Itamar Medical WatchPAT 200 Operation Manual

WatchPAT™200
Operation Manual
Itamar Medical REF OM2196330
Caution: Federal (U.S.) law restricts
this device to sale by, or on the order of, a
physician. Not for pediatric use.
Copyright 2002-2016 By Itamar Medical Ltd.
WatchPAT™ and PAT® are trademarks of Itamar Medical, Ltd.

This manual and the information contained herein are confidential and are the sole property
of Itamar Medical Ltd. Only Itamar Medical Ltd. or its licensees have the right to use
this information. Any unauthorized use, disclosure or reproduction is a direct violation of
Itamar Medical’s proprietary rights.
DISCLAIMER
Itamar Medical Ltd. shall not be held responsible in any manner for any bodily injury
and/or property damage arising from operation or use of this WatchPAT™200 device other
than that which adheres strictly to the instructions and safety precautions contained herein
and in all supplements hereto and according to the terms of the warranty provided in the
License Agreement in Appendix C.
Itamar Medical Ltd.
9 Halamish St., P.O. Box 3579
Caesarea Ind. Park, 3088900, Israel
Tel: International + 972-4-617-7000, US 1-888-7ITAMAR
Fax + 972 4 627 5598
www.itamar-medical.com
This product and/or method of use, is covered by one or more of the following US patents: 6319205,
6322515, 6461305, 6488633, 6916289, 6939304, 7374540, as well as any pending US patent applications and
corresponding patents and/or applications filed in other countries.
ISO 9001:2008 and EN ISO 13485:2012
See appendix D for contact information of the regulatory authorized representative
WatchPAT™200 System i Operation Manual
Edition
Date
Description
Chapter
Pages
Resp.
0
March 2008
Preliminary
All
All 1
June 2008
Sleep Stages and AHI
All
All
2
July 2008
ISO logo, list of standards, Medes
address, pictures
All
All
Bonita
3
Feb 09
Updating:
Itamar Medical address
List of standards
Labeling
-, 11
1.6
1.10.1
i, 47
3
7
Orit
4
Oct 09
Update for bracelet and multi-night
support
Bonita
5
July 2011
Standards, Preparing for use,
Specifications table and minor
wording changes.
SpO2 accuracy
App. G
3, 35, 47
Bonita
6
March 2012
New PCB version with new
connectors
Fixed typos and syntax errors.
Changed figures:
1, 2, 6, 12, 13,
15, 17, 18, 19, 21,
23, 24, 25, 28, 35,
37, App. B
3, 5, 8, 1013, 16, 17,
19, 22, 24,
26-29, 3235, 38, 45,
47, 49
Bonita
7
Feb 2013
Updating standards,
Device compliance, symbols, Labels,
specifications
1.5-1.7
1.11-1.12
10
2-4, 7-8, 48
Orit Kelner
8
May 2013
Updated for Itamar SBP sensor
Updating standards
1.1, 1.2, 1.4, 2.1,
2.2, 3.5, 4.1. 7.1,
App. A
1.6
1, 2, 9, 11,
13, 24, 26,
37, 50-54
3
Bonita
9
July 2013
Updating WATCHPAT™ device label
1.11, 1.12
7-8
Orit Kelner
10
Sep 2013
Updating logo of ISO symbol
Updating PAT® probe – Bottom tab
optional
-,
7.4, 8.3
i ,
40, 44
Bonita Dean
11
Feb 2014
Clarification of exclusion criteria
1.3 2 Efrat Litman
12
March 2014
Updating zip code
QR Code
-,
App C
App H
i ,
63
70
Ido Abraham
13
July 2014
Notes – trademarks
latest version of this OM,
Added trademark symbol
Clarifying 2nd exclusion criteria
Updating Standards, symbols/labels
Clarification of instruction: Attaching
the probe, applying SBP
Update SBP Cleaning instructions
Temp./humidity parameters update
Medes and Itamar’s addresses
Update QR code table
-,
All
1.3
1.6, 1.11, 1.12
7.4, 8.3 (6), ,
App A
6.1, 6.6
10, App. A
App. C, App. D, App. H
i
ii
All
2
3, 7-8
40, 45,
51
31, 32
49,51
65, 66 ,i
71
Ido Abraham
Shelly Ron
Bonita Dean
Record of Editions
WatchPAT™200 System ii Operation Manual
14
Feb 2015
Updating Caution and Warning
symbols
Adding warning
Adding Environmental protection
section
Adding cross reference
Updating Manufacturing Declarations
Adding Nonin Module specifications
Adding spare parts list
All
1.9
1.11
2.1
App.F
App.G
App.I
All
5
7
10
67-70
71
74
Orit Kelner
15
July 2015
Add FDA #K to section 1.14
1.14
8
Efrat Litman
16
Jan 2016
Updating company logo
Updating - Symbols Used on the
Product Labels, adding WEEE symbol
-
1.12
- 8 Orit Kelner
Note: Latest version of the WatchPAT™ system Operation Manual is available at:
http://www.itamar-medical.com/Support/Downloads.html
WatchPAT™200 System iii Operation Manual

Table of Contents
1 GENERAL INFORMATION ..................................................... 1
1.1 Intended use / Indications for Use ......................................................... 1
1.2 Restrictions for Use ................................................................................ 1
1.3 Exclusion Criteria .................................................................................... 2
1.4 Data Generated by the WatchPAT™200 Device ................................... 2
1.5 Equipment Classification ....................................................................... 2
1.6 Quality Assurance System: ISO 9001 & ISO 13485 .............................. 3
1.7 CE and CSA Compliance ........................................................................ 4
1.8 Conventions Used in this Manual .......................................................... 4
1.9 Warnings, Cautions and Notes .............................................................. 5
1.10 Safety Precautions .................................................................................. 6
1.11 Environmental protection ....................................................................... 7
1.12 Symbols Used On the Product Labels .................................................. 7
1.13 WatchPAT™200 Device Labels .............................................................. 8
1.14 FDA information ...................................................................................... 8
2 OVERVIEW ............................................................................. 9
2.1 System Description ............................................................................... 10
2.2 User Interaction with the WatchPAT™ Device Keys .......................... 11
2.3 WatchPAT™ Device Function .............................................................. 13
2.4 Built-In Self-Diagnostic Procedures .................................................... 14
3 PREPARATION FOR SLEEP STUDY ...................................19
3.1 Charging the Battery ............................................................................. 19
3.2 Preparing the Oximetry Sensor ........................................................... 20
3.3 Preparing the Snore and Body Position Sensor ................................ 22
3.4 Preparing the Wrist Strap ..................................................................... 22
3.5 Mounting the WatchPAT™ Device on the Wrist Strap ....................... 23
3.6 Replacing the PAT® Probe.................................................................... 23
3.7 Preparing the WatchPAT™ Device for a New Study .......................... 24
3.8 Testing the WatchPAT™ Device .......................................................... 24
3.9 WP200 Device Self-diagnostic Test Results and Trouble-shooting . 24
3.10 Packing the Carrying Case ................................................................... 25
4 OPTIONAL FUNCTIONS .......................................................26
4.1 Using the Integrated Snore & Body Position Sensor ......................... 26
4.2 Tamper-Proof Testing with WatchPAT™ Device ................................ 26
4.3 Multi-night study ................................................................................... 29
5 DATA DOWNLOAD AND ANALYSIS ...................................30
6 MAINTENANCE .....................................................................31
WatchPAT™200 System iv Operation Manual

6.1 Cleaning ................................................................................................. 31
6.2 Cleaning the WatchPAT™ Device........................................................ 31
6.3 Cleaning the Oximetry Sensor ............................................................. 31
6.4 Cleaning the Wrist Strap ...................................................................... 32
6.5 The PAT® Probe ..................................................................................... 32
6.6 The Snore & Body Position Sensor ..................................................... 32
6.7 Handling ................................................................................................. 32
6.8 Replacing the Oximetry Sensor ........................................................... 32
6.9 Replacing the PAT® Probe Cable ......................................................... 33
6.10 Replacing the Battery ........................................................................... 34
6.11 Setting the Time and Date of the WatchPAT™ Device ...................... 35
6.12 Storing the WatchPAT™ Device .......................................................... 36
7 APPLYING THE WATCHPAT™ DEVICE ..............................37
7.1 Preparing for Use of the WatchPAT™ Device .................................... 37
7.2 Applying the WatchPAT™ Device ....................................................... 38
7.3 Applying the Oximetry Sensor ............................................................. 39
7.4 Attaching the PAT® Probe .................................................................... 40
7.5 Switching On the WatchPAT™ Device ................................................ 42
7.6 When You Wake Up .............................................................................. 42
7.7 Important Notes..................................................................................... 42
8 PATIENT TRAINING – GUIDELINES ....................................44
8.1 Walk Through the Process of Using the WatchPAT™ Device .......... 44
8.2 Product Introduction ............................................................................. 44
8.3 Applying the WatchPAT™ Device ....................................................... 44
8.4 Switching on the WatchPAT™ Device ................................................ 45
8.5 Removing the WatchPAT™ Device ..................................................... 45
8.6 Patient Training ..................................................................................... 46
8.7 Review Safety, General and Functional Issues .................................. 46
9 TROUBLESHOOTING GUIDE ...............................................47
9.1 Operator Error Messages ..................................................................... 47
9.2 Patient Error Messages ........................................................................ 48
10 SPECIFICATIONS .................................................................49
APPENDIX A: WP200 INTEGRATED SNORING + BODY
POSITIONING SENSOR OPERATING INSTRUCTIONS ..................50
APPENDIX B: TAMPER-PROOF TESTING WITH THE WP200 .......55
APPENDIX C: LICENSE AGREEMENT ...........................................59
APPENDIX D: REGULATORY REPRESENTATIVE.........................65
APPENDIX E: DESCRIPTION OF THE WATCHPAT™200 PROBE 66
WatchPAT™200 System v Operation Manual

APPENDIX F: MANUFACTURING DECLARATION ACCORDING TO
IEC 60601-1-2 ....................................................................................67
APPENDIX G: SPO2 ACCURACY IN THE WP200 ...........................71
APPENDIX H: TRAINING RESOURCES ...........................................73
APPENDIX I: SPARE PARTS LIST ...................................................74
WatchPAT™200 System vi Operation Manual

List of Figures
Figure 1 – Packed Device ................................................................................... 11
Figure 2 – WatchPAT™ Device with Sensors ................................................... 11
Figure 3 – The Buttons and Display .................................................................. 12
Figure 4 – Service Ports and Peripherals .......................................................... 13
Figure 5 – WatchPAT™ Wrist with Oximetry Module ....................................... 13
Figure 6 – Charging the WatchPAT™ device .................................................... 19
Figure 7 – Oximetry Sensor ................................................................................ 21
Figure 8 – Preparing the Nonin Oximetry Sensor ............................................ 21
Figure 9 – Wrist Strap ........................................................................................ 22
Figure 10 – Disconnecting the Probe ................................................................ 23
Figure 11 – Probe Disconnected ........................................................................ 23
Figure 12 – A WatchPAT™ Device Fully Prepared ........................................... 24
Figure 13 - The Snoring and Body Position Sensor ......................................... 26
Figure 14 – WatchPAT™ Device with Tamper-Proof Bracelet ......................... 27
Figure 15 – Bracelet on Patient's Hand ............................................................. 27
Figure 16 – WatchPAT™ Device with Cable for Bracelet ................................ 28
Figure 17 – WatchPAT™ Device with Bracelet ................................................. 28
Figure 18 – Bracelet and WatchPAT™ Device on a Patient’s Hand ................ 28
Figure 19 – Cut the Bracelet at the Specified Location .................................... 29
Figure 20 – Case for a 3 Night Multi-Night Study ............................................. 29
Figure 21 – Replacing Oximetry Sensor ............................................................ 33
Figure 22 – PAT® Probe with Screw ................................................................... 34
Figure 23 – Replacing the PAT® Probe .............................................................. 34
Figure 24 – Replacing the Battery ...................................................................... 35
Figure 25 – Finger Designation .......................................................................... 38
Figure 26 – Putting On the Wrist Strap .............................................................. 38
Figure 27 – Wearing the WatchPAT™ Device ................................................... 38
Figure 28 – Removing Adhesive Cover ............................................................. 39
Figure 29 – Positioning Oximetry On Ring Finger ............................................ 39
Figure 30 – Fold Top Flap and Short Flap ......................................................... 39
Figure 31 – Wrap The Long Flap ........................................................................ 39
Figure 32 – Flexiwrap Line Indication ................................................................ 40
Figure 33 – Placing Finger in PAT® Probe ......................................................... 41
Figure 34 – Removing TOP Tab ......................................................................... 41
Figure 35 – Removing BOTTOM Tab ................................................................. 41
Figure 36 – Wearing the WP200 – Ready for Sleep .......................................... 41
List of Tables
Table 1 – Operator Troubleshooting .................................................................. 47
Table 2 – Patient Troubleshooting ..................................................................... 48
Table 3 – WatchPAT™200 Device Specifications ............................................. 49
WatchPAT™200 System vii Operation Manual

1 GENERAL INFORMATION
This manual is part of the WatchPAT™200 system.
1.1 Intended use / Indications for Use
The WatchPAT™200 (WP200) device is a non-invasive home care device for use with
patients suspected to have sleep related breathing disorders. The WP200 is a diagnostic
aid for the detection of sleep related breathing disorders, sleep staging (Rapid Eye
Movement (REM) Sleep, Light Sleep, Deep Sleep and Wake), snoring level and body
position. The WP200 generates a peripheral arterial tonometry ("PAT®") Respiratory
Disturbance Index ("PRDI"), Apnea-Hypopnea index ("PAHI"), PAT sleep staging
identification (PSTAGES) and optional snoring level and body position discrete states
from an external integrated snoring and body position (SBP) sensor. The WP200’s
PSTAGES and SBP provide supplemental information to its PRDI/PAHI. The
WP200’s PSTAGES and SBP are not intended to be used as the sole or primary basis
for diagnosing any sleep related breathing disorder, prescribing treatment, or
determining whether additional diagnostic assessment is warranted.
The WatchPAT™200 device is not indicated for children less than 17 years old.
1.2 Restrictions for Use
1. The WP200 should be used only in accordance with physician’s instructions. For
exclusion criteria see Section 1.3.
2. Only qualified medical personnel may authorize the use of the WP200.
3. Qualified medical personnel must instruct the patients how to attach and use the
WP200 prior to use.
4. In the event of equipment malfunction all repairs should be executed by authorized
Itamar Medical Ltd. personnel or licensed service agents.
5. The eligibility of a patient for a PAT® study is entirely at the discretion of a
physician, and is generally based upon the patient’s medical status.
6. The WP200 system in whole, or in part, may not be modified in any way.
7. The WP200 is used as an aid for diagnostic purposes only, and should not be used
for monitoring.
8. Only suitably trained and qualified personnel should be authorized to prepare the
WP200 equipment prior to use.
9. The WP200 Operation Manual should be carefully studied by the authorized
operators, and kept where it is easily accessible. Periodic review of the Manual is
recommended.
10. Itamar Medical Ltd. makes no representation whatsoever, that the act of reading the
Manual renders the reader qualified to operate, test or calibrate the system.
11. The tracings and calculations provided by the WP200 system are intended as tools
for the competent diagnostician. They are explicitly not to be regarded as a sole
incontrovertible basis for clinical diagnosis.
WatchPAT™200 System 1 Operation Manual

12. In the event that the system does not operate properly, or if it fails to respond to the
controls in the manner described in this Manual, the operator should refer to the
Troubleshooting section. If necessary, contact our service office to report the
incident, and to receive further instructions.
13. The step by step instructions for the patient should be carefully followed when
attaching the unit to the patient.
14. The WP200 device is not indicated for children less than 17 years old.
1.3 Exclusion Criteria
The WatchPAT™200 device should not be used in the following cases:
1. Use of one of the following medications: alpha blockers, short acting nitrates (less
than 3 hours before the study).
2. Permanent pacemaker: atrial pacing or VVI without sinus rhythm.
3. Sustained* non-sinus cardiac arrhythmias.
* In cases of patient having accumulative time of regular R-R intervals of less than 1.5
hours, the WP200 will not have sufficient valid PAT® signal as required to generate a sleep
report.
1.4 Data Generated by the WatchPAT™200 Device
The WatchPAT™200 device generates a PAT® respiratory disturbance index (“PRDI”)
and its derivative, the PAT® Apnea-Hypopnea Index (“PAHI”) and PAT® sleep staging
identification ("PSTAGES"). The PAHI and PRDI are estimates of conventional RDI
and AHI values and REM, DEEP SLEEP, LIGHT SLEEP, and WAKE stages
identification that are produced by polysomnography (“PSG”). The WatchPAT™200
device also generates optional acoustic decibel detector used for snoring level and
body position discrete states from an external integrated snoring and body position
(SBP) sensor.
1.5 Equipment Classification
The WatchPAT™200 device is a Class IIa medical device under MDD 93/42/EEC,
2007/47/EC Annex IX rule 10.
WatchPAT™200 System 2 Operation Manual
STANDARD
#
1.
Medical electrical equipment – Part 1: General requirements
for basic safety and essential performance
IEC 60601-1
2.
Medical electrical equipment – Part 1-2: General
requirements for basic safety and essential performance Collateral standard: Electromagnetic compatibility Requirements and tests
IEC 60601-1-2
3.
Medical Device Software – Software Life Cycle Processes
IEC 62304
4.
Medical electrical equipment - Part 1-4: General
requirements for safety – Collateral Standard:
Programmable electrical medical systems
IEC 60601-1-4
5.
Medical electrical equipment -- Part 1-11: General
requirements for basic safety and essential performance -Collateral standard: Requirements for medical electrical
equipment and medical electrical systems used in the home
healthcare environment
IEC 60601-1-11
6.
Degrees of protection provided by enclosures (IP Code) –
IP22
IEC 60529
7.
Quality management systems - requirements
ISO 9001:2008
8.
Medical devices. Quality management systems.
Requirements for regulatory purposes
EN ISO 13485:2012
9.
Medical devices - Quality management systems Requirements for regulatory purposes (Health Canada)
CAN/CSA-ISO 13485
:2003
10.
Medical devices. Application of risk management to
medical devices
ISO 14971
11.
Medical devices. Symbols to be used with medical device
labels, labelling and information to be supplied. General
requirements
ISO 15223-1
12.
Symbols for use in the labelling of medical devices
EN 980
13.
Graphical symbols for electrical equipment in medical
practice
IEC TR 60878
14.
Graphical symbols - Safety colours and safety signs -Registered safety signs; refer to instruction manual/ booklet
ISO 7010-M002
15.
Information supplied by the manufacture with medical
devices
EN 1041
16.
Biological evaluation of medical devices - Part 1:
Evaluation and testing
ISO 10993-1
17.
Medical devices - Application of usability engineering to
medical devices
BS EN 62366
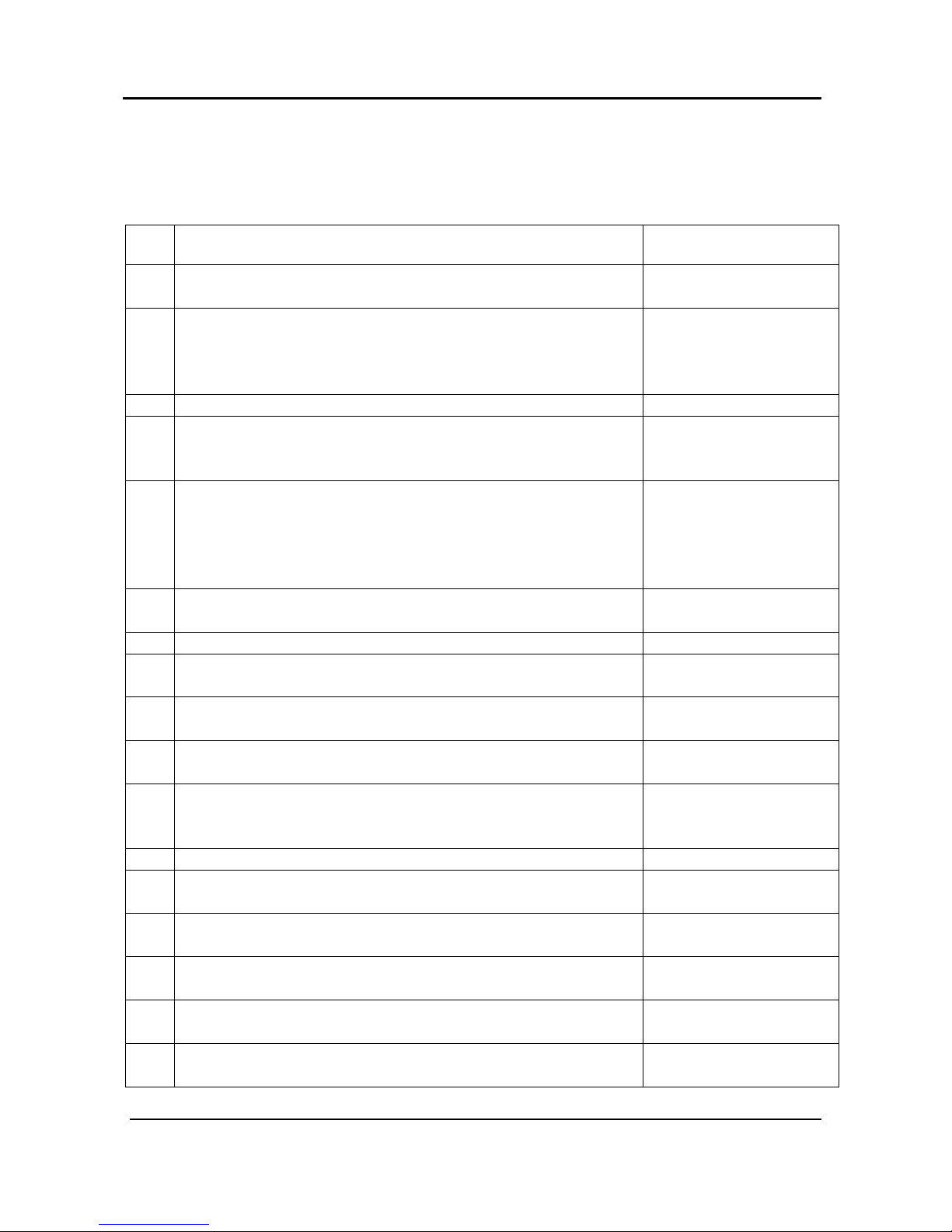
1.6 Quality Assurance System: ISO 9001 & ISO 13485
The Itamar Medical WatchPAT™200 device is compliant to the following standards.
WatchPAT™200 System 3 Operation Manual
18.
Medical Device Directive
MDD 93/42 EEC
MDD 2007/47/EC
19.
FDA Quality Systems Regulation (QSR)
21 CFR part 820
20.
UL standard for safety
UL 60601-1
21.
CSA Standard for safety
CSA 22.2 No.601.1
22.
Canadian Medical Devices Regulation
SOR/98-282
The product complies with the CE mark according to
MDD (Medical Device Directive) and related
standards.
The unit is marked with the CE logo.
The product is certified by CSA.
Warnings are used to identify conditions or actions, which - if the
instructions are ignored - may violate patient safety, or cause
damage/malfunction to the system, resulting in non-recoverable loss of
data.
Les avertissements sont utilisés pour identifier les conditions ou les
actions qui- si elles sont ignorées- peuvent porter atteinte à la sécurité
des patients ou causer des dommages au système et résulter à une
perte irréversible des données.
Cautions are used to identify conditions or actions, which could cause
interference with data acquisition and/or impair study results.
Les précautions sont utilisées affin d’identifier les conditions ou les
actions qui peuvent interférer avec le ramassage de données et
provoquer des résultats équivoque.
1.7 CE and CSA Compliance
1.8 Conventions Used in this Manual
Note: Throughout this document, the references WatchPAT™ and WP200 device are
used to refer to the WatchPAT™200 device.
WatchPAT™200 System 4 Operation Manual
Notes are used to identify an explanation, or to provide additional
information for purposes of clarification.
Les notes sont utilisées pour identifier les explications et pour donner des
informations supplémentaires dans le but de clarifier.
1.9 Warnings, Cautions and Notes
The WatchPAT™200 device is internally powered from a 4.2 V battery.
The WatchPAT™200 device is portable with continuous operation.
The WatchPAT™200 device uses BF patient applied parts.
The WatchPAT™200 device uses UL listed power supply (USA & Canada only).
The power supply is used in a non-patient environment only.
The WatchPAT™200 device should only be transported in its original case.
There are no serviceable parts inside the WatchPAT™200 device.
Do not use WatchPAT™200 device for non-indicated purpose, such as oximetry for
ICU patient monitoring or alarm device.
Environmental conditions during transportation & storage: See Specifications section.
Environmental conditions during operation: See Specifications section.
Sleep professionals (other than patients) using the WatchPAT™200 system should
read the Operation Manual.
WatchPAT™200 System 5 Operation Manual
WARNINGS
Use only the AC adapter provided (5V DC, 5W maximum capacity power
supply). Only authorized personnel may charge the WatchPAT™200. Failure to
heed this warning may cause permanent damage to the equipment.
Do not let the unit get wet.
Avoid placing food or water on any part of the system.
In the event of fire use only fire extinguishers approved for use on electrical
fires.
Handle unit with care. This unit is sensitive to extreme movements and to
falling.
Do not attempt to connect or disconnect any part of the unit.
Do not try to introduce any foreign object into the unit.
The WatchPAT™200 MUST be charged ONLY after being removed from
the patient!
The WatchPAT™200 MUST be removed from the patient BEFORE
connecting it to a PC!
The Adult Flex Pulse Oximetry Sensor may cause skin sensitivity to the
patient. Discontinue use of the NONIN double-backed adhesive tape strips if the
patient exhibits allergic reactions to the adhesive material.
AVERTISSEMENTS
Utiliser seulement un 5V DC, 5W alimentation d'énergie. Seul les techniciens
autorisés peuvent charger la montre PAT. Ignorer cet avertissement peut
causer des dommages irréparables a l’équipement. Ne pas mouiller l’unité.
L’unité est sensible au mouvement extrême est à la chute. L’utiliser avec
précaution. Ne pas essayer de brancher ou débrancher une des parties de
l’unité.
Ne pas introduire un objet étranger a l’intérieur de l’unité.
Le système WatchPAT™200 doit être rechargé uniquement après avoir été
retiré de la main du patient.
Il est impératif de retirer le système WatchPAT™200 de la main du patient
avant de le relier a l'ordinateur pour faire fonctioner les programmes.
L’Oxymètre pur adulte ‘’ Flex Pulse‘’ peut produire des sensibilités
dermatologique aux patients.
1.10 Safety Precautions
WatchPAT™200 System 6 Operation Manual
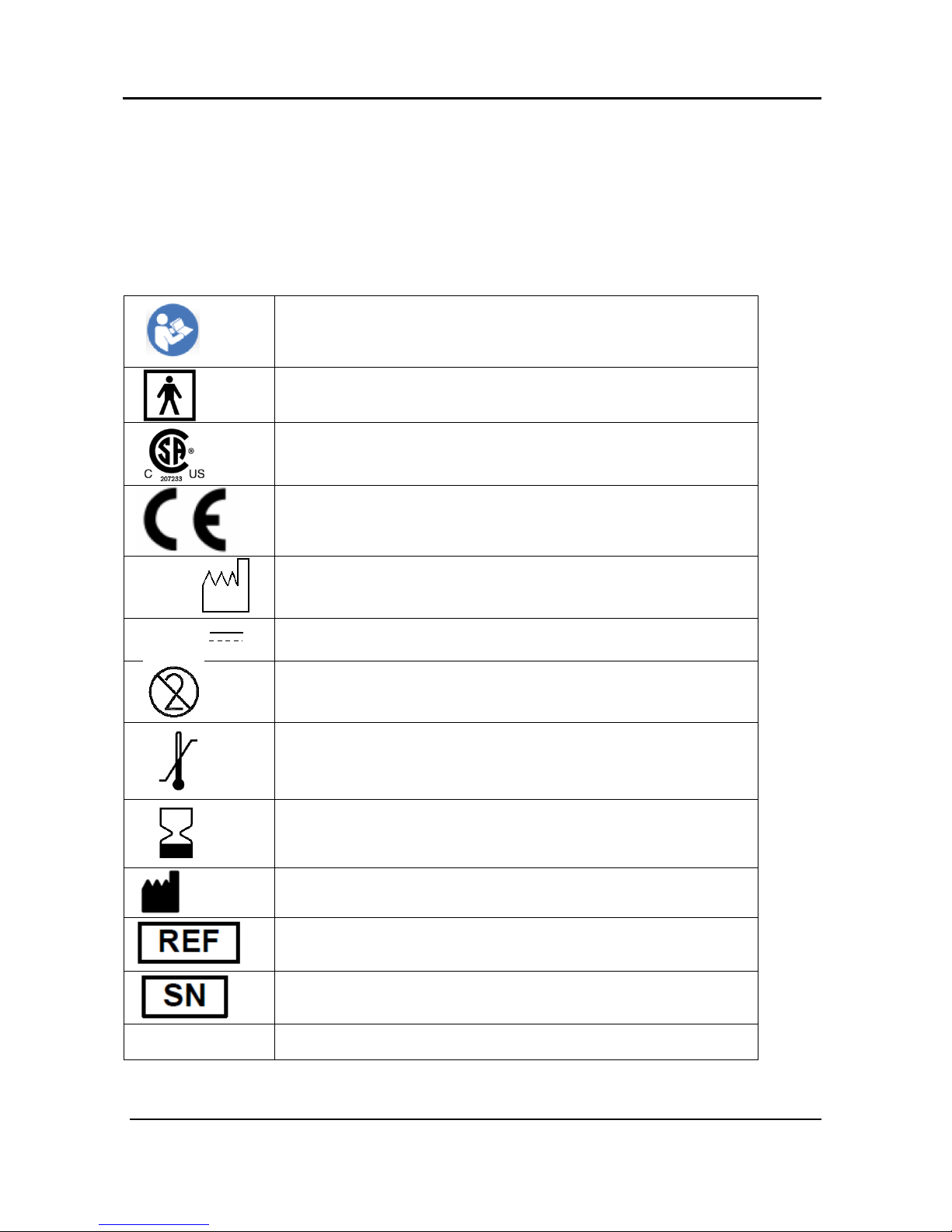
Follow instructions for use
Type BF applied part
The product is certified by CSA
The product complies with the CE mark according to MDD
(Medical Device Directive) and related standards.
The product is marked with the CE logo.
Date of manufacture
Battery Operating Voltage
Single use, do not re-use
Temperature limit
Use-by date
Medical device Manufacturer
Catalogue Number
Serial Number
IP22
Ingress protection
2014
3.7V DC
1.11 Environmental protection
The WatchPAT™200 device including its accessories shall be treated in accordance with
the local laws and regulations for proper waste treatment.
1.12 Symbols Used On the Product Labels
WatchPAT™200 System 7 Operation Manual
According to the WEEE Directive 2012/19/EU, all waste
electrical and electronic equipment (EEE) should be
collected separately and not disposed of with regular
household waste. Please dispose this product and all of its
parts in a responsible and environmentally friendly way.
Located on WatchPAT™200 device
Located on WatchPAT™200 device
Located on Nonin module
1.13 WatchPAT™200 Device Labels
1.14 FDA information
(WP200S-3) and under K081982, trade name Watch-PAT 200S-2 (WP200S-2).
The WatchPAT200 is cleared by the FDA under K102567, trade name Watch-PAT 200S-3
WatchPAT™200 System 8 Operation Manual

2 OVERVIEW
Obstructive sleep apnea syndrome (OSAS) is considered a major public health
problem. The prevalence of the syndrome is estimated at 2% to 5% in the adult
population. It is characterized by recurrent events of complete or partial obstruction of
the upper airways during sleep, often leading to hypoxemia, and/or arousals associated
with sympathetic nervous system activation. The diagnosis and assessment of the sleep
apnea patient is based on the Respiratory Disturbance Index (RDI), the number of
Apneas, Hypopneas and Respiratory Effort Related Arousals (RERA) per hour of
sleep, along with sleep architecture. The common consequences of this sleep disruption
are daytime sleepiness, poor daytime performance and increased vulnerability to
accidents. Cardiovascular complications such as systemic/pulmonary hypertension,
ischemic heart disease and arrhythmias are the major sequel of OSAS in the adult
population.
The WatchPAT™ device is worn on the wrist and is utilizing a plethysmographic
based finger–mounted probe, to measure the PAT® (Peripheral Arterial Tone) signal.
The PAT® signal is a measurement of the pulsatile volume changes in the fingertip
arteries which reflects the relative state of the arterial vasomotor activity, and thus
indirectly the level of sympathetic activation. Peripheral arterial vasoconstriction,
which mirrors sympathetic activation, is shown as attenuation in the PAT® signal
amplitude. The PAT® signal is recorded continuously and stored on an embedded
micro SD card, together with data from a built-in pulse-oximetry sensor (mounted on
an adjacent finger) and an actigraph (embedded in the WatchPAT™200 device).
Following the sleep study, the recordings are automatically downloaded and analyzed
in an offline procedure using the proprietary zzzPAT software.
The zzzPAT algorithms use the four WatchPAT™ channels (PAT®, Pulse Rate,
Oxygen saturation and actigraphy) for the detection of sleep related breathing disorders
and sleep staging (Rapid Eye Movement (REM), Light Sleep, Deep Sleep and Wake).
The zzzPAT uses WatchPAT™ device snoring and body position channels (SBP) to
generate snoring level and body position discrete states. The use of SBP is optional and
according to physician preference.
The software issues comprehensive reports of the study, with statistics and graphic
presentation of the results. The whole night data can be viewed and the automatically
detected events can be revised manually.
WatchPAT™200 System 9 Operation Manual

2.1 System Description
The WatchPAT™ system is comprised of the following items:
WatchPAT™ system that includes:
o Embedded actigraph
o Embedded pulse oximeter
o Embedded CPU and electrical circuit card
o Embedded micro SD card drive
o Rechargeable Lithium Ion Battery
o LCD display
PAT® probe
PAT® probe connection cable
Pulse oximeter sensor – with single use adhesive pads
Wrist Strap
Snore and Body Position sensor – optional
Cable for Tamper-Proof Bracelet - optional
AC adapter
USB cable
Step-by-Step Reference Guide (to be used in conjunction with Section 7)
Quick Reference Cards (to be used in conjunction with Section 8)
Carrying case
WatchPAT™200 System 10 Operation Manual

Optional Snore & Body
Position sensor
Oximetry sensor
PAT® probe
Figure 1 – Packed Device
Figure 2 – WatchPAT™ Device with Sensors
An additional item required for the operation of the system is the zzzPAT kit. zzzPAT
is a proprietary PC software for initializing the study, retrieving, analyzing and
displaying the data. For more information, refer to the zzzPAT Operation Manual.
2.2 User Interaction with the WatchPAT™ Device Keys
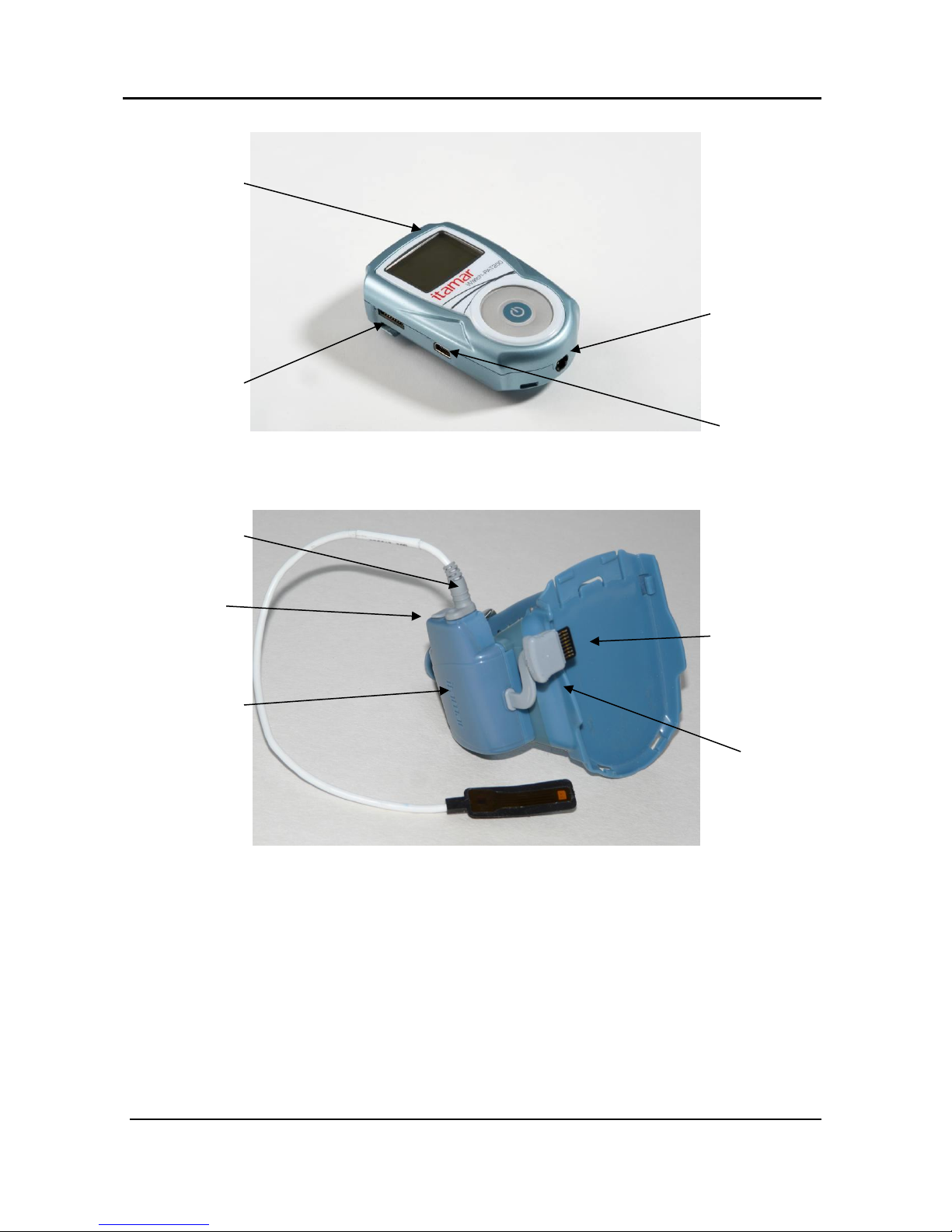
The WatchPAT™ device has the following keys (see Figure 3):
Central On/Enter key to power on the WatchPAT™ device (the only
key visible to the patient)
Outer ring containing four keys (left, right, up, down) that may be used
by the Operator for entering the diagnostic mode and navigating through
the diagnostic menu. These keys are hidden from the patient.
WatchPAT™200 System 11 Operation Manual

ON/ENTER
DOWN
LEFT
LCD
RIGHT
UP
Figure 3 – The Buttons and Display
LCD Display
The display is used for reading status and error messages. The display is divided to
three sections: Title, Info and Status.
Title (first line): Current operational mode and time
o PATIENT mode while recording night study
o DIAGNOSTIC mode while testing device
o PC HOST while connecting to PC
o CHARGER mode while connecting to AC adapter
Info (2nd-5th line): Specific information depending on operational
mode
Status (last line): Message indicating device status depending on
operational mode
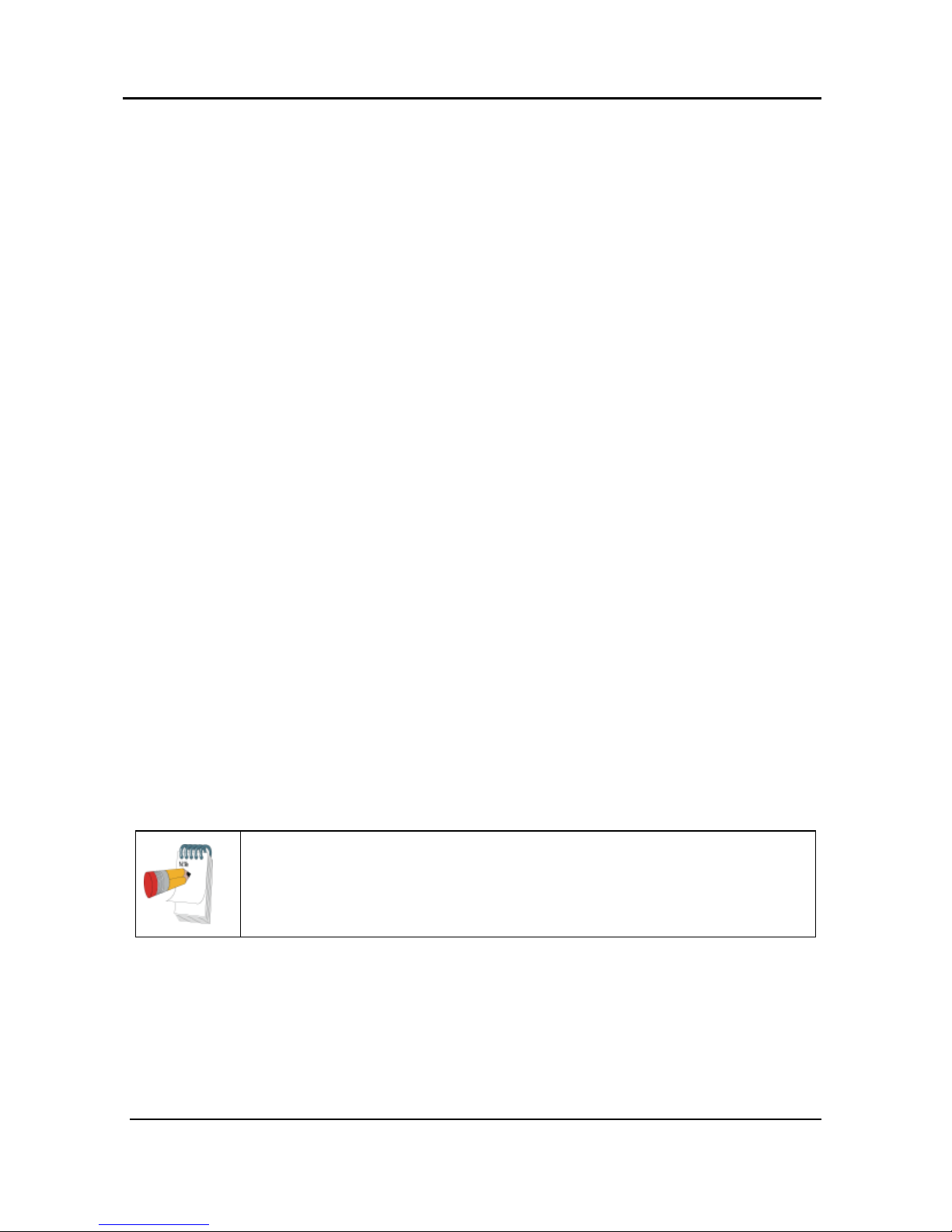
Service Ports and Peripherals
The WatchPAT™ device has 4 ports that are used either for sensor connections or for
servicing and charging (see Figure 4).
The oximetry module port is used for connecting the oximetry module.
The oximetry module has 2 additional ports: one for connecting the
oximetry sensor and one for connecting the bracelet (see Figure 5).
The PAT® probe port is used for connecting the PAT® probe
A port for connecting the optional Snore & Body Position sensor
The USB port is used for charging or connecting to the PC
WatchPAT™200 System 12 Operation Manual
Oximetry Module connector
Oximetry sensor
Oximetry module
Wrist strap
Oximetry module port
PAT® probe port
USB port for charging
and communication
Port for optional Snore
& Body Position
sensor
Bracelet port
Figure 4 – Service Ports and Peripherals
Figure 5 – WatchPAT™ Wrist with Oximetry Module
2.3 WatchPAT™ Device Function
The WatchPAT™ device records the following channels:
PAT® Signal
Oxygen saturation
Actigraphy (movement)
Acoustic decibel detector for Snoring evaluation (optional)
Body Position (optional)
WatchPAT™200 System 13 Operation Manual
Note
In all times, the current time is shown in the upper right hand corner of the
LCD display.
The overnight sleep study data is stored on an embedded micro SD card in the
WatchPAT™ device. After the study is recorded, the data is downloaded from the
WatchPAT™ device through the USB cable using the zzzPAT software. The zzzPAT
software, utilizing automatic algorithms, detects respiratory and other events that occurred
during sleep as well as periods of REM, deep sleep, light sleep and wakefulness. The pulse
rate signal is derived from the PAT® signal and used in the automatic analysis. The
software issues comprehensive detailed reports of the study. The whole night data can be
viewed on the PC screen and the automatically detected events can be revised manually.
An optional tamper-proof patient identification function is available using a custom bracelet
whose presence during the night verifies that the identified patient is indeed the one
sleeping with the device (see section Tamper-Proof Testing with WatchPAT™ Device).
The patient normally sleeps only one night with the WatchPAT™ device unless an optional
multi-night option is selected which enables an up to 3 nights study with the same device
(see Multi-night study section).
2.4 Built-In Self-Diagnostic Procedures
2.4.1 Operator Tests
The WatchPAT™ device contains a comprehensive built-in self-diagnostic procedure.
This procedure is available to the operator and hidden from the patient. The procedure
can be accessed it the UP and DOWN keys (see Figure 3) are pressed simultaneously
after the device is powered ON (during the first 30 seconds only after the device is
powered ON). The procedure performs the following tests:
Device Test – tests the WatchPAT™ device for errors before performing a
night study (make sure all probes are connected before initiating this test)
Oximetry Sensor Test – verifies oximetry sensor is connected and shows
average saturation
The Device test is the default test. Once the device test has passed you should also run
the oximetry sensor test.
To run the self-diagnostic procedure:
Press the ENTER button (Center key) for 2 seconds till the Itamar medical logo
appears on the LCD screen
Immediately press the UP + DOWN keys (see Figure 3) simultaneously for 1
second
WatchPAT™200 System 14 Operation Manual
DIAGNOSTIC 22:40
2.2140 20-Jul-08
*device test (30001)
oxi test
end testing
Select test ↑↓
DEVICE TEST 22:50
ID=111-11-1111
sbp=missing
<-Back
TEST PASSED 2:54
The following screen will be displayed:
First line displays title and current time
Second line displays current embedded S/W version (2.2139) and current date
Third line displays option for running device test (serial number of device in
parenthesis)
Fourth line displays option for running oximetry sensor test
Fifth line indicates option for end testing (turn device off). If no test is selected
within 3 minutes the WatchPAT™ device will automatically shut down
The Up & Down keys (↑↓) navigate between the lines.
An asterisk will indicate current selection. When moving the ↑↓ keys, the asterisk
will move to indicate the current selection. Press the central Enter key to make the
desired selection.
It is recommended that you perform the Device and OXI test every time you prepare the
WatchPAT™ device for a night study.
2.4.2 Device Test
At the completion of the device test, a TEST PASSED indicates that the device is
ready for the night study.
WatchPAT™200 System 15 Operation Manual

DEVICE TEST 22:50
ID=111-11-1111
oxi=mod missing
pat=missing
<-Back More->
TEST FAILED 2:54
At the completion of the device test, a TEST FAILED indicates a problem that
should be taken care of before the device is released for a night study.
The following are the possible error, warning or information messages:
File error: not loaded, missing – the study file was not loaded or somehow
the file was deleted
File error: used x/3 x=1..3 – only when multi-night option is selected
Battery error: low – needs charging
Probe error: used, missing, bad – connect an unused probe
Oximetry error: module missing - connect oximetry module
Hardware (H/W) error: error code - contact customer support
SBP (Snore and Body Position sensor) warning: sensor missing – does not
affect PASSED status
RTC (Real Time Clock) warning: faulty – indicates problem with internal
clock but does not affect PASSED status
Bracelet error: missing – the study file was chosen with the bracelet option
but the bracelet is not connected during the device test
Information messages:
o multi-night=on - when a multi night study is required
o bracelet=on - when a study with tamper-proof patient
identification bracelet is required
More-> indicates that there are more error/warning messages and will be displayed if the
Right (->) button is pressed.
<-Back will move to the previous screen if the Left (<-) button is pressed.
2.4.3 Oximetry Test
For the oximetry test make sure the sensor is attached to the finger. At the end of the
test the saturation and/or any error message will be displayed:
WatchPAT™200 System 16 Operation Manual
OXI TEST 22:50
SaO2=98%
Attach to finger
<-Back
Testing…
OXI TEST 22:50
SaO2=N/A
oxi=mod missing
Attach to finger
<-Back
Testing…
PATIENT 22:51
Please wait
Testing…
PATIENT 22:51
GOOD NIGHT!!!
Time elapsed=9:50
Recording…
The possible oximetry error messages are:
Oximetry error: module or sensor missing - connect oximetry module and
sensor.
SaO2= Not Available (N/A) - attach sensor to finger.
The blood saturation is continuously updated, therefore wait approximately for one
minute for the saturation to stabilize when testing.
<-Back will move to the previous screen if the Left (<-) button is pressed.
2.4.4 Patient Test
When the patient turns on the WatchPAT™ device by pushing the On/Enter key (center
button) for about 2 seconds a self-diagnostic test is automatically performed and the
following screen is displayed:
If the WatchPAT™ device passes this self-diagnostic test, the following screen will be
displayed:
WatchPAT™200 System 17 Operation Manual
 Loading...
Loading...