
FOR QUANTITATIVE MEASUREMENT
OF OSMOLARITY OF OCULAR TISSUES
Osmolarity System
USER MANUAL
osdcare.com
2 3
1. ABOUT THIS MANUAL 4
1.1. Warnings and Precautions 4
2. ESSENTIAL PRESCRIBING INFORMATION 5
2.1. Device Description 5
2.2. Intended Use 5
2.3. Indications 5
2.4. Contraindications 5
2.5. General Safety Instructions 6
3. DESCRIPTION OF COMPONENTS 7
3.1. Identifying System Components 7
3.2. I-PEN® Osmolarity System 7
3.3. I-PEN® Osmolarity Test Sensor 7
4. PERFORMING AN OSMOLARITY MEASUREMENT 8
4.1. Prepare the I-PEN® for Use 8
4.1.1. Insert the Battery 8
4.2. Remove the Single-Use-Sensor from Package 9
4.3. Insert the Single-Use-Sensor 9
4.4. Turn on the Device 10
4.5. Taking a Reading 10
4.5.1. Tips for Use 10
4.6. Eject the Single-Use-Sensor 11
5. EXPECTED RESULTS 11
5.1. Reference Tear Osmolarity Values 11
6. CLEANING AND MAINTENANCE 12
6.1. Cleaning 12
6.1.1. I-PEN® Device 12
6.1.2. Single-Use-Sensors 12
6.2. Maintenance 12
6.2.1. Troubleshooting 12
7. OPERATING AND STORAGE CONDITIONS 13
8. TECHNICAL SPECIFICATIONS 13
9. ELECTROMAGNETIC EMISSIONS 14
10. ELECTROMAGNETIC IMMUNITY 15
10.1. Recommended Separation Distances 17
10.1.1. Applicable Standards 17
11. LABELS AND SYMBOLS 18
11.1. Labels 18
11.2. Symbols 18
I-PEN® User Guide | TABLE OF CONTENTSI-PEN® User Guide
I-PEN
®
I-PEN® is a trademark of I-MED Pharma Inc. Other registered trademark
or trademarks are the property of their respective owners.
CONTACT INFORMATION
Customer satisfaction is an I-MED Pharma Inc. priority.
To help us in providing you with the best possible product and support,
please send us your comments and suggestions.
I-MED Pharma Inc.
1601 St-Regis Blvd.
Dollard-des-Ormeaux, QC
Canada H9B 3H7
Authorized Representative in the EU:
Medes Ltd.
5 Beaumont Gate, Shenley Hill, Radlett
Herts WD7 7AR United Kingdom
Tel.: +423 663 169205
Fax: +44 1923 859 810
E-mail: medes@arazygroup.com
www.medeseurope.com
Marking by the CE symbol indicates compliance of this device as a
Class 1 medical device with a measuring function, with the Medical
Device Directives 93/42/ EEC as amended by 2007/47/CE.
4 5
This manual provides the information necessary to operate the I-PEN® system in a safe and efficient
manner. Please read and thoroughly understand this manual before operating the system. If any part of
this manual is not clear, contact customer support for clarication.
Three types of special messages appear in this User Manual:
A WARNING indicates the possibility of injury, death or other serious adverse reactions
associated with the use or misuse of the device.
A CAUTION indicates the possibility of a problem with the device associated with its use
or misuse. Such problems include device malfunctions, device failure, damage to the device
or damage to other property.
A NOTE provides other important information.
1.1. WARNINGS AND PRECAUTIONS
1. ABOUT THIS MANUAL
2.1. DEVICE DESCRIPTION
2. ESSENTIAL PRESCRIBING INFORMATION
The I-PEN® Osmolarity System is a diagnostic testing device for the quantitative measurement of
osmolarity (concentration of dissolved, active particles in solution) of ocular tissues in normal and Dry
Eye Disease patients. The I-PEN® is for professional in vivo diagnostic use only.
When either the quantity or quality of secreted tears is compromised (known as aqueous deficient or
evaporative Dry Eye Disease), increased rates of evaporation lead to a concentrated tear lm (increased
osmolarity) that places stress on the corneal epithelium and conjunctiva.
The I-PEN® Osmolarity Single-Use-Sensor, in conjunction with the I-PEN® Osmolarity System, provides
a quick and simple method for determining tear osmolarity using impedance measurements of the
saline concentration of the extracellular uid contained in the eyelid tissue. To perform a test, attach a
new Single-Use-Sensor onto the System Reader and touch the tip of the Single-Use-Sensor to the inner
tissue of the lower eyelid.
After several seconds of contact with the eyelid tissue, the I-PEN® will display a quantitative tear
osmolarity test result on the liquid crystal display (LCD). The I-PEN® Osmolarity System simplies the
osmolarity determination process by eliminating the need to transfer tear fluid samples and reducing
the risk of evaporation.
The I-PEN® Osmolarity Test utilizes an impedance measurement to provide an assessment of osmolarity
of the conjunctival tissues surrounding the eye.
2.2. INTENDED USE
The I-PEN® Osmolarity System is a device for the quantitative measurement of osmolarity
(concentration of dissolved, active particles in tissue immersed in solution) of human tears in normal
and Dry Eye Disease patients.
The I-PEN® Osmolarity System should be used only by a trained clinician or under the supervision of
a trained clinician.
2.3. INDICATIONS
The I-PEN® Osmolarity Device is indicated for use in the diagnosis of certain ocular surface disorders
which affect the osmolarity of the tear lm on the surface of the eye.
2.4. CONTRAINDICATIONS
There are no contraindications known at this time.
I-PEN® User Guide | ABOUT THIS MANUAL I-PEN® User Guide | ESSENTIAL PRESCRIBING INFORMATION
6 7
3.1. IDENTIFYING SYSTEM COMPONENTS
3.2. I-PEN® OSMOLARITY SYSTEM
3.3. I-PEN® OSMOLARITY TEST SENSOR
The gures which follow illustrate the components of the I-PEN® System.
The I-PEN® is a portable hand held battery- operated unit that calculates and displays the osmolarity test
result. The unit includes a small display screen that shows the osmolarity test result.
Each Single-Use-Sensor is a single- use, individually packaged unit, designed to work in conjunction with
the I-PEN®. The Single-Use-Sensor does not contain chemicals or reagents.
Unit cover
Ready button
On/Off
switch
WARNING: Do not use a Single-Use-Sensor that is physically damaged.
CAUTION: Do not use the Single-Use-Sensors past the expiration date.
CAUTION: Single-Use-Sensors are for single use only.
2.5. GENERAL SAFETY INSTRUCTIONS 3. DESCRIPTION OF COMPONENTS
WARNING: Changes or modications not expressly approved by I-MED Pharma Inc. can affect
the safety and effectiveness of the system and will void the system’s warranty.
WARNING: Use only indoors, in a clean, dry environment.
WARNING: Do not use a Single-Use-Sensor that is physically damaged.
WARNING: The system contains no user-serviceable components.
WARNING: Medical device regulations restrict the operation of the application to trained and
qualied personnel.
WARNING: Do not test patients who have used eye drops within two hours prior to testing.
WARNING: Do not test patients wearing makeup on eyelids.
WARNING: Do not test patients within 10 minutes after removal of eye makeup.
WARNING: Do not test patients after ocular surface staining.
WARNING: Do not test patients after invasive ocular diagnostic testing.
WARNING: Do not test patients within 10 minutes after a slit lamp examination.
WARNING: Do not test a patient who has been crying.
CAUTION: The information contained in this Manual is intended for the sole and exclusive use of
the Company’s customers. Any other unauthorized use of this Manual or any of the information
it contains is prohibited.
CAUTION: Refer all service problems to a qualied I-MED Pharma Inc. representative only.
CAUTION: Replace the device if the LCD is cracked, unreadable, has missing pixels, or is
otherwise damaged.
CAUTION: Replace the device if a “beep” is not heard after turning it on.
CAUTION: Check the operation of the device prior to use. Replace if damaged.
CAUTION: Replace the device if the casing or battery cover is lost or damaged.
CAUTION: Single-Use-Sensors are for single use only.
CAUTION: Do not use the Single-Use-Sensors past the expiration date.
CAUTION: The device is to be used within a clinical facility environment only.
CAUTION: The device is for professional in vivo diagnostic use only.
LCD display
Battery
cover
I-PEN® User Guide | GENERAL SAFETY INSTRUCTIONS I-PEN® User Guide | DESCRIPTION OF COMPONENTS

1 2 3
8 9
4. PERFORMING AN OSMOLARITY MEASUREMENT
WARNING: Do not test patients who have used eye drops within two hours prior to testing.
WARNING: Do not test patients wearing makeup on eyelids.
WARNING: Do not test patients within 10 minutes after removal of eye makeup.
WARNING: Do not test patients after ocular surface staining.
WARNING: Do not test patients after invasive ocular diagnostic testing.
WARNING: Do not test patients within 10 minutes after a slit lamp examination.
WARNING: Do not test a patient who has been crying.
4.1. PREPARE THE I-PEN® FOR USE
WARNING: Use only indoors, in a clean, dry environment.
In order to prepare for a test, place the battery in the System Reader, and insert a Single-Use-Sensor.
WARNING: Do not use a Single-Use-Sensor that is physically damaged.
4.3. INSERT THE SINGLE-USE-SENSOR
First 4 remove the unit cover, then 5 insert the disposable Single-Use-Sensor
CAUTION: Replace the device if a “beep” is not heard after turning it on.
CAUTION: It is important to visually inspect the Single-Use-Sensor before use. In the case of
suspected contamination, or if the expiration date is expired, replace the Test Sensor.
CAUTION: Do not touch the gold nodes while inserting the Single-Use-Sensor.
4.1.1. INSERT THE BATTERY
CAUTION: This device uses a battery type CR2032 only.
CAUTION: Replace the device if the casing or battery cover is lost or damaged.
The battery compartment can be accessed by removing the battery cover, as shown below.
I-PEN® User Guide | PERFORMING AN OSMOLARITY MEASUREMENT I-PEN® User Guide | PERFORMING AN OSMOLARITY MEASUREMENT
4.2. REMOVE THE SINGLE-USE-SENSOR FROM PACKAGE
1. Tear along the dotted line to separate the attached 1, wrapped Single-Use-Sensors.
2. Grasping the bottom rmly with one hand 2, with the other hand tear in the direction of the pre-cut
section to expose the end of the Single-Use-Sensor 3 to be inserted into the I-PEN® device .
4 5

6
7
10 11
Push the Ejector button and the Single-Use-Sensor
will be ejected.
You may now discard the
Single-Use-Sensor.
The LCD will display a test result.
See the next Section for a discussion of how to
interpret the measurement results.
4.6. EJECT THE SINGLE-USE-SENSOR
5. EXPECTED RESULTS
I-PEN® test results are displayed on the LCD in units of mOsms/L. No calculations are required. The
chart below shows some typical test results and their possible interpretation. All such interpretations are
subject to the review of the physician or other medical professional.
I-PEN® User Guide | PERFORMING AN OSMOLARITY MEASUREMENT I-PEN® User Guide | EXPECTED RESULTS
1. Do not immerse the tip of the Single-Use-Sensor in the lower tear meniscus.
2. If the reading on the LCD screen shows an “Error”, you may attempt another reading in the same eye by
pressing the Ready button.
4.5.1. TIPS FOR USE
4. Approach at a 30-45 degree angle from horizontal and gently lower the end of the Single-Use-
Sensor on to the conjunctiva on the inside of the lower eyelid.
5. When correctly placed, the tip of the Single-Use-Sensor should be depressing the surface slightly
so that both gold nodes are in good contact with the conjunctiva.
6. The I-PEN® will take make an audible beep after several seconds and display the reading
on the LCD screen.
4.5. TAKING A READING
1. Ask the patient to gently squeeze their eyelids shut
for 30-60 seconds prior to taking a reading.
2. Position the tip of the disposable Single-Use-Sensor
just above the lower eyelid with the LCD screen
facing upwards.
3. Turn on the I-PEN
®
as indicated in section 4.4 only
when you are ready to take the reading by pushing
the on/off switch to the on position.
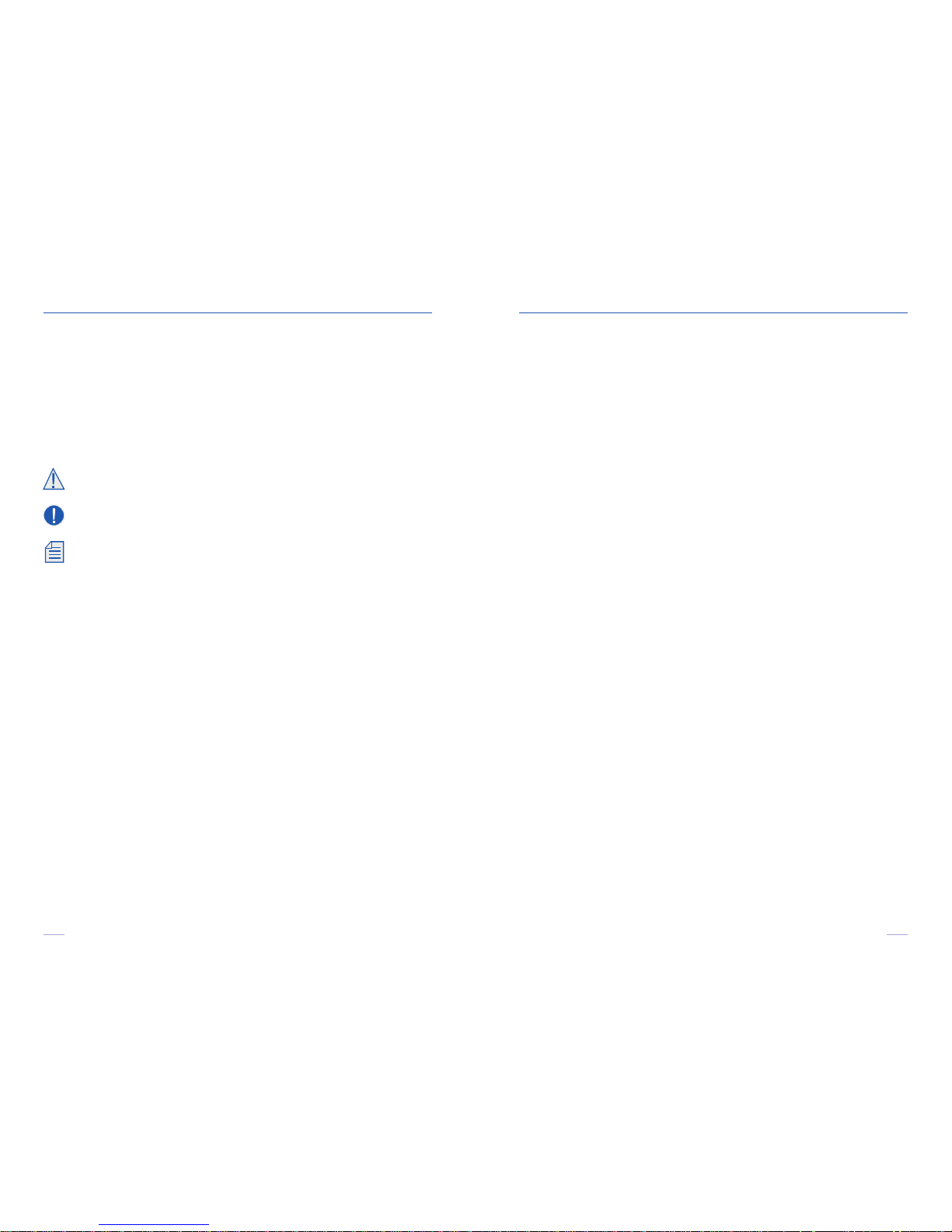
4.4. TURN ON THE DEVICE
Push the On/Off switch 6. You should hear a
“beep” and the LCD display 7 should display
the “I-PEN Ready” message.
CAUTION: Replace the device if a “beep” is not heard after turning it on.
NOTE: In order to conserve battery life, the I-PEN® is programmed to enter Sleep Mode
automatically thirty seconds after it powers up. In doing so, this can invalidate the SUS and
a new SUS should be inserted.
CAUTION: Do not use the unit if the Reader does not display “I-PEN Ready”.
DRY EYE SEVERITY SCALE
380365350335
320
305290275
Normal
< 290
Marginal
290 -310
Mild
310-330
Moderate
330 -350
Severe
> 350
5.1. REFERENCE TEAR OSMOLARITY VALUES
Reading in mOsms/L
(Use result from eye with
highest reading)
Variance Between Right &
Left Eye
Interpretation
<290 Normal Patient
290-310
≤
7 Normal Patient
290-310
≥
8 Dry Eye Disease Patient
>310 Dry Eye Disease Patient

12 13
6. CLEANING AND MAINTENANCE 7. OPERATING AND STORAGE CONDITIONS
6.1. CLEANING
6.2. MAINTENANCE
6.1.1. I-PEN® DEVICE
6.1.2. SINGLE-USE-SENSORS
6.2.1. TROUBLESHOOTING
The I-PEN® can be cleaned with a damp cloth or alcohol wipe as required. When cleaning, it is
important to keep the electronic contacts of the control unit and Reader dry. The electronic contacts
and docking port should also be kept free of dust and dirt.
To ensure warranty coverage and reliable system operation, defective system components should
be serviced and/or replaced exclusively by I- MED Pharma Inc. authorized personnel, and replacement
parts should be those specied by the manufacturer. It is important to use and store the device within
the environmental conditions shown in the table below.
Single-Use-Sensors are for use on a single eye. Never reuse or try to clean a Single-Use-Sensor.
Single-Use-Sensors are for single use only. Never reuse or try to clean a Single-Use-Sensor.
The I-PEN® Osmolarity System is designed to work without direct service or preventive maintenance.
If quality checks fail, contact I-PEN® Customer Support.
Battery should be replaced when “Low Bat” indication appears on the screen.
Condition Possible Cause Recommended Action
The “I-PEN Ready” message
does not display
Battery not installed.
Device malfunction.
Verify that the correct type of battery is
installed, and that the battery is fresh.
Contact I-PEN® customer support.
A “beep” is not heard when
the device is turned on
Device malfunction. Contact I-PEN® customer support.
“Low Bat” indication appears
on screen
Battery close to
end-of-life.
Replace battery.
Screen goes dim
Battery close to
end-of-life.
Replace battery.
Transport and Storage Temperature
2°–35°C/36°–95°F
Transport and Storage Relative Humidity
10–85% non- condensing
Transport and Storage Altitude
0–2,000 meters
Operating Temperature
15°– 30°C/59–86°F
Operating Altitude
0–2,000 meters
Operating Relative Humidity
10–85% non- condensing
NOTE: If the Recommended Action does not solve the problem,
contact I- MED Pharma Inc. Customer Support.
Single-Use-Sensors may be ordered on line at www.imedpharma.com or by calling your
representative at I- MED Pharma Inc. Tel. Number: (800) 463- 1008 or (514) 685- 8118.
I-PEN® User Guide | CLEANING AND MAINTENANCE I-PEN® User Guide | OPERATING AND STORAGE CONDITIONS | TECHNICAL SPECIFICATIONS
8. TECHNICAL SPECIFICATIONS
Calibration
No calibration required
Degree of Protection Against Electric Shock
BF Type applied part
Size (not including probe holder)
W 140mm
L 223mm
H 140mm
Weight
50 gm
Battery
CR2032
Frequency
80 Hz
Peak Voltage
± 1.5V
Current Source
Max100 μA AC
Sinus Distortions
5%
WARNING: Cleaning uids should never be used on the Single-Use-Sensor.

14 15
9. ELECTROMAGNETIC EMISSIONS 10. ELECTROMAGNETIC IMMUNITY
NOTES
•
The I-PEN® requires special precautions with regard to electromagnetic compatibility.
•
It must be installed and prepared for use as described in Section 4. Performing an Osmolarity
Measurement.
•
Certain types of mobile telecommunication devices such as mobile telephones are likely to interfere
with the I-PEN®.
•
The recommended separation distances in this paragraph must therefore be complied with.
•
The I-PEN® must not be used near or on top of another device. If this cannot be avoided, it is necessary
– before clinical use – to check the equipment for correct operation under the conditions of use.
•
The use of accessories other than those specied or sold by I- MED Pharma Inc. as replacement parts
may have the consequence of increasing the emissions or decreasing the immunity of the unit.
•
I-PEN® is intended for use in the electromagnetic environment specied in the following tables.
This is not a life- sustaining device.
•
The user and/or installer of the unit must ensure that it is used in such an environment.
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions
The I-PEN® is intended for use in the electromagnetic environment specied below. The customer or the
user of the I-PEN® device should assure that it is used in such an environment.
Emissions Test Compliance
Electromagnetic Environment -
Guidance
RF emissions
Test: CISPR 11
Group 1
The I-PEN® uses RF energy only for
its internal function. Therefore, its RF
emissions are very low and are not likely
to cause any interference in nearby
electronic equipment.
RF emissions
CISPR 11
Class B
Harmonic emissions
IEC 61000- 3- 2
Not applicable
Voltage uctuations/icker
emissions IEC 61000- 3-3
Not applicable
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The I-PEN® is intended for use in the electromagnetic environment specied below. The customer or the
user of the I-PEN® device should assure that it is used in such an environment.
Immunity Test
IEC 60601-1-2 Test
Level
Compliance
Level
Electromagnetic
Environment- Guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±8 kV contact
±8 kV air
Floors should be wood,
concrete or ceramic tile.
If oors are covered with
synthetic material, the
relative humidity should be
at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV
for power supply lines
±1 kV
for input/ Output lines
Not applicable
Surge
IEC 61000-4-5
±1 kV differential mode
±2 kV common mode
Not applicable
Voltage uctuations/
icker emissions IEC
61000- 3-3
<5 %UT
(>95 %dip in UT)
for 0.5 cycle
40 %UT
(60 %dip in UT)
for 5 cycles <5 %UT
70 %UT
(30 %dip in UT)
for 25 cycles
<5 %UT <5 %UT
(>95 %dip in UT)
for 5 s
Not applicable
Power frequency
(50/60 Hz) magnetic
eld IEC 61000-4-8
3 A/m 3 A/m
Power frequency magnetic
elds should be at levels
characteristic of a typical
public low-voltage power
supply network that supplies
buildings used for domestic
purposes, commercial or
hospital, clinic environment.
I-PEN® User Guide | ELECTROMAGNETIC EMISSIONS I-PEN® User Guide | ELECTROMAGNETIC IMMUNITY

16 17
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity
The I-PEN® is intended for use in the electromagnetic environment specified below. The customer or the
user of the I-PEN® device should assure that it is used in such an environment.
Immunity Test IEC 60601-1-2
Test Level
Compliance
Level
Electromagnetic EnvironmentGuidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms 150kHz to
80MHz
3 V/m
80MHz to 2.5GHz
Not applicable
3 V/m
Portable and mobile RF
communications equipment should
be used no closer to any part of the
I-PEN®, including cables, than the
recommended separation distance
calculated from the equation
applicable to the frequency of the
transmitter.
Recommended separation distance
d = 1.17√P
d = 1.17√P 80 M Hz t o 800 MHz
d = 2.3√P 800 MHz t o 2.5 GHz
Where P is the maximum output
power rating of the transmitter
in watts (W) according to the
transmitter manufacturer and d
is the recommended separation
Distance in meters (m).
Field strengths from xed R F
transmitters, as determined by
an electromagnetic site survey,a
should be less than the compliance
level in each frequency range
Interference may occur in the
vicinity of equipment marked with
the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures objects and people.
a
Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the
electromagnetic environment due to xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld
strength in the location in which the I-PEN® is used exceeds the applicable RF compliance level above, the I-PEN® should be observed
to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or
relocating the I-PEN®.
10.1. RECOMMENDED SEPARATION DISTANCES
10.1.1. APPLICABLE STANDARDS
Recommended separation distances between portable and mobile RF communications
equipment and the I-PEN
®
The I-PEN® is intended for use in an electromagnetic environment in which radiated radio frequency disturbances
are controlled. • The user and/or installer of the unit can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile radio frequency communications equipment (emitters) and the
I-PEN®, according to the maximum output power of the equipment, as recommended in the table below.
Separation distance according to the frequency
of transmitter (m)
Rated maximum output
power of transmitter (W)
80MHz to 800MHz
d = 1.17 √P
800MHz to 2.5GHz
d = 2.3 √P
0.01 0.12 0.23
0.1 0.37 0.73
1 1.17 2.3
10 3.7 7.3
100 11.7 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be
estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures, objects, and people.
The following list of standards applies to the I-PEN® Osmolarity Device:
•
IEC/EN/UL 60601-1: 2005 (3rd Ed.), Medical electrical equipment,
part 1: General requirements for basic safety and essential performance.
•
IEC/EN 60601-1-2: 2007, Medical Electrical Equipment- Part 1-2: General requirements
for safety – Collateral standard: Electromagnetic compatibility – Requirements and tests.
•
IEC 62304: 2006, Medical Device Software – Software Lifecycle Processes.
•
ISO 15223: 2012, Medical devices – Symbols to be used with medical device labels,
labeling and information to be supplied – Part 1: General requirements.
•
ISO 10993- 1: 2009, Biological Evaluation for Medical Devices,
part 1 – Evaluation and testing within a risk management process.
I-PEN® User Guide | ELECTROMAGNETIC IMMUNITY I-PEN® User Guide | ELECTROMAGNETIC IMMUNITY
18 19
11. LABELS AND SYMBOLS
11.1. LABELS
OSMOLARITY SYSTEM
11.2. SYMBOLS
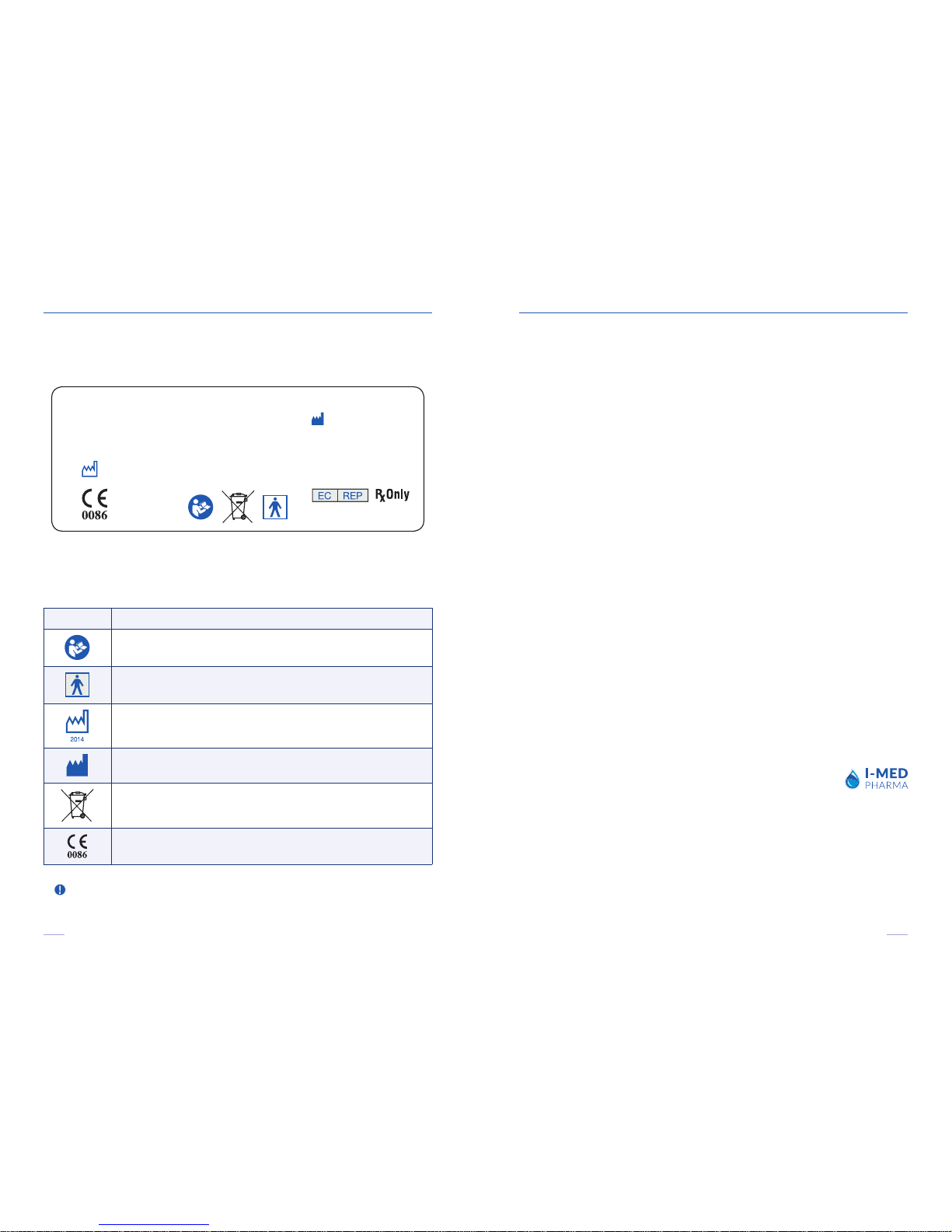
Symbol Symbol meaning
Attention: Consult Accompanying Document
BF type applied part
Month/Year of Manufacture
Manufactured by
Special Requirements for Waste of Electrical and Electronic Equipment (WEEE Directive)
CE Compliance (Medical Device Directive)
A number of internationally recognized symbols are found on the I-PEN® unit.
These relate to safety requirements and standards and are briey reviewed below.
REF: 600
SN: nn-xxxx
mm-yyyy
LifeCare Ltd.
2 Zipori Street
Tiberias 1424602
Israel
PRT-14-200 Rev. 01
CAUTION: At the end of its useful life, the system must be disposed of in accordance with
local law and/or code concerning electrical and electronic equipment.
Manufactured by:
Life Care Ltd
2 Zipori Street
Tiberias 1424602
Isræl
Distributed by:
1601 St-Regis Bl vd.
Dollard-des-Ormeaux, QC
Canada H9B 3H7
Tel : ( 514 ) 68 5-8 118
Tol l f ree: (800) 463-1008
Fax: (5 14) 6 85-8 998
Email: info@imedpharma.com
Website: www.imedpharma.com
I-PEN® User Guide I-PEN® User Guide | LABELS AND SYMBOLS

I-MED Pharma Inc.
1601 St-Regis Blvd.
Dollard-des-Ormeaux, QC
Canada H9B 3H7
Tel: (514) 68 5-8118
Toll free: (800) 463-1008
Fax: (514) 685-8998
info@imedpharma.com
SEE THE DIFFERENCE
Product #IPUMEN 0718
 Loading...
Loading...