IRIDEX 65600 Users Manual

IRIDEX IQ 577
Operator Manual
™

IRIDEX IQ 577™ Operator Manual
15510-EN Rev A
© 2009 by IRIDEX Corporation. All rights reserved.
IRIDEX, the IRIDEX logo, IRIS Medical, OcuLight, EndoProbe and SmartKey are registered trademarks;
BriteLight, CW-Pulse, DioPexy, EasyFit, EasyView, FiberCheck, G-Probe, IQ 532, IQ 577, IQ 810, LongPulse,
MicroPulse, MilliPulse, OtoProbe, PowerStep, Symphony, TruFocus, and TruView are trademarks of
IRIDEX Corporation. All other trademarks are the property of their respective holders.

Contents
1 Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Compatible Delivery Devices . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Pulse Types . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Warnings and Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
IRIDEX Corporation Contact Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
2 Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Unpacking the System . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Choosing a Location . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Connecting the Components. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
3 Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Front Panel Controls. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Powering the Laser On and Off . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Treating Patients . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Using the IQ 577 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
4 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
General Problems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
Error Messages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
5 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Inspecting and Cleaning the Laser . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Inspecting and Cleaning the Footswitch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Verifying the Power Calibration. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
6 Safety and Compliance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Protection for the Physician. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Protection for All Treatment Room Personnel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
Safety Compliance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
Labels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
Symbols (As Applicable) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
7 Wireless Footswitch and EMC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Setting Up the Wireless Footswitch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Testing the Batteries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
EMC Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
EMC Requirements for Console and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . 35
15510 Rev A iii

Contents
iv 15510 Rev A
1
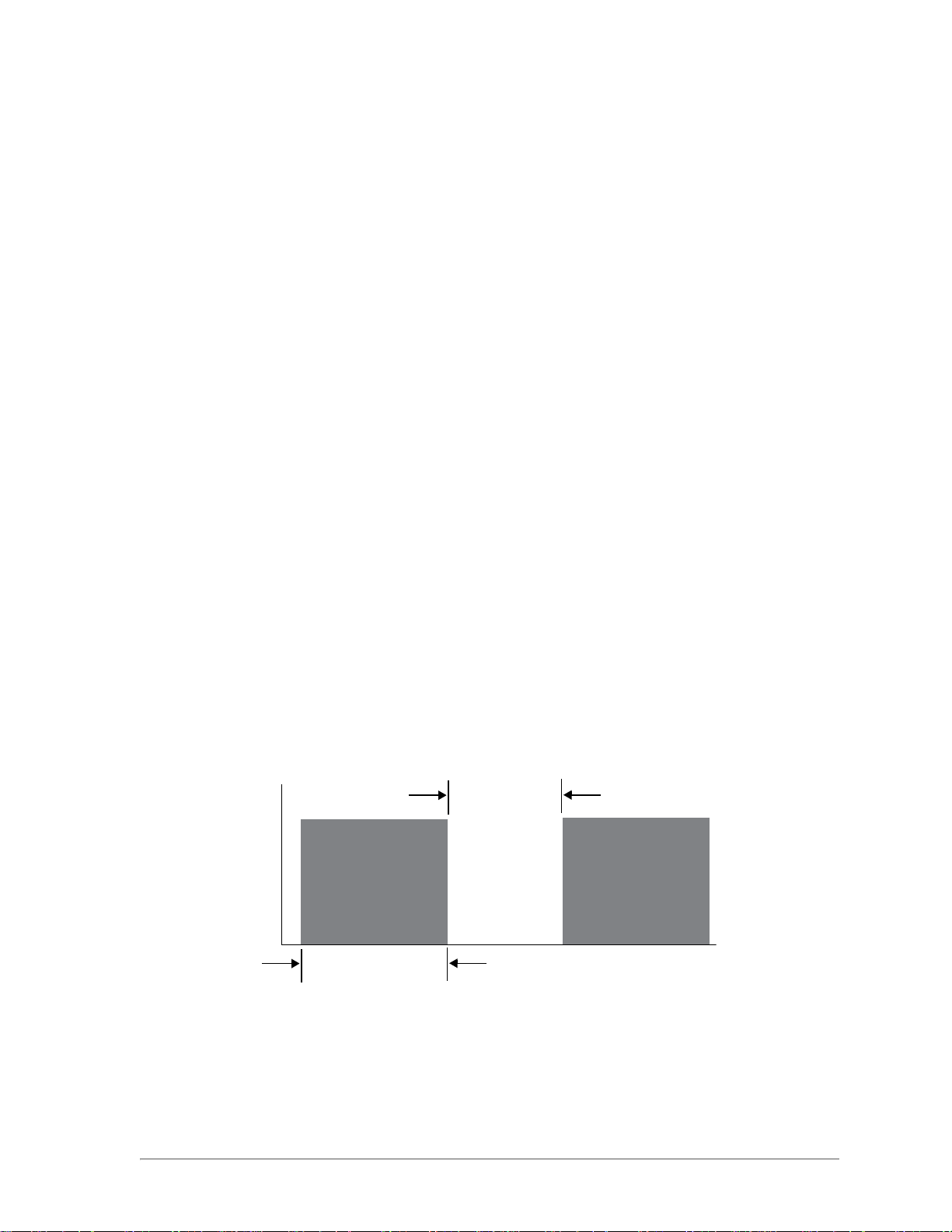
Interval
Pulse Duration
Time (ms)
Power (W)
Introduction
Improper use of the laser system can result in adverse effects. Follow the instructions for use described
in this operator manual.
Compatible Delivery Devices
These IRIDEX delivery devices are compatible with the IQ 577 laser systems:
•EndoProbe
• Slit Lamp Adapters (SLA)
• Laser Indirect Ophthalmoscopes (LIO)
NOTE: Refer to the appropriate delivery device manual for indications for use, contraindications, precautions,
®
and adverse effects information.
Pulse Types
The IQ Laser System is capable of delivering a continuous wave laser pulse in 2 modes: CW-Pulse™
and MicroPulse™.
CW-Pulse
15510 Rev A Introduction 1
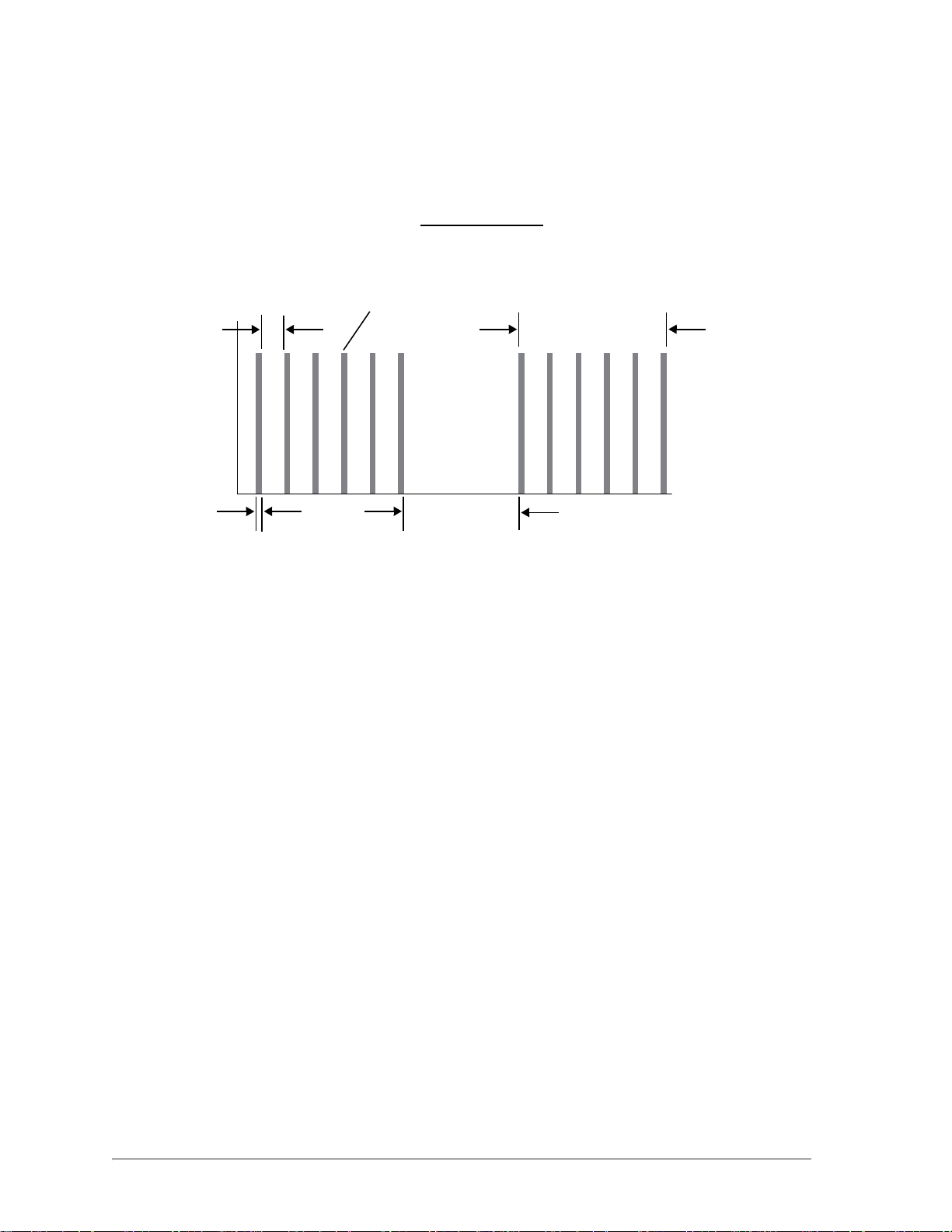
MicroPulse
P Duration
x
P
Duration
P
Interval
+
100
Duty Cycle =
Pulse Envelope
Interval
P
Interval
MicroPulse
Pulse Envelope
Duration
Time (ms)
Power (W)
P
Duration
MicroPulse (P) is a laser delivery consisting of a group of microsecond bursts.
2 IRIDEX IQ 577™Operator Manual 15510 Rev A

Indications for Use
This section provides information on the use of the IQ 577 in clinical specialties. Information is
provided by specialty and includes procedural recommendations along with specific indications and
contraindications. This information is not intended to be all-inclusive and is not intended to replace
surgeon training or experience. The regulatory information provided is applicable only in the United
States. If you use the IQ 577 for indications not included herein, you will be subject to 21 CFR Part 812,
the Food and Drug Administration’s Investigational Device Exemption (IDE) regulations. For
information regarding the regulatory status of indications other than those listed in this manual,
contact IRIDEX Regulatory Affairs.
IRIDEX does not make recommendations regarding the practice of medicine. References in literature
are provided as a guide. Individual treatment should be based on clinical training, clinical observation
of laser tissue interaction, and appropriate clinical endpoints.
The IRIDEX IQ 577 and the handpieces, delivery devices, and accessories that are used with it to
deliver laser energy in either CW-Pulse™ or MicroPulse™ mode are intended for soft and fibrous
tissue, including osseous tissue incision, excision, coagulation, vaporization, ablation and vessel
hemostasis in Ophthalmology.
Ophthalmology
Indicated for use in photocoagulation of both interior and posterior segments, including:
• Retinal photocoagulation, panretinal photocoagulation and intravitreal endophotocoagulation of
vascular and structural abnormalities of the retina and choroids, including:
– Proliferative and nonproliferative diabetic retinopathy
– Choroidal neovascularization
– Branch retinal vein occlusion
– Age-related macular degeneration
– Retinal tears and detachments
– Retinopathy of prematurity
• Iridotomy, iridectomy, and trabeculoplasty in angle closure glaucoma and open angle glaucoma
P
ROCEDURAL RECOMMENDATIONS
The user is directed to review the operating instructions for the compatible delivery devices prior to
treatment.
ECHNIQUE
T
The laser energy is recommended to be administered via the EndoProbe optical fiber delivery
handpiece which is utilized intra-ocularly.
L
ASER SETTINGS
Beginning at low power with short duration exposures, the surgeon should note the surgical effect and
increase power, power density, or exposure duration until the desired surgical effect is obtained. The
following table is intended to provide guidance only for treatment settings, which are not prescriptive
for any condition. The operative needs of each patient should be individually evaluated based on the
15510 Rev A Introduction 3
indication, treatment location, and on the patient’s medical and wound healing history. If uncertain of
expected clinical response, always start with a conservative setting and increase the setting in small
steps.
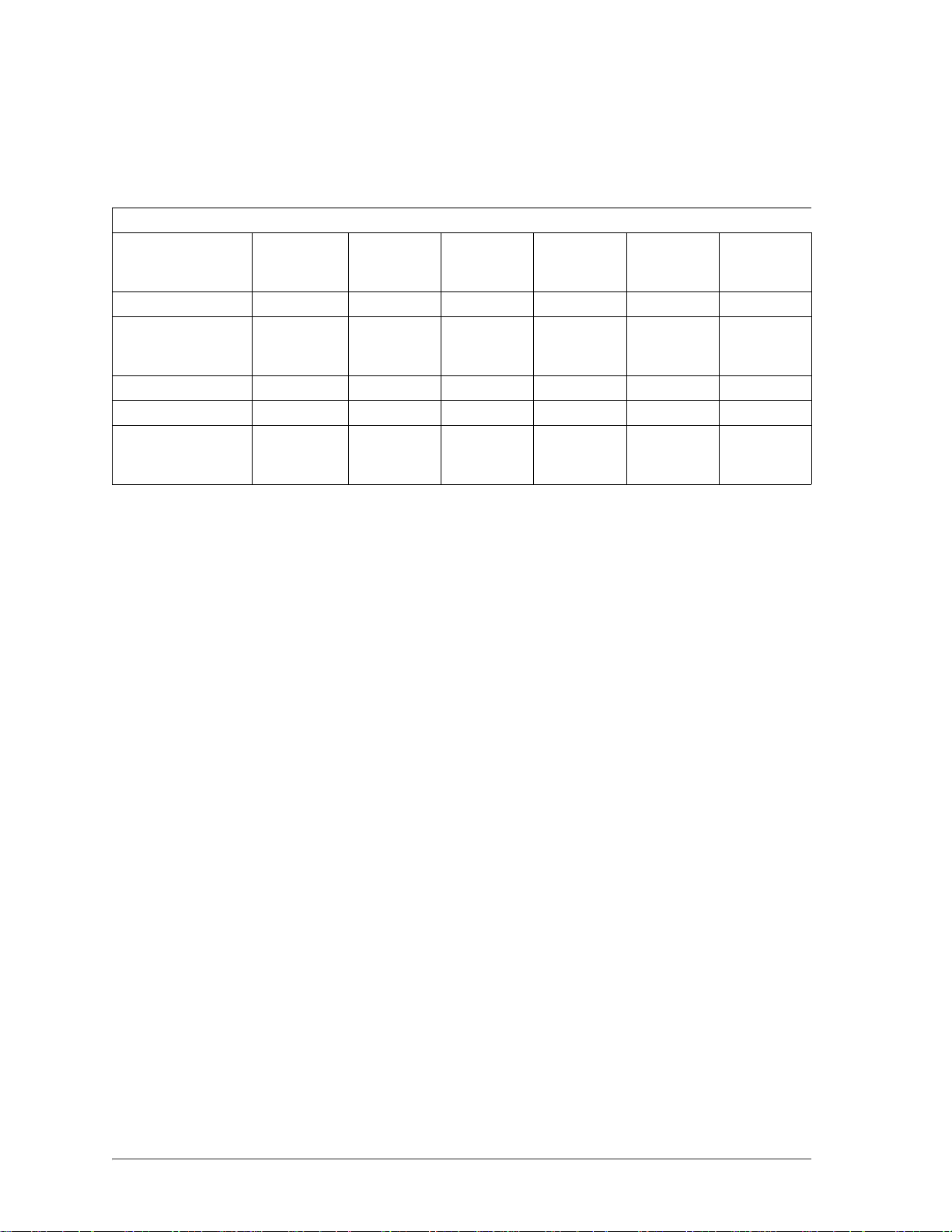
Ophthalmic Treatment Parameters
Treatment Delivery
Devices
Trabeculoplasty SLA 1.5–2.0 0.05–0.5 1–10 100–500 100–500
Retina Grid/Focal SLA, LIO,
EndoProbe,
OMA
Trabeculoplasty SLA 0.5–2.0 N/A N/A 100–500 50–200
Iridotomy SLA, LIO 0.2–2.0 N/A N/A 100–300 50–200
Retina Grid/Focal SLA, LIO,
EndoProbe,
OMA
S
PECIFIC WARNINGS AND PRECAUTIONS
Power (W) MicroPulse
Length (ms)
1.0–2.0 0.05–0.5 1–10 100–1000 50–100
0.1–2.0 N/A N/A 100–1000 100–1000
MicroPulse
Interval (ms)
Exposure
Duration
(ms)
Spot Size
(µm)
It is essential that the surgeon and attending staff be trained in all aspects of this procedure. No
surgeon should use these laser products for ophthalmic surgical procedures without first obtaining
detailed instructions in laser use. Refer to “Warnings and Cautions” for more information. Proper eye
protection for 577 nm light must be utilized. Follow the Eye Protection Policy at your facility.
S
PECIFIC COMPLICATIONS AND RISKS
None known specific to ophthalmology use at this time.
PECIFIC CONTRAINDICATIONS
S
None known specific to ophthalmology use at this time.
4 IRIDEX IQ 577™Operator Manual 15510 Rev A

M.B. Parodi, S .Spasse, P. Iacono, G. DiStefano, T. Canziani; Subthreshold Grid Laser Treatment of Macular Edema
Secondary to Branch Retinal Vein Occlusion With Micropulse Infrared (810 Nm) Diode Laser; Ophthalmology Volume 113,
Number 12, December 2006.
T J Desmettre, S R Mordo, D M Buzawa and M A Mainster; Micropulse and continuous wave diode retinal
photocoagulation: visible and subvisible lesion parameters; British Journal of Ophthalmology 2006;90;709-712; originally
published online 10 Mar 2006.
JK Luttrull, DC Musch and CA Spink; Subthreshold diode micropulse panretinal and photocoagulation for proliferative
diabetic retinopathy., Eye online publication, February 2007.
AM Fea, A Bosone, et. al. Micropulse diode laser trabeculoplasty (MDLT): A phase II clinical study with 12 months
follow-up. Clinical Ophthalmology advance online publication, 9 Apr 2008.
Brancato R, Carassa R, Trabucchi G.; Diode Laser Compared With Argon Laser for Trabeculoplasty American Journal of
Ophthalmology 112:50-55, 1991.
Moriarty A, McHugh J, ffytche T, Marshall J, Spalton D, Moriarty B.; Diode Laser Trabeculoplasty (DLT) versus Argon
Laser Trabeculoplasty (ALT) in Primary Open-Angle Glaucoma Scientific Poster #52. AAO. San Francisco, CA. October,
1994.
Wong JS, Chew P, Chee C; Comparison of Corneal Transmissibility of 810 nm Diode Laser With 448 nm Argon Laser;
Diode Laser Peripheral Iridoplasty/Iridotomy in Acute Angle Closure Glaucoma; [ARVO Abstract]. Invest
Ophthalmology Vis Sci. 39(4): S472. Abstract nr2162, 1998
Akduman L, Olk RJ. “Diode Laser (810 nm) versus Argon Green (514 nm) Modified Grid Photocoagulation for Diffuse
Diabetic Macular Edema”, Ophthalmology 104:1433-1441, 1997.
15510 Rev A Introduction 5

Warnings and Cautions
WARNIN G S :
Lasers generate a highly concentrated beam of light that may cause injury if improperly used. To
protect the patient and the operating personnel, the entire laser and the appropriate delivery system
operator manuals should be carefully read and comprehended before operation.
Never look directly into the aiming or treatment beam apertures or the fiber-optic cables that deliver the
laser beams with or without laser safety eyewear.
Never look directly into the laser light source or at laser light scattered from bright reflective surfaces.
Avoid directing the treatment beam at highly reflective surfaces such as metal instruments.
Ensure that all personnel in the treatment room are wearing the appropriate laser safety eyewear.
Never substitute prescription eyewear for laser safety eyewear.
CAUTIONS:
US federal law restricts this device to sale by or on the order of a healthcare practitioner licensed by the
law of the State in which he/she practices to use or order the use of the device.
Use of controls or adjustments or performing of procedures other than those specified herein may result
in hazardous radiation exposure.
Do not operate the equipment in the presence of flammables or explosives, such as volatile anesthetics,
alcohol, and surgical preparation solutions.
Laser plume may contain viable tissue particulates.
When no delivery device is attached to the system, ensure that the fiber ports are closed.
6 IRIDEX IQ 577™Operator Manual 15510 Rev A

IRIDEX Corporation Contact Information
0086
IRIDEX Corporation
1212 Terra Bella Avenue
Mountain View, California 94043-1824 USA
Telephone: (800) 388-4747 (US only)
(650) 962-8100
Fax: (650) 962-0486
Technical Support: (650) 962-8100
(800) 388-4747 (US only)
techsupport@iridex.com
Medical Devices Consultants International Limited
Arundel House
1 Liverpool Gardens
Worthing, West Sussex BN11 1SL
United Kingdom
Warranty and Service. Each laser system carries a standard factory warranty. The warranty covers all
parts and labor required to correct problems with materials or workmanship. This warranty is void if
service is attempted by anyone other than certified IRIDEX service personnel.
WARNIN G : Use only IRIDEX delivery devices with the IRIDEX laser system. Use of a non-IRIDEX delivery
device may result in unreliable operation or inaccurate delivery of laser power. This Warranty
and Service agreement does not cover any damage or defect caused by the use of non-IRIDEX
devices.
NOTE: This Warranty and Service statement is subject to the Disclaimer of Warranties, Limitation of
Remedy, and Limitation of Liability contained in IRIDEX’s Terms and Conditions.
WEEE Guidance. Contact IRIDEX or your distributor for disposal information.
15510 Rev A Introduction 7

2
Keys
Power cord
Footswitch
Laser
Remote Control
(optional)
Wireless Footswitch
(optional)
Setup
Unpacking the System
Make sure you have all components that were ordered. Check components for damage before use.
NOTE: Contact your local IRIDEX Customer Service representative if there are problems with your order.
Appearance and type of components may vary based on the system ordered.
• Laser (also “Console”) • Operator Manual (not shown)
• Power cord (U.S. configuration shown) • Laser warning sign (not shown)
• Keys • Optional accessories (not all shown)
•Standard footswitch
Choosing a Location
Choose a well-ventilated location within the specified operating range of the console.
8 IRIDEX IQ 577™Operator Manual 15510 Rev A

Place the laser system on a table or on existing operating room equipment. Allow at least 5 cm (2 in.) of
clearance on each side.
In the U.S., this equipment must be connected to an electrical supply source at 120V or 240V with a
center tap.
To ensure that all local electrical requirements can be met, the system is equipped with a hospitalgrade (green dot) three-wire grounding plug. When choosing the location, ensure that a groundingtype AC outlet is available; it is required for safe operation.
The power cord included in the packaging is appropriate for your location. Always use an approved
three-wire grounding cord set. Do not alter the power inlet. To ensure proper grounding, follow local
electrical codes before installing the system.
CAUTIONS:
Do not defeat the purpose of the grounding pin. This equipment is intended to be electrically grounded.
Contact a licensed electrician if your outlet prevents you from inserting the plug.
Do not position or use the system near open flames.
Connecting the Components
CAUTION: Do not connect two footswitches to the laser console.
NOTES:
Refer to the appropriate delivery device manual for specific connection instructions.
The Auxiliary Output contact supports low voltage electrical signaling circuits of up to five amps and
24 volts AC or DC. Ensure that all wiring conforms to local electrical codes.
15510 Rev A Setup 9
 Loading...
Loading...