass ..................................................................................................................23
Metal .................................................................................................................23
Items Not To Be Processed ....................................................................................23
Do Not Process in the EXPRESS Cycle ...........................................................24
Do Not Process in the DUO Cycle ....................................................................24
Guidelines for Preparing Items to Be Sterilized ....................................................25
Cleaning, Rinsing, and Drying ...............................................................................25
Packaging and Loading ..........................................................................................27
Instrument Trays................................................................................................27
Tray Mats ..........................................................................................................27
Packaging ..........................................................................................................27
Loading .............................................................................................................28
Chemical Indicators ..........................................................................................29
Special Considerations for Flexible Endoscopes ..............................................29
STERRAD® 100NX® User’s Guide 1

Chapter 4. Operation ....................................................................................... 31
Before You Start .....................................................................................................31
Start and Warm-up .................................................................................................31
Biological Indicators ..............................................................................................31
Login ......................................................................................................................32
Entering Load Information ....................................................................................33
Enter Load Item Data ........................................................................................33
Loading the Chamber .............................................................................................36
Selecting and Starting a Cycle ...............................................................................40
System Ready Screen ........................................................................................41
Inserting a Cassette ...........................................................................................41
Cycle in Progress ...................................................................................................43
Canceling a Cycle .............................................................................................44
Cycle Completed ....................................................................................................45
Processing a Sterilized Load ..................................................................................46
Inspecting Chemical Indicators .........................................................................46
Processing Biological Indicators .......................................................................46
Chapter 5. Troubleshooting ............................................................................. 49
Running Diagnostics ..............................................................................................49
System Message Table ...........................................................................................50
Temperature Messages ......................................................................................50
Messages Not In This Table ..............................................................................50
Call Your ASP Representative ...............................................................................54
Chapter 6. Sterilizer Overview ......................................................................... 55
Sterilizer Cycles .....................................................................................................55
Sterilizer Features ..................................................................................................56
Cassette .............................................................................................................57
Cassette Disposal Box .......................................................................................58
Touch Screen and Speaker ................................................................................58
Chamber ............................................................................................................59
Printer ................................................................................................................60
Touch Screen Data Entry ..................................................................................61
Chapter 7. Maintenance ................................................................................... 63
Automatic Maintenance ........................................................................................63
Automatic Lamp Adjustment ............................................................................63
Manual Maintenance ..............................................................................................64
2 STERRAD® 100NX® User’s Guide

Disposing of Cassettes ...........................................................................................64
Removing a Cassette Disposal Box ..................................................................65
Replacing the Printer Paper ...................................................................................66
Cleaning the Sterilizer Exterior .............................................................................69
Cleaning the Hydrogen Peroxide Monitor Detector Lens .....................................70
PCMCIA Card Handling and Replacement ...........................................................71
Data Transfer Using a Memory Stick................................................................72
Sterilizer Disposal ..................................................................................................73
Chapter 8. Reports and Files ........................................................................... 75
Displayed Reports .................................................................................................75
Cycle History ....................................................................................................75
Printed Reports .......................................................................................................77
Short Report ......................................................................................................77
Parametric Report..............................................................................................77
Long Report ......................................................................................................77
Chapter 9. Access Levels and Supervisor Tasks ..............................................79
Overview ................................................................................................................79
Access Levels .........................................................................................................79
Additional Utilities Menu ......................................................................................80
Date and Time Settings ..........................................................................................81
Set Date .............................................................................................................82
Set Time ............................................................................................................82
Time Zone .........................................................................................................82
Date Format .......................................................................................................82
Time Format ......................................................................................................82
Cancel/Done ......................................................................................................82
System Confi guration.............................................................................................83
Access Control Option ......................................................................................83
IMS ....................................................................................................................83
Vacuum Units ....................................................................................................83
Load Data Entry Option ....................................................................................84
Load Removal Option .......................................................................................84
Notepad Option .................................................................................................84
Auto Send Network Files ..................................................................................84
Alarm Volume ...................................................................................................84
Backlight Conservation (Minutes) ....................................................................84
Language Selection ...........................................................................................84
Sterilizer Settings ..............................................................................................85
Printer Settings ..................................................................................................86
Transfer Settings ...............................................................................................87
Cancel/Done ......................................................................................................87
STERRAD® 100NX® User’s Guide 3

User Administration ...............................................................................................88
Add User ...........................................................................................................89
Modify User ......................................................................................................90
Upload User Data ..............................................................................................91
Cassette Functions .................................................................................................93
Dispose Cassette................................................................................................94
Peroxide Clearance............................................................................................95
Network ..................................................................................................................96
Diagnostics .............................................................................................................96
Diagnostic T ests ................................................................................................97
Service Functions ...................................................................................................97
File Management ...................................................................................................98
Calibration Files ................................................................................................98
Diagnostic Files .................................................................................................98
Upload File .............................................................................................................99
Input/Output Doors ..............................................................................................100
Product Options ...................................................................................................100
Appendix A. Sterilizer Specifi cations ............................................................... 103
Appendix B. Consumables, Accessories, and Additional Parts ........................ 107
Appendix C. User’s Network Connection Information Guide ............................ 109
4 STERRAD® 100NX® User’s Guide

Chapter 1. Introduction
Introduction
How to Use This Guide
If you are a STERRAD® 100NX® Sterilizer operator, you must read
the “Safety Information, “ the “Introduction,” “Load Preparation,” and
“Operation” chapters prior to operating the sterilizer. This “Introduction”
explains the features and parts of the sterilizer. “Load Preparation” explains
how to prepare and package instruments for processing. “Operation” explains
how to operate the sterilizer and obtain optimal results.
If you are a supervisor overseeing the STERRAD
you should read the entire user’s guide and pay particular attention to the
chapter featuring “Access Levels and Supervisor Level Tasks.” This chapter
describes tasks and options that are only available through “Supervisor
Level” access.
Introduction 1
®
100NX® Sterilizer,
Intended Use
The STERRAD® 100NX® Sterilization System is a general purpose, low
temperature sterilizer which uses the STERRAD
inactivate microorganisms on a broad range of medical devices and surgical
instruments.
When used as directed by the instructions in this user’s guide, the
STERRAD
nonmetal medical devices at low temperatures. Please review “How to
Determine What Can Be Sterilized in the STERRAD
in the “Load Preparation” chapter along with the cycle information to make
sure you follow the directions for processing items in each type of cycle.
STERRAD® 100NX® User’s Guide 5
®
100NX® Sterilization System will sterilize both metal and
®
100NX® Process to
®
100NX® Sterilizer”

1 Introduction
The STERRAD® Sterilization Process
The STERRAD® 100NX® Sterilizer sterilizes medical devices by diffusing
hydrogen peroxide vapor into the chamber and then electromagnetiy exciting
the hydrogen peroxide molecules into a low-temperature plasma state. The
combined use of hydrogen peroxide vapor and plasma safely and rapidly
sterilizes medical instruments and materials without leaving toxic residue.
All stages of the sterilization cycle operate within a dry environment at a low
temperature, and thus the cycle is not damaging to compatible instruments
that are sensitive to heat and moisture.
The STERRAD
devices, and can also sterilize instruments that have diffi cult-to-reach
(diffusion-restricted) spaces, such as hinges on forceps. Refer to the “Safety
Information” chapter for more information on device safety.
The sterilizer consistently provides a Sterility Assurance Level (SAL) of 10
as defi ned by U.S. Food and Drug Administration (FDA) and international
standards, for clinical use on all allowed substrates within the limits of
the claims for materials and geometries when used in accordance with the
directions in this user’s guide.
®
100NX® Sterilizer can be used for both metal and nonmetal
-6
,
If You Have Questions
If you have questions about the STERRAD® 100NX® Sterilizer or questions
about which items may be safely sterilized by the STERRAD
please call your local Advanced Sterilization Products (ASP) Representative
or visit our website at www.aspjj.com.
®
Process,
6 STERRAD® 100NX® User’s Guide

Chapter 2. Safety Information
Safety Information
Safety Information 2
Your safety is of primary concern to Advanced Sterilization Products (ASP).
This chapter provides information on safely using the STERRAD
Sterilizer. You must read and understand the safety information in
this chapter before operating the sterilizer. Always pay attention to the
warnings, cautions and notes throughout this user’s guide. This information
is for your safety and to ensure that you receive the most benefi t from the
safe operation of your STERRAD
®
100NX® Sterilization System.
Personal Safety and First Aid
WARNING! HYDROGEN PEROXIDE IS CORROSIVE.
Concentrated hydrogen peroxide is corrosive to skin, eyes, nose, throat, lungs, and
the gastrointestinal tract. Always wear chemical resistant latex, PVC (vinyl), or nitrile
gloves while removing items from the sterilizer following a cancelled cycle or if any
moisture is noted on items in the load following a completed cycle.
WARNING! HYDROGEN PEROXIDE IS AN OXIDIZER.
Hydrogen peroxide is strong oxidizing agent and poses a hazard for fi re, explosion,
or container rupture. Avoid allowing hydrogen peroxide to contact organic materials,
including paper, cotton, wood, or lubricants. Do not use or store near heat or open
fl ame. Shoes, clothing, or other combustible material that have come into contact
with hydrogen peroxide must be immediately and thoroughly rinsed with water to
avoid a potential fi re hazard. In case of fi re, use only water to extinguish.
®
100NX®
WARNING! RISK OF EYE INJURY.
Direct hydrogen peroxide contact with eyes can cause irreversible tissue damage. If
contact with eyes occurs, hold the eyes open and fl ush with large amounts of water
for at least 15-20 minutes. Remove contact lenses, if present, and then continue
rinsing the eyes. Consult a physician immediately after fl ushing the eyes.
STERRAD® 100NX® User’s Guide 7
2 Safety Information
WARNING! RISK OF SKIN INJURY.
Direct hydrogen peroxide contact with the skin can cause severe irritation. Wear
chemical resistant latex, PVC (vinyl), or nitrile gloves when handling new, used, or
ejected cassettes, items from a cancelled cycle, or items that have moisture present
after a completed cycle. Immediately take off contaminated clothing and rinse
thoroughly with water to avoid potential fi re hazard and wash before re-use.
WARNING! RISK OF RESPIRATORY IRRITATION.
Inhalation of hydrogen peroxide mist can cause severe irritation of lungs, throat,
and nose. If inhalation occurs, move to the person to fresh air. If the person is not
breathing, call for emergency medical attention, or an ambulance, then give artifi cial
respiration, preferably mouth-to-mouth, if possible. Consult a physician immediately.
WARNING! CONCENTRATED HYDROGEN PEROXIDE IS TOXIC.
Ingestion of hydrogen peroxide may be life-threatening. If swallowed, call a “poison
control” center or physician immediately for treatment advice. Have the person drink
plenty of water if the person is able to swallow. Do not give anything by mouth to an
unconscious person. Do not induce vomiting unless instructed to do so by the poison
control center or physician.
WARNING! HEATED STERILIZATION SURFACES.
At the end of a cycle, the interior of the sterilizer may be hot. Do not touch the inside
of the chamber or door with your bare or gloved hands. Allow the sterilizer to cool
before touching interior surfaces.
WARNING! AVOID EXPOSURE TO ULTRAVIOLET LIGHT.
The hydrogen peroxide monitor uses an ultraviolet light source located inside the
chamber behind the door. To avoid eye injury, do not stare directly at the ultraviolet
light source for an extended period of time.
WARNING! HYDROGEN PEROXIDE MAY BE PRESENT.
If white residue is visible on the load, this is residue from the hydrogen peroxide
stabilizer. Wear chemical resistant latex, PVC (vinyl), or nitrile gloves when
removing a load with visible white residue. White residue can be minimized by
making sure regular Planned Maintenance procedures are performed on your system.
The system will inform you when Planned Maintenance is due. Please schedule your
PM service in a timely manner.
8 STERRAD® 100NX® User’s Guide
Safety Information 2
WARNING! RISK OF BREATHING DIFFICULTIES.
On rare occasions, the outlet fi lter on the vacuum pump can prematurely fail. If this
occurs, you may see mist or what some users have described as “haze” or “smoke”
in the room where the sterilizer is operating. The chemical composition of the mist
is primarily airborne mineral oil with trace amounts of other compounds. Oil mist
exposure may, theoretically, pose an increased risk to people with certain respiratory
conditions, such as asthma, and they should take special precautions not to be
exposed to the mist. If you observe these conditions, personnel should leave the room
as a precaution and discontinue use of the STERRAD
repaired. Personnel should avoid working in the room until the mist has cleared.
®
System until the system is
Please note that all STERRAD
ventilated environment (a minimum of 10 air exchanges per hour).
®
Sterilizers should be used and installed in a well
Personal Protective Equipment
WARNING! HYDROGEN PEROXIDE MAY BE PRESENT.
Wear chemical resistant latex, PVC (vinyl), or nitrile gloves whenever handling a
load after a cycle cancellation. Hydrogen peroxide liquid may be present on the load
or in the chamber.
Device Safety
WARNING! RISK OF INJURY OR DAMAGE TO STERILIZER.
The STERRAD® 100NX® Sterilizer should not be used stacked with other equipment.
CAUTION: RISK OF DAMAGE TO LOAD.
Metal objects must not come into contact with the chamber walls, the door, or the
electrode. Contact with the walls, door, or electrode could damage the sterilizer or the
metal objects.
STERRAD® 100NX® User’s Guide 9

2 Safety Information
CAUTION: KNOW WHAT YOU CAN PROCESS.
Before processing any item in the STERRAD® 100NX® Sterilizer, make sure
you know how the STERRAD Sterilization Process will affect the item. Read,
understand, and follow the medical device manufacturers’ instructions for their
products. This guide lists certain types of items and materials that can be safely
processed in certain cycle choices. Make sure you understand the parameters of each
cycle type before processing your items. This guide is not intended to replace any
medical device manufacturers’ instructions. If you have questions, or if you are in
doubt about the materials in your devices, contact the medical device manufacturer or
your ASP Customer Representative for more information.
CAUTION: RISK OF VIOLATION OF WARRANTY.
Improper processing may limit our liability for damage to processed instruments.
Improper processing may also violate your instrument warranty.
CAUTION: RISK OF DAMAGE TO LOAD – METAL OBJECTS.
Metal objects must not come into contact with the chamber walls, the doors, or the
electrode. Contact with the walls, doors, or electrode could damage the sterilizer or
the metal objects.
CAUTION: RISK OF DAMAGE TO LOAD – VENTING CAPS.
Take special care to confi rm that venting caps are placed according to the
manufacturers’ instructions. Venting caps are intended to prevent damage to fl exible
scopes that are being exposed to a vacuum, regardless of the sterilant used.
CAUTION: RISK OF DAMAGE TO LOAD – IMMERSION CAPS.
You must remove the water-resistant immersion cap (if present) prior to processing
in the sterilizer. If the immersion cap is not removed prior to processing in the
STERRAD
to properly vent.
CAUTION: KNOW WHAT YOU CAN PROCESS – FLEXIBLE
ENDOSCOPES.
Prior to processing fl exible endoscopes in the STERRAD® 100NX® Sterilizer, you
must read, understand, and follow the medical device manufacturer’s instructions
for use for the particular scope to be processed. Please contact the medical device
manufacturer for more information on what can be processed in the STERRAD
100NX
CAUTION: RF COMMUNICATIONS EQUIPMENT.
Portable and mobile RF communications equipment can affect medical electrical
equipment.
®
100NX® Sterilizer, it will damage the fl exible scope due to the inability
®
Sterilizer.
®
10 STERRAD® 100NX® User’s Guide

Safety Information 2
Guidance And Declaration-Electromagnetic Emissions
The STERRAD® 100NX® Sterilizer is intended for use in the electromagnetic environment specifi ed below.
Assure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment - Guidance
RF emissions CISPR 11 Group 1
RF emissions CISPR 11 Class A
Harmonic emissions
IEC 61000-3-2
Voltage fl uctuations/fl icker
emissions IEC 61000-3-3
Class A
Complies
System is confi gured with FCC ID: AXJ100NXRFID
or FCC ID: AXJ02532480 or contains FCC ID: AXJ02532481
System is confi gured with IC Certifi cation Number:
10207A-100NXRFID
or IC Certifi cation Number: 10207A-02532480 or
contains IC Certifi cation Number 10207A-02532481
The STERRAD® 100NX® Sterilizer uses RF energy
only for its internal function. Therefore, its RF
emissions are very low and are not likely to cause any
interference in nearby electronic equipment.
The STERRAD® 100NX® Sterilizer is suitable for
use in all establishments other than domestic and
those directly connected to the public low-voltage
power supply network that supplies buildings used for
domestic purposes.
WARNING! (PART 15.21) RISK OF NON-COMPLIANCE.
Changes or modifi cations not expressly approved by Advanced Sterilization Products
could void the user’s authority to operate the equipment. Manufacturer is not
responsible for any radio or TV interference caused by unauthorized modifi cations to
this equipment.
STERRAD® 100NX® User’s Guide 11

2 Safety Information
FCC Rules and Industry Canada (IC) Regulatory Information
Compliance Statement (Part 15.19)
The equipment device complies with Part 15 of the FCC Rules. Operation
is subject to the following two conditions: (1) This device may not cause
harmful interference, and (2) This device must accept any interference
received including interference that may cause undesired operation.
Compliance Statement (Part 15.105(b))
Note: This equipment has been tested and found to comply with the limits for
a Class A digital device, pursuant to part 15 of the FCC Rules. These limits
are designed to provide reasonable protection against harmful interference
when the equipment is operated in a commercial environment. This
equipment generates, uses, and can radiate radio frequency energy and, if
not installed and used in accordance with the instruction manual, may cause
harmful interference to radio communications. Operation of this equipment
in a residential area is likely to cause harmful interference in which case the
user will be required to correct the interference at his own expense.
This device complies with Industry Canada license-exempt RSS standard(s).
Operation is subject to the following two conditions: (1) this device may
not cause interference, and (2) this device must accept any interference,
including interference that may cause undesired operation of the device.
Class A digital device notice “CAN ICES-3 (A)/NMB-3(A)”
RF Radiation Exposure Statement
This equipment complies with the FCC/IC radiation exposure limits set forth
for portable transmitting devices operation in a controlled environment. End
users must follow the specifi c operating instructions to satisfy RF exposure
compliance.
The equipment should only be used where there is normally at least 20cm
separation between the antenna and all person/user.
This transmitter must not be co-located or operation in conjunction with any
other antenna or transmitter.
Any changes or modifi cations not expressly approved by the party
responsible for compliance could void the user’s authority to operate this
equipment.
12 STERRAD® 100NX® User’s Guide

Warnings, Cautions, and Notes
Warnings and cautions are accompanied by symbols surrounded by a triangle or a
square and are printed in the text in bold. Warnings indicate events or conditions that
can result in serious injury or death. Cautions indicate events or conditions that can
result in severe damage to the equipment.
Notes are printed in italics and have a checkmark in front of the word “Note.” Notes
highlight specifi c information about the proper use and maintenance of the sterilizer.
Safety Information 2
STERRAD® 100NX® User’s Guide 13

2 Safety Information
Symbols
Hot surfaces present.
Do not touch without protection.
Hazardous chemical present.
Use personal protective equipment.
Corrosive chemical present.
Use personal protective equipment.
Oxidizing chemical present.
Avoid exposure, contact, or ingestion.
Use personal protective equipment.
WEEE Symbol
Toxic chemical present.
Avoid exposure, contact, or ingestion.
Ultraviolet (UV) light hazard.
Do not look at the light without UV eye protection.
High voltage hazard.
I/O On/Off.
Alternating current.
14 STERRAD® 100NX® User’s Guide

Chapter 3. Load Preparation
Load Preparation
Load Preparation 3
The STERRAD® 100NX® Sterilizer is designed for sterilization of both
metal and nonmetal medical devices at low temperatures. The STERRAD
sterilization process is a multiphase sterilization process that utilizes
a combination of exposure to hydrogen peroxide vapor and plasma to
safely sterilize medical instruments and materials without leaving toxic
residue. Because the cycle operates within a dry environment and at low
temperatures, it is especially suitable for instruments sensitive to heat and
moisture.
CAUTION: KNOW WHAT YOU CAN PROCESS.
Before processing items in the sterilizer, make sure you know how the STERRAD
Sterilization Process will affect the item. When constructing your load, the total
weight of the load to be sterilized should not exceed the load requirements for the
specifi c cycle. If you have questions, or if you are in doubt about the materials
in your devices, contact the medical device manufacturer or your ASP Customer
Representative for more information.
CAUTION: RISK OF VIOLATION OF WARRANTY.
Improper processing may limit our liability for damage to processed instruments.
Improper processing may also void your instrument warranty.
®
®
Load Weight Requirements
The weight of the items to be sterilized must conform to the weights used for
validating the sterilizer processes. These weights are listed in the following
table. The weight of the load depends on the cycle selected and whether one
or both shelves are used.
STANDARD Cycle 9.7 kg (21.4 lbs) total weight 1 or 2 shelves
DUO Cycle 6.0 kg (13.2 lbs) total weight 1 or 2 shelves
EXPRESS Cycle 4.9 kg (10.7 lbs) total weight Bottom shelf only
FLEX Cycle 9.7 kg (21.4 lbs) total weight 1 or 2 shelves
STERRAD® 100NX® User’s Guide 15
Cycle T ype Weight Shelves

3 Load Preparation
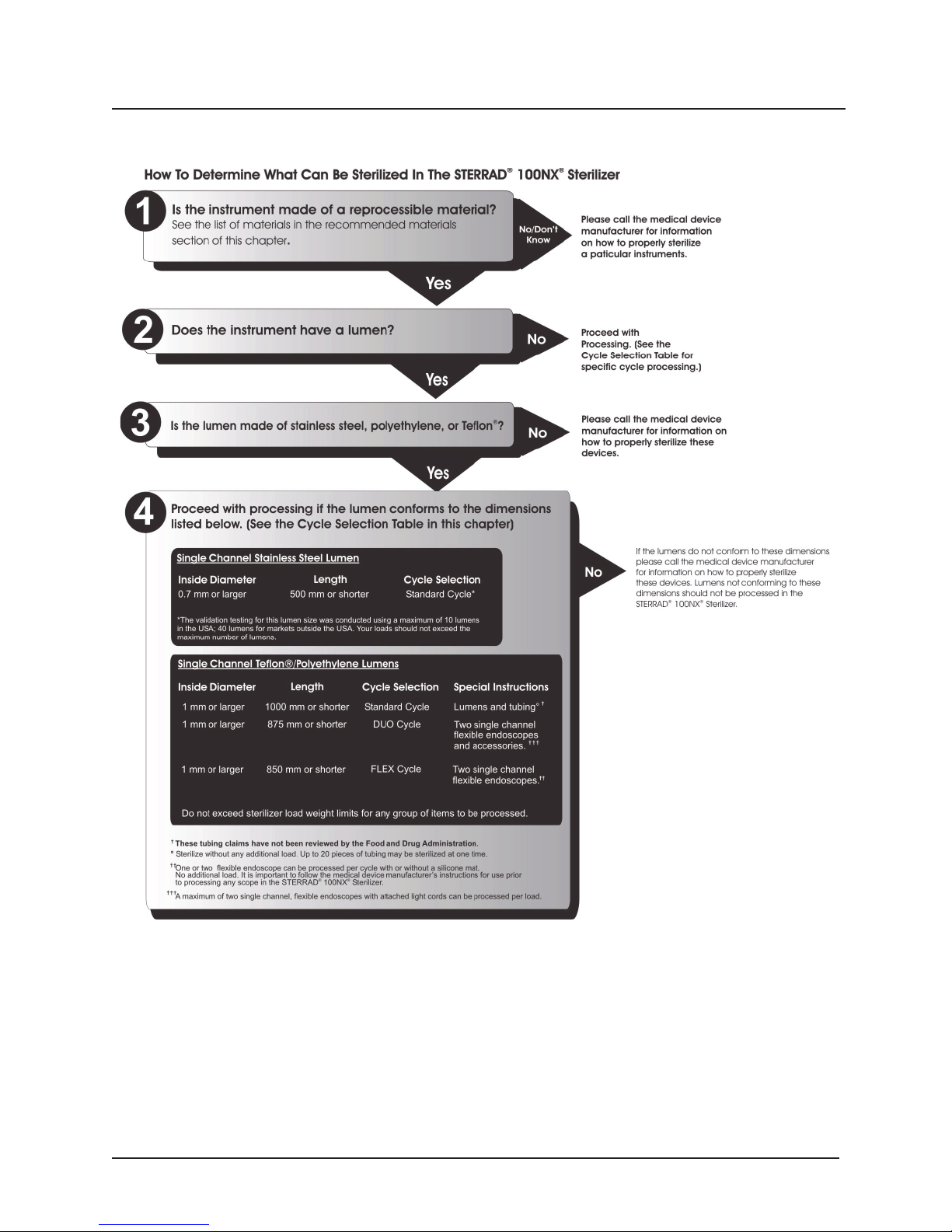
STANDARD Cycle Processing
The STERRAD® 100NX® Sterilizer can sterilize instruments which have
diffusion-restricted spaces, such as the hinged portion of forceps and scissors.
Medical devices with the following materials and dimensions can be
processed in the STERRAD
Single channel stainless steel lumens with an inside diameter of 0.7 mm
®
100NX® Sterilizer STANDARD cycle:
or larger and a length of 500 mm or shorter.†
Processing Tubing
ASP has validated the processing of non-reusable polyethylene and
®
Tefl on
(polytetrafl uoroethylene) medical grade tubing with the
dimension and cycles listed below. (These tubing claims have not been
reviewed by the Food and Drug Administration (FDA) as the FDA does
not classify tubing as medical devices.):
An inside diameter of 1 mm or larger and a length of 1000 mm
or shorter can be processed in the STERRAD
STANDARD cycle.
*
®
100NX® Sterilizer
DUO Cycle Processing
Medical devices, including many fl exible endoscopes with accessory devices
such as light cords and cameras with the following material and dimensions
can be processed in the STERRAD
Single channel polyethylene and Tefl on
fl exible endoscopes with an inside diameter of 1 mm or larger and a
length of 875 mm or shorter.
®
100NX® DUO Cycle.
®
(polytetrafl uoroethylene)
Cameras.
Accessory light cords.
Flexible endoscopes without lumens.
Note: Do not include more than 2 fl exible endoscopes per
load.
†The validation testing for this lumen size was conducted using a maximum of 10 lumens in
the USA; 40 lumens for markets outside the USA. Your loads should not exceed the validated
maximum number of lumens.
*Sterilize without any additional load. Up to 20 pieces of tubing may be sterilized at one time.
16 STERRAD® 100NX® User’s Guide

Load Preparation 3
EXPRESS Cycle Processing
The following types of medical devices can be sterilized in the EXPRESS
Cycle:
General medical devices requiring surface sterilization, or sterilization of
mated titanium and stainless steel surfaces.
Rigid or semi-rigid endoscopes without lumens; for example da Vinci
endoscopes.
FLEX Cycle Processing
®
Medical devices, including most fl exible endoscopes, with the following
materials and dimensions can be processed in the STERRAD
®
100NX®
Sterilizer FLEX cycle:
Single channel polyethylene and Tefl on
®
(polytetrafl uoroethylene)
fl exible endoscope with an inside diameter of 1 mm or larger and length
of 850 mm or shorter.
Flexible endoscopes without lumens.
**
Note: Do Not process more than 2 fl exible endoscope per
load.
Check the medical device manufacturer’s instructions before loading any
item into the STERRAD
®
100NX® Sterilizer.
**One or two fl exible endoscopes can be processed per sterilization cycle. No additional load.
STERRAD® 100NX® User’s Guide 17

3 Load Preparation
Cycles and Materials Processing
CAUTION: RISK OF DAMAGE TO LOAD OR STERILIZER.
Do not attempt to sterilize items or materials that do not comply with the guidelines
specifi ed in this user’s guide. Consult the medical device manufacturer’s instructions
or call your ASP Representative to determine if an item can be sterilized by the
STERRAD
®
100NX® Sterilization System.
This chapter includes cycle information regarding recommended items,
materials, and some typical devices that can be sterilized in each of the cycles
on the STERRAD
you need materials information.
®
100NX® Sterilizer. Please refer to these pages whenever
Check the medical device manufacturer’s instructions before loading any
item into the STERRAD
There is a wide variety of materials and devices that can be sterilized in the
STERRAD
Representative or visit our website at www.aspjj.com. Information may also
be obtained from the device manufacturer.
®
100NX® Sterilizer. For more information contact your local ASP
®
100NX® Sterilizer.
18 STERRAD® 100NX® User’s Guide

Load Preparation 3
STERRAD® 100NX® User’s Guide 19
3 Load Preparation
20 STERRAD® 100NX® User’s Guide

Load Preparation 3
STERRAD® 100NX® User’s Guide 21

3 Load Preparation
Recommended Materials
There is a wide variety of materials and devices that may be sterilized in the
sterilizer. The materials listed below are commonly found in medical devices
and represent typical classes of materials used to construct medical devices.
The items marked with an asterisk (*) may have limited life after repeated
sterilization.
Please contact your ASP Representative for more information. Information
may also be obtained from the device manufacturer.
Thermoplastics
Ethylvinyl Acetate (EV A)
Kraton
Liquid Crystal Polymer (LCP)
Polyacetal (Delrin
®
Polymers
®
acetal resin)*
Polyamide (Nylon)*
Polycarbonate
Polyetheretherketone (PEEK)
Polyetherimide (ULTEM
Polyethylene
Polymethyl methacrylate (PMMA)*
Polyphenylene sulfone (Radel
Polypropylene
Polystyrene
Polytetrafl uoroethylene (Tefl on
Thermoplastic Elastomers
Santoprene™
®
Polymers)
®
)*
®
)
22 STERRAD® 100NX® User’s Guide

Thermosetting Elastomers
Silicone
Polyurethane
PVC
Glass
Glass
Metal
Aluminum
Brass
Gold
Stainless steel
Load Preparation 3
Titanium
Items Not To Be Processed
Single use items for which the manufacturer does not recommend
resterilization.
Liquids and powders.
Items or materials that absorb liquids.
Items made of materials that contain cellulose, such as cotton, paper or
cardboard, linens, huck towels, gauze sponges, or any item containing
wood pulp
Paper instrument count sheets or lot stickers.
Items with hinged/mated nylon surfaces.
Instruments and devices that cannot withstand a vacuum and are labeled
for gravity steam sterilization methods only.
Items whose design permits the surfaces to collapse onto each other
unless some method is used to keep the surfaces separated.
Devices with dead-end lumens.
STERRAD® 100NX® User’s Guide 23

3 Load Preparation
Devices with internal parts, such as sealed bearings, that cannot be
immersed, may present diffi culties in cleaning and should not be
processed in the STERRAD
®
100NX® Sterilizer.
Implants for which the manufacturer has not specifi cally recommended
sterilization in the STERRAD
Do Not Process in the EXPRESS Cycle
Items made of nylon cannot be processed in the EXPRESS Cycle.
Items made of Kraton
Items made of Polyurethane cannot be processed in the EXPRESS Cycle.
Items with mated Delrin
®
cannot be processed in the EXPRESS Cycle.
®
surfaces cannot be processed in the EXPRESS
Cycle.
Items with mated anodized aluminum surfaces cannot be processed in the
EXPRESS Cycle.
Items with mated Radel
®
surfaces cannot be processed in the EXPRESS
Cycle.
Items with mated Ultem
®
surfaces cannot be processed in the EXPRESS
Cycle.
Items with lumens cannot be processed in the EXPRESS Cycle.
Do Not Process in the DUO Cycle
®
100NX® Sterilizer.
Items with mated anodized aluminum surfaces cannot be processed in the
DUO Cycle.
24 STERRAD® 100NX® User’s Guide

Load Preparation 3
Guidelines for Preparing Items to Be
Sterilized
Note: All items must be cleaned, rinsed, and thoroughly
dried before being placed in the STERRAD
Sterilizer. Loads containing moisture may cause cycle
cancellations.
Cleaning, Rinsing, and Drying
Cleaning and sterilization are two separate processes. Proper cleaning of
instruments and devices is a critical and necessary step prior to sterilization.
All items including accessories must be thoroughly cleaned, rinsed, and
dried before loading into the sterilizer.
Carefully inspect all instruments, devices, and accessories for cleanliness
and dryness prior to packaging. If visible soil is present, the item must be
re-cleaned and dried prior to sterilization. If moisture is present, dry the
item thoroughly prior to sterilization.
Carefully inspect all instruments, devices, and accessories for fl aws
or damage prior to packaging. Items with fl aws or damage should be
replaced or repaired before using.
®
100NX®
Note: Periodic careful inspection of items after repeated
exposure to disinfectant/cleaner/sterilant is necessary,
due to the potential damaging effects of the chemical
agents.
Cleaning is necessary to remove organic and inorganic soil and debris from
equipment. This process also removes many microorganisms from the
surface of the items. Sterilization then inactivates all remaining spores and
live microorganisms.
Clean your devices according to the medical device manufacturers’
instructions. You must remove all blood, tissue, and soil from items using
appropriate detergents, cleansers and/or methods.
Rinse items thoroughly to remove detergent or cleanser residue. Use
treated water that is of a quality that ensures hard water stains do not
occur. Failure to remove all organic materials or detergents may result in
the formation of light-colored residue on the devices. If residue is visible,
you should clean, rinse, dry, and resterilize the device prior to use.
STERRAD® 100NX® User’s Guide 25

3 Load Preparation
WARNING! POSSIBLE RESIDUAL HYDROGEN PEROXIDE CONTACT!
Failure to ensure that instruments are completely dry before they are processed in
the STERRAD
on the surface of the load after the cycle is complete. This may cause contact burns
when the surface of the load is handled.
Dry all items thoroughly. An acceptable method for drying is to blow
compressed gas through the lumen until no moisture exits the distal end
of the device. Please ensure that any method used to dry the devices is
in accordance with the manufacturers’ instructions for use or contact
the device manufacturer to obtain appropriate and safe procedures. It
is necessary to remove moisture from all parts of the items. Only dry
items should be loaded into the sterilization chamber to prevent cycle
cancellation.
®
sterilizer may result in residual hydrogen peroxide being present
Some complex reusable medical devices may require disassembly for
proper cleaning and sterilization. It is very important that you follow
the device manufacturers’ recommendations concerning cleaning and
sterilization. In the absence of STERRAD
please contact the relevant medical device manufacturer.
®
System-specifi c instructions,
WARNING! POSSIBLE NON-STERILE DEVICE!
Loads containing moisture may result in either a non-sterile device or cycle
cancellation. Wear chemical resistant gloves when handling items from any load
containing moisture.
26 STERRAD® 100NX® User’s Guide

Packaging and Loading
If you choose to package the instruments (highly recommended), proper use
and preparation of trays, pouches, and instruments can minimize or prevent
cycle cancellations and positive biological indicator (BI) results due to load
related problems. All instruments must be cleaned, rinsed, and thoroughly
dried before loading into the sterilizer.
Special considerations for loading and processing fl exible endoscopes are
presented at the end of this chapter.
Instrument Trays
Only STERRAD
Trays, are recommended for use in the STERRAD
These instrument trays are specially designed to allow diffusion of
hydrogen peroxide and plasma around every item in the load.
®
Instrument Accessories and APTIMAX® Instrument
Load Preparation 3
®
100NX® Sterilizer.
Tray Mats
Packaging
Instrument trays should only be padded with STERRAD
®
Mats or polypropylene sterilization wrap. Never use linen, cellulose, or
any materials listed in the “Items Not To Be Processed” section.
Follow the Instructions for Use included with the STERRAD
Instrument Mats to determine the number of mats that can be used at one
time in the chamber. Do not use more than the recommended amount of
mat material in the chamber at any time.
Cycle Name Square Centimeters Square Inches
STANDARD 2250 349
DUO 2774 430
FLEX 2250 349
EXPRESS 1387 215
Do not use foam pads in instrument trays as they may absorb the
hydrogen peroxide.
Use only STERRAD
wrap and Tyvek
®
Sterilizer-compatible polypropylene sterilization
®
pouches.Tyvek® Pouches and Rolls with STERRAD®
Chemical Indicators are the only pouches and rolls available on the
market that are validated by ASP. They are the only pouches and rolls
validated by ASP for effi cacy and stability.
Instrument
®
STERRAD® 100NX® User’s Guide 27

3 Load Preparation
Do not use paper pouches or sterilization wraps containing cellulose or
cotton.
Do not use any wraps or packaging that are not approved by ASP or
materials listed in the “Items Not To Be Processed” section. In the USA,
use only FDA-cleared polypropylene wraps.
Properly arrange the items or the scope in a tray to ensure adequate
diffusion of hydrogen peroxide throughout the load.
Place peel pouches on edge, if possible. Arrange them so that the
transparent side of a pouch faces the opaque side of the next pouch. Do
not stack pouches on top of each other.
Do not stack instruments inside the trays. Do not stack trays. Do not
stack trays within trays. Do not wrap instruments within a wrapped tray.
Loading
If you are using rigid containers cleared by the FDA for use in the
STERRAD
®
100NX® Sterilizer, follow the Instructions for Use provided
by the rigid container manufacturer. Verify that the rigid containers are
cleared for use in each sterilization cycle. Remember the following:
Do not stack instruments inside the containers.
Do not stack containers.
Do not stack containers within containers.
Do not wrap instruments within the containers.
Place STERRAD
®
Chemical Indicator Strips inside trays and pouches as
needed.
Do not allow any item to touch the walls of the sterilization chamber,
door, or electrode.
STANDARD and FLEX Cycles Loading Preparation
The STERRAD
were validated using a load weight of 4.9 kg (10.7 lbs) per shelf. When
constructing your load, the total weight of the load to be sterilized should
not exceed 9.7 kg (21.4 lbs).
®
100NX® Sterilizer STANDARD and FLEX Cycles
DUO Cycle Loading Preparation
The STERRAD
28 STERRAD® 100NX® User’s Guide
®
100NX® Sterilizer DUO Cycle was validated using
a total load weight of 6.0 kg (13.2 lbs). When constructing your load,
the total weight of the load to be sterilized should not exceed 6.0 kg
(13.2 lbs).
 Loading...
Loading...