Inova Labs XYC100B-P4L User Manual

Operator’s Manual
FOR USE WITH MODEL:
XYC100B-P4L
®
®
Manufactured & Distributed by
www.InovaLabs.com

Copyright © 2015 Inova Labs, Inc. All rights reserved.
No part of this document may be reproduced or
transmitted in any form or by any means, electronic,
mechanical, photocopying, recording, or otherwise,
without prior written permission from Inova Labs Inc.
Inova Labs Inc.
3500 Comsouth Drive
Suite 100
Austin, TX 78744 USA
Phone: 1.512.617.1700
Toll-Free: 1.800.220.0977
www.InovaLabs.com
Represented in Europe by:
QNET BV
Hommerterweg 286
6436 AM Amstenrade
The Netherlands

TABLE OF CONTENTS
Introduction 2
Application/Indications For Use 2
Symbol Descriptions 3
Warnings 3
Contraindications 4
Adverse Events/Hazards 4
Standard Package Contents 5
User Controls 6
Part Names 8
Operating Instructions 9
Battery Life Timetable 12
Battery Recharge Timetable 12
Attach & Remove Nasal Cannula Instructions 13
Repressurization Technology 14
Operating Procedure 14
Normal Operation Indicators 15
Alarm Indicators 16
Carry Case Configuration Instructions 17
Flying With Your POC 17
Routine Cleaning and Maintenance 18
Service Life 19
Technical Support 19
Disposal 19
Specifications 20
Oxygen Concentration Over Altitude and Flow Rate 20
Accessories 21
Warranty 22
EMC Information 26

INTRODUCTION
This Operator’s Manual will provide familiarity with the LifeChoice®
Activox® Portable Oxygen Concentrator (POC) model XYC100B-P4L and
its accessories. Be sure to read all of the enclosed information in its entirety
before using the device.
The device is an internally powered, Type BF device when powered by
the Internal Battery and a Class II, Type BF device when connected to the
external AC Power Supply, DC Power Supply or rechargeable External
Battery. The essential performance of the device is to provide oxygen
at a volume that remains within tolerance (the tolerance was defined
based on technical judgment from within the manufacturer’s expertise
in this specific medical application). In addition, the device’s ability to
detect certain error conditions (such as low purity or no breath) and create
an alarm is also considered a part of essential performance.
Operation with the DUO2 Stationary Base Concentrator
The Activox 4L can be purchased as part of the Inova Labs Activox DUO2
system. The system is composed of an Actiovox 4L POC and the DUO2
stationary base concentrator. If your POC came with the Activox DUO2
system, please refer to the Activox DUO2 Operator’s Manual for POC
operating and charging instructions.
The POC and stationary base can be disconnected and used independently
yet it is recommended they be connected. When connected:
•ThePOCandPOCexternalbatterycanbechargedbythestationary
base.
•ThePOCcanbeusedtomutetheaudiblealarmsofthestationarybase.
•ThePOCwillstoresomestationarybaseoperatingdataforuseby
service technicians.
•ThePOCwillprovidethetotaloperatinghoursofthestationarybase.
•ThePOCcannotbepowered“on”.
APPLICATION/INDICATIONS FOR USE
This manual applies to the LifeChoice Activox POC XYC100B-P4L.
INDICATIONS FOR USE: The LifeChoice Activox Oxygen Concentrator
is used on a prescriptive basis by adult patients who are diagnosed as
requiring supplemental oxygen. This oxygen concentrator will provide
supplemental, high concentration oxygen to these patients. It is not lifesupporting nor life-sustaining. It may be used continuously in a home,
institution or travel environment. The LifeChoice Activox is also portable.
This device should be used only when prescribed by a physician.
2
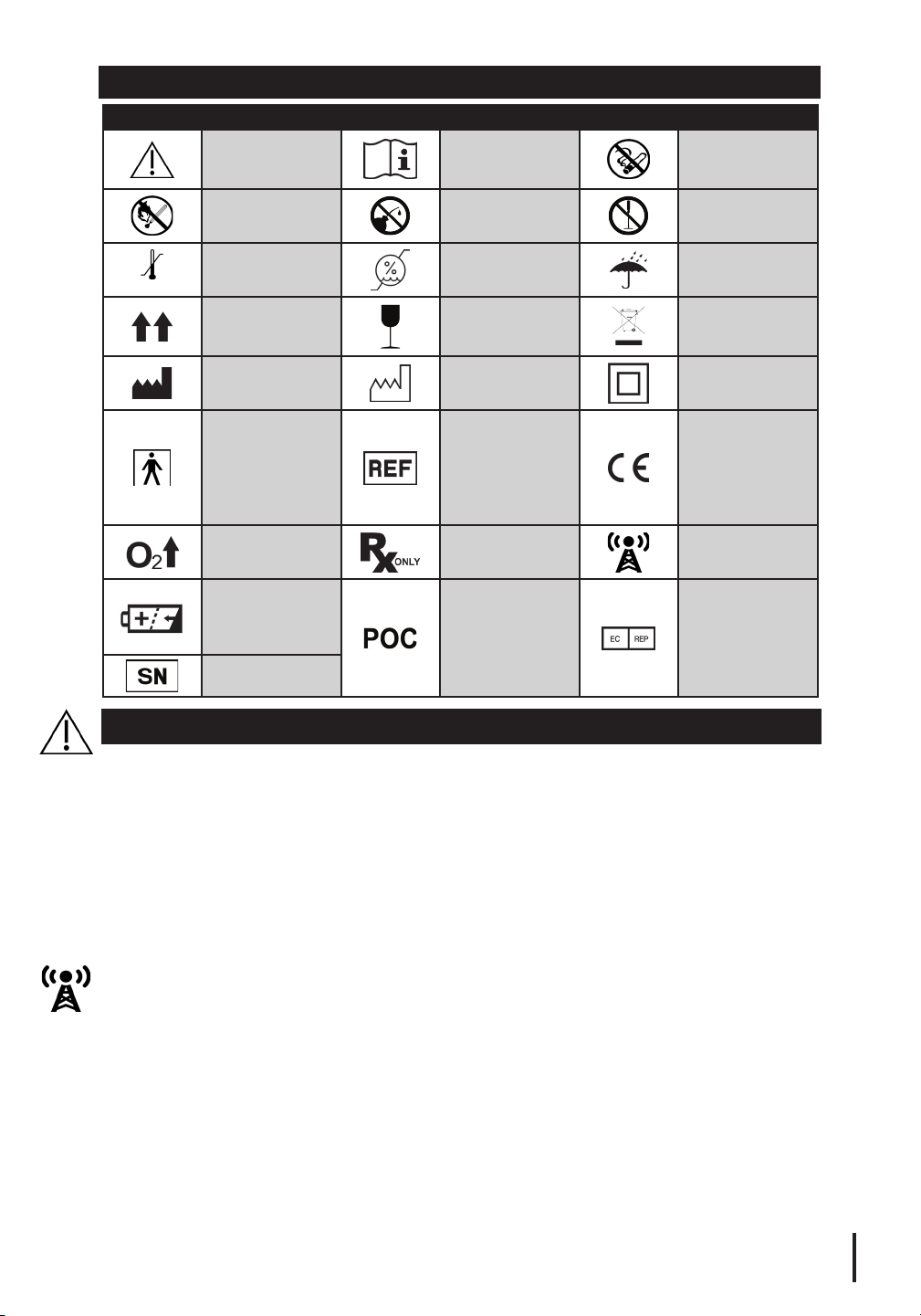
SYMBOL DESCRIPTIONS
Symbol Description Symbol Description Symbol Description
Caution
Consult
instructions for
use
No smoking
No open flame No oil or grease
Temperature
Manufacturer
Type BF applied
has conductive
Rechargeable
Serial number
WARNINGS
limit
This side up
part
Device that
contact with
patient
Gas flow
battery
Humidity
limitation
Fragile, handle
with care
Date of
manufacture
Catalogue
number
Prescription
only
Portable
Oxygen
Concentrator
Connection
Do not
disassemble
Keep dry
Compliant with
WEEE
Class II
equipment
CE Marking
of Conformity
Representative
Radio frequency
Authorized
representative
in the European
Community
1. U.S. Federal law restricts this device to sale by or on the order of a physician.
2. It is the responsibility of the patient and/or provider to make back-up
arrangements for an alternative oxygen supply.
3. Availability of an alternate source of oxygen is required in case of power
outage or mechanical failure.
4. The device is to be operated in the approved carry case provided.
5. The device should be located as to avoid pollutants or flames.
6. Portable and mobile RF communications equipment can affect medical
electrical equipment.
7. The device should not be used adjacent to or stacked with other equipment
other than the the Activox DUO2.
8. When traveling by air, the device and External Battery must be transported
as carry-on (not checked) baggage.
9. The device and External Battery contain lithium-ion batteries that are subject
to special shipping regulations. If shipping either the device or External
Battery, notify the shipper that the shipment will contain lithium-ion batteries.
10. In the event of a battery’s cell leaking, do not allow the liquid to come in
contact with the skin or eyes. If contact has been made, wash the affected
area with copious amounts of water and seek medical advice.
3
CONTRAINDICATIONS
1. The device is not intended to be life-sustaining or life-supporting.
2. In certain circumstances, oxygen therapy can be hazardous. Please
seek medical advice before using this device.
3. The device is designed to provide a flow of high purity oxygen
up to 4 LPMeq pulse. The device should only be used by patients
prescribed oxygen therapy within this range.
4. As the device will alarm through audio and visual indicators, patients
who are unable to communicate discomfort, hear, see and/or
understand the alarms may require additional monitoring.
ADVERSE EVENTS/HAZARDS
Inova Labs Inc. assumes no liability for persons choosing not to
adhere to manufacturer’s recommendations. Failure to adhere to the
statements below may impair performance of the device and will void
all warranties.
1. DO NOT use oil, grease or petroleum-based products on or near
the device as the use of such products may damage the electronic
components of the device.
2. DO NOT use power supplies or accessories other than those that
came with the device as the use of non-specified accessories may
impair performance.
3. DO NOT allow smoking or open flames within 10 ft. (3 m) of the device
as the device produces enriched oxygen gas which accelerates
combustion.
4. DO NOT operate the device in the accessory bag or any other
enclosed bag as improper ventilation will impair performance.
5. DO NOT submerge or expose the device to liquids as it may
damage the electronic components of the device.
6. DO NOT operate or expose the device to temperatures and humidity
levels outside of the specified operational environment conditions listed
in the Specifications section on page 19. Extreme temperatures and
humidity levels may damage the device.
7. DO NOT press the Control Panel buttons or screen with any hard,
sharp and/or small object as it may damage the surface.
8. DO NOT dismantle, open or shred secondary cells or batteries.
9. DO NOT expose cells or batteries to heat or fire and avoid storage in
direct sunlight.
4

STANDARD PACKAGE CONTENTS
2
1
3
7
6
4
LifeChoice Activox 4L POC
1
Model identified on device and
packaging labels. See .
4-Way Carry Case
2
Use as a backpack, shoulder bag,
waist pack or briefcase
Adjustable Straps
3
For use with 4-Way Carry Case
An optional External Battery (not included
in the Standard Package) is available for
purchase.
*Nasal cannula may not be included. Based on international requirements.See
your doctor for compatibility of other accessories (CPAP, BiPAP, etc.).
DC Power Supply
4
AC Power Supply
5
Standard 7-Foot (2-meter)
6
Single Lumen Nasal
Cannula*
7
Accessory Bag
5
5
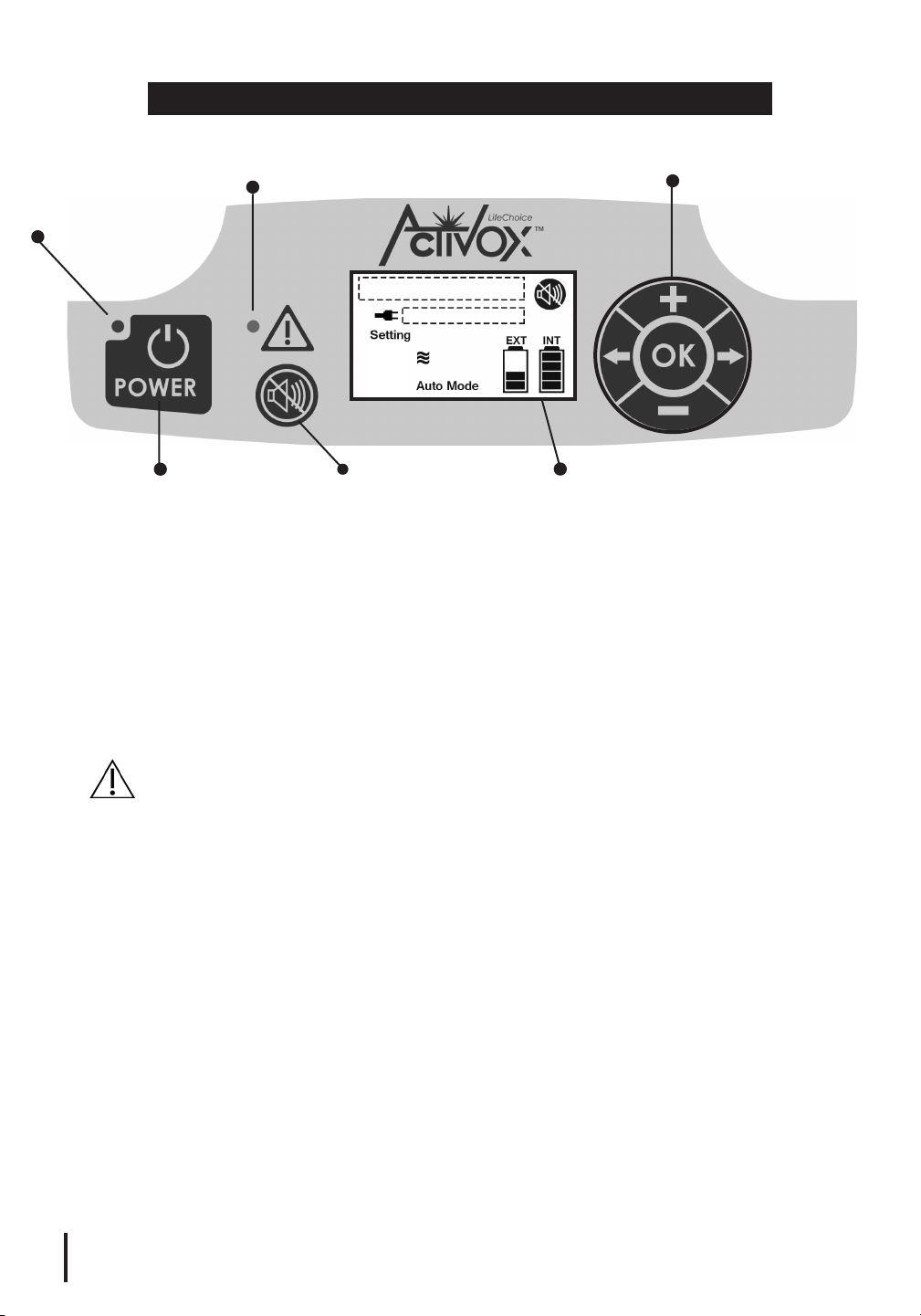
USER CONTROLS
Alarm Indicator
Power Indicator
Control Panel
Navigation Controls
4
Power Button
Mute Button
Alarm Indicator: A red LED will illuminate and an audible signal (tone)
will sound if there is a change in operating status or a condition occurs
that may need response (alarm).
Display Screen: Displays operational indicators. (Reference Display
Screen diagram on page 7.)
Mute Button: Disables audible alarm signals during operation. If an
alarm has been muted, the Mute symbol will appear on the Display
Screen. (Reference Display Screen diagram on page 7.)
CAUTION: Please use the Mute function appropriately as it silences
important audio signals regarding the status of the device.
Navigation Controls: The Plus, Minus, Right Arrow, Left Arrow and OK
buttons enable navigation within operating menus.
Plus/Minus Buttons: Adjust the Pulse Setting (1, 2, 3 or 4 LPMeq).
OK Button: Press once to illuminate the screen. Press and hold
down to display the serial number and hours of operation.
Display Screen
Right/Left Arrow Buttons: Press once to illuminate the screen.
Service personnel will also use these buttons to access maintenance
menus for troubleshooting.
Power Button: To turn on, briefly press the Power Button. To turn off,
press and hold down the Power Button until you hear a tone.
Power Indicator: A green LED will illuminate when the POC is turned
on and in use.
6
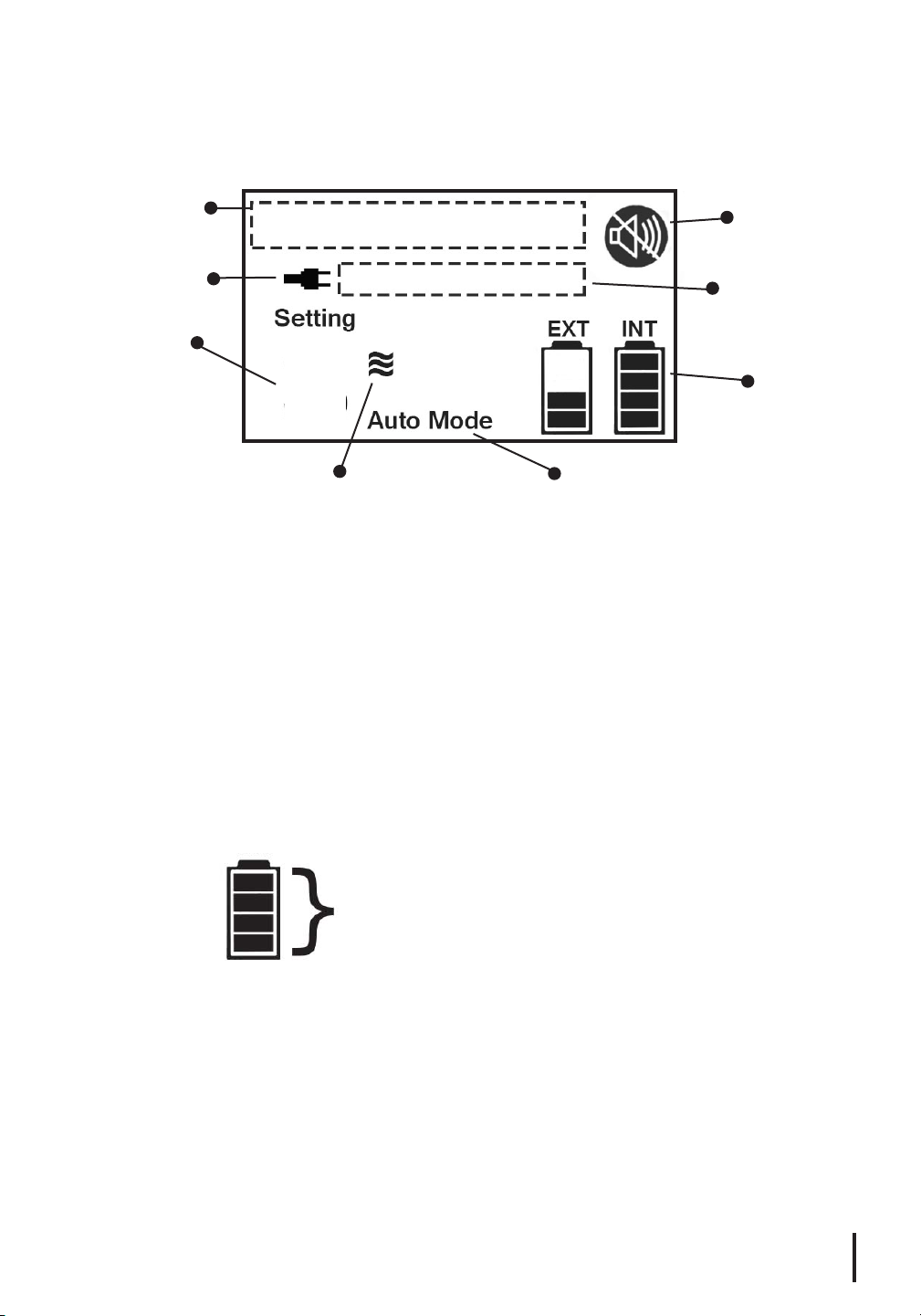
Display Screen
Message Field
External
Power Symbol
Flow Setting
Level
PULSE-WAVE Breath
Detection Symbol
Auto Mode Setting: There are two inhalation sensitivity modes on
the device: Active and Rest, which automatically adjust based on your
breathing patterns. The activated Auto Mode setting will appear on the
display.
External Power Symbol: This symbol is displayed only when the unit
is connected to an external power supply (AC or DC).
Flow Setting Level: Represents the selected Pulse Setting (1, 2, 3 or 4
LPMeq). Use the Plus and Minus Buttons to adjust the Pulse Setting up
or down.
Mute Symbol
Operating Status
Field
INT and EXT
Battery Bars
4
Auto Mode Setting
INT and EXT Battery Bars: Represent the charging level of the Internal
(INT) and External (EXT) Batteries.
100%
75%
50%
25%
Message Field: Displays the title of an alarm if activated. (Reference
Alarm Indicators section on page 15.)
Mute Symbol: Appears only when the Mute Button has been pressed.
Operating Status Field: This field will indicate if the device is
“Running”, “Charging Internal” or “Charging External”. When the
batteryisfullycharged,“ChargingInternal”or“ChargingExternal”
will disappear from the display.
PULSE-WAVE® Breath Detection Symbol: Appears when a breath is
detected and the device delivers a pulse of oxygen.
Each Battery Bar is divided into 4 segments
that represent 25% charge levels. As the
charge level of the device increases, more
segments will appear until full.
7
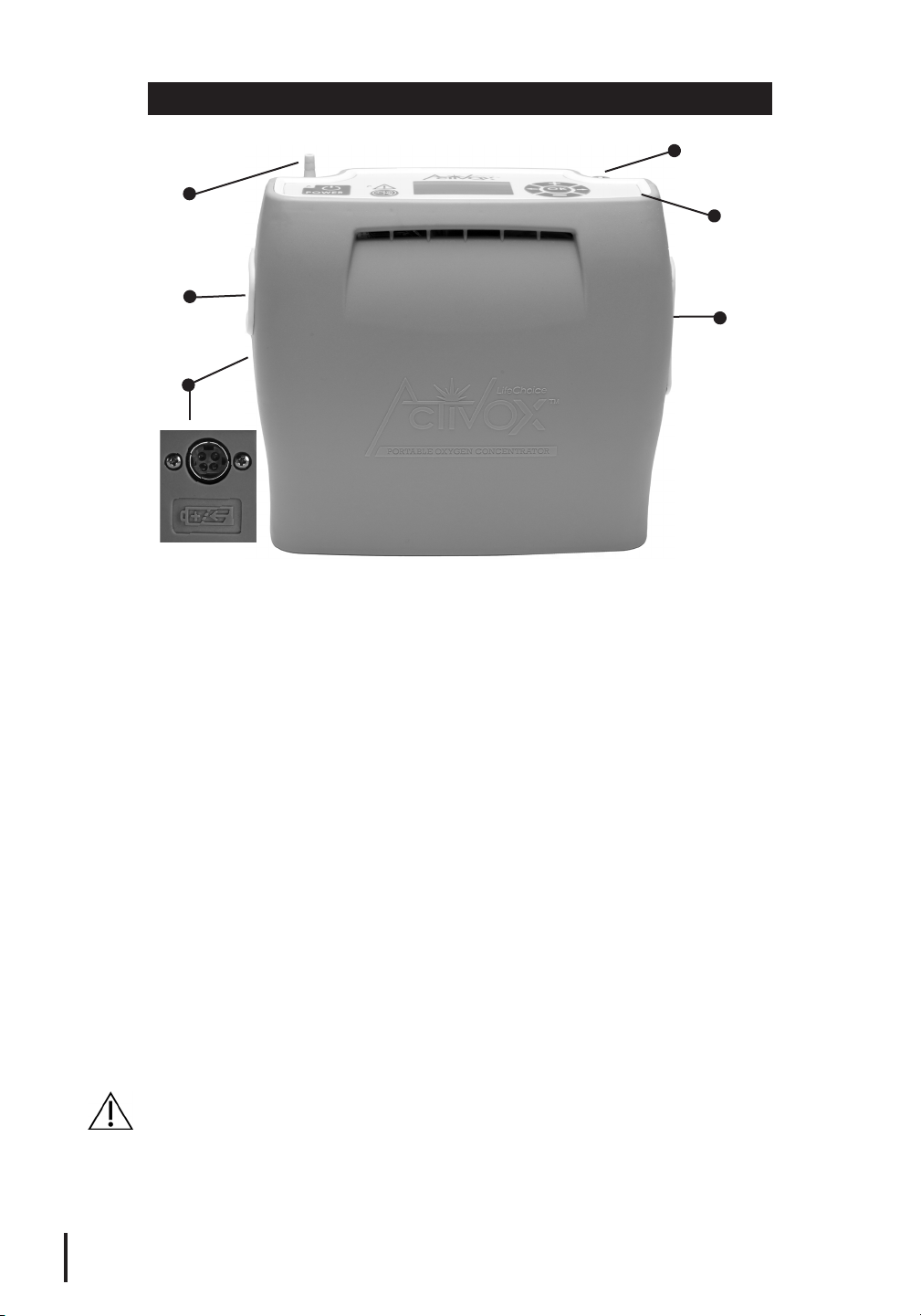
Cannula
Nozzle Fitting
Fan Outlet
Vent
External
Battery Port
Cannula Nozzle Fitting: Connect the nasal cannula to this fitting at the top
of the device.
PART NAMES
Charger Port
Control Panel
Fan Inlet Vent
Charger Port: Connect the AC or DC Power Supply to this port on the
device.
Control Panel: All user controls are located on this panel. (Reference User
Controls section on page 6 for details.)
External Battery Port: Connect the External Battery, if purchased, to this
port. The flat end of the External Battery plug should be facing upwards
when plugging into the port on the device.
Fan Inlet Vent: Cooling air is drawn in through this opening.
Fan Outlet Vent: Processed air is exhausted through this opening.
Nasal Cannula: A standard single lumen nasal cannula or equivalent must
be used with the device to provide oxygen from the concentrator.† The
maximum length recommended for use is 7-feet (2-meter). For a replacement
cannula, please contact your local medical equipment provider. Follow
cleaning and care instructions provided with the nasal cannula.
CAUTION: Use of some accessories and/or service equipment not specified
for use with this oxygen concentrator may impair the performance.
†
Nasal cannula may not be included. Based on international requirements. See your doctor for
compatibility of other accessories (CPAP, BiPAP, etc.).
8
 Loading...
Loading...