ivWatch M400 User Manual

MODEL 400
TO AID IN INTRAVENOUS INFILTRATION DETECTION
User Manual
Rx Only

SCLAIMER
DI
ivWatch, LLC, verifies that the content in this document is correct and
accurate, reflecting the features of the product available at the time
of writing. However, the content is subject to change without notice.
Typographical errors; changes to screens or images; or other, minor
device changes may occur that should not affect the understanding
or operation of this device. All dimensions and values are approximate.
NOTE : For the most current release of this manual, refer to the online
version on the ivWatch website at www.ivwatch.com/manuals.
TRADEMARKS AND PATENTS
ivWatch® is a registered trademark of ivWatch, LLC.
All rights reserved. © ivWatch, LLC, 2016
Part No. ML-0000866 REV.06
01 DEVICE DESCRIPTION 4
CONTENTS
How Monitoring and 6
Detection Work
Package Contents 7
Safety Information 8
Indications 8
Intended Use 8
Contraindications 8
Warnings 9
Precautions 10
02 INSTRUCTIONS FOR USE 12
Using the Monitor 13
Setting Up a Monitoring Run 14
Monitoring a Patient 20
IV Monitoring: Watches and Warnings 21
Patient Monitoring Using Battery Power 23
Ending a Monitoring Run 24
Continuing a Monitoring Run 25
with an Existing Patient
Turning Off the Monitor 30
History and Event Data 31
For more information, please contact:
ivWatch, LLC
1100 Exploration Way, Suite 209
Hampton, VA 23666
Tel: (855) 489-2824
Fax: (757) 645-4760
www.ivWatch.com
NOTE : For the most current release of this manual,
refer to the on line version on the ivWatch website
at www.ivwatch.com/manuals.
!
CAUTION : Federal law (USA) restricts this
device to sale by or on the order of a physician.
03 TROUBLESHOOTING 32
Testing the Sensor 33
Troubleshooting Table 35
Error Message Table 36
04 APPENDICES 40
Options Screen 41
Preparing the System for Reuse 44
Specifications 45
Symbols on the Product or Package 54
Repair and Maintenance 55
Disposal 55
MRI Safety Information 56
2
3

MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
01
The ivWatch® Model 400 provides
continuous monitoring of peripheral
IVs to aid in the detection of conditions
that may indicate an intravenous (IV)
infiltration event. The monitoring
system is an adjunct to the health care
practitioner and is in no way intended to
replace regular assessment of the IV site
or any other standardized practice for IV
administration and management.
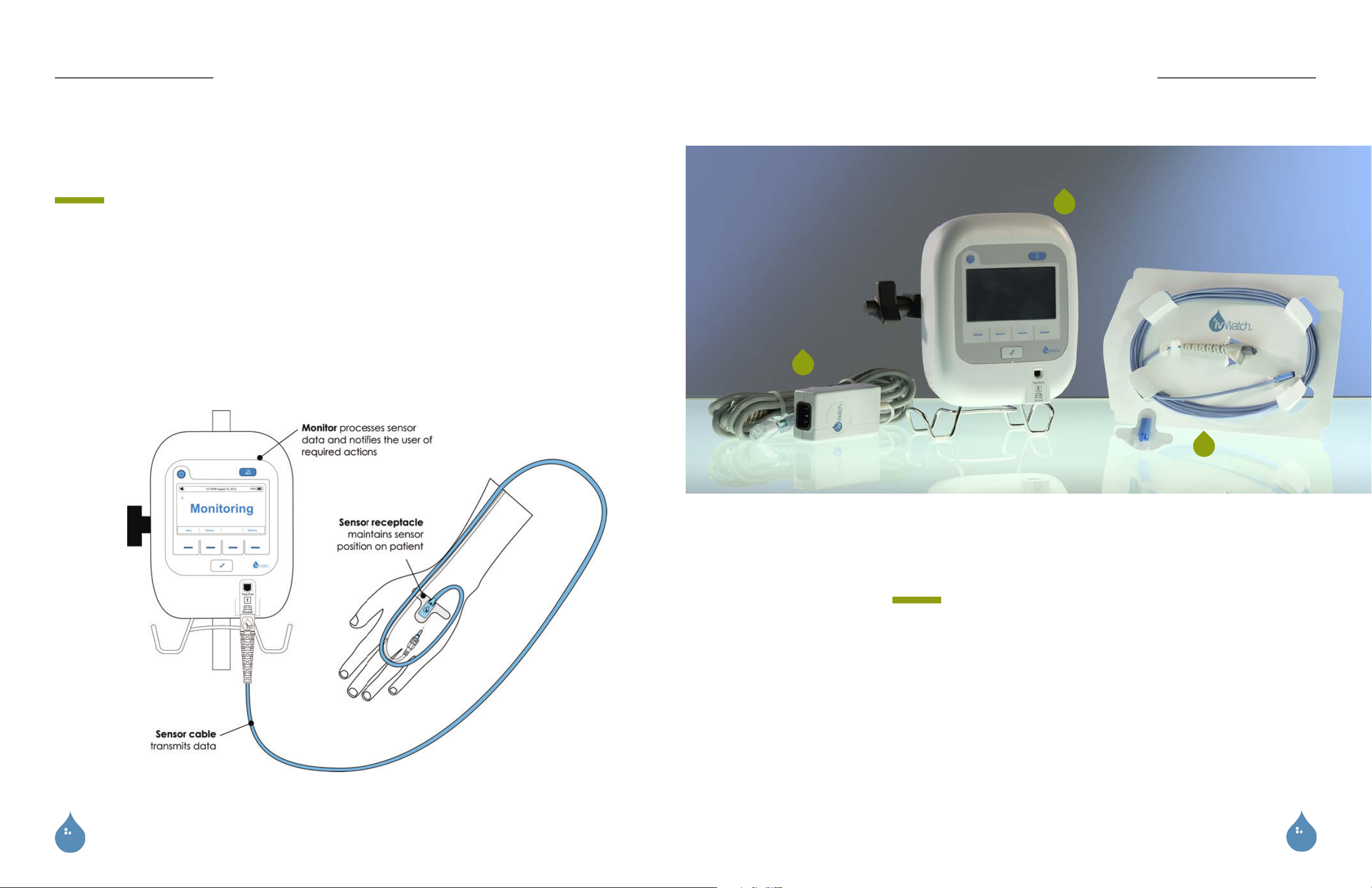
The complete system includes the
monitor, a reusable sensor cable, an
AC adapter, and a disposable adhesive
sensor receptacle used to position and
DEVICE
DESCRIPTION
DEVICE DESCRIPTION 5
HOW MONITORING AND 6
DETECTION WORK
PACKAGE CONTENTS 7
SAFETY INFORMATION 8
INDICATIONS 8
INTENDED USE 8
WARNINGS 9
PRECAUTIONS 10
secure the sensor on the patient.
DEVICE DESCRIPTION
5
MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
DEVICE DESCRIPTION DEVICE DESCRIPTION
HOW MONITORING
AND DETECTION WORK
The monitoring system uses visible and near-infrared (IR) light to measure slight
changes in the optical properties of tissue near the IV insertion site. A sensor placed
adjacent to the IV insertion site on the patient’s hand or forearm takes measurements.
The monitor provides notification (audible and visual) when it detects changes
consistent with IV fluid leaking and pooling in the tissue adjacent to the IV.
1
2
SYSTEM COMPONENTS
1. Patient Monitor
2. Adapter and Power Cord
3. Sensor Cable and Receptacle
4. Reference Card (not shown)
3
6 7

MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
DEVICE DESCRIPTION DEVICE DESCRIPTION
SAFETY INFORMATION
WARNINGS
USE OF THE DEVICE AS AN AID TO DETECTION OF SUBCUTANEOUS IV INFILTRATION
The ivWatch Model 400 must only be used as an adjunct to regular assessments of IV placement by clinicians.
The device cannot serve as a substitute for regular clinician assessment, and should not be used in the absence of standard clinical
supervision and procedures that are typically utilized (i.e. when the device is not used) for detection of subcutaneous infiltrations
and extravasations.
EXPLOSIVE ENVIRONMENTS
Do not plug in or use the monitor in an environment that contains concentrations of flammable gas (eg, anesthesia), vapors, or dust.
The following indications, warnings, and precautions are presented by
topic and should be reviewed in their entirety prior to using this monitor.
INDICATIONS
The ivWatch Model 400 is indicated for the detection of subcutaneous infiltrations and extravasations of 10cc or less of optically
clear, uncolored infusates, as an adjunctive device to the clinical evaluation in the hospital setting of patients 18 years old or greater
with peripherally-inserted IVs (PIVs) on the forearm or dorsal aspect of the hand.
The device is indicated to assess patients for subcutaneous infiltrations and extravasations but should not serve as a substitute for
regular clinician assessment of the PIV site. The ivWatch 400 is indicated for use by physicians, or under the direction of a physician,
who have been trained in the use of the ivWatch Model 400.
INTENDED USE
The ivWatch Model 400 is intended for use in monitoring IV infusion of optically clear, uncolored fluids at sites on the forearm or the
dorsal aspect of the hand in adult patients. The user profile is health care practitioners who are experienced in IV administration and
management and located at hospitals and similar medical care facilities.
CONTRAINDICATIONS
The ivWatch Model 400 is not intended for use with power injectors or for monitoring peripheral IV infusions of colored, dark or
cloudy fluids (for example, TPN, rifampin, and multi-vitamin “banana bags”). The system is not validated for use in pediatrics.
The ivWatch sensor should not be placed over tattooed, scarred, or bruised tissue. Neither the ivWatch Patient Monitor or Sensor
Cable should not be taken into an MR environment.
OXYGEN-RICH ENVIRONMENTS
Do not use the monitor in oxygen-rich environments. Note that this statement applies to oxygen enriched environments, such as
oxygen tents. It is not meant to apply to patients on breathing tubes.
IMMERSION IN LIQUID
Do not immerse the monitor or sensor cable in liquid. The monitor must be disconnected and the sensor removed from the patient
prior to patient bathing to prevent electrical shock. Other than for bathing, the monitoring run should be stopped only when the IV
is removed.
INDOOR USE
The monitoring system is designed for indoor use and should not be exposed to extreme temperatures, humidity, or moisture.
See “Specifications” on page 46 for additional information.
IV INSERTION SITE
This monitoring system was designed for peripheral IV insertion site infiltration monitoring on the forearm or the dorsal aspect
of the hand. Monitoring has not been tested for other IV placement locations.
MAGNETIC RESONANCE IMAGING (MRI)
The monitor and sensor cable pose a safety hazard if brought into the MRI environment. The receptacle contains no ferromagnetic
materials. Disconnect the sensor cable and monitor prior to taking the sensor receptacle into the MR environment. See “MRI Safety
Information” on page 56 for more information.
PEDIATRIC USE
The ivWatch Model 400 has not been cleared by the FDA for use in pediatric populations.
USE OF INTACT SKIN ONLY
The ivWatch sensor receptacle and sensor cable should only make contact with intact skin.
CHANGES OR MODIFICATIONS TO PRODUCT
Changes or modifications to the ivWatch Model 400 not expressly approved by ivWatch could void the user's
authority to operate the equipment.
8 9

MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
MODEL 400 PATIENT MONITOR
USER MANUAL /
01 DEVICE DESCRIPTION
DEVICE DESCRIPTION DEVICE DESCRIPTION
PRECAUTIONS
IV INFUSIONS OF OPTICALLY CLEAR, UNCOLORED FLUIDS
Use this monitoring system only for monitoring infusions of optically clear, uncolored fluids; examples of these include saline and
saline-based solutions, sugar solutions (D5W), Lactated Ringer’s solution, and colorless crystalloid solutions. The device has not
been tested for monitoring infusions of dark, colored or cloudy fluids (for example, TPN, rifampin, and multi-vitamin “banana bags”).
Dark, colored or cloudy fluids block light and may reduce the system’s sensitivity.
CABLE POSITIONING
Route the sensor cable and power cord to reduce the possibility of equipment or patient entanglement. Place excess cable length so
that it does not pose a hazard.
CLEANING
Do not immerse the monitor or the sensor in liquid. Always disconnect the monitor from the power supply prior to cleaning. Clean the
system components as directed in “Preparing the System for Reuse” on page 44.
COMPATIBILITY
Use only components that are manufactured by ivWatch, LLC. Monitors, sensors, and receptacles made by other manufacturers have
not been tested and may reduce the system’s sensitivity.
DISPOSAL
Dispose of the packaging and material components according to local regulations.
EQUIPMENT MODIFICATION
Do not modify components of the monitoring system (eg, remove the ground pin on the electrical plug). Modifications could result
in increased electrical hazard, unknown changes in product performance, and potential risk to the user and patient.
EXCESSIVE LIGHT
Use the monitoring system in normal to low-light conditions. Detection of infiltration events depends on the transmission of light
through the patient’s skin; as such, excessive ambient light over the sensor area may activate a notification on the monitor to change
the lighting conditions. Failure to change the lighting conditions may degrade the system’s performance.
LIGHT-BLOCKING BARRIERS
Do not place a dressing under the sensor receptacle. Light-blocking barriers (eg, bandage) between the patient’s skin and the sensor
may reduce the system’s sensitivity.
PATIENT MOVEMENT
Minimize patient movement during a monitoring run to reduce the possibility of sensor displacement.
PRESCRIPTION ONLY
Federal law (USA) restricts this device to sale by or on the order of a physician.
REUSE
Do not reuse any of the components originally provided as a sterile product.
SENSOR CABLE DAMAGE
Do not kink or compress the sensor cable. Kinking or compressing the cable could damage the sensor, activating a notification on
the monitor to test the sensor cable. Kinking or compressing the cable could also result in the exposure of glass fibers which can pose
a safety risk.
SENSOR INTERFERENCE
Sensor placement should not be on the same arm as a blood pressure cuff or arterial blood pressure measurement device.
STERILIZATION
Do not sterilize any components of the monitoring system. Sterilization may damage or reduce the system’s sensitivity.
TATTOOED, SCARRED, OR BRUISED TISSUE
Do not place the sensor receptacle over tattooed, scarred, or bruised tissue as these conditions may reduce the system’s sensitivity.
TRAINED HEALTH CARE PRACTITIONER
Do not rely solely on the monitoring system for IV monitoring. All monitoring runs should be managed by a trained health care
practitioner who is experienced in IV administration and management. The monitoring system is not intended to replace IV
monitoring by a trained health care practitioner.
STACKING THE IVWATCH MODEL 400 MONITOR WITH OTHER EQUIPMENT
Do not use the ivWatch Model 400 adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, observe the
ivWatch Model 400 to verify normal operation in the configuration in which it will be used.
10 11
MODEL 400 PATIENT MONITOR
USER MANUAL /
02 INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
The monitor should be fully charged prior to its first use. Inspect the components
for visible damage prior to use. If desired, test the sensor cable
(see “Testing the Sensor” on page 33) to see if the sensor is damaged.
02
INSTRUCTIONS
FOR USE
INSTRUCTIONS FOR USE 13
USING THE MONITOR 13
SETTING UP A MONITORING RUN 14
MONITORING A PATIENT 20
IV MONITORING WATCHES AND WARNINGS 21
PATIENT MONITORING USING BATTERY POWER 23
ENDING A MONITORING RUN 24
USING THE MONITOR
The monitor attaches to a standard IV pole; the monitor may be placed on the pole at a height of up to 7 feet. A grounded wall outlet
is required to recharge the monitor battery.
NOTE : The power port and USB port are on the back of the monitor. See “Specifications” on page 45 for additional information about the
monitor ports and connectivity.
CONTINUING A MONITORING RUN 25
WITH EXISTING PATIENT
TURNING OFF THE MONITOR 30
HISTORY AND EVENT DATA 31
The monitor can be used remotely on battery power for limited periods of time, but during normal use, the monitor should be
powered by the medical-grade AC power supply. See “Patient Monitoring Using Battery Power” on page 23 for more information.
INSTRUCTIONS FOR USE
13
MODEL 400 PATIENT MONITOR
USER MANUAL /
02 INSTRUCTIONS FOR USE
MODEL 400 PATIENT MONITOR
USER MANUAL /
02 INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE
SETTING UP A MONITORING RUN
Begin a monitoring run after the IV catheter has been placed in
accordance with facility protocols for IV administration and management.
IMPORTANT NOTE : Allow the patient monitor to run in monitoring mode for at least 1 minite before starting the infusion.
This allows the monitor to take critical baseline readings.
TO SET UP A MONITORING RUN, COMPLETE THE FOLLOWING STEPS:
1. Use the clamp to securely mount the monitor on the IV pole.
2. Position the monitor so that the display is easily viewed.
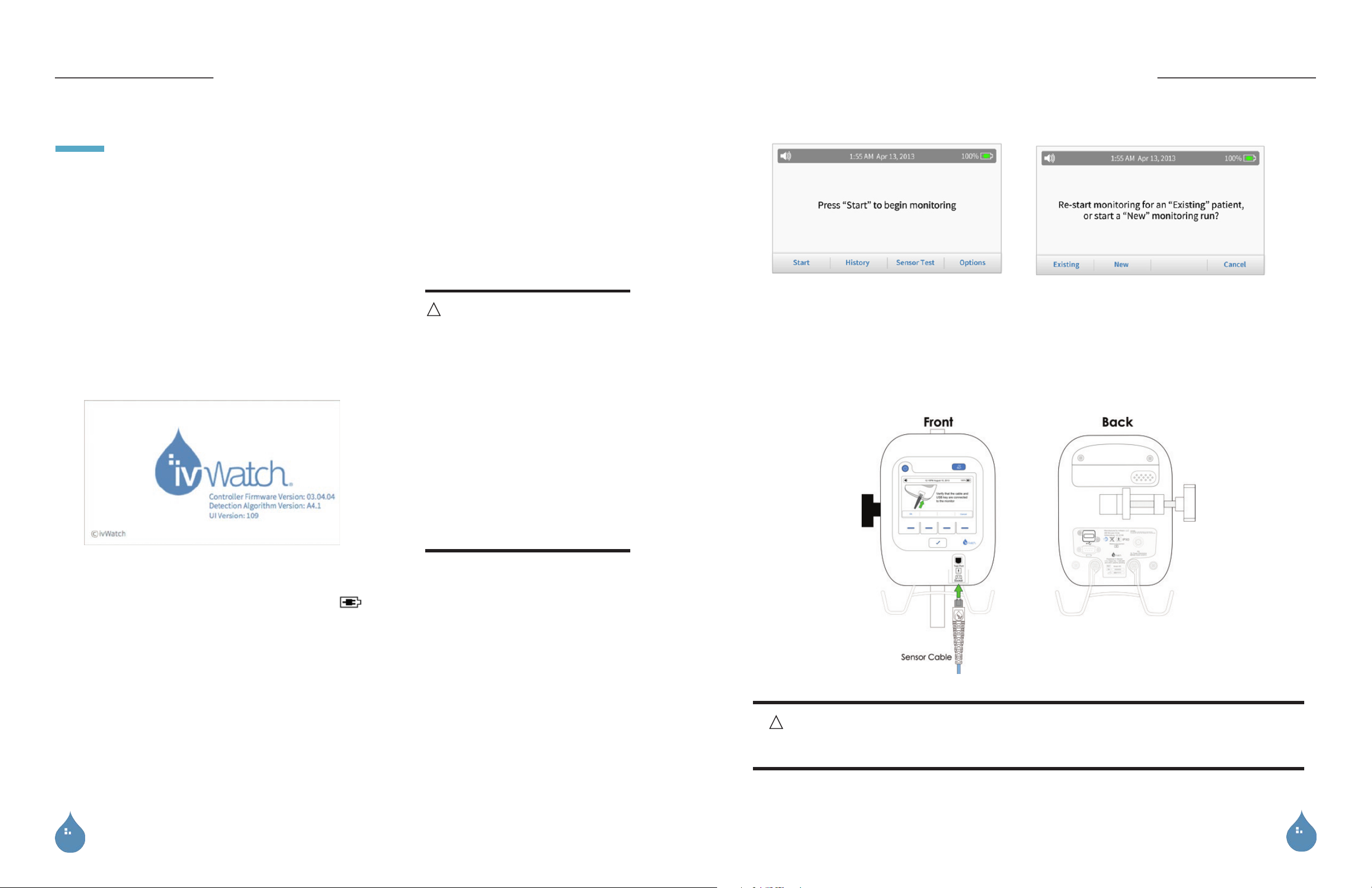
3. Press Power on the front of the monitor. The Start-up screen
appears briefly.
Start-up Screen
CAUTION :
!
• Use this monitoring system only with
optically clear fluids, uncolored fluids. Refer
to the Precautions section of this manual for
examples of colored, dark or cloudy fluids
that should not be used with the monitoring
system as they may reduce the system’s
sensitivity.
• Do not place a dressing under the sensor
receptacle. Light-blocking barriers (eg,
bandages and occlusive dressings) between
the patient’s skin and the sensor may reduce
the system’s sensitivity.
• Do not place the sensor over tattooed,
bruised, or scarred tissue as these
conditions may reduce the system’s
sensitivity.
6. Press the Start key to begin the monitoring run.
NOTE : See “Continuing a Monitoring Run with an Existing Patient” on page 25 if the monitoring run is for an existing patient.
8. Confirm that the patient is new by pressing the Yes key.
9. Connect a sensor cable to the monitor (as shown on the display).
7. Press the New key to start a new monitoring run.
4. If the monitor does not turn on, insert the power plug into the power port on the back of the monitor and connect the electrical
plug to a grounded wall outlet. Verify that the battery symbol
NOTE : During normal operation, the monitor should be plugged into a power source, but for ambulatory activities, a fully charged
monitor can be used on battery power for extended periods of time. See “Patient Monitoring Using Battery Power” on page 23.
The volume and display brightness settings are demonstrated when the monitor is turned on.
NOTE : These and other settings can be changed on the Options screen. (Follow the instructions on the display, or see “Options” on
page 41).
5. Check the time, date, and battery status as displayed on the top of the Home screen.
NOTE : The date and time should be verified prior to beginning a monitoring run. The time cannot be changed after a monitoring
run has started. The
Time & Date
option is not available during a monitoring run to prevent errors in data collection.
shows that the monitor is charging.
!
CAUTION : If you are using a new sensor cable (one that was just removed from the packaging), be sure to remove any
clear plastic caps covering the optical interface of the fibers prior to inserting that end of the sensor cable into the sensor
port on the monitor.
14 15
MODEL 400 PATIENT MONITOR
USER MANUAL /
02 INSTRUCTIONS FOR USE
MODEL 400 PATIENT MONITOR
USER MANUAL /
02 INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE
a. If the cable has been damaged (or if damage is suspected), press Cancel to return to the Home screen.
b. From the Home screen, press Sensor Test to test the integrity of the sensor. (Follow the instructions on the display, or
see “Testing the Sensor” on page 33.)
c. Resume the monitoring run setup at step 9 with a working sensor cable.
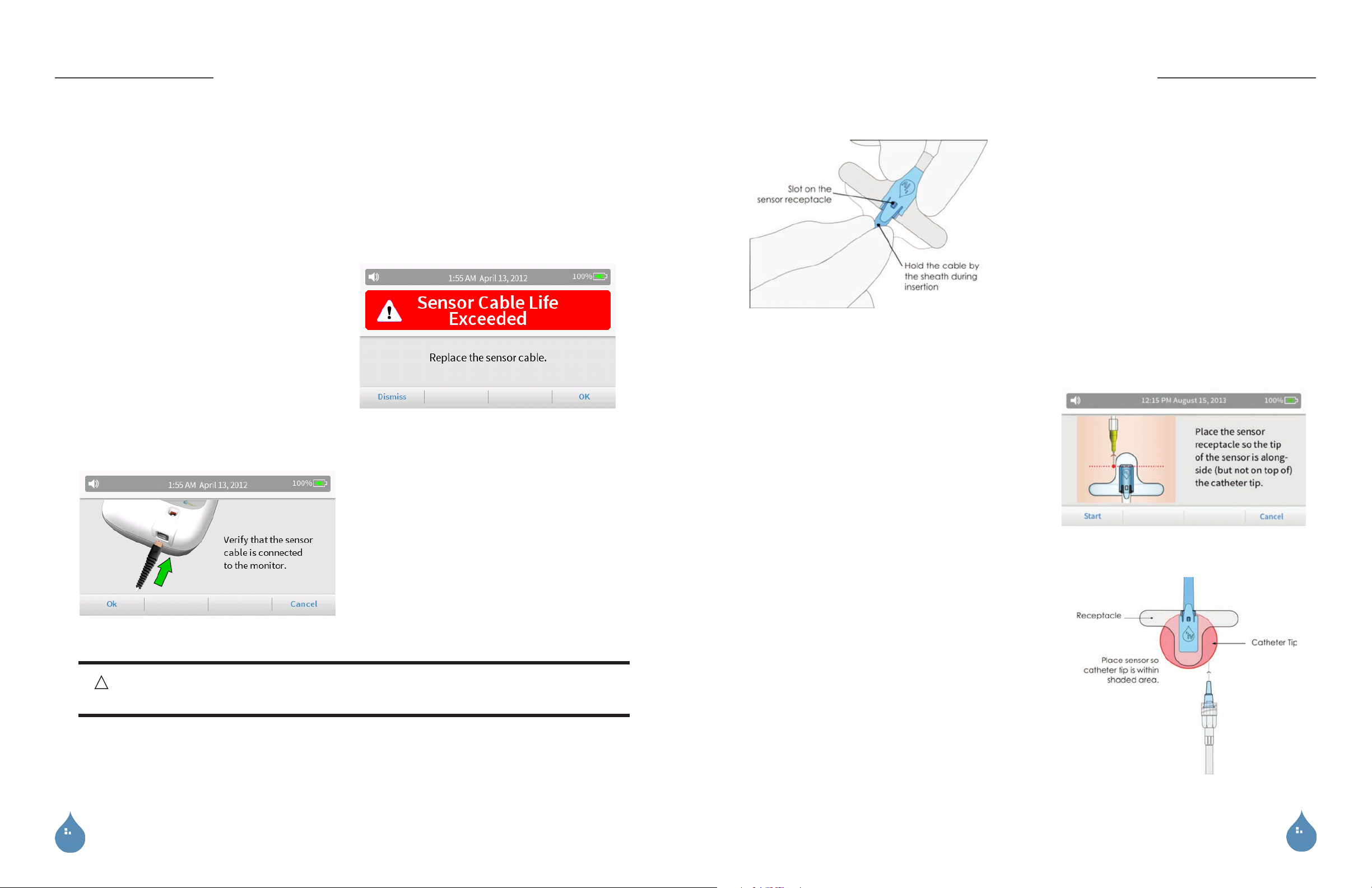
NOTE : In order to maximize the sensitivity of the
ivWatch system, each sensor cable is limited to 10
catheter line days (or 240 hours) of monitoring. The
system tracks the amount of time that a sensor cable
has been used for monitoring. Whenever a monitoring
run is started, the system performs a check to see if the
cable has exceeded its usage limit. When the system
detects that a sensor cable has exceeded its usage limit,
a Sensor Cable Life Exceeded error will be displayed.
Replace the sensor cable if prompted by this error.
NOTE : Ensure that the tab on the sensor snaps into the
slot of the sensor receptacle.
12. Placing the sensor:
a. Properly route the sensor cable from the monitor to the
patient.
b. Position the sensor receptacle adjacent to the catheter
tip (as shown on the display). Do not stretch the
receptacle during application.
11. Insertion of the sensor into the sensor receptacle:
a. Ensure that the sensor is clean prior to use. If
necessary, wipe the sensor head with isopropyl
alcohol and allow it to dry.
b. Using proper sterile technique, open a new sterile
ivWatch sensor receptacle.
c. Remove the sensor receptacle from the package.
d. Hold the sensor cable by the sheath to prevent
damage to the cable during insertion.
e. Fully insert the sensor tip into the sensor receptacle.
10. Press OK to confirm sensor cable connection.
!
CAUTION : Route the sensor cable to reduce the possibility of equipment or patient entanglement. Place excess cable
length so that it does not pose a hazard.
c. Position the sensor receptacle so that the tip of the
catheter is located in the shaded area shown in the
figure to the right.
NOTES : Avoid placement of the sensor directly over a large
vein for best performance. The sensor receptacle should not
be removed and replaced. If it must be removed, use a new
sensor receptacle for that patient.
16 17
 Loading...
Loading...