Ivy Biomedical Systems 3000 Series, 3000T Operation Manual

Part No. 2718-55-16
OPERATION MANUAL
© 2010 IVY Biomedical Systems Inc. All Rights Reserved.
Part No. 2718-04-16
Cardiac Trigge r Monitor
Model 3000 Series

OEM OM3000
User Responsibility
This product will perform in conformity with the description thereof contained in this Operation Manual and
accompanying labels and/or inserts, when assembled, operated, maintained and repaired in accordance with the
instructions provided. This product must be checked periodically. A defective Product should not be used. Parts that
are broken, missing, plainly worn, distorted or contaminated should be replaced immediately. Should such repair or
replacement become necessary, IVY Biomedical Systems, Inc. recommends that a telephone call or written request
for service advice be made to IVY Biomedical Systems, Inc. Service Department. This product or any of its parts
should not be repaired other than in accordance with instructions provided by IVY Biomedical Systems, Inc. trained
personnel. The product must not be altered without the prior written appro val of IVY Biomedical Systems, Inc.
Quality Assurance Department. The user of this Product shall have the sole responsibility for any malfunction, which
results from improper use, faulty maintenance, improper repair, damage or alteration by anyone other than IVY
Biomedical Systems, Inc.
CAUTION: US Federal law restricts this device to sale by or on the order of a licensed medical practitioner.
Ivy Biomedical Systems, Inc. has declared that this product conforms with the Eurpean Council Directive 93/42/EEC
Medical Device Directive when its used in accordance with the instructions provided in the Operation and
Maintenace Manual.
Ivy Biomedical Systems, Inc.
11 Business Park Drive
Branford, Connecticut 06405. USA
(203) 481-4183 (800) 247-4614 FAX (203) 481-8734
www.ivybiomedical.com e-mail:sales@ivybiomedical.com
21 January 2011
2718-04-16 Rev.07
This page is intentionally le ft blank.
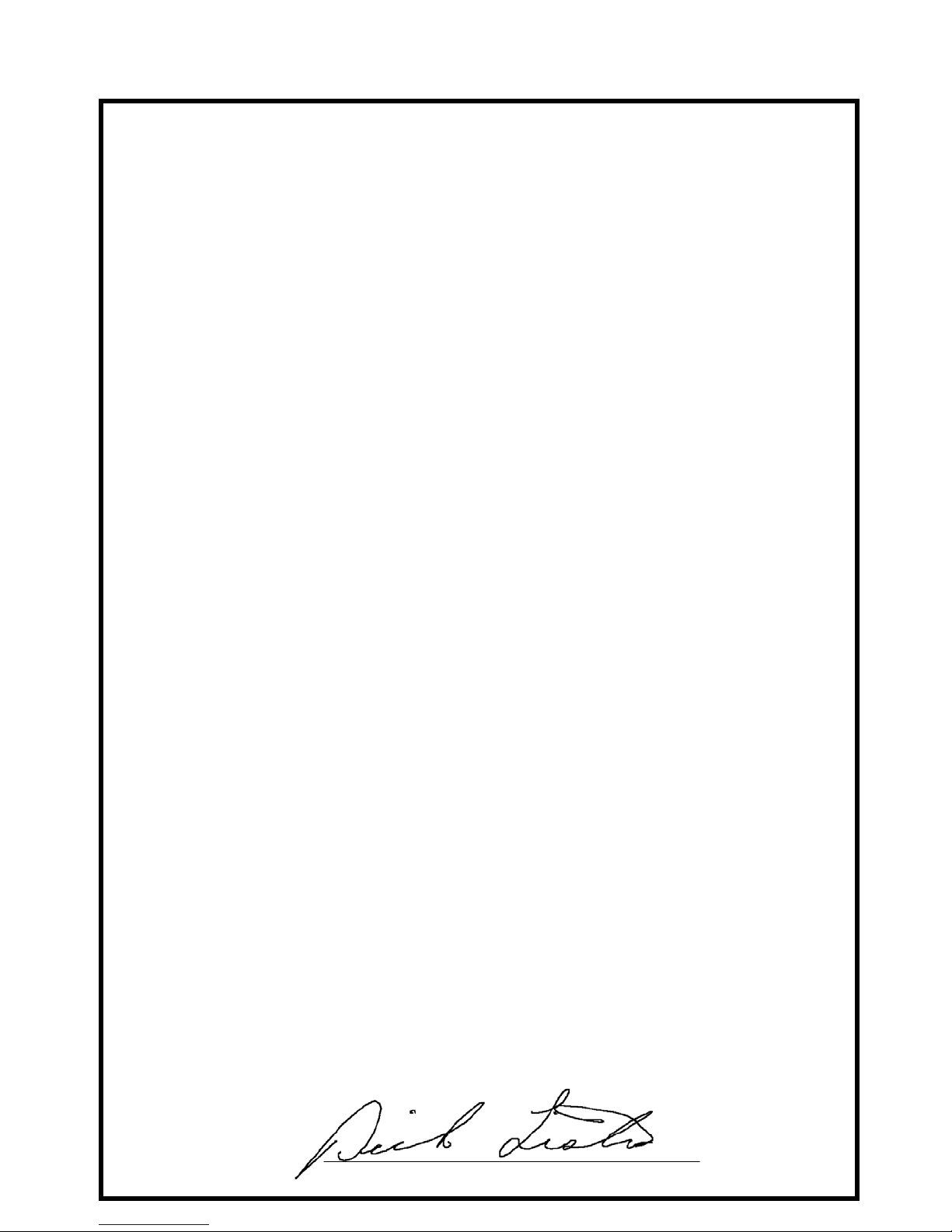
Declaration of Conformity
Manufacturer: Ivy Biomedical Systems, Inc.
11 Business Park Drive
Branford, CT 06405
Authorized Representative: Cavendish Scott Ltd.
Starlings Bridge, Nightingale Road
Hitchin, Herts, SG5 1FW, England
Type of Equipment: Physiological Monitors
Models: 3000 (Series)
We, Ivy Biomedical Systems, Inc., hereby declare that the devices mentioned above
comply with the Swedish National Board of Health and Welfare Regulation and
guidelines on medical devices LVFS 2003:11 (M) 28 October 1994 – transposing
European Medical Devices Directive 93/42/EEC.
Date of Validity: March 30, 2010
Classification: IIb According to rule No. 10
Conformity Assessment
Procedure: Annex II
Notified Body: Intertek SEMKO AB Notified Body No. 0413
Name of Authorized Signatory: Dick Listro
Position held in Company: Director of Regulatory
Signature
This page is intentionally le ft blank.

Table of Contents
Table of Contents
WARRANTY ...................................................................................................................................................... iii
INTRODUCTION ................................................................................................................................................ 1
SAFETY ................................................................................................................................................................ 2
Electrical ................................................................................................................................................................ 2
Explosion ............................................................................................................................................................... 2
Patient Connections .............................................................................................................................................. 3
MRI ........................................................................................................................................................................ 3
Pacemakers ............................................................................................................................................................ 3
Electrosurgery Protection .................................................................................................................................... 3
Defibrillation Protectio n ...................................................................................................................................... 3
EMC ....................................................................................................................................................................... 3
Electromagnetic Compatibility IEC 60601-1-2:2001 ............................................................................... 3
Description of Warning Labels ............................................................................................................................ 8
MONITOR DESCR I PTION ................................................................................................................................ 9
Classification ......................................................................................................................................................... 9
Control and Indicator s ....................................................................................................................................... 10
Basic Keys .............................................................................................................................................. 10
Programmable Keys ............................................................................................................................... 10
Menu Structure (with Polarity Control and Simulator option) ............................................................... 11
Menu Structure (without Polarity Control and Simulator option) .......................................................... 12
Display ................................................................................................................................................... 13
Alarms .................................................................................................................................................... 14
Rear Panel .............................................................................................................................................. 14
Fuse Ratings ........................................................................................................................................... 15
MONITOR SETUP ............................................................................................................................................ 16
Set up the instrument for operation .................................................................................................................. 16
Change Mains Voltage ....................................................................................................................................... 16
Set the Language ................................................................................................................................................. 16
Set Time, Date, and Audio ................................................................................................................................. 16
Trace Speed ......................................................................................................................................................... 17
Default Settings ................................................................................................................................................... 17
ALARM MESSAGES ........................................................................................................................................ 18
ECG MONITORING ......................................................................................................................................... 19
Safety Considerations ......................................................................................................................................... 19
Patient Connections ............................................................................................................................................ 20
ECG Waveform Amplitude (Size) ..................................................................................................................... 20
Lead Selection ..................................................................................................................................................... 21
AC Power Filter .................................................................................................................................................. 22
ECG Filter ........................................................................................................................................................... 22
Alarm Limits ....................................................................................................................................................... 23
Pacemaker ........................................................................................................................................................... 23
Model 3000 Operation Manual i

Table of Contents
RECORDER OPERATION .............................................................................................................................. 24
Changing Paper .................................................................................................................................................. 24
Recorder Modes .................................................................................................................................................. 25
Recorder Speed ................................................................................................................................................... 26
Example Printout ................................................................................................................................................ 26
SYNCHRONIZED OUTPUT (TRIGGER) ...................................................................................................... 27
The Synch Pulse .................................................................................................................................................. 27
Trigger-Mark Display ........................................................................................................................................ 27
Polarity Control Option ..................................................................................................................................... 28
MONITOR TESTING ........................................................................................................................................ 29
ECG Simulator (Optional) ................................................................................................................................. 29
MAINTENANCE AND CLEANING ................................................................................................................ 30
Monitor ................................................................................................................................................................ 30
Patient Cables ..................................................................................................................................................... 30
Preventive Maintenance ..................................................................................................................................... 30
ACCESSORIES .................................................................................................................................................. 31
ECG ..................................................................................................................................................................... 31
Disposal ................................................................................................................................................................ 31
SPECIFICATIONS ............................................................................................................................................ 32
ii Model 3000 Operation Manual

WARRANTY
WARRANTY
All products manufactured by Ivy Biomedical Systems, Inc. under normal use, are warranted to be free from defects
in material and workmanship and to operate within published specifications, for a period of one year from date of
original shipment.
All accessories such as patient cables and lead wires, supplied by Ivy Biomedical Systems, Inc. under normal use,
are warranted to be free from defects in material and workmanship and to operate within published specifications, for
a period of 90 days from date of original shipment.
If an examination by Ivy Biomedical Systems, Inc. discloses such product(s) or component part(s) to have been
defective, then Ivy’s obligation is limited at Ivy’s option, to repair or replacement.
When a product or products need to be returned to the manufacturer for repair or examination, contact customer
service personnel at Ivy Biomedical Systems, to obtain a Return Material Authorization number (RMA #) and the
correct packing instructions:
Customer Service
Telephone: (203) 481-4183 or (800) 247-4614.
Fax: (203) 481-8734.
E-mail: ivybio@ivybiomedical.com
All products being returned for warranty repair shall be shipped prepaid to:
Service Department
Ivy Biomedical Systems, Inc.
11 Business Park Drive.
Branford, CT. 06405. USA.
Ivy will prepay the shipment of the repaired or replacement product to customer at Ivy’s expense.
Model 3000 Operation Manual iii
WARRANTY
This page is intentionally le ft blank.
iv Model 3000 Operation Manual

INTRODUCTION
INTRODUCTION
This manual is to provide information on the correct use of the Model 3000 Cardiac Trigger Monitor. It is up to the
user to ensure that any applicable regulations regarding the installation and operation of the mo nitor are ob served.
The model 3000 is Med ic a l E le c trical Equipment intended to monitor patients under medical supervision. The model
3000 must be operated by trained and qualified medical personnel only.
Using This Manual
We recommend that you read this manual before operating the equipment. This manual is written to include all
options. If your monitor does not include all options, menu selections and display data for those options will not
appear on your monitor.
Use the Monitor Description section for general descriptions of controls and displays. For details on the use of each
option, refer to the section of the manual dealing with the appropriate option.
Boldface type is used in text to refer to the labeling on user controls. Special brackets [ ] surround menu
selections used with the programmable keys.
Manufacturer’s Responsibility
The manufacturer of this equipment is responsible for the effects on safety, reliability, and performance of the
equipment only if:
• Assembly operations, extensions, re-adjustments, or repairs are carried out by persons authorized by the
manufacturer
• The electrical installation complies with all applicable regulations
• The equipment is used in accordance with the instructions in this manual
Incorrect operation or failure of the user to maintain the monitor in accordance with proper maintenance procedures
relieves the manufacturer or his agent from all responsibility for consequent non-compliance, damage, or injury.
Ivy Biomedical Systems, Inc.
11 Business Park Drive
Branford, Connecticut 06405
(203) 481-4183 or (800) 247-4614
fax (203) 481-8734
e-mail: techline@ivybiomedical.com
This manual explains how to set up and use the Model 3000. Important safety information is located throughout the
manual where appropriate. READ THE ENTIRE SAFETY INFORMATION SECTION BEFORE YOU OPERATE
THE MONITOR.
Model 3000 Operation Manual 1
SAFETY
SAFETY
Electrical
This product is intended to be operated from a mains power source of nominally 100 to 230V~, 47 to 63 Hz and
Maximum AC Power consumption: 35VA.
WARNING: Before this monitor is plugged into any power source verify visually that the line selector switch on
the rear panel displays the appropriate voltage range for your location.
WARNING: To prevent electrical hazards to all personnel, this monitor must be properly grounded. Connect the
monitor only to a three-wire, grounded, hospital grade receptacle. The three-conductor plug must be inser t ed into a
properly wired three-wire receptacle; if a three-wire receptacle is not available, a qualified electrician must install
one in accordance with the governing electric code.
WARNING: Do not under any circumstances remove gr ounding conductor from the power plug.
WARNING: The power cable supplied with this equipment provides for this protection. Do not attempt to defeat
this protec t ion by modifying the cable or by using ungrounded adapters or extension cables. The power cord and
plug must be intact and undamaged. To disconnec t the equipment fro m the mains power unplug the power cord.
WARNING: Do not connect to an electrical outlet controlled by a wall switch or dimme r .
WARNING: If there is a ny doubt about the integrity of the protective ground conducto r arrangement, d o not
operate the monitor until the AC power source protective conductor is fully functional.
WARNING: Do not place the monitor in any position that may cause it to fall on the patient. Do not lift the monitor
by the power supply cord or patient cable.
WARNING: Electric shock hazard! Do not remove covers or panels. Refer service to qualified service
personnel.
WARNING: To avoid electrical shock, disconnect the monitor from its power so urce before changing fuses.
Replace fuses only with same type and rating T.5A, 250V (Metric 5x20mm).
WARNING: Do not clean monitor while it is on and/or plugged into a power source.
WARNING: If unit is accidentally wet, discontinue use until dry and then test unit for proper o peration before reuse
on a patient.
WARNING: This unit uses a co mmon isolation path fo r the ECG leads . Do not connect any non-isolated
accessories to the ECG input when connected to a patient, as this may compromise the safety of the unit. When
attached to other devices, insure that the total chassis leakage currents of all units do not exceed 300 μA.
Explosion
DANGER: Explosion hazard! Do not use this equipment in the presence of flammable anesthetics or other
flammable substance in combination with air, oxygen-enriched environment or nitrous oxide.
2 M odel 3000 Operation Manual

SAFETY
Patient Connec tions
Patient connections are electrically isolated. For a ll c onnections use isolated probes. Don’t let patient connections
contact other conductive parts, including ground. See i ns tructions for patient connec t ions in this manual.
Carefully route patient cables to reduce the possibility of patient entanglement or strangulation.
Leakage current is limited internally by this monitor to less than 10 μA. However, always consider cumulative
leakage current that can be caused by o ther equipment used on the patient at the same time as this monitor.
To ensure that the leakage current protection remains within the specifications, use only the patient cables specified
in this manual. This monitor is supplied with protected lead wires. Do not use cables and leads with unprotected
lead wires having exposed conductors at the cable end. Unprotected lead wires and cables may pose an
unreasonable risk of adverse health consequences or death.
Line isolation monitor transients may resemble actual cardiac waveforms and thus inhibit heart rate alarms. To
minimize this problem, ensure proper electrode placement and cable arrangement.
If an alarm condition occurs while the alarms are set to off, neither visual or audio alarms will be present.
MRI
The model 3000 should not be used within the magnet i c field during Magnetic Resonanc e Imaging.
Pacemakers
Rate meters might continue to count the pacemaker rate during occurrences of cardiac arrest or some arrhythmias.
Do not rely on rate meter alarms. Keep pacemaker patients under close surveillance.
Electrosurgery Protection
This equipment is protected against electrosurgery potentials To a vo id the potential of electrosurgery burns at
monitoring sites, ensure proper connection of the electrosurgery return circuit as described by the manufacturer’s
instructions. If improperly connected, some electrosurgery units might allow energy to return through the ECG
electrodes.
Defibrillation Protection
This equipment is protected against 360 J defibrillator discharge.. The monitor is internally protected to limit current
through the elec trodes to prevent injury to the patient and damage to the equipment as long as the defibrillator is
used in confor manc e with the manufacturer’s instructions.
EMC
This equipment has been certified to be protected to emissions and immunity according to IEC-60601-1-2.
Electromagnet ic Compatibility IE C 60601-1-2:2001
CAUTION: Medical Equipment needs special precautions regarding EMC and needs to be installed and put into
service according to the EMC information provided in the Operation Manual.
Model 3000 Operation Manual 3

SAFETY
Minimum amplitude of patient physiological signal
depending upon the type of patient cable.
Patient cable part number
Minimum amplitude
590406
1.5 mV
590317
0.5 mV
590323
0.5 mV
CAUTION: Portable and mobile RF communications equipment can affect medical electrical equipment.
WARNING: The model 3000 should not be used adjacent to or stacked with other equipment and that if adjacent or
stacked use is necessary, the Model 3000 should be observed to verify normal operation in the configuration in
which it will used.
Accessories
WARNING: The use of accessories other than those specified below may result in increased emissions or decreased
immunity of the equipment.
Part Number Description
590406 Three lead patient cable
590407 Set of three lead wires
590317 Low noise three lead patient cable
590318 Set of three radiotranslucent lead wires
590323 Low noise thre e le a d patient cable with 1kohm resistors.
The minimum amplitude or value patient physiological signal is 0.5 mV (AAMI EC-13 3.2.6.1).
WARNING: The use of the Model 3000 below the following amplitude values may cause inaccurate results:
4 M odel 3000 Operation Manual
 Loading...
Loading...