
IMEDIPLUS INC.
510(k) Submission
Electronic Stethoscope
DS3011A
Section 13
Document Title: Electronic Stethoscope DS3011A User
Manual
File Name: 002_Electronic Stethoscope DS3011A User
Manual
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
2 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
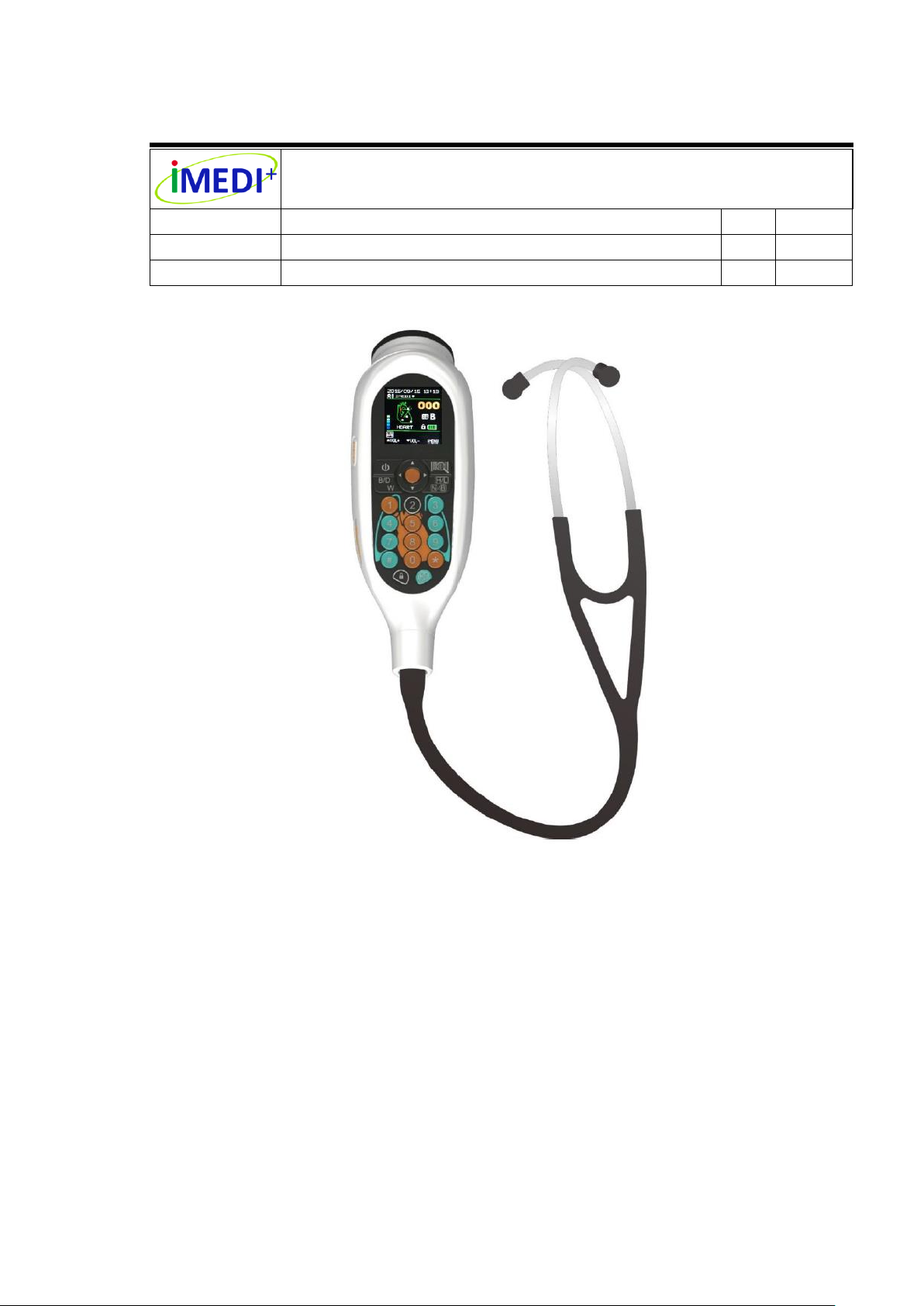
Feature
Auscultation for heart, Anterior/Posterior chest, neck, bowel, limbs arteries, veins and internal
organs.
Heart rate detection.
Built-in barcode reader for 1D (One dimensional) barcode scanning.
Real time recording and playing of auscultation sounds.
Recording multiple sounds in one patient and up to 600 10-second auscultation sound tracks.
Micro SD memory card for file transmission of auscultation sound tracks.
Friendly design for operation with organ mapping oriented interface
Clear acoustic performance
With 24X auscultation sound amplify ability and 10 volume level adjustable.
Ergonomic Design
Easy to use.
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
3 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Content
1. Introduction .................................................................................................................. 4
2. Safety Information ...................................................................................................... 4
2.1 Symbol Definitions ................................................................................................. 4
2.2 Important Safety Information .............................................................................. 6
3. Product Description ................................................................................................... 8
4. Intended Use ................................................................................................................. 9
5. Operator Profile ........................................................................................................... 9
6. Patient Privacy ............................................................................................................. 9
7. Instructions for Use ................................................................................................. 10
7.2 Insert Battery .......................................................................................................... 11
7.3 Position Headset ................................................................................................... 12
7.4 Adjust Headset for Comfort .............................................................................. 12
7.5 Function Buttons .................................................................................................. 13
7.6 OLED display shows information ................................................................... 15
7.7 Schematic of Menu Page.................................................................................... 16
7.8 Operation Description ......................................................................................... 16
8. PhonoMagics Use ..................................................................................................... 31
8.1 Main Recording Screen ...................................................................................... 33
8.2 Profile Screen ......................................................................................................... 34
8.3 Review Screen ....................................................................................................... 34
9. Assembly Parts List ................................................................................................. 36
10. Accessory Information ....................................................................................... 37
11. Mechanical Dimension ........................................................................................ 38
12. Cleaning ................................................................................................................... 39
13. Warranty ................................................................................................................... 39
14. Troubleshooting .................................................................................................... 40
15. Maintenance and Repair ..................................................................................... 41
16. Transportation, Storage, and Disposal ......................................................... 42
17. Organ Positions Correspond to Recorded File Name ............................. 43
18. Appendix: Guidance and Manufacturer’s Declaration ............................. 45
19. EMC Compliance ................................................................................................... 48
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
4 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Explanation of Safety Markings and Symbols
Warning
Consult instructions for use.
1. Introduction
Thank you for choosing the IMEDIPLUS Electronic Stethoscope DS3011A.
This useful stethoscope will be a great aid in your auscultation.
It has several smart and friendly design, which makes it easy to use and properly
fit the needs for users. The design included an easy-to-use interface that enable
the users to approach the patient with one hand. Rapid identification of recording
number and user’s identities and 2-step operation for marking the source of the
sound make the valid label while you capture the sound signals.
The ear-tips are comfortable to wear with the soft texture. As well, it could provide
a good tightness for reduction of environmental noise to offer a good sound quality
for users.
IMEIDIPLUS Electronic Stethoscope DS3011A was innovated from a group of
healthcare professionals. Therefore, the user experience is an important factor of
designing the stethoscope. We appreciate for your adoption of IMEDIPLUS
DS3011A and look forward to your valuable feedback.
2. Safety Information
Please read, understand, and follow all safety information contained in these
instructions prior to using this electronic stethoscope. Retain these instructions for
future reference.
2.1 Symbol Definitions
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
5 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
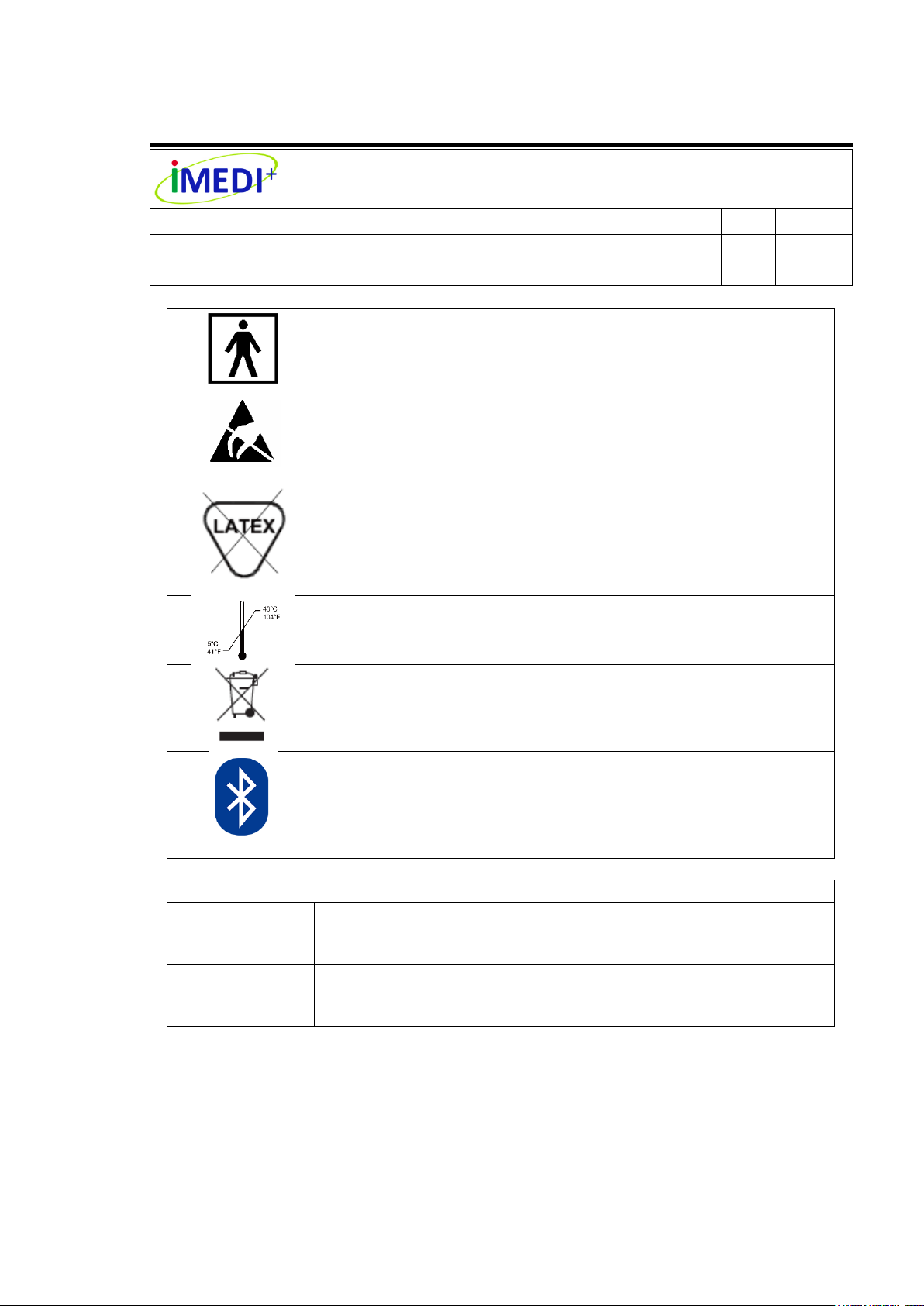
Indicates Type BF Equipment: The equipment provides
protection against electrical shock and electrical current
leakage. Applied parts are considered to be the complete
chest-piece with diaphragm.
For ESD sensitivity connector the testing exemption.
This product and packing does not contain natural rubber
latex.
Temperature Limit
This product contains electrical and electronic components
and must not be disposed of using standard refuse collection.
Please consult local directives for disposal of electrical and
electronic equipment.
This product uses wireless Bluetooth communication.
Explanation of Signal Word Consequences
NOTICE
Indicates a hazardous situation, which, if not avoided, may
result in property damage.
WARNING
Indicates a hazardous situation, which, if not avoided, could
result in minor injury and/or property damage.
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
6 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
NOTICE
To reduce the risks associated with environmental contamination follow
applicable regulations in local when disposing of this stethoscope. Properly
dispose of, or recycle, spent batteries.
No modification of this device is allowed. Use only authorized IMEDIPLUS
service personnel to repair this electric stethoscope. If user modify by self,
solely responsibility for the consequence.
The DS3011A is MR unsafe. Do not use the DS3011A in Magnetic
Resonance Imaging (MRI) environment.
WARNING
To reduce the risks associated with an incorrect result, personal injury
and equipment damage, stethoscope shall be stored and operated by
medical professionals only as instructed in this manual.
To reduce the risks associated with infection follow all cleaning and
disinfecting instructions included in this manual. Establish and follow a
cleaning and disinfecting schedule.
To reduce the risks associated with a damage of ear canal, please hold
tight the device to avoid sudden falling and make sure that the soft sealing
ear-tips are snapped firmly into position.
2.2 Important Safety Information
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
7 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
WARNING
To reduce the risks associated with very strong electromagnetic fields,
avoid using the stethoscope near strong radio frequency signals or portable
and/or mobile RF devices. The stethoscope might be damaged. If you hear
sudden or unexpected sounds, move away from any radio transmitting
antennas.
To reduce the risk associated with an electrical shock, do not use the
stethoscope on patients without the stethoscope’s diaphragm cover in place.
DS3011A contains a Bluetooth Class 2 wireless data link. The maximum
radio frequency field strength generated by the stethoscope is below three
volts per meter, a level that is considered safe to use with other medical
devices. However, audio, video, and other similar equipment may cause
electromagnetic interference. If such devices are encountered and cause
interference, immediately move DS3011A away from that device and/or turn
the Bluetooth feature OFF.
To reduce the risks associated with a damage of user’s eyes, do not
looking into the illumination beam of barcode reader.
To reduce the risks associated with a damage of stethoscope, please
put the device into the pocket of physician gowns to avoid sudden falling,
when you put the device hanging on the neck.
Do not use the unauthorized accessories, which would be caused hazard.
The accessories use only from IMEDIPLUS provided.
Do not immerse the stethoscope in a liquid or subject it to any
sterilization process. The device might be damaged.
Before first use, battery must be charged continuously for at least 8 hours.
Failure to do so may shorten the battery's lifetime.
Do not use the DS3011A in Magnetic Resonance Imaging (MRI)
environment. Because of DS3011A contain conductive, metallic and
magnetic materials. Those materials include headset, wire, connectors and
inductors, which are assembled in DS3011A.
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
8 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
3. Product Description
The IMEDIPLUS Electronic Stethoscope DS3011A picks up the sounds from the
heart, lung, anterior/posterior chest, abdomen, neck, limbs, arteries, veins and
other internal organs from a patient’s body. After detection and amplification, the
sounds are transferred to the user’s ears via an active speaker and passive sound
tubes. It could also identify the recording number by 1-D barcode reader, indicate
the sound location by intuitive keypad, and simultaneously record the sounds from
different sites.
The one-hand user interface includes a full-color OLED display and an intuitive
keypad at the anterior part, a barcode reader at the posterior part, a chest-piece
at the superior part, a tube connector for output of sounds at the inferior part, and
a recording button at the left part. Sound processing is operated with the aid of a
digital signal processor.
In order to transmit sounds to the “PhonoMagics” APP, the stethoscope and device
must be connected via Bluetooth, and in order to fully use certain functions, the
mobile device must be connected to the internet via cellular data connection or WiFi.
The DS3011A uses a Bluetooth Class 2 wireless data link. The Bluetooth range
will be reduced when objects (walls, furniture, people, etc) are between the
DS3011A and a paired mobile device. To improve Bluetooth connection, reduce
the distance and/or allow a line of sight between the DS3011A and mobile device.
It is highly recommended that users of the (PhonoMagics Dashboard and)
PhonoMagics use device and networking security features to protect patient data
created and stored using this software, in addition to security features embedded
in the system. Please consult your institution’s technical services to implement
appropriate security measures.
The DS3011A could also exchange audio data with an external personal
computing device using micro SD card. Every single audio file stored in micro SD
card was labeled with the user’s ID, recording number and indicated position. And
the recorded audio data can only be replayed by DS3011A, but cannot be replayed
by personal computing device.
The DS3011A does not incorporate any off-the-shelf (OTS) software.
DS3011A Document For SGS Certification
The DS3011A operates on one (1) NP-120 lithium battery with an included power
management system to prolong the battery life.
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
9 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
4. Intended Use
The IMEDIPLUS Electronic stethoscope DS3011A is intended for the detection,
amplification and recording of sounds from the heart, lung, anterior and posterior
chest, abdomen, neck, limbs, arteries, veins and other internal organs with
selective frequency ranges. The stethoscope chest-piece is designed for
application to child, adolescent and adult patients. It is used for any subject
undergoing a physical examination and intended only for medical diagnostic
purpose in clinic or hospital.
5. Operator Profile
The IMEDIPLUS Electronic Stethoscope DS3011A is designed to be used by
anyone who wishes to listen to sounds as described in the Intended Use section
above. The user manual provides complete information on how to operate the
DS3011A so that no additional operating training is required.
6. Patient Privacy
The privacy of patient health information may be protected by state, federal, or
international/foreign laws that regulate how such information can be used, stored,
transmitted, and disclosed. The IMEDIPLUS system employs security features
that are compliant with HIPAA policies. Third party access may be prohibited to
such information without obtaining written authorization from the patient.
The user is fully responsible for understanding and following all laws that regulate
storage, transmission, and disclosure of any electronic patient data through the
use of software. If the user becomes unable to comply with a law or restriction that
applies to use and disclosure of such data, the user should not proceed to collect
or save such information.
This application may require entry of individually identifiable health information in
order to function. Records are stored and recalled through the use of patient name,
date of birth, and/or patient ID #. By entering this information, the user assumes
any and all risks of and liabilities incurred with using or transmitting such
information.
DS3011A Document For SGS Certification
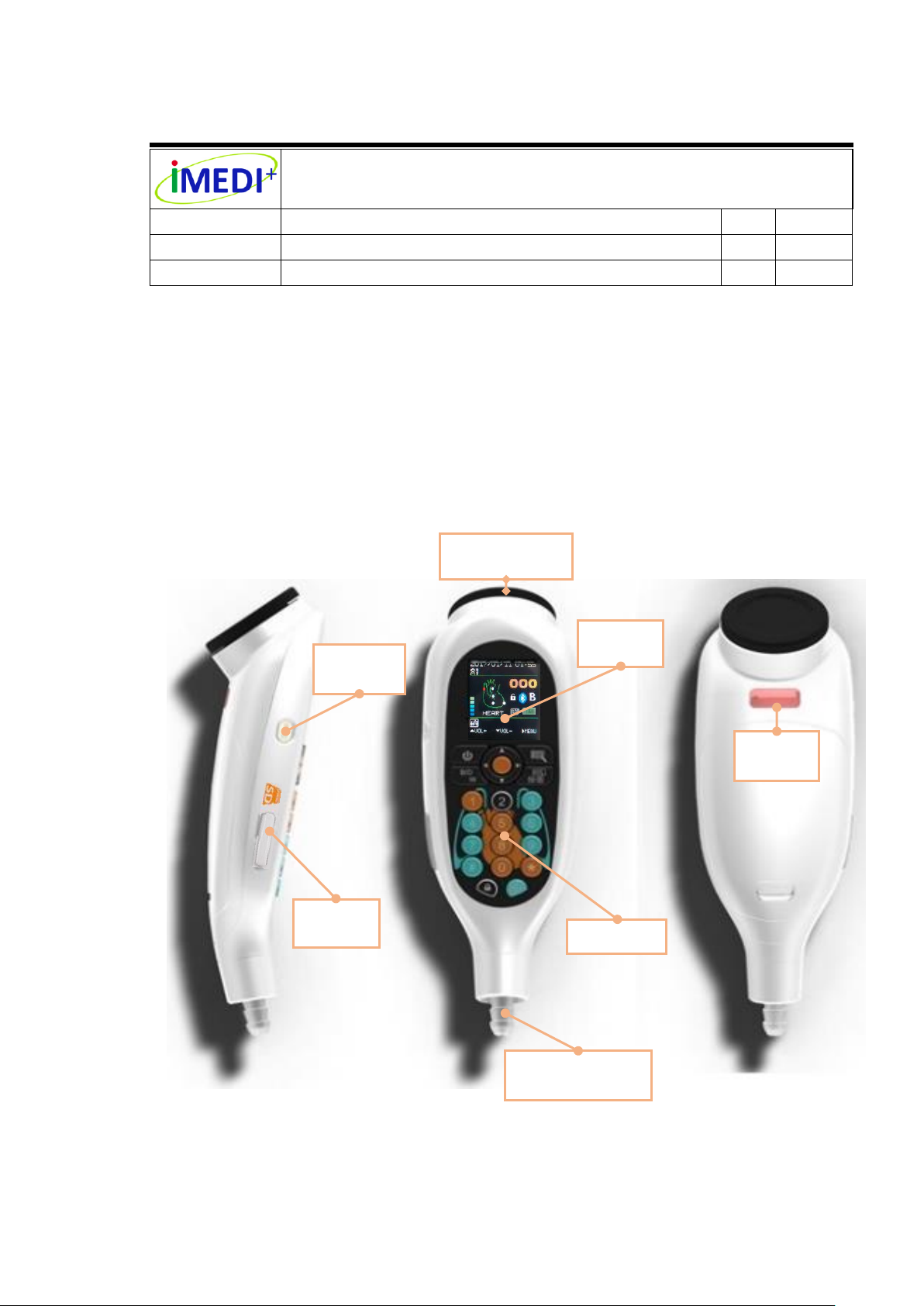
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
10 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Chest-piece
(Microphone)
OLED
Screen
Keypad
Audio Output
eartube Connector
Barcode
Reader
Micro SD
Card Slot
Recording
Button
7. Instructions for Use
Please read through the user manual carefully before using the product and
operate it according to the user manual. It is advised that you should keep this
manual for reference anytime.
7.1 Stethoscope Interface
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
11 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
7.2 Insert Battery
Insert one NP-120 lithium battery (provided in package) into the IMEDIPLUS
Electronic Stethoscope DS3011A.
Unlock of the battery cap, and then remove the battery cap, as follows.
Insert the battery with correct direction, as follows.
Remount the battery cap, as follows.
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
12 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
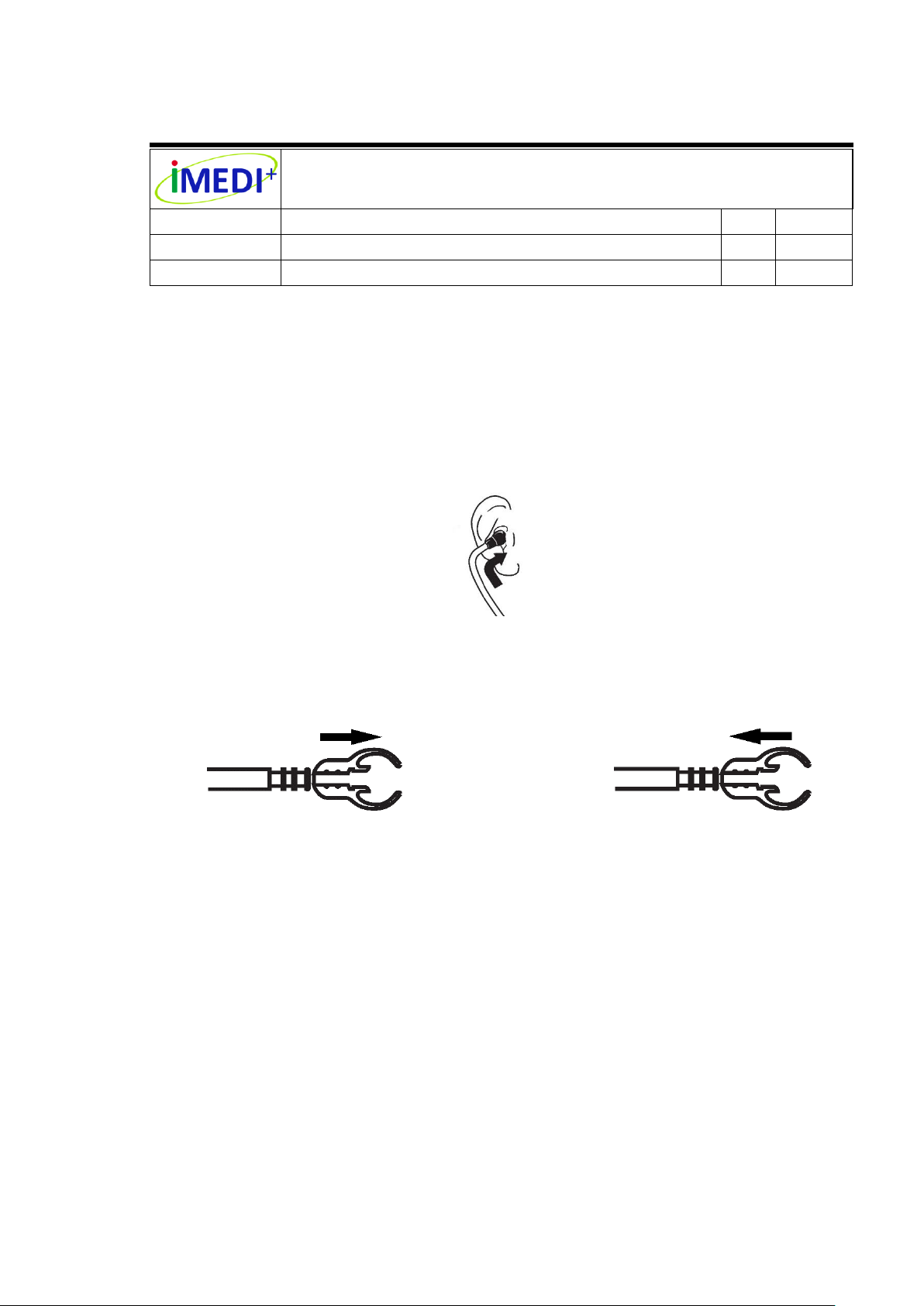
Eartube
Ear-tip
Ear-tip
Eartube
Remove Ear-tip
Apply Ear-tip
7.3 Position Headset
The ear-tips should point in the forward direction as you insert them into your ear
canals. When ear-tips are properly positioned, diaphragm will face towards your
body, as follows.
The user can pull ear-tips away from the eartube to remove the ear-tips, as follows.
The user can push ear-tip firmly onto eartube to apply new ear-tips, as follows.
7.4 Adjust Headset for Comfort
To reduce the spring tension in the headset, hold each eartube at the bend part
near the ear-tips and gradually pull apart until fully extended (180 degrees), as
follows.
To increase spring tension, grasp the headset with one hand where the metal
eartube enter the plastic tubing, and squeeze until the plastic tubing on one
eartube touches the other. Repeat as necessary, as follows.
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
13 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Reduce Tension
Increase Tension
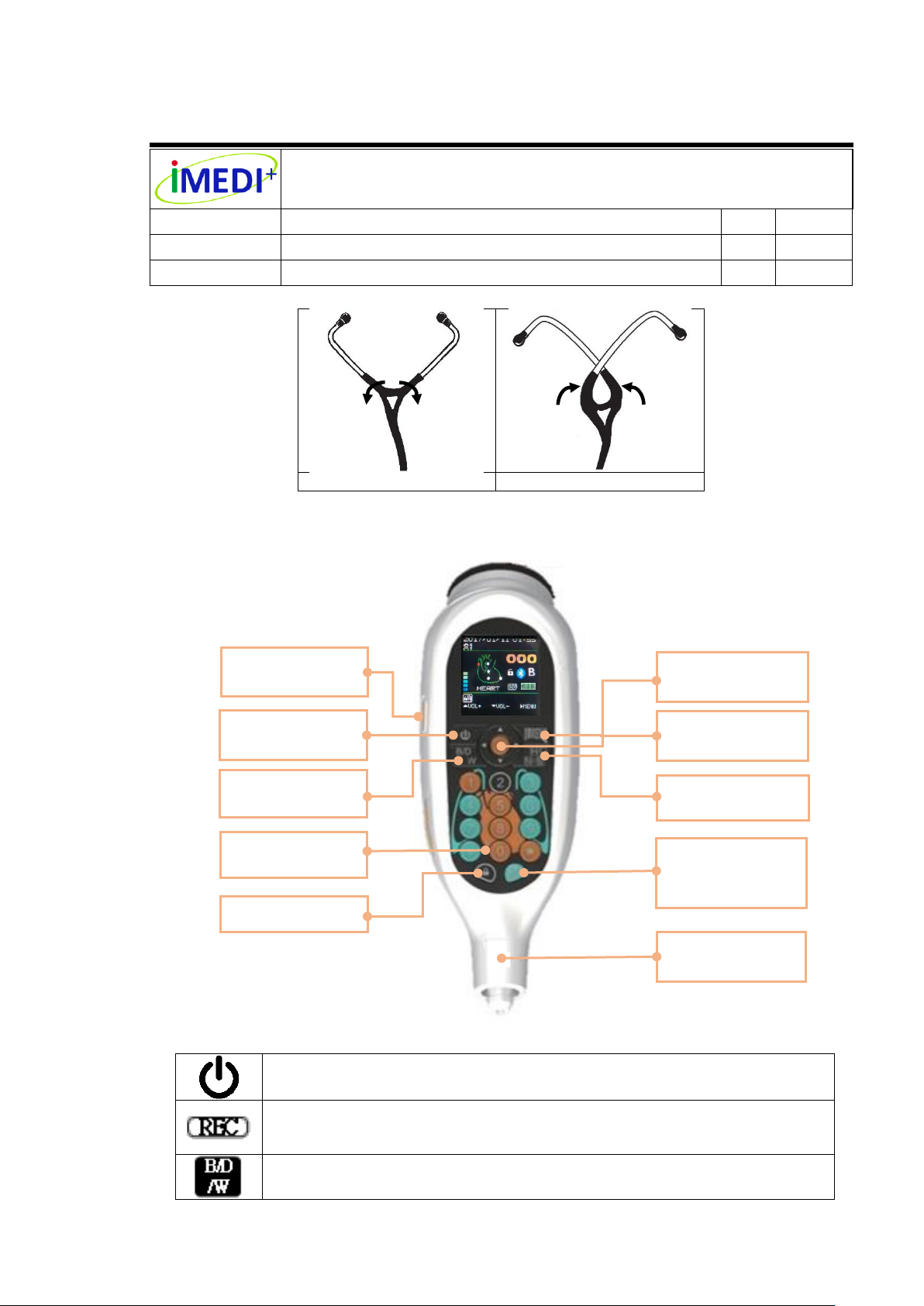
Power Button
To turn on the device or enter the sleep mode.
Recording Button
During auscultation (in the HOME page), press recording button to record
auscultation sounds of 10 seconds.
Filter Modes Switch
Lock Button
Filter Modes
Switch (B/D/W)
Power
Button
Four-way and
OK Button
Barcode
Reader Button
Organ Position
Anterior Chest /
Posterior Chest
Switch
Audio Output &
Tube Connector
Recording
Button
7.5 Function Buttons
Number Keys
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
14 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
During auscultation (in the HOME page) and playback the recorded sound track,
this button can select one of the auscultation filter mode, including “Bell”,
“Diaphragm”, and “Wide” modes.
Number Keys
During auscultation (in the HOME page), the “Number” keys on the intuitive
keypad illustrated with heart and lung are used to choose the auscultation position.
For “DATE SET” page, Number keys are used to set the date and time.
Lock Button (Lock/Unlock Switch)
During auscultation (in the HOME page), the “Lock” button is used to “lock” most
of the function keys, besides Recording Button. When pressing the button again,
you will “unlock” the locked status.
Four-way Button and OK Button (Red key)
The “Four-way” button is used for selection.
The “Right” and “Left” keys are used to enter or exit the pages.
The “Up” and “Down” keys are used to adjust the sound amplification level.
Using the “Up” and “Down” can move the indicator upward and downward.
The “OK” button(Red key) is to set and confirm the selection item
Barcode Reader Button
For one-dimensional barcode scanning.
Organ Position Switch
The “Organ Position Switch” is used to change the organ positions among “Heart
and Lung”, “Neck”, and “Bowel”.
A/P CHEST Image Switch (Anterior and Posterior Chest)
A/P CHEST Image Switch is used to change the organ images between “Anterior
Chest” and “Posterior Chest”.
DS3011A Document For SGS Certification
Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
15 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
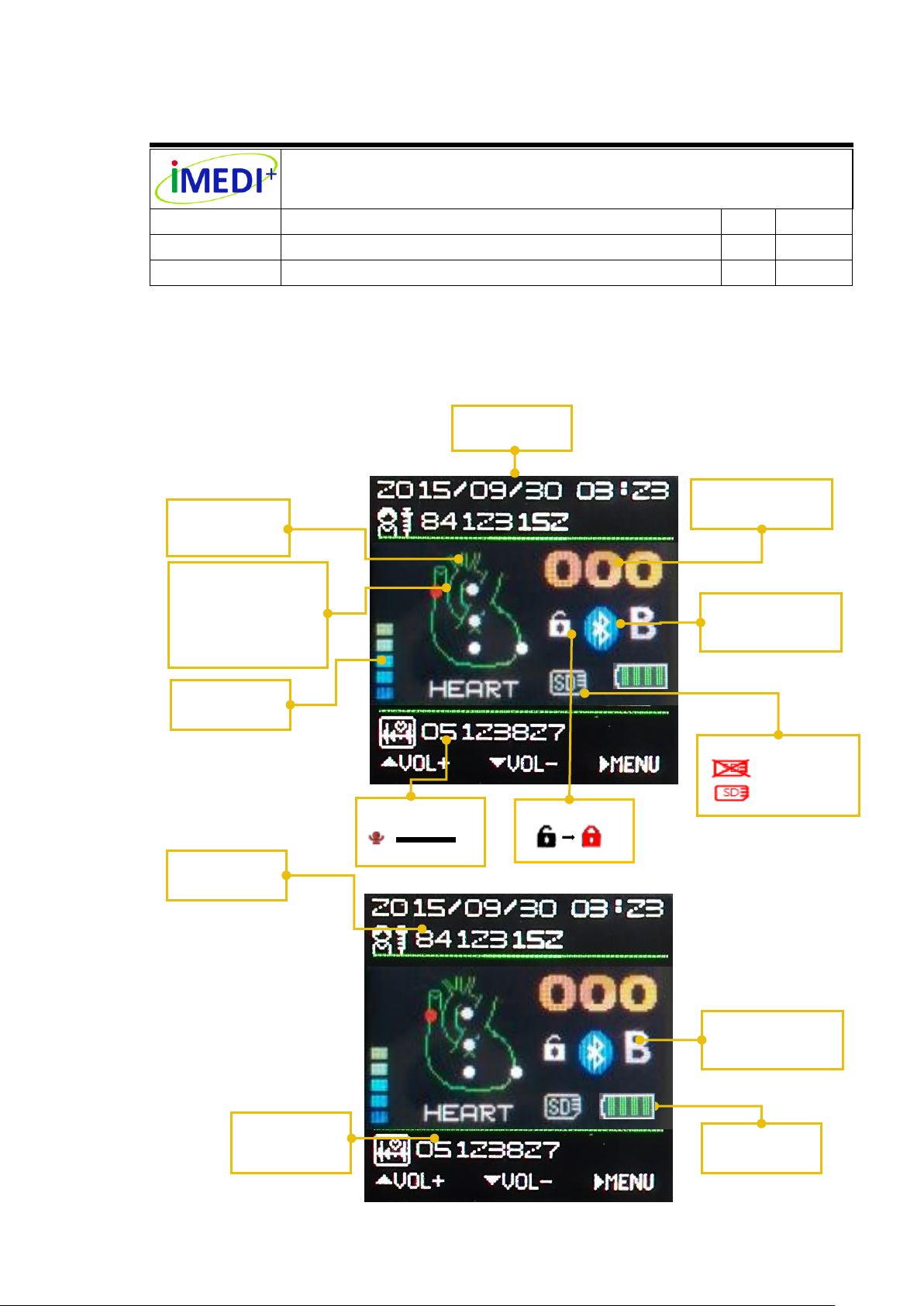
Volume Level
Date and Time
User’s ID
Position
Auscultation
Recording)
Recording Tips
Heart Rate
Modes B/D/W
Battery Status
Key Lock Icon
Unlock
Lock
Micro SD Card Tip
No micro SD Card
Micro SD Card Full
Blinking
Number
7.6 OLED display shows information
Organ
Position (Red Point
is Under
Auscultation and
Bluetooth
Recording
DS3011A Document For SGS Certification
Auscultation

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
16 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
7.7 Schematic of Menu Page
7.8 Operation Description
Power Button (Switch On / Sleep Mode)
Press the “Power Button” continuously for 3 seconds to turn on the device
or enter the sleep mode.
The device will automatically enter sleep mode after idling for 5 minutes.
The idle time can be defined by the user on the “Setup Page”. (Reference
to the functions of this user manual.)
Password Setting
When turn on the device, enter the password to log in to the device
DS3011A Document For SGS Certification
application. The default password for use is “000000”.
Create a personal secure 6-digit password by selecting “SETUP” option.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
17 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Navigate to the UPDATE PWD screen after selecting the “SETUP” option.
Next, select “UPDATE PWD” > INPUT PASSWORD. Follow the
instructions on the screen to create and save a 6 -digit password. You will
need to enter your PIN twice for verification purposes.
Detection of The Patient’s Heart Rate
Place the stethoscope close to the patient’s heart sound positions. Within
10 seconds, the screen will display the patient’s heart rate and the data
will be updated every two seconds. The heart rate ranges from 30 bpm
(beat per minute) to 180 bpm (beat per minute). When taking the
stethoscope away from the patient, the heart rate will display 000 within 5
seconds.
IMPORTANT!
If the ambient sound is too noisy or the measuring position is incorrect,
inaccurate heart rate may happen.
WARNING: To avoid the damage of ears, do not tap hard or scratch
the chest-piece with diaphragm while wearing the eartips with the
stethoscope powered on.
Organ Position
Using function keys to select the organ position.
Using number keys to select the auscultation position under auscultation
and recoding.
Red point is the auscultation position under auscultation and recording
(Indicated position)
Every single audio file stored in micro SD card was labeled with the
indicated position.
DS3011A Document For SGS Certification
Organ position switch among “Heart/Lung”, “Neck”, and “Bowel”

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
18 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Organ Position Switch is used to change the organ position among “Heart and Lung”, “Neck”,
and “Bowel”.
Press the key with image of “Heart” or “Anterior Chest” on the keypad to switch and select organ
position
Using the “Heart /Lung” organ position, it could be quickly switched
between “Heart” and “Anterior Chest” for the selection of organ position.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
19 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Press the function button to switch the position to “Anterior Chest” or “Posterior Chest”.
When you pick up the sound of right upper lung filed at the front, you shall select the “Anterior Chest”
area and then press the number key “4” which stands for “RUL(Right Upper Lobe) illustrated on the
When you pick up the sound of left upper lung filed at the back, you shall select the “Posterior Chest”
area and then press the number key “4” which stands for “LUL(Left Upper Lobe) illustrated on the
intuitive keypad.
Press function button to select the filter mode.
The “B” mode, “D” mode, and “W” mode will cycle in turn when you press this button.
Bell Mode
Diaphragm Mode
Wide Mode
Organ position switch between “Anterior Chest” and “Posterior Chest”
intuitive keypad.
Filter Mode Selection (B/D/W Modes)
The stethoscope allows the users to select these three different filter modes
(B/D/W modes) for diverse application to different clinical scenario. Which can
emphasize the specific patient sounds of interest.
Using the “Bell” mode, the sound frequency below 390 Hz is amplified, and
the sound frequency from 20 to 200 Hz is emphasized.
Using the “Diaphragm” mode, the sound frequency below 630 Hz is
amplified, and the sound frequency from 100 to 500 Hz is emphasized.
Using the “Wide” mode, the sound frequency below 1000 Hz is amplified.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
20 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
To switch the mode to show the sound wave, press the “OK” button while listening the sound.
10 sec Recording
Press function button to record the auscultation sounds.
Real-time monitoring sound wave
The stethoscope can show the sound wave on screen during hearing the
sound in different mode.
Recording the Auscultation Sound
While recording the auscultation sound, every single audio file of the sound
track stored in the micro SD card will be labelled with recording number
(Identity), user’s ID (Identity), and indicated position. The user could also
exchange audio data with an external personal computing device using the
micro SD card (Maximum to 600, 10-second sound tracks)
Press the Recording Button to record the auscultation sound for 10
seconds after selecting the auscultation position with recording number
and user’s ID.
The recorded auscultation sound track will be saved in the micro SD card
which can store up to 600 sound tracks. Don’t remove the micro SD card
during recording.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
21 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Press the “Right” button to enter the “MENU” page.
the “PLAYBACK” page.
MENU Page
Playback Page
PLAY LIST page
Playing Back a Sound Track
When you enter the “MENU” page, selecting “PLAY” will help you enter the
“PLAY LIST” page.
Press the Right Button to enter the “MENU” page and then press “OK”
Button to enter the “PLAY LIST” page.
“PLAY LIST” provides the latest 50 sound tracks with auscultation position.
Press the “Up” or “Down” button to select the sound track which you want
to play back. Then press the “OK” button to play the one you select.
The sound track you select could be transferred to the user’s ears via an
active speaker and passive sound tubes. The sound track will loop
continuously and demonstrate on the screen.
While listening the sound track, the sound can be listened in different filter
mode by pressing the button.
Press the “Left” button to stop playing the sound track and return to the
“PLAY LIST” page.
Press the “OK” button to enter the “PLAYLIST” page.
The sound track you select will loop continuously and demonstrate on the screen when you enter
Adjust Sound Amplification Level
The IMEDIPLUS Electronic Stethoscope DS3011A sound level could be
amplified in 10 increments up to 24X amplification of a non-electronic
(Cardiology-level) stethoscope. Level 0 is equal to silent mode. Level 10 is
equal to 24X amplification of a non-electronic stethoscope. The greater the
DS3011A Document For SGS Certification
amplification, the more bars you will see on the screen.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
22 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Press the “UP” and “DOWN” button to adjust sound amplification level.
The greater the amplification, the more bars you will see on the screen.
Volume +
Volume -
Press the “UP” button to increase the volume level until the desired level
is achieved.
Press the “Down” button to decrease volume level until the desired level
is achieved.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
23 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Unlocked
Locked
Press function button to lock or unlock keypad.
Lock /Unlock
Press the “Lock” Button to lock or unlock keypad to avoid inadvertently
touching other keys during auscultation.
When the keypad is locked, only the “Recording Button” can be used.
Press the “Barcode Reader” button to set the user’s ID
(Identity) and recording number (Identity)
Setting the user’s ID and recording number before you select the auscultation
position and pick up the sound for clinical auscultation. Every single audio file
of sound track will be labelled with the recording number, user’s ID, and
auscultation position for digital sound track database storage and build-up. It
will be helpful to follow up your patient with valid auscultation records with lots
of correlated clinical evidence for advanced study and personalized care. After
your successful setting, both of the user’s ID and recording number will show
on the “Home” page as following illustration.
Setting the recording number
On the “HOME” page, press the “Barcode Reader” button could identify
the recording number. After your setting, the recording number will show
in the screen as follows.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
24 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Recording number
Press the “Barcode Reader” button to identify the recording number.
User’s
ID
Press the “Barcode Reader” button to read and set the “User’s ID” for the following auscultation.
Select USER ID
WARNING: Looking at the beam light of barcode reader is prohibited
to avoid damage to your eyes.
IMPORTANT!
• The recording number is the series number of clinic or hospital, it did not
include any patient’s personal information.
Setting the user’s ID
We could set the user’s ID after going to the “MENU” page and please select
“USER ID SET” to go to the “USER ID SET” Page. You can use Barcode
reader to set the user’s ID. After your setting, the user’s ID will show in the
screen as follows.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
25 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
WARNING: Looking at the beam light of barcode reader is prohibited
to avoid damage to the eyes.
IMPORTANT!
• The 1D (one dimensional) barcode reader can only read the 1D (one
dimensional) barcode.
• The barcode of the subject should be placed within the effective range of
the red light emitted from the barcode reader.
• Continuously reading the barcode of the subject may lead to failure. An
interval of two seconds between one scan to the next scan is
recommended.
• Symbologies: 1D (one dimensional) barcode types, such as Code128,
UCC/EAN 128, Code 39, UPCA, UPCE, EAN8, EAN13, Codabar,
Plessey, Code 93 and ISSN etc. can be support.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
26 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Set Date
Press the “Right” button to enter the “MENU” page.
Use the “Down” and “Up” button to control the indicator. When selecting the “Date SET” item, press
Use the “Four-Way” button to control the indictor with number keys for setting the date and time
and then press the “OK” button to save your setting.
Select DATE SET
DATE SET page
Set Time
Setting Date and Time
On the “DATE SET” page, using the number keys to set the current date
and time is very significant. We will label the audio file with the date and
time for storage and database build-up.
the “OK” Button to enter DATE SET page.
Set The Idle Time-Out before DS3011A Goes To Sleep Under
the SLEEPING MODE In the SETUP Page. Set The Filter Option
Group for the “BDW Switch” or the “BD Switch”.
SLEEPING MODE: To avoid the possibility of the user forgetting to turn off
the DS3011A or leaving it exposed to unauthorized personnel, the user can
set an idle time-out that will automatically make the DS3011A go to sleep if
the DS3011A is not used for a specified amount of time, including “2 MIN (2
minutes)”. “5 MIN (5 minutes)”, and “15 MIN (15 minutes)”.
FILTER Option Group: There are two filter option group. One group is “BDW”
Switch, the other one group is “BD” Switch. Under the “BDW” switch group,
the “B” mode filter, “D” mode filter, and “W” mode filter will cycle in turn as you
press the “B/D/W Mode Switch” button. Under the “BD” switch, the “B” mode
filter and the “D” mode filter will cycle in turn as you press the “B/D/W Mode
Switch” button.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
27 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Select “SETUP” in the
“MENU” page to enter
Set up “SLEEP MODE”
and “FILTER” group in the
Press the “Right” button to
Using the “Up” and “Down”
buttons to move the indicator
to “FACTORY RESET” and
press the “OK” button to go to
the “FACTORY RESET” page.
If you do need to restore to
factory setting, you can
select “YES” to reset. If not,
you can select “NO” and go
back to the “HOME” page
Press the “Right”
button to go to the
enter the “MENU” page.
the “SETUP” page.
“SETUP” page.
Reset To Factory Setting
The stethoscope can be reset to factory setting by using “FACTORY RESET”.
After entrance the “FACTORY RESET” page, you can select to restore the
factory setting if you select “YES” and press the “OK” button. This function
can help you default the unexpected condition and reset to factory setting.
“MENU” page.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
28 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Parameters
Default
Idle time of “Sleeping Mode” before
going to sleep
5MIN (5 minutes)
Filter mode group
“BDW” group
Auscultation organ position
HEART
Filter Mode
B Mode
Amplification level
Level 5
Recording number
Empty
User ID
NULL
Select “PRODUCT INFO”
to go to the “PRODUCT
Press the “Right” button to
The “PRODUCT INFO”
page will show the software
version, product ID and
Bluetooth Media Access
Control Address on the
Factory Default Setting Table
Product Information
The stethoscope show the software version and product name on the
“PRODUCT INFO” page.
go to the “MENU” page.
Connection
First, enable Bluetooth on the selected mobile device. On the Android
device go to Settings > Bluetooth > and tap the slider to turn Bluetooth ON.
Then, navigate to the Menu screen by clicking on the top right tab “Connect”
in the App. Navigate to Bluetooth menu, select the SSID (Bluetooth Media
Access Control Address) and pair with the device.
DS3011A Document For SGS Certification
The mobile device is now ready to record sounds from the DS3011A. If
Bluetooth pairing is unsuccessful, the Bluetooth mark will flash on the OLED
INFO” page
page.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
29 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
screen of DS3011A and no sounds will be recorded. If the Bluetooth
connection is successful the mark will keep in bright blue of the device.
System requirement
The mobile app software can be used on ASUS ZenPad10 with Android™ 6.0
and greater than 30MB of free memory.
DS3011A uses Bluetooth Class 2; mobile devices used must be compatible
with Bluetooth Class 2.
*ZenPad10 are registered trademarks of ASUS, Inc.
*Bluetooth is a registered trademark of Bluetooth SIG, Inc.
Using The Micro SD Card To Transfer Audio Files To An
External Device
Removing the micro SD card from the electronic stethoscope can transfer
auscultation sound tracks of audio files to an external device.
IMPORTANT!
• Please turn off the system before removing the micro SD card.
• Please do not delete the built-in files in the micro SD card arbitrarily.
• Having a backup of your DS3011A is helpful. When the SD card is full
capacity, you need to take a copy of your SD card to an external computing
device and then empty the SD card for the coming storage of audio files.
• For the stability of the operation, please backup and empty the micro SD
card periodically as regular maintenance.
• In addition to audio files, do not store any other files in order to avoid
unnecessary interference.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
30 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
80~100% Battery Power
60~80% Battery Power
40~60% Battery Power
15~40% Battery Power
0~15% Low Battery Power
Battery Power Indication
Battery life is indicated by an icon in the screen. IMEDIPLUS Electronic
Stethoscope DS3011A has a rechargeable 2300mAh NP-120 lithium-ion
battery provides approximate 24 hours of continuous use. The battery power
loss and the use of barcode reader are highly correlated. As the battery power
decreases, the icon will change as follows:
When the battery power is running out, the screen will display the “0~15%
Low Battery Power” located on the right lower area of the screen to remind
the user about the current state of the battery power. When the “0~15% Low
Battery Power” displays in the screen, the user should replace the battery or
charge the battery as soon as possible.
IMPORTANT!
• When the battery is completely no battery power, the IMEDIPLUS
Electronic Stethoscope DS3011A will automatically turn off. All the
recorded sound tracks and the settings of the DS3011A will be saved
before it turns off.
Charge the Battery
Taking out the Battery from the electronic stethoscope and using the battery
charger, which is one of the DS3011A accessories, to charge the battery.
Putting the adaptor plug into the battery charger, the charging indicator will
display green.
When the battery is placed in the charger, the charger will begin to charge
the battery
The charging indicator will display the charging states. Under charging
states, the indicator will display red. When the battery is fully charged, the
screen will show a green light.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
31 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
WARNING: Do not use the unauthorized battery and charger to
prevent unexpected hazards
IMPORTANT!
• Please turn off the system before taking out the battery.
• Make sure the battery is placed correctly on the battery charger before
charging the battery.
Other Operating Considerations
Operating range is 32ºF to 104ºF (0ºC to 40ºC), 15 to 93% relative
humidity.
Maximum operating altitude is 2000m.
Maximum expected service life is 5 years.
Storage and transport range is -4ºF to 158ºF (-20ºC to 70ºC), 0 to 93%
relative humidity.
To keep the life of your electronic stethoscope, please avoid operating
under extremely hot and extremely cold condition.
Don’s use solvents and oils to prevent unexpected hazards.
Remove the battery if the electronic stethoscope will not be used for
several months.
Failure to follow care and maintenance recommendations could result in
damage to the internal components of the IMEDIPLUS Electronic
Stethoscope DS3011A. Internal damage could cause malfunction of the
product, ranging from a slight decrease in auditory response to complete
failure of the product.
If you experience any problems with the IMEDIPLUS Electronic
Stethoscope DS3011A, do not attempt to repair it yourself. Please notify
our customer service center for directions on shipping and receiving.
8. PhonoMagics Use
Installation for Android system
Open the Android App Store using a supported iPad model. Ensure that the
device is connected to the internet. Follow the instructions to download the App
and wait until it has finished installing.
DS3011A Document For SGS Certification
Logging in

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
32 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Open the PhonoMagics on the mobile device. When prompted, enter your
existing password. The password is “000000” for factory setting.
Bluetooth Pairing
Bluetooth must be enabled in the tablet’s Bluetooth settings in order to use
Core with the PhonoMagics.
First, enable Bluetooth on the selected mobile device. On the Android device
go to Settings > Bluetooth > and tap the slider to turn Bluetooth ON.
Then, open the PhonoMagics and navigate to the PhonoMagics menu screen
by clicking on the top right tab “Connect” on the home screen. Navigate to
Bluetooth menu, select the SSID (Bluetooth Media Access Control Address)
and pair with the device.
The mobile device is now ready to record sounds from the DS3011A. If
Bluetooth pairing is unsuccessful, the Bluetooth mark will flash on the OLED
screen of DS3011A and no sounds will be recorded. If the Bluetooth
connection is successful the mark will keep in bright blue of the device.
Setting up a Password
Create a secure 6-digit password by logging in to the application. Navigate to
the Menu screen by selecting the icon on the top left of the Pad App home
screen.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
33 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Settings
Connect to Device
Search IMA File
Change Password
Connect
Bluetooth connection
button
Connect Status
Bluetooth connect or
not.
Audio waveform
Retrieved from the
DS3011A
Organ Mark
Indicate the listening
position.
Next, select Change Password. Follow the instructions on the screen to create
and save a 6 -digit password. You will need to enter your original password
and new password twice for verification purposes.
Capture & Save Recordings using the Android App
Open the PhonoMagics and log in. Ensure that the DS3011A Device is paired
to the pad device (See Section 8).
In the home screen, use DS3011A and press Recording Button to being
recording. The recording process will last 10 seconds. Once it is finished, the
recording process will stop automatically. When recording is finished, the
home screen will appear another windows to select to save the recording or
not.
8.1 Main Recording Screen
The Main Recording Screen allows users to view audio data captured by
DS3011A, begin the recording process, retrieve patient specific data, or
adjust settings. Audio data is represented in real-time as a
phonocardiogram. A period of recording is 10-second intervals.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
34 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Delete
Delete the recording.
Search Bar
Search recording numbers
Recording List
Recording Data
Recording numbers,
heart rate, filter mode,
date and time.
Organ Mark
Audio waveform &
8.2 Profile Screen
Selecting the Search IMA File icon on the main screen brings up the
recording list and search bar. From this page, users may access
previously assigned recordings or add a new recording to the patient’s
record history.
8.3 Review Screen
More information on a specific recording can be viewed by tapping on a
recording listing. This screen displays a waveform of the recording, options
to playback the sound, and notes. Notes may be added by any user with
access to the patient’s profile.
The location of
sound.
DS3011A Document For SGS Certification
Modify Recording
Playback and stop.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
35 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
IMPORTANT!
The audio quality of the sounds play through the pad speaker or audio jack may
not performed actual sounds quality, to hearing the best sounds quality, it is highly
recommended that a DS3011A eartube is used to listen to the record sounds.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
36 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
1. DS3011A Device: It consists of chest-
piece with diaphragm, screen, intuitive
keypad and eartube connector of audio
output.
2. DS3011A Headset: Including eartube ,
connector, and ear-tips
3. Micro SD Card
4. Rechargeable Lithium-ion Battery: NP-
120 2300mAh
5. Battery Charger: Including Adaptor and Charger.
9. Assembly Parts List
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
37 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Part no
Part Name
Q'ty
B002011100002
Power adaptor
1 SET
B002011101003
Battery Charger
1 SET
B002028000002
Adaptor cable
1 EA
A014063001026
Sanyo Li-ion Battery UF553450Z 1S2P
3.7V 2300mAh, Model:UF553450Z
1 SET
B002032008001
SD Card
1 SET
F0002005A1S00
Headset
1 SET
B051052301016
DS301-SILICONE- Diaphragm
1 EA
WARNING: Do not use the unauthorized accessories which would
lead to unexpected hazards. All accessories only from
IMEDIPLUS provided could be used. For more
information, please refer to “Accessory Information
section”.
10. Accessory Information
The following items can be purchased as a replacement for your device.
Please contact our distributors or authorized service centers for inquiries.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
38 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Item
Description
Outline
Dimensions (mm)
Net
weight
L W H
1
Device
191.3
64.3
45.9
304
2
Rechargeable
Lithium-ion
Battery
53.5
35.5
11.5
55
3
Headset
660
135
25
107
4
Diaphragm
44
5
3
11. Mechanical Dimension
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
39 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
12. Cleaning
Cleaning of stethoscope should be done between each patient use.
WARNING: To reduce the risk of infection, cleaning of stethoscope
should be done between each patient use.
Cleaning the Chest-piece
Under normal conditions, it is unnecessary to remove the diaphragm for
cleaning. The diaphragm can be cleaned by using an alcohol wipe. If
necessary, please remove the diaphragm, carefully follow the instructions
mentioned bellow.
Diaphragm Removal: With the diaphragm sided up, using a thumbnail to
lift the underside portion of the diaphragm out of its designated groove,
and peel off the chest-piece. The groove that holds the diaphragm in place
can be cleaned by sliding the edge of an alcohol swab around the groove.
All parts of the chest-piece can be wiped down with alcohol.
Diaphragm Reassembly: Once the diaphragm is completely dry, insert
the diaphragm into the groove of the rim, starting at one point, and run
your finger around the diaphragm until it is seated back in the groove.
Cleaning Other Parts of the Stethoscope
Ear-tips, eartube and chest-piece can be wiped clean with alcohol. Ear-tips
may be removed for the advanced cleaning and disinfection.
WARNING: Do not immerse the stethoscope in a liquid or subject it
to any sterilization process. The device might be damaged.
13. Warranty
Your IMEDIPLUS Electronic Stethoscope DS3011A is warranted against any
defects in material and manufacture for a period of a year. If a material or
manufacturing defect is discovered during the warranty period, repairs will be
made without charge upon the return of the instrument to IMEDIPLUS, except
in cases of obvious abuse or accidental damage.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
40 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Item
Questions
Answer
1.
No “Powered”, after “Turn ON”.
Please check battery whether
properly installed, and then try
again after reinstall the battery.
2.
No “Powered”, after “Turn ON”.
Please replace a new one
battery, and then try again after
reinstall battery.
3.
No “auscultation sound”, after “Turn
ON”.
Please check headset whether
properly installed, and then try
again after reassembly headset.
4.
No “auscultation sound”, after “Turn
ON”.
Please use the “amplification
level control” button to adjust
amplification level properly.
5.
The screen displays “micro SD card
read error” , after “Turn ON”
Check the micro SD card is
properly installed or damaged. If
fails after you’re trying, please
call local service for help.
6.
The screen displays “micro SD card
read” blinkingly.
The micro SD card could save
the maximum amount of 600
auscultation sound tracks.
Please remove the micro SD
card, and empty the micro SD
card after backup the sound
tracks. The system will work
normally after insert the micro
SD card into the device again.
7.
When the stethoscope is operating,
the system will shut down
automatically if you remove or insert
the micro SD card.
In order to protect the data in the
micro SD card, the stethoscope
will go to sleep mode
automatically when you remove
or insert the micro SD card.
8.
The screen displays “FILE CHK ERR”,
when the system entered PLAYBACK
page.
The system can check the
integrity of sound file. If the file
format is incorrect, please takeout the micro SD card and format
it.
9.
The screen displays “DATE
WARNING” after “Turn ON”
This stands for unsetting of the
date and the time. Please refer to
the section "Setting Date”
14. Troubleshooting
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
41 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
(setting date section 6-8) to set
date.
Item
Questions
Answer
10.
The screen displays “ILLEGAL DATE”
when you set the date.
Please make sure the date is
legal.
11.
After the barcode reader scanning,
the screen displays empty or “Invalid
symbol”.
Please make sure that the
subject’s barcode is placed
within the range of the red light of
the barcode reader. Don’t move
the barcode when you are
scanning.
Please make sure that the
barcode messages don’t have
anyone of the symbols: ", *, /, :, ?,
<, >, \ and |.
12.
The screen displays “KEY Scanning
Err” when you press the keypad.
Please reinsert the battery, and
then make sure the error
message disappears.
13.
The device has no response when
you are operating the device.
Please reinsert the battery, and
then make sure the device has
response when you are
operating.
IMPORTANT!
• If you have tried all of the solutions to the questions and that still failed to
solve your problem, please call local service branches for assistance.
15. Maintenance and Repair
For maintenance or repair Services, please register your name, physical
address, e-mail address, and phone number with your IMEDIPLUS Electronic
Stethoscope DS3011A.
NOTICE: No modification of this device is allowed. Use only IMEDIPLUS
DS3011A Document For SGS Certification
service personnel to repair this electric stethoscope. If user modify
by himself, the user will take all of the responsibility for the
consequence.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
42 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
If you have any questions or comments, please feel free to contact
IMEDIPLUS Customer Service Center.
In the U.S.A:
U.S. Agent Contact Name: Mr. JAMES WANG
U.S. Agent Full Address: 31 Trillium Lane, San Carlos, California, 94070,
UNITED STATES
U.S. Agent Email: jwjwang5@gmail.com
U.S. Agent Tel: +1-650-8629968
http://www.imediplus.com
In the Taiwan (R.O.C.):
IMEDI PLUS Inc.
Address: 2F, 12, ShengYi Rd. Sec. 2, Chupei City, Hsinchu County 30261,
Taiwan (R.O.C.)
Email: service@IMEDIPLUS.com
Tel: +886-3-658-7700
Fax +886-3-658-9535
http://www.imediplus.com
16. Transportation, Storage, and Disposal
Transportation and Storage
General transportation of the unit should correspond to the conditions
outlined in the 'Other Operating Considerations' section of this manual.
The IMEDIPLUS Electronic Stethoscope DS3011A needs to be sent to an
authorized service center for inspection and repair. The storage
environment conditions must be follow the 'Other Operating
Considerations' section of this manual.
Disposal
You shall properly dispose of the DS3011A and follow the local regulations.
The Lithium-ion battery must be disposed of separately or recycled from
regular waste.
NOTICE: To reduce the risks associated with environmental contamination,
we need to follow the applicable regulations in local when disposing
DS3011A Document For SGS Certification
of this stethoscope. The Lithium-ion battery must be disposed of
separately or recycled from regular waste.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
43 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Organ
Organ and Auscultation Position
Indication
Corresponding
Recorded File Name
File Name
Descriptions
Numeric
Key
Saved
Name
HEART
1
##A
HEART
##AAortic;
##PPulmonic;
#SASecond
Aortic;
##TTricuspid;
##MMitral
5
##P
8
#SA
0
##T
*
##M
NECK
1
RUN
NECK
1. The first character
R/L means
Right/Left; M
means Middle.
2. The second
character U/L
means
Upper/Lower; M
means Middle.
3. The third
character
abbreviation “N”
correspond to
NECK organ.
4
RMN
7
RLN
3
LUN
6
LMN
9
LLN
BOWEL
1
RUB
BOWEL
1. The first character
R/L means
Right/Left; M
means Middle.
2. The second
character U/L
means
Upper/Lower; M
means Middle.
3. The third
character
abbreviation “B”
correspond to
BOWEL organ.
2
MUB
3
LUB
4
RMB
5
MMB
6
LMB
7
RLB
8
MLB
9
LLB
17. Organ Positions Correspond to Recorded File Name
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
44 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Organ
Organ and Auscultation Position
Indication
Corresponding
Recorded File Name
File Name
Descriptions
Numeric
Key
Saved
Name
A-CHEST
(AnteriorCHEST)
4
RUA
A-CHEST
1. The first character
R/L means
Right/Left; M
means Middle.
2. The second
character U/L
means
Upper/Lower; M
means Middle.
3. The third
character
abbreviation “A”
correspond to ACHEST organ
7
RMA
#
RLA
3
LUA
6
LMA
9
LLA
P-CHEST
(Posterior
-CHEST)
4
LUP
P-CHEST
1. The first character
R/L means
Right/Left; M
means Middle.
2. The second
character U/L
means
Upper/Lower; M
means Middle.
3. The third
character
abbreviation “P”
correspond to PCHEST organ.
7
LMP
#
LLP
3
RUP
6
RMP
9
RLP
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
45 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Declaration – Electromagnetic Emissions
The IMEDIPLUS Electronic Stethoscope DS3011A is intended for use in the electromagnetic
environment specified below. The customer or the user of the DS3011A should assure that it is
used in such an environment.
Emissions test
Compliance
Electromagnetic environment –guidance
RF emissions CISPR 11
Group 1
The DS3011A uses RF energy only for its
internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
equipment
RF emissions CISPR 11
Class A
The DS3011A is suitable for use in all
establishments other than domestic and
those directly connected to the public lowvoltage power supply network that supplies
buildings used for domestic purposes.
Harmonic emissions IEC
61000-3-2
Class A
Voltage fluctuations/flicker
emissions IEC 61000-3-3
Compliance
Declaration – Electromagnetic Immunity
The IMEDIPLUS Electronic Stethoscope DS3011A is intended for use in the electromagnetic
environment specified below. The customer or the user of DS3011A should assure that it is
used in such an environment.
Immunity test
IEC 60601 test level
Compliance level
Electromagnetic
environment–
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be
wood, concrete or
ceramic tile. If floors
are covered with
synthetic material,
the relative humidity
should be at least
30%.
Electrical fast
transient/ burst IEC
61000-4-4
± 2 kV for power supply
lines
± 1 kV for input/output
lines
± 2 kV for power
supply lines
Not applicable
Mains power quality
should be that of a
typical commercial or
hospital environment.
Surge IEC 610004-5
± 1 kV differential mode
± 2 kV common mode
± 1 kV differential
mode
Not applicable
Mains power quality
should be that of a
typical commercial or
hospital environment.
Power frequency
(50/60 Hz)
magnetic field
IEC61000-4-8
3 A/m
3 A/m
The DS3011A power
frequency magnetic
fields should be at
levels characteristic
of a typical location
in a typical
commercial or
hospital environment.
18. Appendix: Guidance and Manufacturer’s Declaration
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
46 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Declaration – Electromagnetic Immunity – Continued
The IMEDIPLUS Electronic Stethoscope DS3011A is intended for use in the electromagnetic
environment specified below. The customer or the user of DS3011A should assure that it is
used in such an environment.
Immunity test
IEC 60601 test level
Compliance level
Electromagnetic
environment–
guidance
Voltage dips, short
interruptions and
voltage variations
on power supply
lines IEC61000-411
< 5% UT (>95% dip in
UT) for 0.5 cycle
40% UT (60% dip in UT)
for 5 cycle
70% UT (30% dip in UT)
for 25 cycle
< 5% UT (>95% dip in
UT) for 5 sec
< 5% UT (>95% dip
in UT) for 0.5 cycle
40% UT (60% dip in
UT) for 5 cycle
70% UT (30% dip in
UT) for 25 cycle
< 5% UT (>95% dip
in UT) for 5 sec
Mains power quality
should be that of a
typical commercial or
hospital environment.
If the user of the
DS3011A requires
continued operation
during power mains
interruptions, it is
recommended that
the DS3011A be
powered from an
uninterruptible power
supply or a battery.
NOTE UT is the a.c. mains voltage prior to application of the test level.
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 Vrms
3 V/m
Portable and mobile
RF communications
equipment should be
used no closer to any
part of the DS3011A,
including cables,
than the
recommended
separation distance
calculated from the
equation applicable
to the frequency of
the transmitter.
Recommended
separation distance:
d= 1,2 √𝑃
d = 1,2 √𝑃 80 MHz to
800 MHz
d = 2,3 √𝑃 800 MHz
to 2,5 GHz
Where P is the
maximum output
power rating of the
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
47 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
transmitter in watts
(W) according to the
Declaration – Electromagnetic Immunity – Continued
The IMEDIPLUS Electronic Stethoscope DS3011A is intended for use in the electromagnetic
environment specified below. The customer or the user of DS3011A should assure that it is
used in such an environment.
Immunity test
IEC 60601 test level
Compliance level
Electromagnetic
environment–
guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 Vrms
3 V/m
transmitter
manufacturer and d
is the recommended
separation distance
in meters (m).
Field strengths from
fixed RF transmitters,
as determined by an
electromagnetic site
survey, a should be
less than the
compliance level in
each frequency
range. b
Interference may
occur in the vicinity
of equipment marked
with the following
symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be considered.
If the measured field strength in the location in which the DS3011A is used exceeds the
applicable RF compliance level above, the DS3011A should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such
as re-orienting or relocating the DS3011A.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
DS3011A Document For SGS Certification

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
48 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Recommended Separation Distances Between Portable and Mobile RF Communications
Equipment and the IMEDIPLUS Electronic Stethoscope DS3011A
The DS3011A is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the DS3011A can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile
RF communications equipment (transmitters) and the DS3011A as recommended below,
according to the maximum output of the communications equipment.
Rated maximum
output power of
transmitter, P [W]
Separation distance according to frequency of transmitters, d
[m]
150 kHz to 80 MHz
d = 1,2 √𝑃
80 MHz to 800 MHz
d = 1,2 √𝑃
800 MHz to 2,5 GHz
d = 2,3 √𝑃
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73 1 1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
affected by absorption and reflection from structures, objects and people.
WARNING: To reduce the risks associated with the strong
electromagnetic fields, we shall avoid using the
electronic stethoscope near the strong radio
frequency signals or portable and/or mobile RF
devices. The electronic stethoscope might be
damaged, if you hear sudden or unexpected sounds.
Please immediately move away from any radio
transmitting antennas to protect from damage.
19. EMC Compliance
FCC Intentional Radiator Certification
iMED+ / CaRDIaRT Electronic Stethoscope Model DS3011A
FCC ID: 2AM7NDS3011A
DS3011A Document For SGS Certification
This device complies with Part 15 of the FCC rules. Operation is subject to
the following two conditions: (1) This device may not cause harmful

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
49 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
interference, and (2) this device must accept any interference received,
including interference that may cause undesirable operation.
This equipment has been tested and found to comply with the limits for a Class
B digital device, pursuant to part 15 of the FCC rules. These limits are
designed to provide reasonable protection against harmful interference in a
residential installation.
This equipment generates, uses and can radiate radio frequency energy and,
if not installed and used in accordance with the instructions, may cause
harmful interference to radio communications. However, there is no guarantee
that interference will not occur in a particular installation. If this equipment
does cause harmful interference to radio or television reception, which can be
determined by turning the equipment off and on, the user is encouraged to try
to correct the interference by one or more of the following measures:
-Reorient or relocate the receiving antenna.
-Increase the separation between the equipment and receiver.
-Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
-Consult the dealer or an experienced radio/TV technician for help.
You are cautioned that changes or modifications not expressly approved by
the part responsible for compliance could void the user’s authority to operate
the equipment.
Industry Canada Intentional Radiator Certification
IC: 23024-DS3011A
Industry Canada Statement
This device complies with Industry Canada's licence-exempt RSSs. Operation
is subject to the following two conditions: (1) this device may not cause
interference, and (2) this device must accept any interference, including
DS3011A Document For SGS Certification
interference that may cause undesired operation of the device.

Document Title:
Electronic Stethoscope DS3011A User Manual
Document No.:
DS3011A-SDUS001-01302
Page:
50 of 50
Product Name:
Electronic Stethoscope DS3011A
Ver.:
A1
510(k) File Name:
002_Electronic Stethoscope DS3011A User Manual
Date:
2017/6/23
Avis d’industrie Canada
Le present appareil est conforme aux CNR d'Industrie Canada applicables
aux appareils radio exempts de licence. L'exploitation est autorisee aux deux
conditions suivantes: (1) l'appareil ne doit pas produire de brouillage, et, and
(2) l'utilisateur de l'appareil doit accepter tout brouillage radioelectrique subi,
meme si le brouillage est susceptible d'en compromettre le fonctionnement.
Industry Canada Class B Emission Compliance Statement
This Class B digital apparatus complies with Canadian ICES-003.
Avis de conformite a la reglementation d'Industrie Canada.
Cet appareil numerique de la classe B est conforme a la norme NMB-003 du
Canada.
Déclaration d'exposition aux radiations:
Cet équipement est conforme aux limites d'exposition aux rayonnements IC
établies pour un environnement non contrôlé. Cet équipement doit être
installé et utilisé avec un minimum de 20 cm de distance entre la source de
rayonnement et votre corps.
NCC
根據 NCC 低功率電波輻射性電機管理辦法規定:
第十二條 經型式認證合格之低功率射頻電機,非經許可,公司、商號或使用者均不得擅
自變更頻率、加大功率或變更原設計之特性及功能。
第十四條 低功率射頻電機之使用不得影響飛航安全及干擾合法通信;經發現有干擾現象
時,應立即停用,並改善至無干擾時方得繼續使用。前項合法通信,指依電信法規定作
業之無線電通信。低功率射頻電機須忍受合法通信或工業、科學及醫療用電波輻射性電
機設備之干擾。
DS3011A Document For SGS Certification
 Loading...
Loading...