iReliev iRenew Plus ET-8080 Instruction Manual

iRenew™ Plus Pain Relief & Recovery
TENS + EMS System
Instruction Manual
Model # ET-8080

INDICATIONS AND CONTRAINDICATIONS
Read this instruction manual before operation. Be sure to comply with all “CAUTIONS” and “WARNINGS” in this manual.
Failure to follow and implement according to the use and operating instructions can cause harm to the user or device.
The device is intended for over-the-counter use. If you have medical questions we strongly encourage you to consult with your physician regarding indications for use of this device.
What is TENS?
TENS stands for transcutaneous electrical nerve stimulation. This TENS unit is intended to deliver electrical current to
electrode pads applied to your skin to relieve pain associated with sore or aching muscles.
What is EMS?
EMS, stands for electrical muscle stimulation. This electric muscle stimulator, is used to stimulate healthy muscles in
order to improve muscle strength and performance.
Indications for Use
The iRenew™ Plus is a muscle stimulator for active treatment as per the following intended use:
• For temporary relief of pain associated with sore and aching muscles in the lower back due to
strain from exercise or normal household and work activities. (Choose TENS Modes P1 through P7)
• For temporary relief of pain associated with sore and aching muscles in the upper and lower
extremities (arm and/or leg) due to strain from exercise or normal household and work activities.
(Choose TENS Modes P1 through P7)
• For symptomatic relief and management of chronic, intractable pain and relief of pain associated
with arthritis. (Choose TENS Mode P8)
• For use by healthy adults for the stimulation of healthy muscles in order to improve or facilitate
performance. (Choose EMS Modes P1 through P6)
www.iReliev.com2

TABLE OF CONTENTS
INDICATIONS AND CONTRAINDICATIONS...................................................................................................................................... 2 & 4
WARNINGS AND PRECAUTIONS………………………............................................................................................................................... 4-10
WHAT’S INCLUDED..………………………....……………………………....................................................................................................................11
DEVICE FEATURES...………………………....……………………………................................................................................................................... 12
ABOUT RECHARGEABLE BATTERY………………………………………........................................................................................................... 13
CHARGING THE BATTERY................................................................................................................................................................... 14-15
STEP BY STEP OPERATION GUIDE FOR TREATMENT ……..…………………....................................................................................... 16
Connecting the Lead Wires to Device.............................................................................................................................
Connecting Electrode Pads to Lead Wires.………………………...........................................................................................
Removing Electrode Pads From Plastic Film………........................................................................................................
Placement of Electrode Pads ………………………………………………………………….......................................................................
Turning On & Off the Device …………………………………….......................................................................................................
Selecting the Treatment Time.…………………………..............................................................................................................
Selecting TENS or EMS....….…………………………………………………...........................................................................................
Selecting the Therapy Modes (TENS P1-P8 or EMS P1-P6).......................................................................................
Selecting the Intensity Level....….……………………………………................................................................................................
SPECIAL FEATURES…………………………………………………...........................................................................................................................19
PAD PLACEMENTS................................................................................................................................................................................20-21
TENS PROGRAMS………………………………………………….............................................................................................................................. 22
EMS PROGRAMS…………………………………………………................................................................................................................................ 23
CARE AND MAINTENANCE………………….............................................................................................................................................. 24
TROUBLESHOOTING.………..………………………......................................................................................................................................... 25
TECHNICAL SPECIFICATIONS……………………………………………………...................................................................................................... 26-27
ELECTROMAGNETIC COMPATIBILITY ……….....................................................................................................................................28
FCC INFORMATION ………………………………………………………………............................................................................................................. 33
WARRANTY……………………………….............................................................................................................................................................34
REGISTER YOUR DEVICE …………………................................................................................................................................................. 36
REGISTRATION...................................................................................................................................................................................... 37
www.iReliev.com 3

Contraindications
• Do not use this device if you have a cardiac pacemaker, implanted debrillator or any other im-
planted metallic or electronic device. Such use could cause electric shock, burns, electrical interfer-
ence or death.
• Do not use this device if you have undiagnosed chronic pain.
• Do not use this device if you are pregnant. The safety of TENS or Electronic Muscle Stimulation
(EMS) over a pregnant uterus has not been determined or established.
• Do not use this device if you have cancer. The effects of electronic stimulation on cancerous
tissue is unknown.
• Do not use this device if you are under medical supervision for cognitive dysfunction as you may
not be able to comply with safety and operating instructions.
• Do not use this device if it is in close proximity to shortwave or microwave diathermy equipment.
• Do not wear the device or place electrode pads over areas where drugs/medicines are adminis-
tered (short-term or long-term) by injection (e.g. hormone treatment).
• Do not use if you have epilepsy.
• Do not use if you have recently undergone a surgical procedure.
• Do not use following acute trauma or fracture in case of critical ischemia of the limbs.
WARNINGS AND PRECAUTIONS
Warnings
• If you are under the care of a Physician, consult with your Physician before using this system.
• The long-term effects of this system are not known.
• Do not place the electrode pads on or close to your heart.
• Do not place the electrode pads and apply stimulation around or close to your neck, throat area
or carotid arteries. Severe spasm of the muscles may occur and the contractions may be strong
enough to close the throat or cause difculty in breathing. Stimulation over the throat could also
have an adverse effect on hearing or blood pressure.
www.iReliev.com4

• Do not use the electrode pads over or close to sores on the skin.
• Do not place the electrode pads on the front or sides of the neck, or across the heart (one elec-
trode pad on the front of the chest and one on the back). Do not place on the genital region or on
the head as such risk is considered inappropriate areas of the body for the use of this device.
• Do not place the electrode pads over any recent scars, broken or inflamed areas of infection or
susceptibility to acne, thrombosis or other vascular problems (e.g. varicose veins), or any part of
the body where feeling is limited.
• Do not place the electrode pads over areas of injury or restricted movement.
• Do not use this system while sleeping.
• Do not use this system if you feel numbness.
• Do not use this system in or close to water.
• Do not apply stimulation across the chest because the introduction of electrical current into the
chest may cause rhythm disturbances to the heart, which could be lethal.
• Do not use electrode pads over or close to cancerous lesions.
• Use the electrode pads only on normal, healthy, clean and dry skin. Do not use the electrode pads
on open wounds, rashes or over swollen, red, infected or inflamed skin.
• If you have ever had back surgery, consult your Physician before using this system.
• Electronic monitoring equipment (such as ECG and ECG alarms) may not operate properly when
electrical stimulation is in use.
• Avoid use on areas of injury or restricted movement such as fractures or sprains.
• Avoid placing the electrode pads over metal implants.
• Do not use in the bath or shower and other environments of elevated humidity (e.g. sauna,
hydrotherapy, etc).
• Do not use the device in an environment where flammable or explosive fumes may exist.
• User should never operate potentially dangerous machinery such as power saws, automobiles, exercise
equipment etc. during electrical stimulation.
• Application of electrodes pads near the thorax may increase the risk of cardiac brillation.
www.iReliev.com 5

Wait Before Using the Unit:
• At least 6 weeks after the birth of your baby. You must consult your doctor before use.
• One month after an IUD contraceptive device (e.g. coil) has been tted. You must consult your
doctor before use.
• At least 3 months after having a cesarean section. You must consult your doctor before use.
• Until the heavy days of your period have nished. Vigorous abdominal exercise or muscle stimu-
lation is not recommended during this time.
Precautions
• Read instruction manual before using this system for the rst time.
• Keep this instruction manual available whenever you use this system.
• The system is intended for personal use on healthy adults only.
• The safety of using the system during pregnancy or birth has not been established.
• The effectiveness of the system depends greatly on a person’s individual physical condition. It
may not always be effective for every user.
• The safety of neuromuscular stimulation during pregnancy has not been established.
• Use caution when and/or if:
- Sensory nerve damage is present by a loss of normal skin sensation.
- Use caution prior to using this device on patients suspected of having heart disease.
- Use caution for patients with suspected or diagnosed with epilepsy when using this
device.
- Use caution following recent surgical procedures when muscle contraction may disrupt
the healing process.
- Use caution when there is a tendency to hemorrhage, such as following acute trauma
or fracture.
- Over a menstruating or pregnant uterus.
- User may experience skin irritation due to electrical stimulation or the conductive
medium. Discontinue stimulation and consult a clinician. Irritation may be reduced by
www.iReliev.com6

an alternative conductive medium or an alternative electrode pad placement. Isolated cases of
skin irritation may occur at the site of electrode pad placement following long-term application.
• This unit should not be used while driving, operating machinery or during any activity in which
involuntary muscle contractions may place the user at undue risk of injury.
• Some users may experience skin irritation or hypersensitivity due to the electrical stimulation or
the conductive medium. Seek the advice of a clinician.
• Keep this device out of reach of children. If the user is a child, make sure he/she is properly super-
vised during electrical stimulation.
• Application of moderate heat (thermal wrap) to muscles as well as moistening skin prior to
treatment improves treatment efcacy and the use of cold packs on treated muscles after
treatment is likewise recommended.
• This unit should only be used with iReliev® brand electrode pads and accessories.
• The device is not intended for medical use, for the treatment of any medical condition or for any
permanent physical changes.
• Contact ExcelHealth Inc. or an authorized reseller if your unit is not working correctly. Do not use in
the meantime.
• An effective session should not cause discomfort.
• For rst time users, muscle stimulation can be an unusual sensation. We recommend that you
begin in a seated position with low stimulation intensity settings to familiarize yourself with the
sensation before progressing to higher intensity settings.
• The electrode pads must not be connected to other objects.
• Do not overexert yourself while using muscle stimulation. Any workout should be at a comfort-
able level for you.
• Do not place electrode pads over jewelry or body piercings.
Use caution and consult your Physician before using this system if any of the following conditions apply
to you:
• You have any serious illness, diagnosis or injury not mentioned in this guide.
www.iReliev.com 7

• You have recently undergone a surgical procedure.
• You take insulin for diabetes.
• You use the unit as part of a rehabilitation program.
• If you suspect or have been diagnosed with a heart problem.
• If you suspect or have been diagnosed with epilepsy.
• If you have a tendency to bleed internally following an injury.
• If you recently have had surgery or have ever had surgery on your back.
• If areas of skin lack normal sensations, such as skin that tingles or is numb.
• During menstruation or during pregnancy.
• Some people may feel skin irritation or experience a very sensitive feeling in the skin due to elec-
trical stimulation. If this occurs, stop using this system and consult your Physician.
• If skin under one or more electrode pads feels irritated after using the stimulator for a long period
of time, use this device for a shorter period of time.
• Minor redness at the point stimulation placement is a normal skin reaction. It is not considered a skin irrita tion, and it will normally disappear within 30 minutes after the electrode pads are removed. If the
redness does not disappear after 30 minutes from the removal of electrode pads, do not use the
stimulator again until excessive redness has disappeared.
• Turn off the stimulator if the stimulation feels unpleasant or does not provide pain relief.
• Keep your system out of the reach of children.
• Use your stimulator only with iReliev® brand electrode pads and accessories.
• Do not use this system when driving, operating machinery, or when swimming.
• Before removing the electrode pads, be sure to power off device to avoid unpleasant stimulation.
After Strenuous Exercises or Exertion:
• Always use lower intensity to avoid muscle fatigue.
Important:
• Effectiveness is highly dependent upon user’s selection of therapy program. Please refer to a
clinician qualied in the management of pain or rehabilitation.
www.iReliev.com8

• Do not use this system at the same time as any other device which transfers an electrical cur-
rent into the body (e.g. another muscle stimulator).
• Stop using your unit if you are feeling light-headed or faint. Consult a doctor if this happens.
• Do not touch the electrode pads or metal studs while the unit is switched on.
• Do not use this system if you are wearing a belly button ring. Remove ring before use of this device.
• Use this device with only the leads and electrode pads provided for use by iReliev®. Other accessories may
not be compatible with your device and could degrade the performance of this device and minimum safety
precautions listed in this instruction manual. Use only the electrode pad placements and stimulation settings
prescribed by your Doctor.
• This device is for external use only.
• Choking may result from a child swallowing a small part that has become detached from the device.
Note: If you have any doubts or have medical questions about using this system, please consult your doctor.
Electrode Pad Precautions
• To reposition the electrode pads during a session, always pause the program currently running,
reposition the electrode pads, and then restart the program.
• The electrode pads are for single person use only.
• Do not plunge the electrode pads into water.
• Do not apply solvents of any kind to the electrode pads.
• Always ensure the unit is OFF before removing the electrode pads.
• Apply the whole surface of the electrode pads rmly to the skin. Do not use electrode pads that
do not adhere properly to the skin.
• If your skin is red under the electrode pad after a session, do not start another session in the
same area until your redness has completely disappeared.
www.iReliev.com 9

Adverse Reactions
• You may experience skin irritation and/or minor burns due to prolonged use of the electrode pads applied to
your skin.
• You may experience potential allergic reactions to accessible materials used in the electrode pads.
• Do not apply electrode pads to your head, throat, face or genitals. You may experience headaches and
other painful sensations during or following the application of electrical stimulation near your eyes, throat
head and face.
• You should stop using the device and should consult with your physician if you experience any ad-
verse reactions from the device.
Conditions That May Affect Your System
Since this device is a battery-operated electronic system, its output performance and safety may be affected greatly
in extreme humidity. Therefore, it is very important to keep the system device(s) dry to ensure the safety and performance of the device.
• User of this system must be at least 16 years old.
• This system is for indoor home-use.
• This system may be used daily with no operation time limit but it is recommended to not exceed
60 minutes per day.
• If there is any other problem, please consult ExcelHealth or return the device to an authorized
iReliev® reseller. Do not try to repair a defective device.
• WARNING: No modication of this system is allowed.
• WARNING: Use of non-iReliev® brand accessories may negatively affect the system’s performance.
• WARNING: Do not stack and store this system close to other equipment.
www.iReliev.com10

www.iReliev.com 11
WHAT’S INCLUDED
Package Content
1. iRenew™ Plus TENS + EMS Device x 1
2. Belt Clip & Holster x 1
3. 3.5” x 5” XL Electrode Pads x 2
4. 2” x 2” Electrode Pads x 4
5. AC Adapter and USB Charging Wire (23.5”)
6. Lead Wires x 2
7. Tote Bag x 1
1. 2. 3. 4.
6. 7.
5.
www.iReliev.com
12
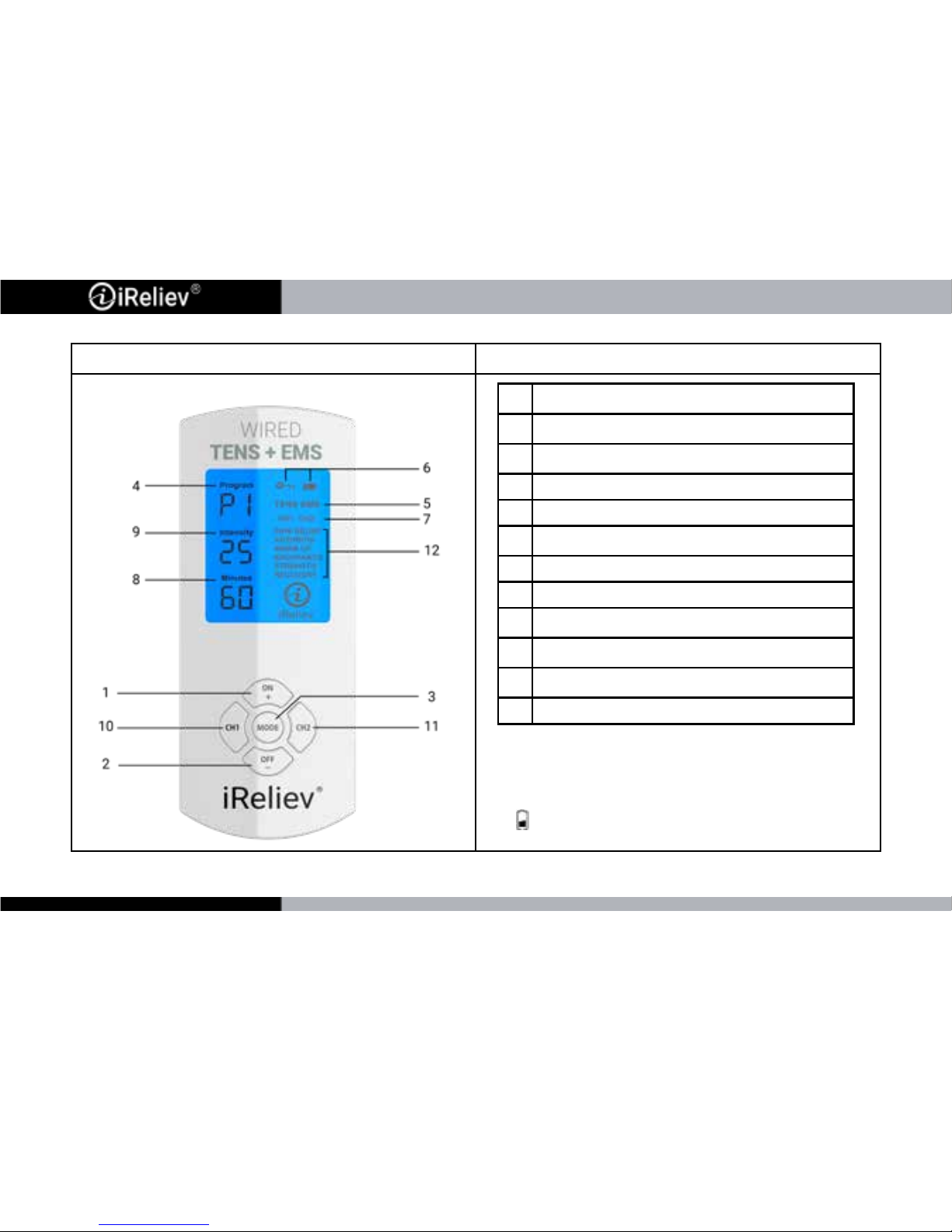
DEVICE FEATURES DESCRIPTION
Indicators and Buttons:
1. LED backlight will display for 10 seconds upon
turning on.
2. When charging, the device will shut off
automatically.
3. If symbol is displayed, battery power is low.
1
Power ON/Adjust/Increase Key
2
Power OFF/Adjust/Decrease Key
3
Mode Navigation Key
4 Program Mode
5 Therapy Type (TENS or EMS)
6
Battery Status/ Lock Function Indicator
7 Channel Indicator
8 Therapy Minutes Remaining
9
Intensity Level (For CH1 or CH2)
10
Channel 1 Key (CH1)
11
Channel 2 Key (CH2)
12 Program Mode Function
 Loading...
Loading...