JKH Health TENS1 User Manual

Manual of Electronic Pulse Stimulator
‐ 1 ‐
PL-029K8B
Operation Manual
PL-029K8B Edition V1.0

Table of Contents
Introduction......................................................................................................................................3
Indications for Use...........................................................................................................................3
Safety Warning................................................................................................................................ 4
Contraindications.............................................................................................................................4
Warnings..........................................................................................................................................4
Precautions.......................................................................................................................................4
Adverse Reactions........................................................................................................................... 5
Environmental Condition for Transport and Storage...................................................................... 5
Symbols interpretation.....................................................................................................................6
Safety Test Standards...................................................................................................................... 6
Electromagnetic Compatibility and FCC statement........................................................................ 6
Product Specifications................................................................................................................... 11
Setup.............................................................................................................................................. 13
Recommended Use Positions........................................................................................................ 13
Operating Instruction..................................................................................................................... 14
App Aplication...............................................................................................................................17
Cleaning and Maintenance............................................................................................................ 20
Disposals........................................................................................................................................20
Trouble Shooting........................................................................................................................... 21
Contact Information.......................................................................................................................22
‐ 2 ‐

Introduction
Electronic Pulse Stimulator delivers electric pulses generated to the user’s skin through the electrodes.
The portable and compact device has multiple modes of different pulse frequencies, covering
Transcutaneous Electrical Nerve Stimulation (TENS) and Powered Muscle Stimulation (PMS). It
includes operating elements of ON/OFF button, intensity increase/mode selection button, and intensity
decrease/timer selection button, and could be attached and detached to the electrode through the two
snap-on connectors.
Indications for Use
TENS
To be used for temporary relief of pain associated with sore and aching muscles in the shoulder, waist,
back, arm, and leg, due to strain from exercise or normal household and work activities.
It is also intended for symptomatic relief and management of chronic, intractable pain and relief of pain
associated with arthritis.
PMS
To be used for the improvement of muscle tone and firmness, and for strengthening muscles in the
arms, abdomen, legs, and buttocks.
It is also intended to temporarily increase local blood circulation in the lower extremity.
-3-

Safety Warning
Contraindications
Do not use this device on patients who have a cardiac pacemaker, implanted defibrillator, or other
implanted metallic or electronic device, because this may cause electric shock, burns, electrical
interference, or death.
Do not use this device on patients whose pain syndromes are undiagnosed.
Warnings
Do not apply stimulation over the patient’s neck because this could cause severe muscle spasms
resulting in closure of the airway, difficulty in breathing, or adverse effects on heart rhythm or blood
pressure.
Do not apply stimulation across the patient’s chest, because the introduction of electrical current into
the chest may cause rhythm disturbances to the patient’s heart, which could be lethal.
Do not apply stimulation over, or in proximity to, cancerous lesions.
Do not apply stimulation when the patient is in the bath or shower.
If you have one of the following conditions, please consult with your physician before purchasing or
using this device.
Acute disease, malignant tumor, infective disease, pregnant, heart disease, high fever, abnormal blood
pressure, lack of skin sensation or an abnormal skin condition, any condition requiring the active
supervision of a physician.
Precautions
Do not use this device while driving.
Do not use this device while sleeping.
Do not use this device in high humidity areas such as a bathroom.
Keep the device away from wet, high temperature and direct-sunlight place.
Keep this device out of reach of children.
Stop using this device at once if you feel pain, discomfort, dizziness or nausea and consult your
physician.
Do not attempt to move the electrode pads while the device is operating.
Do not use the device around the heart, on the head, mouth, pudendum or blemished skin areas.
Do not apply stimulation of this device in the following conditions:
(1) across the chest because the introduction of electrical current into the chest may cause rhythm
disturbances to the heart, which could be lethal;
(2) over painful areas. Please consult with your physician before using this device if you have painful
areas;
(3) over open wounds or rashes, or over swollen, red, infected, or inflamed areas or skin eruptions (e.g.,
phlebitis, thrombophlebitis, varicose veins). Apply stimulation only to normal, intact, clean, healthy
skin;
(4) in the presence of electronic monitoring equipment (e.g., cardiac monitors, ECG alarms). The
electronic Stimulator may not operate properly when the electrical stimulation device is in use;
(5) while operating machinery, or during any activity in which electrical stimulation can put you at risk
of injury;
(6) on children.
Be aware of the following.
(1) to consult with your physician before using this device. The simulation with the device may:
i. cause lethal rhythm disturbances to the heart in susceptible individuals, and,
-4-

ii. disrupt the healing process after a recent surgical procedure;
(2) that the device is not effective for pain of central origin, including headache;
(3) that the device is not a substitute for pain medications and other pain management therapies;
(4) that the device has no curative value;
(5) that the device is a symptomatic treatment and, as such, suppresses the sensation of pain that would
otherwise serve as a protective mechanism;
(6) that the long-term effects of electrical stimulation are unknown;
(7) that the user may experience skin irritation, burns or hypersensitivity due to the electrical
stimulation or electrical conductive medium (gel);
(8) if the user has suspected or diagnosed epilepsy, the user should follow precautions recommended by
his or her physician;
(9) to use caution if the user has a tendency to bleed internally, such as following an injury or fracture;
(10) use caution if stimulation is applied over the menstruating uterus;
(11) use caution if stimulation is applied over areas of skin that lack normal sensation;
(12) stop using the device if the device does not provide pain relief; and,
(13) use this device only with the leads, electrodes, and accessories that the manufacturer recommends.
(14) Do not share the use of the electrode pads with others.
(15) Do not use the device while it’s charging.
(16) The device contains the lithium battery. If overheating of the device occurred during the charging,
stop the charging or operation immediately and report to the distributor/seller.
(17) Dispose of the battery-containing device according to the local, state, or federal laws.
The long-term effects of electrical stimulation are unknown.
Since the effects of stimulation of the brain are unknown, stimulation should not be applied across the
head, and electrodes should not be placed on opposite sides of the head.
The safety of electrical stimulation during pregnancy has not been established.
Some patients may experience skin irritation or hypersensitivity due to the e lectrical stimulation or
electrical conductive medium (gel).
Patients with suspected or diagnosed heart disease should follow precautions recommended by their
physicians.
Patients with suspected or diagnosed epilepsy should follow precautions recommended by their
physicians.
Use caution if stimulation is applied over the menstruating or pregnant uterus.
Adverse Reactions
Patients may experience skin irritation and burns beneath the stimulation electrodes applied to the skin;
Patients may experience headache and other painful sensations during or following the application of
electrical stimulation near the eyes and to the head and face.
Patients should stop using the device and should consult with their physicians if they experience
adverse reactions from the device.
Environmental condition for transport and storage
Normal working ambient temperature: 10~40°C
Normal working ambient humidity: 30~80%
Store and transport ambient temperature: -10 ~50°C
Store and transport ambient humidity: 30~90%
-5-
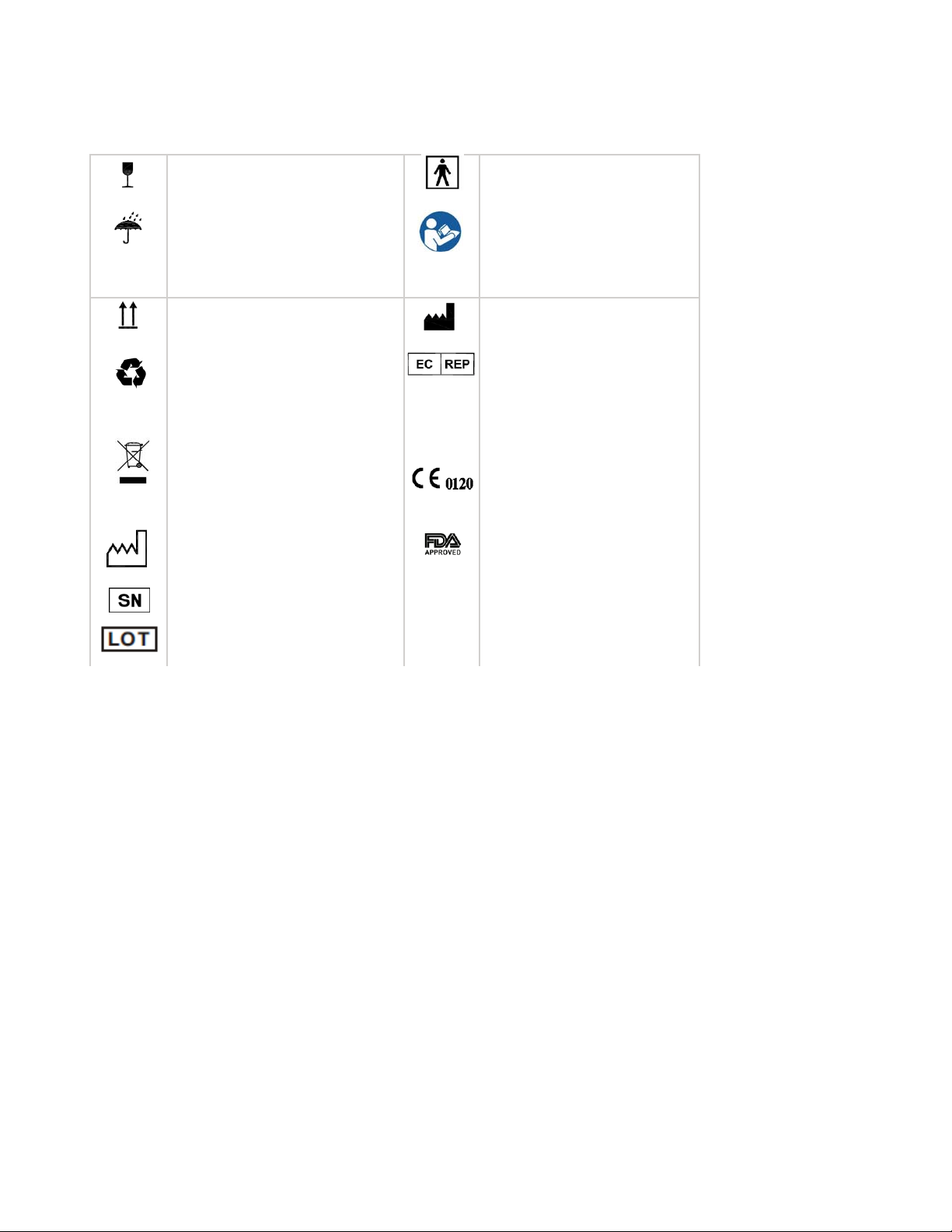
Symbols interpretation
Fragile, handle with care Type BF applied part
Keep the product in the dry
place
Away from water and rain.
This way up Manufacturer
Product package should be
recycled.
Unrecyclable
Date of manufacture
Serial number
Batch code
IP22
CAUTION, Avoid injury.
Read and understand
owner’s manual before
operating this product.
Symbol for "AUTHORISED
REPRESENTATIVE IN
THE EUROPEAN
COMMUNITY"
CE marking, Certificate
issued by SGS.
FDA register
IP code of the device
The Electronic Pulse Stimulator is compliant with:
Medical Devices Directive 93/42/EEC
IEC 60601-1:2005+A1:2012/EN 60601-1:2006 Medical electrical equipment - Part 1: General
requirements for basic safety and essential performance
IEC 60601-1-2:2007/EN 60601-1-2:2007 Medical electrical equipment - Part 1-2: General
requirements for safety - Collateral standard: Electromagnetic compatibility - Requirements and
tests
IEC 60601-2-10:2012/EN 60601-2-10:2000+A1:2001 Medical electrical equipment - Part 2-10:
Particular requirements for the safety of nerve and muscle simulators
Electromagnetic Compatibility and FCC Compliance Statement
(1) This product needs special precautions regarding electromagnetic compatibility (EMC) and needs to
be installed and put into service according to the EMC information provided, and this unit can be
affected by portable and mobile radio frequency (RF) communications equipment.
(2) Do not use a mobile phone or other devices that emit electromagnetic fields, near the unit. This may
result in incorrect operation of the unit.
(3) Caution: This unit has been thoroughly tested and inspected to assure proper performance and
-6-
operation!
(4) Caution: This machine should not be used adjacent to or stacked with other equipment and that if
adjacent or stacked use is necessary, this machine should be observed to verify normal operation in the
configuration in which it will be used
Guidance and manufacturer’s declaration – electromagnetic emission
The device is intended for use in the electromagnetic environment specified below. The customer
of the user of the device should assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment – guidance
The device uses RF energy only for its internal
RF emissions
CISPR 11
RF emission
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Group 1
Class B
Class A
Complies
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in
nearby electronic equipment.
The device is suitable for use in all establishments,
including domestic establishments and those
directly connected to the public low-voltage power
supply network that supplies buildings used for
domestic purposes.
-7-
 Loading...
Loading...