IMEDIPLUS MCE12L001 User Manual

Cardiart Wireless 12-Lead Electrocardiograph Monitor
Owner’s manual
Be sure to read the original manual and follow the instructions before use
2017.09.30 Ver. 1.0

1
Contents
Warning and Precaution ........................................................................................ 2
Chapter 1 Outline .................................................................................................. 4
1. Product introduction ................................................................................ 4
2. Intended use ............................................................................................. 4
3. Principle ................................................................................................... 4
4. Features .................................................................................................... 4
Chapter 2 Composition .......................................................................................... 5
2.1 Appearance .............................................................................................. 5
2.2 Content ..................................................................................................... 6
Chapter 3 Electrode locations ............................................................................... 7
Chapter 4 Installation ............................................................................................ 9
Chapter 5 Software Operation ............................................................................. 11
5.4 VIEW/ Add/ Delete the patient data ............................................. 20
5.5 Select a patient profile to creative a profile for patient report ...... 21
5.6 View a patient report .................................................................... 21
Chapter 6 Environment and Maintenance ........................................................... 22
Chapter 7 Products Specification ........................................................................ 25
Chapter 8 Symbol Description and Manufacturer Information .......................... 26

2
Warning and Precaution
Warnings for product using
1) The product is not intended to diagnose.
2)
Please remove the battery when not using for a long time, to avoid corrosion of the spring
if the battery holder.
3) The product can be used continuously for at least 19 hours under normal use.
4)
Do not use the product near any flammable gas (e.g., high concentrations oxygen,
hydrogen or anesthetics), which can cause an explosion or fire.
5) To avoid electric shock, do not disassemble or modify the product of one’s own.
6) Please contact the manufacturer before connecting the product to other equipment.
7) This product is a portable device, but the safety of the instrument is not able to be
ensured in the transport process with a strong impact or fall.
8)
Avoid the product from water, or the patient and the operator may involve in electric
shock and other dangers.
9) To avoid infection, please follow the instruction bellow
Regularly clean the parts that contact with patients.
Patients of open type infectious disease do not use the product for testing.
10)
When placing the wires or cables, be aware to avoid the possibility of tripping people or
winding the patient's neck.
11)
To ensure using the equipment safely, follow the maintenance procedures contained
inthe original manual.
12)
Improper battery disposal may result in explosion or contamination. Please recycle the
battery in accordance with local regulations, do not throw the battery as common trash
13) Do not use the product with Pacemaker.
Precaution
1)
The conductor parts of the electrode of ECG signal record box and its connector
(including the neutral electrode) should not contact with other conductor parts, including
the earth.
2)
The factory will yearly check whether the ECG signal record box and the cable are
damaged.
3)
Do not use the product with high frequency surgical equipment.
4)
The product is classified as CF type equipment, but not directly used for the patient's
heart.
5)
There will be potential risks when using the product along with installation of cardiac
rhythm or other electrical stimulator at the same time. Pay full attention to changes of the
ECG to ensure safety and to ensure that the leakage current is within the allowable range.
6)
Do not use accessories that are not included in the product system. The use of non-original
accessories may result in inaccurate patient data, damage to equipment, and invalid
warranty.

3
7)
Please use the original standard patient cable, otherwise it will reduce the minimum safety
of the product.
8)
The electrodes is suggested to be placed by professional medical personnel.
9)
To avoid the possible damage, do not contact the product with sharp or hard objects.
10)
Do not expose the patient cable to strong ultraviolet radiation.
11)
Do not stretch the patient cable for this may cause mechanical or electrical malfunction.
Before storing the patient cable should be wrapped as a loose circle.
12)
Avoid placing the patient cable where it may be caught, stretched, or stamped. Otherwise
the results may be inaccurate and may require repair.
13)
Please use the original accessories. The use of non-designated accessories may result in
degradation of equipment performance or unsafety.
14)
Portable and mobile radio frequency (RF) communication equipment will affect the
performance of the product.
15)
Other medical devices (including but not limited to defibrillators, ultrasound instruments,
pacemakers and other stimulators) can be used along with the product, but such
equipment may interfere the signal.

4
Chapter 1 Outline
1. Product introduction
Wireless ECG monitor of IMEDIPLUS (hereinafter referred as the product; model: MCE12L001
) is a compact, lightweight, easy carry, and agile product. The data is
transmitted through Bluetooth, avoided the inconvenience of the traditional data
transmission; also making transmission space and distance more flexible, convenient
and smooth.
The software can be installed on iOS devices, users can view data on iPad / iPhone /
iTouch; this kind of working mode is more flexible than the traditional system
composed of personal, laptop, the record box (with data lines ), and the printer (with
data transmission cable).
2. Intended use
The product is mainly for checking abnormalities of the adults’ heart through
recording ECG by medical staff in hospital or clinic. The product cannot be used as
the only basis for diagnosis.
3. Principle
The product collect the ECG signal through lead wire, initially amplifies the signal
through preamplifier, and filter out the polarization voltage, baseline shift, and high
frequency noise. Amplify the signal with the use of secondary amplification circuit,
and convert the analog signal to digital signal with the A / D converter. Then transmit
the signal to iOS mobile system through Bluetooth, at the same time, display on the
screen for clinicians to diagnose and study.
4. Features
Compact, lightweight, simple operation, and easy carry.
Breakthrough the limitation of traditional ECG (workstations).
Timely information transmission.
Low power-consuming Bluetooth.

5
Chapter 2 Composition
The product is composed of ECG record box, ECG lead wire, electrode patch/ suck ball and
instruction manual.
2.1 Appearance
Power button
Power Indicator
Green light for sufficient power;
Glint for power shortage
Lead wire port
Bluetooth
ECG Signal Record box
Front
Lead wire plug
2 AA 1.5 V batteries
Battery compartment
Battery cover
Back
Caution: If there’s any damage between the connection of the lead wire and signal
record box, please replace with the specified lead wire.

6
2.2 Content
No. Item
Name
Amount
1
ECG Signal record box
1
2
ECG lead cable
1
3
Instruction Manual
1
4
ECG Arm clamp
4
5
ECG Suck ball
1 set
7
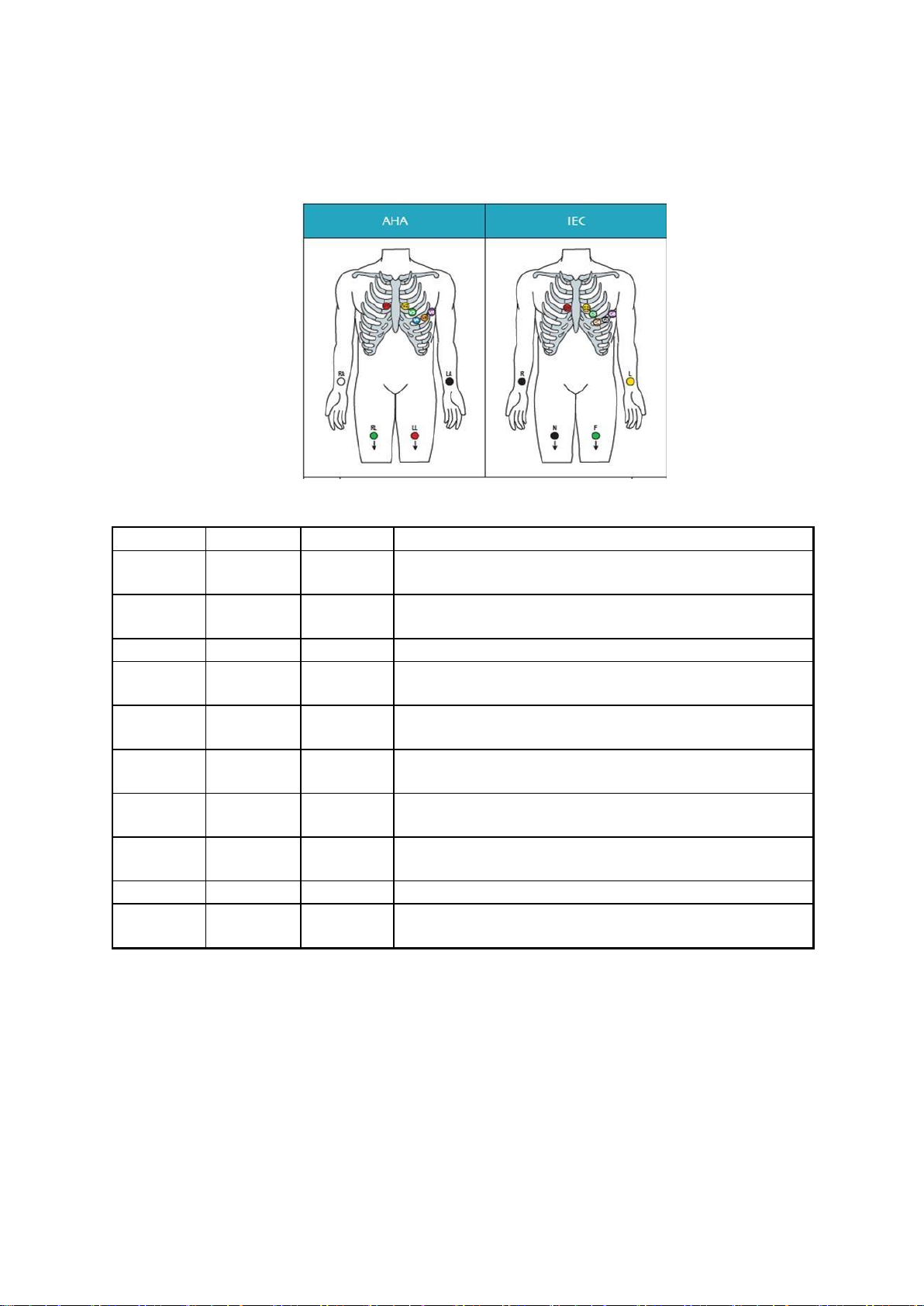
Chapter 3 Electrode locations
Electrode
AHA
IEC
Hookup
A
V1 red
C1 red
The fourth intercostal space to the right of the
sternum
B
V2 yellow
C2
yellow
The fourth intercostal space to the left of the
sternum C
V3 green
C3 green
Midway between electrode B and D
D
V4 blue
C4
brown
The fifth intercostal space in the mid-clavicular
line
E
V5
orange
C5 black
Horizontally even with D in the anterior axillary
line
F
V6
purple
C6
purple
Horizontally even with D in the midaxillary line
G
LA black
L yellow
Left arm (resting ECG) or left shoulder (exercise
test)
J
RA white
R red
Right arm (resting ECG) or right shoulder
(exercise test)
H
LL red
F green
Left foot (resting ECG) or left leg (exercise test)
I
RL green
N black
Right foot (resting ECG) or right leg (exercise
test)
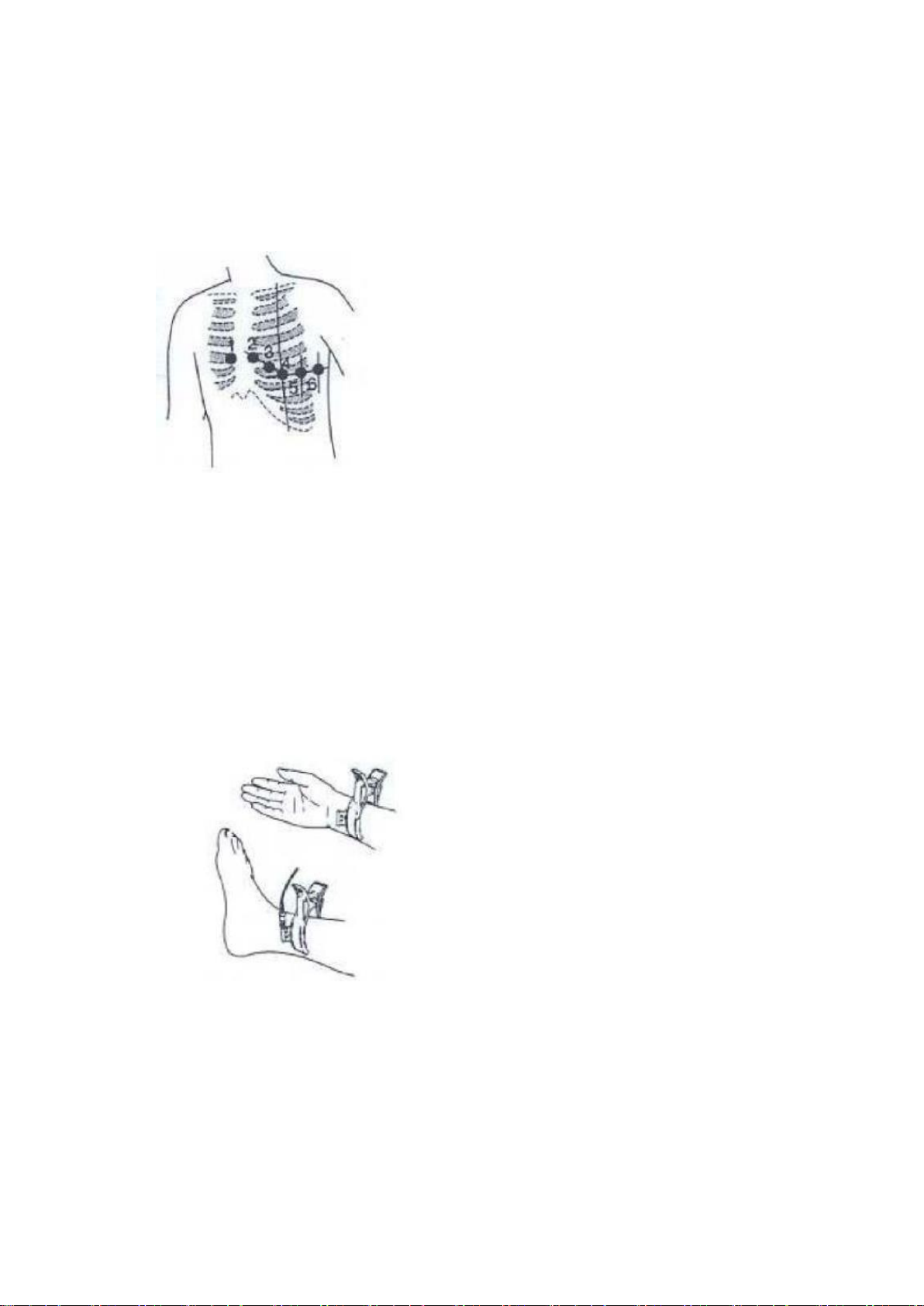
3.1
WILSON standard lead
Electrode loctions
8
3.2 Electrode connection and position
Precordial lead:Coat the patient’s chest with conductive paste or wipe with
alcohol cotton balls in correct position, then attach the lead and
suck ball to the patient in the order as below:
Precordial lead position:
V1:The fourth intercostal space to the right of the sternum
V2:The fourth intercostal space to the left of the sternum
V3:Mid-point between V2 and V4
V4:The fifth intercostal space in the left clavicular midline
V5:The fifth intercostal space in the axillary line V6:
The fifth intercostal space in the axillary midline
Electrode clip:
Limb lead:
1) Clean the skin of the patient
2) Apply conductive paste or wipe with alcohol cotton balls
3) Attach the electrode clip, preferably on the inside of limbs
4) To prevent oxidation and poor connection, please clean the clips after use
Limb leas position:
RA:Right arm
LA:Left arm RL:
Right leg LL:Left
keg
Recommendation:
1) It is recommended that electrodes be placed by medical trained personnel.
2) To obtain correct ECG record, please previously clean the skin.
 Loading...
Loading...