iHealth PO3M User guide [ml]

Wireless Pulse Oximeter
Oxymètre de pouls sans l
Ossimetro wireless per il rilevamento del battito
Pulsioxímetro inalámbrico
Funkgesteuertes Pulsoximeter
Oxímetro de Pulso Wireless
OPERATION MANUAL
Manuel de presentation
Manuale dell’utente
Manual de Introducción
Bedienungsanleitung
Manual de Funcionamento

MODEL PO3M
Wireless Pulse Oximeter
OPERATION MANUAL
TABLE OF CONTENT
EN
SYMBOLS
INTENDED USE
PARTS AND DISPLAY INDICATORS
PARTS AND DISPLAYS
DEVICE DESCRIPTION
CONTRAINDICATIONS
WARNINGS
Notice
USING YOUR PULSE OXIMETER
CARE AND MAINTENANCE
SPECIFICATIONS
TROUBLESHOOTING
IMPORTANT INFORMATION REQUIRED BY THE FCC
IMPORTANT INFORMATION REQUIRED BY THE INDUSTRY CANADA
Manufacturer Information
ELECTROMAGNETIC COMPATIBILITY INFORMATION
1
2
2
3
3
4
4
4
6
9
10
12
13
13
14
14
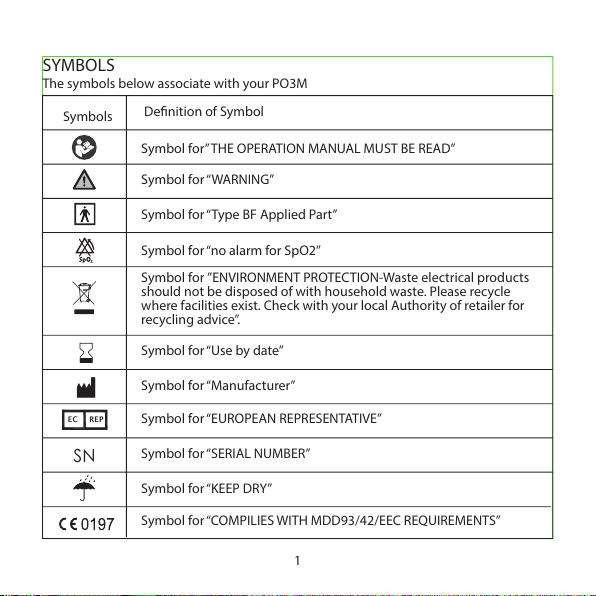
SYMBOLS
The symbols below associate with your PO3M
1
Symbols
Symbol for” THE OPERATION MANUAL MUST BE READ”
Symbol for “WARNING”
Symbol for “Type BF Applied Part”
Symbol for ”ENVIRONMENT PROTECTION-Waste electrical products
should not be disposed of with household waste. Please recycle
where facilities exist. Check with your local Authority of retailer for
recycling advice”.
Symbol for “no alarm for SpO2”
Symbol for “Use by date”
Symbol for “Manufacturer”
Symbol for “SERIAL NUMBER”
Symbol for “EUROPEAN REPRESENTATIVE”
Symbol for “KEEP DRY”
Symbol for “COMPILIES WITH MDD93/42/EEC REQUIREMENTS”
Denition of Symbol
SN
EC REP

INTENDED USE
The PO3M Wireless Pulse Oximeter is a non-invasive device intended for spot-checking
of functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate.
The wireless pulse oximeter is intended to measure blood oxygen saturation and pulse
rate of adults above 16 years old in home and hospital environments (including clinical
use in internist/surgery, anesthesia, intensive care, etc.).
The Wireless Pulse oximeter is not intended for continuous monitoring.
Compatibility
The Wireless Pulse Oximeter PO3M is designed for use with the following devices:
iPod touch 5th generation
iPhone 4S
iPhone 5
New iPad
iPad 4
with an iOS version of V5.0 or higher.
iPhone and iPod touch are trademarks of Apple Inc., registered in the U.S. and other
countries. iPad is a trademark of Apple Inc.
PARTS AND DISPLAY INDICATORS
One (1) Wireless Pulse Oximeter PO3M
One (1) Lanyard
One (1) Operation Manual
One (1) Quick Start Guide
One (1) USB cable
2
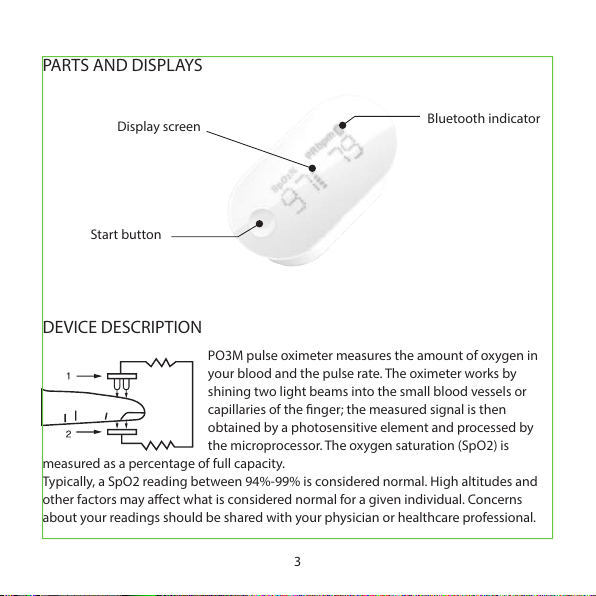
DEVICE DESCRIPTION
PARTS AND DISPLAYS
3
Display screen
Bluetooth indicator
Start button
PO3M pulse oximeter measures the amount of oxygen in
your blood and the pulse rate. The oximeter works by
shining two light beams into the small blood vessels or
capillaries of the nger; the measured signal is then
obtained by a photosensitive element and processed by
the microprocessor. The oxygen saturation (SpO2) is
measured as a percentage of full capacity.
Typically, a SpO2 reading between 94%-99% is considered normal. High altitudes and
other factors may aect what is considered normal for a given individual. Concerns
about your readings should be shared with your physician or healthcare professional.

4
IEC 60601-1: 2005 + CORR. 1 (2006) + CORR. 2 (2007)/EN 60601-1:2006 /AC:2010(
Medical electrical equipment-Part1: General requirements for basic safety and essential
performance)
IEC60601-1-2:2007/EN 60601-1-2:2007 /AC:2010 (Medical electrical equipment -- Part
1-2: General requirements for basic safety and essential performance - Collateral
standard: Electromagnetic compatibility - Requirements and tests)
IEC 60601-1-11 (First Edition): 2010(Medical electrical equipment-Part 1-11: General
requirements for basic safety and essential performance – Collateral Standard:
Requirements for medical electrical equipment and medical electrical systems used in
the home healthcare environment)
ISO 80601-2-61:2011 (Medical electrical equipment-Particular requirements for the
basic safety and essential performance of pulse oximeter equipment for medical use).
CONTRAINDICATIONS
The PO3M Wireless Pulse Oximeter cannot be used on infant babies.
WARNINGS
1.This device is for use on adults only.
2.Certain activities may pose a risk of injury, including strangulation, if the lanyard
should become wrapped around your neck. Use the lanyard with caution.
3.Do not use the device in a magnetic resonance (MR) environment.
1.Do not use the device as the only basis for making medical decisions. It is intended
only to be used as additional information that you can give to your licensed health
care professional.
Notice

5
2.The device might misinterpret excessive movement as good pulse strength. Limit
nger movement as much as possible when using the device.
3.Do not use the device on the same hand/arm when using a blood pressure cu or
monitor.
4.The device has no alarms of blood oxygen saturation and pulse rate, and it will not
sound if the amount of oxygen in your blood is too low or your pulse rate is
abnormal.
If the measurement of SpO2 and pulse rate is not in the normal range, please
contact your health care professional.
5.Do not place the device in liquid or clean it with agents containing ammonium
chloride or products that are not listed in this Operation Manual.
6.Any of the following conditions may reduce the performance of the device:
a)Flickering or very bright light;
b)Excessive Movement;
c)Weak pulse quality (low perfusion);
d)Low hemoglobin;
e)Nail polish, and/or articial nails;
f)Any tests recently performed on you that required an injection of intravascular dyes
7. The device may not work if you have poor circulation. Rub your nger to increase
circulation, or place the device on another nger.
8.The device measures oxygen saturation of functional hemoglobin. High levels of
dysfunctional hemoglobin (caused by sickle cell anemia, carbon monoxide, etc.)
could aect the accuracy of the measurements.
9.Do not use the device in a combustible environment (oxygen enriched environ
ment).
10.Do not use the device outside the specied operating temperature range, and do
not store the device outside the specied storage temperature ranges

6
11.The materials used in the device conform to the biocompatibility and nontoxic
regulations and present no hazard to the body.
12.Use in emergency vehicles with communication systems may aect accuracy of the
measurements.
13.The packaging of the device is recyclable, and it must be collected and disposed
according to the related regulation in the country or region where the package of
the device or its accessories is opened.
14.Any material of the device that may cause pollution must be collected and disposed
according to local rules and requirements.
15.Any single functional tester cannot be used to assess the accuracy of a pulse
oximeter.
16.Do not stare at the lighting LED, as it may irritate your eyes.
17.The device is calibrated to display FUNCTIONAL OXYGEN SATURATION
18.Do not use the device for more than 30 minutes.
19.The wavelength range of pulse oximeter can be especially useful to clinicians
20.Because the pulse oximeter measurements are statistically distributed, only about
two-thirds of pulse oximeter measurements can be expected to fall within ±Arms of
the value measured by a oximeter.
21.The SpO2 accuracy was tested by comparing it to a Co-oximeter and the pulse rate
accuracy was tested by comparing it to a function tester.
USING YOUR PULSE OXIMETER
Before Using Pulse Oximeter
The wireless pulse oximeter may be used when the user is seated, standing or lying
down. The user should not walk or run during measurements and should take care of
not excessively moving the nger where the device is attached and the corresponding
hand and arm.

Charge the battery before rst use
Link the wireless Pulse Oximeter to a USB port of a electrical power source (may be a
PC), and press the “start” button. Then the battery indicator " " will blink, which
means the battery charging is started. When the battery indicator " " stops blinking,
it means the battery has been fully charged.
Download App
Download the app from the App Store and install it. Search for “iHealth MyVitals”. (Your
compatible iOS device should be version 5.0 or later.)
Create User and Cloud
Account
After downloading the
app, register and set up
your user account
following the on-screen
instructions.
7
It is recommended that the user should wash hands before use. Nail polish, especially
dark shades, may aect the accuracy of the measurement and it is suggested that any
polish be removed prior to monitoring.
The device is suitable for using on any nger excluding the thumb. It is preferred to use
the index or middle nger.

8
When setting up your user account, you will also have access to a free, secure iHealth
Cloud account. Go to www.ihealthlabs.com, then click “Sign In” to access your cloud
account from PC or Mac.
Turn Bluetooth “On” under the “Settings” menu of your iOS device (The date and time of
the Pulse Oximeter will be synced with your iOS device upon rst successful
connection). Once your Bluetooth is on, the Pulse Oximeter will connect automatically
when the app is launched and the Bluetooth indicator on the screen of oximeter will
turn on.
Turn Bluetooth “On”
TESTING INSTRUCTIONS
1.Open the clamp of the Pulse Oximeter PO3M, then place the
middle, ring or index nger of your left hand into the rubber
opening of the oximeter with nail side down, as pictured.
2. On the front panel, press the “Start” button once to turn the
oximeter on.
3. Keep your hand still for the reading.
Note: If the pulse signal is too weak to measure, dashes (- - -) will appear on the
display.
4. After a few seconds, your SpO2 reading will appear on the oximeter display screen
and the app if the app is turned on.
5. If the signal strength is too low, switch to another nger and perform the test again.
6. Remove the oximeter from the nger. The oximeter will automatically turn o after 8
seconds.

9
After it has been used for the rst time, the date and time of the Pulse Oximeter PO3M
will be synchronized with your iOS device. It can also be used without being connected
to an iOS device. In this case, the measurement data is stored in the memory and can be
uploaded to the app when the connection is re-established. The Pulse Oximeter PO3M
can store up to 100 measurements. When the memory is full, any new measurement
overwrites the oldest ones.
USING WITHOUT iOS DEVICE
1. Clean the device once per week or more frequently if handled by multiple users.
2. Wipe the device with a soft cloth dampened with rubbing alcohol to avoid cross
infection. Do not pour the alcohol directly on or into the device. Dry with a soft
cloth, or allow to air dry.
3. Avoid dropping this device on a hard surface.
4. Do not immerse the device in water or other liquid, as this will result in damage to
the device.
5. If this device is stored below 0℃, please acclimate the device to room temperature
before use.
6. Do not try to disassemble this device.
7. The PO3M is a precision electronic instrument and must be repaired/serviced by an
accredited iHealth service center.
8. Incorrect replacement of battery by inadequately trained personnel could result in
an unacceptable risk (e.g., excessive temperatures, re or explosion).
9. The patient simulator « Index 2 », made by the Fluke company, can be used to verify
operation of the oximeter.
10.The expected service life of the PO3M is about 5 years.
CARE AND MAINTENANCE
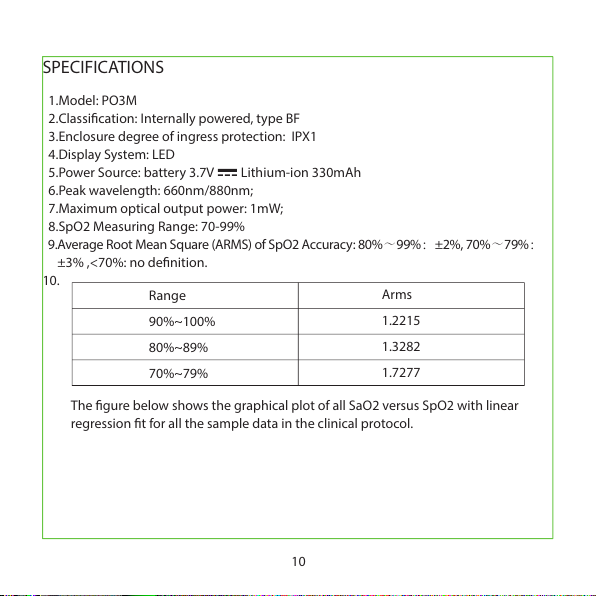
1.Model: PO3M
2.Classication: Internally powered, type BF
3.Enclosure degree of ingress protection: IPX1
4.Display System: LED
5.Power Source: battery 3.7V Lithium-ion 330mAh
6.Peak wavelength: 660nm/880nm;
7.Maximum optical output power: 1mW;
8.SpO2 Measuring Range: 70-99%
9.Average Root Mean Square (ARMS) of SpO2 Accuracy: 80%~99%:±2%, 70%~79%:
±3% ,<70%: no denition.
10.
SPECIFICATIONS
The gure below shows the graphical plot of all SaO2 versus SpO2 with linear
regression t for all the sample data in the clinical protocol.
Range
90%~100%
80%~89%
70%~79%
Arms
1.2215
1.3282
1.7277
10
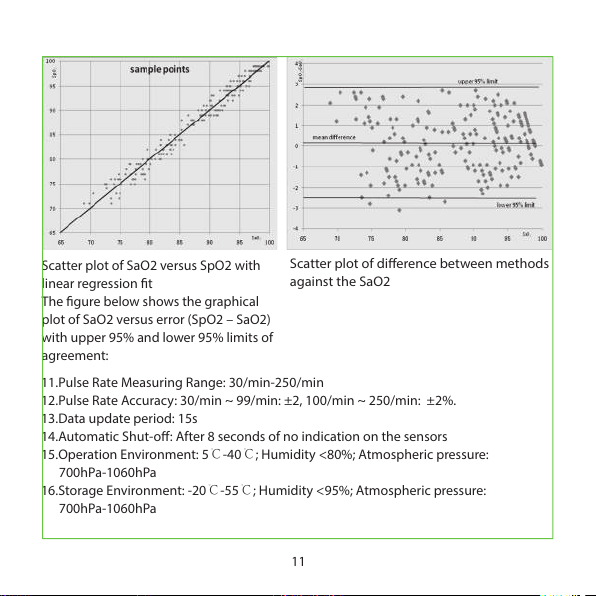
Scatter plot of SaO2 versus SpO2 with
linear regression t
The gure below shows the graphical
plot of SaO2 versus error (SpO2 – SaO2)
with upper 95% and lower 95% limits of
agreement:
Scatter plot of dierence between methods
against the SaO2
11.Pulse Rate Measuring Range: 30/min-250/min
12.Pulse Rate Accuracy: 30/min ~ 99/min: ±2, 100/min ~ 250/min: ±2%.
13.Data update period: 15s
14.Automatic Shut-o: After 8 seconds of no indication on the sensors
15.Operation Environment: 5℃-40℃; Humidity <80%; Atmospheric pressure:
700hPa-1060hPa
16.Storage Environment: -20℃-55℃; Humidity <95%; Atmospheric pressure:
700hPa-1060hPa
11
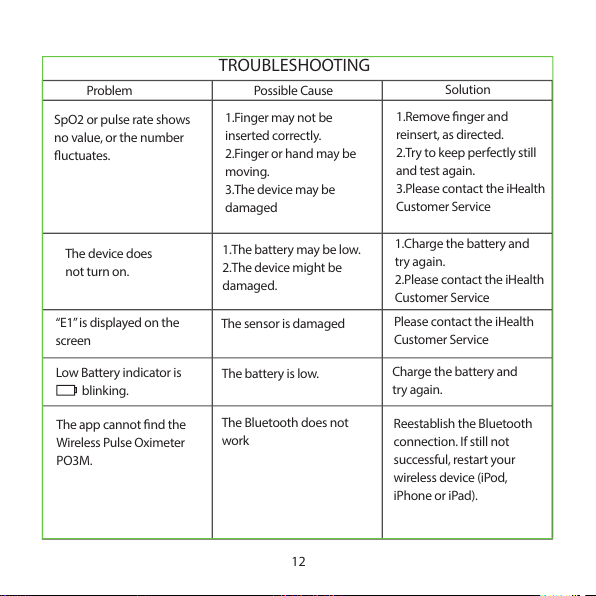
TROUBLESHOOTING
12
Problem Possi ble Cau se
Solution
SpO2 or pulse rate shows
no value, or the number
uctuates.
1.Finger may not be
inserted correctly.
2.Finger or hand may be
moving.
3.The device may be
damaged
1.Remove nger and
reinsert, as directed.
2.Try to keep perfectly still
and test again.
3.Please contact the iHealth
Customer S ervice
The device does
not turn on.
1.The battery may be low.
2.The device might be
damaged.
1.Charge the battery and
try again.
2.Please contact the iHealth
Customer S ervice
“E1” is displayed on the
screen
The sensor is da maged
Please contact the iH ealth
Customer S ervice
Low Batter y indicator is
blinking.
The battery i s low.
Charge the battery and
try again.
The app cannot nd the
Wireless Pulse Oximet er
PO3M.
The Bluetooth do es not
work
Reestablish the Bluetooth
connection. If still not
successful, restart your
wireless device (iPod,
iPhone or iPad).

13
IMPORTANT INFORMATION REQUIRED BY THE FCC
This device complies with Part 15 of the FCC Rules. Its operation is subject to the
following two conditions:
(1) This device may not cause harmful interference.
(2) This device must accept any interference received, including interference that may
cause undesired operation.
Note: This product has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
product generates, uses, and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
particular installation. If this product does cause harmful interference to radio or
television reception, which can be determined by turning the equipment of and on, the
user is encouraged to try to correct the interference by one or more of the following
measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit dierent from that to which the
receiver is connected.
—Consult the dealer or an experienced radio/TV technician for help.
IMPORTANT INFORMATION REQUIRED BY THE INDUSTRY CANADA
Under Industry Canada regulations, this radio transmitter may only operate using an
antenna of a type and maximum (or lesser) gain approved for the transmitter by

Industry Canada. To reduce potential radio interference to other users, the antenna
type and its gain should be so chosen that the equivalent isotropically radiated power
(e.i.r.p.) is not more than that necessary for successful communication.
This device complies with Industry Canada license-exempt RSS standard(s). Operation
is subject to the following two conditions: (1) this device may not cause interference,
and (2) this device must accept any interference, including interference that may cause
undesired operation of the device.
14
Manufacturer Information
ANDON HEALTH CO., LTD.
No. 3 Jinping Street, YaAn Road, Nankai District, Tianjin 300190, China.
Tel: 86-22-60526081
IHealthLabs Europe SARL
3 rue Tronchet, 75008, Paris, France
Tel: +33 1 44 94 04 81
Health is a trademark of iHealth Lab Inc. Bluetooth® associated logos are registered
trademarks owned by
Bluetooth SIG, Inc. and any use of such marks by iHealth Lab Inc. is permitted under license.
Other trademarks and trade names are those of their respective owners.
EC REP
ELECTROMAGNETIC COMPATIBILITY INFORMATION
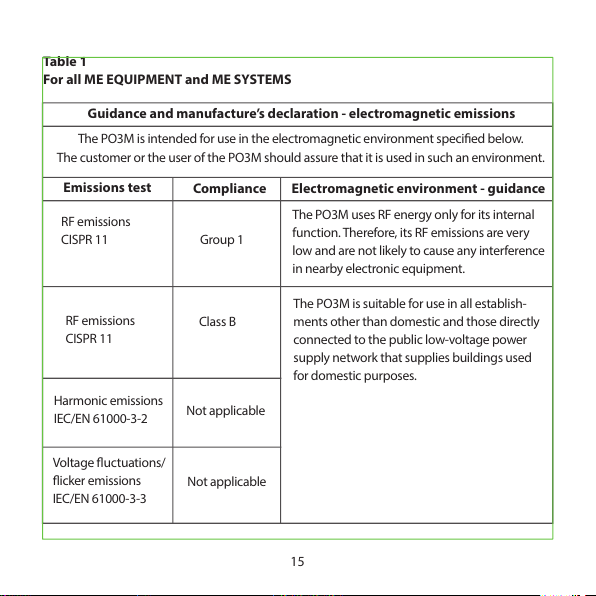
15
Table 1
For all ME EQUIPMENT and ME SYSTEMS
Guidance and manufacture’s declaration - electromagnetic emissions
The PO3M is inten ded for use in the electroma gnetic environment specied bel ow.
The customer or the user of the PO3M should assure that it is used in s uch an environment.
Emissions test
Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
Group 1
The PO3M uses RF energy only for its internal
function. Therefore, its RF emissions are very
low and are not likely to cause any interference
in nearby electronic equipment.
RF emissions
CISPR 11
Class B
The PO3M is suit able for use in all establis h-
ments other than domestic and those directly
connected to the p ublic low-voltage power
supply network that supplies buildings used
for domes tic purposes.
Harmonic emissions
IEC/EN 61000-3-2
Not applicable
Volt age uct uatio ns/
icker emissions
IEC/EN 61000-3-3
Not applicable
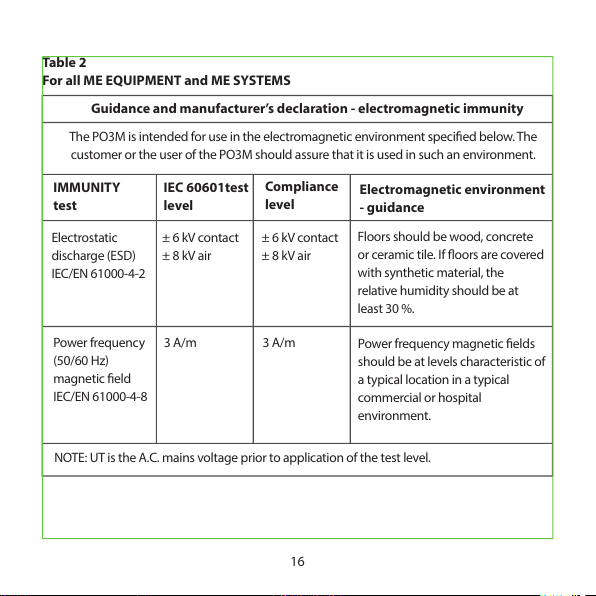
Table 2
For all ME EQUIPMENT and ME SYSTEMS
16
Guidance and manufacturer’s declaration - electromagnetic immunity
The PO3M is inten ded for use in the electroma gnetic environment specied bel ow. The
customer or the user of the PO3M should assure that it is used in such an environment.
IMMUNITY
test
IEC 60601test
level
Compliance
level
Electromagnetic environment
- guidance
Electrostatic
discharge (ESD)
IEC/EN 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be w ood, concret e
or ceramic tile. If oors are covered
with synthetic material, the
relative humi dity should be at
least 30 %.
Power frequen cy
(50/60 Hz)
magnetic eld
IEC/EN 61000-4-8
3 A/m 3 A/m
Power frequen cy mag netic elds
should be at levels characteristic of
a typical location in a typical
commercial or hospit al
environment.
NOTE: UT is the A.C. mains voltage prior to application of the test level.
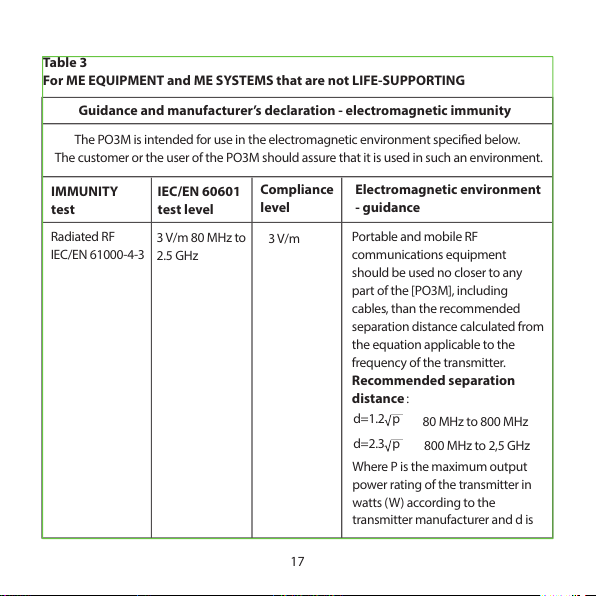
17
Table 3
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacturer’s declaration - electromagnetic immunity
The PO3M is inten ded for use in the electroma gnetic environment specied bel ow.
The customer or the user of the PO3M should assure that it is used in s uch an environment.
IMMUNITY
test
IEC/EN 60601
test level
Compliance
level
Electromagnetic environment
- guidance
Radiated RF
IEC/EN 61000-4-3
3 V/m 80 MHz to
2.5 GHz
3 V/m
Port able an d mobil e RF
communications equi pment
should be used no closer to any
part of the [PO3M], including
cables, than the recommended
separation distance calculated from
the equation applicable to the
frequency of the transmitter.
Recommended separation
distance:
d=1.2 p
80 MHz to 800 MHz
d=2.3 p
800 MHz to 2,5 GHz
Where P is the maximum output
power rating of the transmitter in
watts (W ) according to the
transmitter manufacturer and d is

NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
aected by absorption and reection from structures, objects and people.
A) Field strengt hs from xed transmitters, such as base station s for radio (cellular/c ordless)
telephones and lan d mobile radios, amateur radio, AM and FM rad io broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to xed RF transmitters, an electromagnetic site survey should be
considered. If the m easured eld strength in the locat ion in which the PO3M is used excee ds
the applicable RF compliance level above, the PO3M should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the [PO3M].
B) Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than [V1] V/m.
18
the recommended separation
distance in meters (m).
Field streng ths fro m xed RF
transmitters, as determined by an
electromagnetic site survey,a should
be less than the compliance level in
each frequency range.b
Interference may occur in the vicinity
of equipment marked with the
following symbol:
19
Table 4
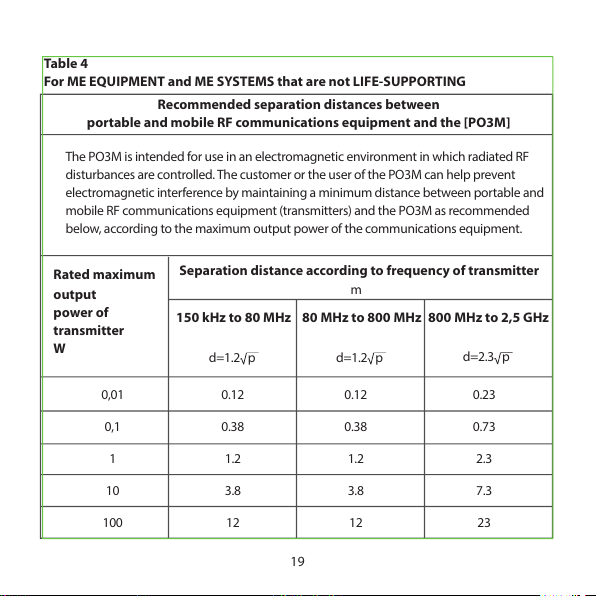
For ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Recommended separation distances between
portable and mobile RF communications equipment and the [PO3M]
The PO3M is inten ded for use in an electromagn etic environment in which radia ted RF
disturbances are controlled. The customer or the user of the PO3M can help prevent
electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the PO3M as recommended
below, according to the maximum output power of the communications equipment.
Rated maximum
Separation distance according to frequency of transmitter
output
power of
transmitter
W
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2,5 GHz
0,01
0,1
1
10
100
0.12
0.38
1.2
3.8
12
0.12
0.38
1.2
3.8
12
0.23
0.73
2.3
7.3
23
d=1.2 p
d=1.2 p
d=2.3 p

For t ransm itter s rate d at a maxi mum o utput power not liste d abov e, the reco mmende d
separation distance d in meters (m) can be determined using the equation applicable to
the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range
applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is
aected by absorption and reection from structures, objects and people.
20

FR
MODÈLE PO3M
Oxymètre de pouls au doigt
MANUEL DE PRÉSENTATION
TABLE DES MATIÈRES
SYMBOLES
UTILISATION PRÉVUE
CONTENU ET VOYANTS D’AFFICHAGE 5
PARTIES COMPOSANTES ET AFFICHAGES
DESCRIPTION DU DISPOSITIF
CONTRE-INDICATIONS
AVERTISSEMENTS
PRÉCAUTIONS
UTILISATION DE VOTRE OXYMÈTRE DE POULS
SOINS ET ENTRETIEN
SPÉCIFICATIONS
DÉPANNAGE
Informations sur le fabricant
INFORMATIONS SUR LA COMPATIBILITE ÉLECTROMAGNÉTIQUE
1
2
2
3
3
4
4
4
6
9
10
11
12
12
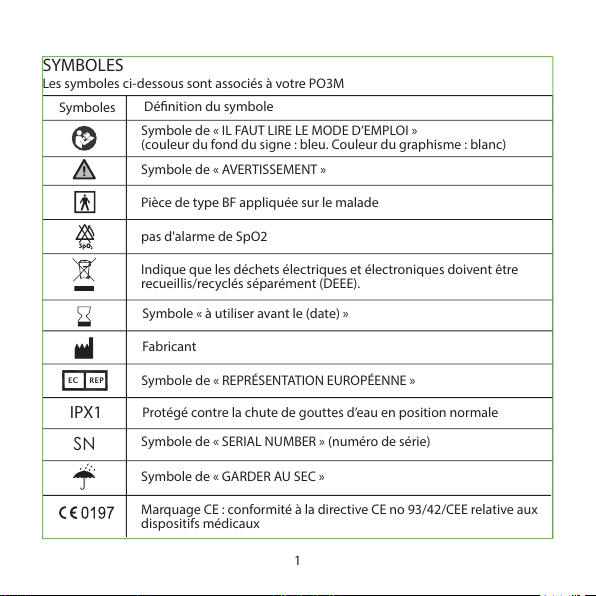
SYMBOLES
Les symboles ci-dessous sont associés à votre PO3M
1
Symboles
Symbole de « IL FAUT LIRE LE MODE D’EMPLOI »
(couleur du fond du signe : bleu. Couleur du graphisme : blanc)
Symbole de « AVERTISSEMENT »
Pièce de type BF appliquée sur le malade
Indique que les déchets électriques et électroniques doivent être
recueillis/recyclés séparément (DEEE).
pas d'alarme de SpO2
Symbole « à utiliser avant le (date) »
Fabricant
Symbole de « SERIAL NUMBER » (numéro de série)
Symbole de « REPRÉSENTATION EUROPÉENNE »
Symbole de « GARDER AU SEC »
Marquage CE : conformité à la directive CE no 93/42/CEE relative aux
dispositifs médicaux
Dénition du symbole
SN
EC REP
IPX1
Protégé contre la chute de gouttes d’eau en position normale

UTILISATION PRÉVUE
L’Oxymètre de pouls sans l PO3M est un dispositif non invasif destiné à ponctuelle-
ment la saturation fonctionnelle en oxygène de l'hémoglobine artérielle (SpO2) et la
fréquence du pouls. Le dispositif portable au doigt est recommandé pour les adultes
au-dessus de 16 ans, à la maison et en milieu hospitalier (y compris pour une utilisation
clinique en médecine interne ou en chirurgie, en cas d’anesthésie, pendant des soins
intensifs, etc.). L’oxymètre de pouls sans l iHealth n’est pas conçu pour un suivi
permanent.
Compatibilité
L’oxymètre de pouls PO3M iHealth est conçu pour être utilisé avec les dispositifs
suivants :
iPod touch 5e génération
iPhone 4S
iPhone 5
Nouvel iPad
iPad 4
avec une version V5.0 d’iOS ou plus récente.
iPhone et iPod touch sont des marques commerciales d’Apple Inc., déposées aux
États-Unis et dans d’autres pays. iPad est une marque commerciale d’Apple Inc.
CONTENU ET VOYANTS D’AFFICHAGE
Un (1) oxymètre de pouls PO3M iHealth
Une (1) dragonne
Un (1) mode d’emploi
Un (1) guide de démarrage rapide
Un (1) câble USB
2

DESCRIPTION DU DISPOSITIF
PARTIES COMPOSANTES ET AFFICHAGES
3
Écran d’achage
Indicateur Bluetooth
Bouton Marche/Arrêt
L’oxymètre de pouls mesure le taux d’oxygène dans votre sang et la fréquence
cardiaque. Il fonctionne en émettant deux rayons
lumineux vers les vaisseaux capillaires du doigt pour
mesurer la teneur en oxygène de votre sang, et en
achant la valeur obtenue sur l'écran. Le degré de
saturation en oxygène (SpO2) s’exprime en pourcentage
de la saturation totale.
En général, une valeur de SpO2 située entre 94 et 99 % est considérée comme normale.
L’altitude et d’autres facteurs peuvent modier ce qui est considéré comme normal
pour une personne donnée. En cas de doutes concernant les résultats que vous
obtenez, demandez conseil à votre médecin ou à tout autre professionnel de la santé.

4
IEC 60601-1 : 2005 + CORR. 1 (2006) + CORR. 2 (2007)/EN 60601-1:2006 /AC:2010
(Appareils électromédicaux, partie 1 : Exigences générales pour la sécurité de base et
les performances essentielles)
IEC60601-1-2:2007/EN 60601-1-2:2007 /AC:2010 (Appareils électromédicaux - Partie 1-2
: Exigences générales pour la sécurité de base et les performances essentielles - Norme
collatérale : Compatibilité électromagnétique – Prescriptions et essais)
IEC 60601-1-11 (Première édition) : 2010 (Appareils électromédicaux – Parties 1-11 :
Exigences générales pour la sécurité de base et les performances essentielles – Norme
collatérale : Exigences pour les appareils électromédicaux et les systèmes électromédi-
caux utilisés dans l'environnement des soins à domicile
ISO 80601-2-61:2011 (Appareils électromédicaux - Exigences particulières pour la
sécurité de base et les performances essentielles pour les oxymètres de pouls à usage
médical).
CONTRE-INDICATIONS
Ne pas utiliser l’oxymètre de pouls sans l PO3M sur les nourrissons.
AVERTISSEMENTS
1.Maintenez l’oxymètre hors de la portée des enfants.
2.Certaines manipulations peuvent comporter des risques, et notamment la
strangulation si la dragonne venait à être passée autour du cou. Utilisez la dragonne
avec précaution.
3.N’utilisez pas le dispositif à proximité d’appareils de résonance magnétique (RM).
PRÉCAUTIONS

5
1.Aux États-Unis cet appareil n’est vendu qu’aux médecins ou sur prescription
médicale.
2.Ne vous servez pas de l’appareil comme seule base pour la prise de décisions
médicales. Il n’est conçu que pour fournir des renseignements complémentaires
dont vous pouvez faire part à votre médecin ou au personnel soignant.
3.L’appareil pouvant interpréter par erreur un excès de mouvement comme un bon
pouls, veillez à maintenir vos doigts aussi immobiles que possible lorsque vous
utilisez l’appareil.
4.N’utilisez pas l’appareil en même temps qu’un brassard de tensiomètre ou un suiveur
de tension sur le même membre.
5.L’appareil n’est pas pourvu d’alarmes ; aucun son ne vous préviendra si le taux
d’oxygène dans votre sang est trop bas, ni si votre pouls est trop rapide ou trop lent.
6.Ne plongez pas l’appareil dans un liquide, et ne le nettoyez pas avec des produits
contenant du chlorure d’ammonium ou ne gurant pas dans la liste qui se trouve
dans ce mode d’emploi.
7.L'une quelconque des situations suivantes peut diminuer la performance de l'appareil :
1.Éclairage vacillant/clignotant ou très intense ;
2.Pouls faible (faible perfusion) ;
3.Faible taux d’hémoglobine ;
4.Vernis à ongles et/ou ongles postiches ; et
5.Tout examen eectué récemment ayant nécessité l’injection de colorants
intravasculaires.
8.Il se peut que l’appareil ne fonctionne pas si vous avez une mauvaise circulation.
Frottez votre doigt pour aviver la circulation, ou essayez sur un autre doigt.
9.L’appareil mesure la saturation en oxygène de l'hémoglobine fonctionnelle. Des taux
élevés d'hémoglobine dysfonctionnelle (causés par une anémie falciforme, le
monoxyde de carbone, et.) peuvent altérer la précision des mesures.

10.N’utilisez pas l’appareil dans un environnement enrichi en oxygène.
11.N’utilisez pas cet appareil en-dehors des plages de températures de service et
d’entreposage spéciées.
12.Les matériaux utilisés dans cet appareil répondent aux exigences de biocompatibil
ité, et ne présentent ni toxicité ni danger pour votre corps.
13.Une utilisation dans des véhicules destinés aux urgences et équipés de systèmes de
communication peut altérer la précision des mesures.
14.Le matériel d’emballage de cet appareil est recyclable. Il doit être recueilli et éliminé
selon la réglementation en vigueur dans le pays ou la région dans lesquels l’appareil
ou ses accessoires sont déballés.
15.Tout matériau composant l’appareil et susceptible de polluer l’environnement doit
être recueilli dans le strict respect des règlements et des exigences locales.
16.On ne peut pas utiliser n’importe quel testeur pour vérier la précision d’un
oxymètre de pouls.
17.Veillez à ne pas regarder xement la LED quand elle s’éclaire, cela pourrait irriter vos
yeux.
18.L’appareil est étalonné de manière à acher la SATURATION FONCTIONNELLE EN
OXYGÈNE
19.N’utilisez pas l’appareil pendant plus de 30 minutes.
20.On ne peut pas utiliser n’importe quel testeur pour vérier la précision d’un
oxymètre de pouls.
6
UTILISATION DE VOTRE OXYMÈTRE DE POULS
Avant d’utiliser l’oxymètre
Pendant l'utilisation de l’oxymètre le patient peut être assis, debout ou couché.
L’utilisateur ne doit ni marcher ni courir pendant la prise de mesures, et ne pas trop

7
bouger le bras ni la main lorsque l'appareil est xé au doigt.
Il est conseillé à l’utilisateur de se laver les mains avant l’utilisation. Les vernis à ongles,
surtout s’ils sont foncés, peuvent nuire à la précision de la mesure. Il est conseillé
d’enlever le vernis avant la mesure.
On peut utiliser le dispositif sur n'importe quel doigt, sauf le pouce. L’idéal est de choisir
l’index ou le majeur.
Chargez la batterie avant la première utilisation
Branchez l’Oxymètre de pouls PO3M sur un port USB, appuyez sur le bouton
Start/Démarrer, et l’indicateur de charge clignotera. Quand ce voyant s’éteint, la
batterie est à pleine charge.
Téléchargez l’application
Téléchargez l’application depuis l’App store et installez-la. Recherchez « iHealth SpO2 ».
(La version de votre dispositif iOS compatible doit être 5.0 ou plus récente).
Créez un utilisateur et un
compte nuage
Après le téléchargement
de l’application,
enregistrez et congurez
votre compte d’utilisateur
en suivant les
instructions qui s’achent
sur l’écran.
 Loading...
Loading...