iHealth BG5-Kit, BG5 User guide
Wireless Smart Gluco-Monitoring
System

OWNER’S MANUAL
For in vitro diagnostic use only
Read instructions before use for self-testing
iHealth® Wireless Smart Gluco-Monitoring System
OWNER’S MANUAL
Table of Contents
INTRODUCTION 1
INTENDED USE 1
IMPORTANT SAFETY INSTRUCTIONS 1
CONTENTS OF THE WIRELESS SMART GLUCO-
MONITORING SYSTEM 2
Parts and Displays 4
Mobile Device Compatibility 6
TEST PRINCIPLE 6
IMPORTANT TEST INFORMATION 6
FIRST TIME SETUP INSTRUCTIONS 7
DATA SYNCING12
REVIEWING SAVED TEST RESULTS ON THE METER 13
CLEANING AND DISINFECTION 13
SIGNS OF POTENTIAL PHYSICAL AND PERFORMANCE
DETERIORATION14
INFORMATION ABOUT ALTERNATE SITE TESTING (AST) 15
What is Alternate Site Testing? 15
What is the Advantage of Alternate Site Testing? 16

When Should You Use Alternate Site Testing? 16
IMPORTANT INFORMATION ABOUT CONTROL
SOLUTION TESTS17
PERFORMING A CONTROL SOLUTION TEST 17
Out-of-Range Results 19
COMPARING GLUCOSE METER TEST RESULTS WITH
LABORATORY RESULTS
While at the Lab
TEST STRIP VIAL LABEL
SPECIFICATIONS
LIMITATIONS OF USE
SYSTEM TROUBLESHOOTING
DISPLAY MESSAGES
TROUBLESHOOTING
WARRANTY INFORMATION
EXPLANATION OF SYMBOLS
IMPORTANT INFORMATION REQUIRED BY THE FCC

INTRODUCTION
Thank you for purchasing the iHealth Wireless Smart GlucoMonitoring System (the iHealth system). This manual provides
important information to help you use the system properly. Before
using this product, please read the Owner’s Manual thoroughly.
INTENDED USE
The iHealth system is intended to be used for:
• Quantitative measurement of glucose in fresh capillary
whole blood samples drawn from the fingertip, palm, forearm,
upper arm, calf, or thigh
• Single person measurement only (it should not be
shared)
• Self-testing outside the body (in vitro diagnostic use) by
people with diabetes at home as an aid to monitor the
effectiveness of diabetes control
The iHealth system should not be used for the diagnosis of
diabetes or screening for diabetes, or for neonatal use.
IMPORTANT SAFETY INSTRUCTIONS
Please read the following information carefully before using the
iHealth system. Always keep these instructions in a safe place for
reference.
• Misuse of the iHealth system can cause electrocution, burns, fire,
and other hazards.
• The meter and lancing device are for single patient use. Do not
use either item on multiple patients.
1

• Do not share the meter or lancing device with anyone, including
other family members.
• Do not place the iHealth system in or near liquid.
• Use the iHealth system only for the purpose described in the
Owner’s Manual.
• Use only accessories that are supplied by the manufacturer.
• Do not use the iHealth system if it has sustained any damage or is
not working properly.
• Keep the iHealth system away from heat at all times. Do not let
the iHealth system come into contact with surfaces that are hot
to the touch.
• Do not block test port or place the iHealth system on soft
surfaces that may block them. Keep test port free from lint, hair,
debris, etc.
• Do not place anything on top of the iHealth system.
• Do not place foreign objects into any opening in the iHealth
system.
• Do not use the meter in a manner not specified by the
manufacturer.
• All parts of the iHealth system are considered biohazards and
can potentially transmit infectious diseases, even after you have
performed cleaning and disinfection.
• Please refer to the resources identified below for detailed
information:
“FDA Public Health Notification: Use of Fingerstick Devices on
More than One Person Poses Risk for Transmitting Bloodborne
Pathogens: Initial Communication” (2010)
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/uc
m224025.htm
2
“CDC Clinical Reminder: Use of Fingerstick Devices on More than
• Travel Case
• USB Charging Cable
• iHealth Control Solution
l
One Person Poses Risk for Transmitting Bloodborne Pathogens”
(2010) http://www.cdc.gov/injectionsafety/FingerstickDevicesBGM.html
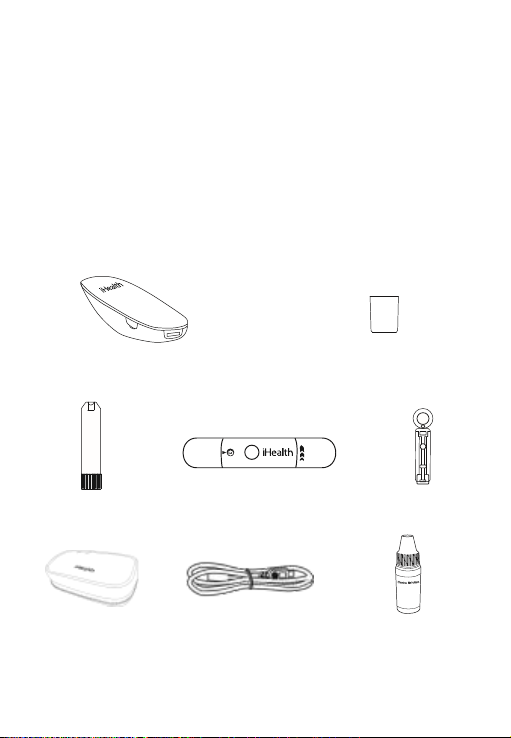
CONTENTS OF THE WIRELESS SMART GLUCOMONITORING
SYSTEM
Package contents vary from country to country. Please refer to the
package contents listed on the package you purchased.
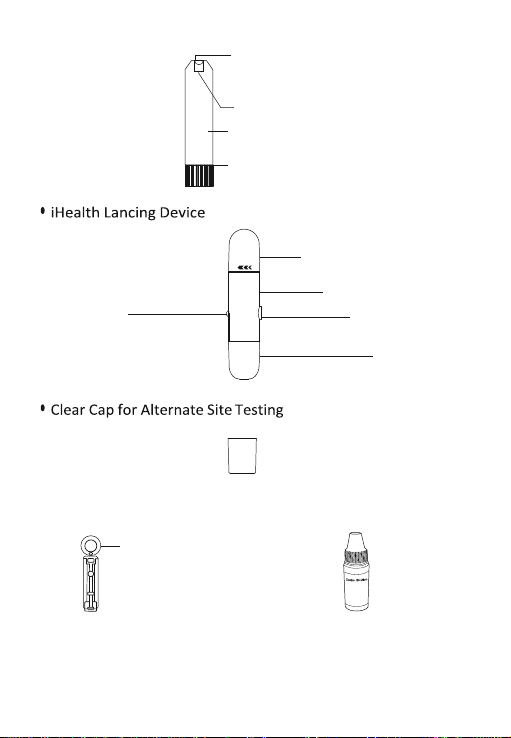
• iHealth Smart Glucose Meter • Clear Cap for Alternate
(the meter) Site Testing
• iHealth Test Strip • iHealth Lancing Device • Lancet
3
• Owner’s Manual • Quick Start Guide
Note: If any items printed on the package are missing from your
package or the package appears to have been opened prior to
your use, please call iHealth Labs Customer Service at 1-855-
8167705.
Parts and Displays
• iHealth Blood Glucose Meter(the meter)
Appears when the battery is low.
Located at the back side of the
meter. It is used to automatically
remove the test strip.
4
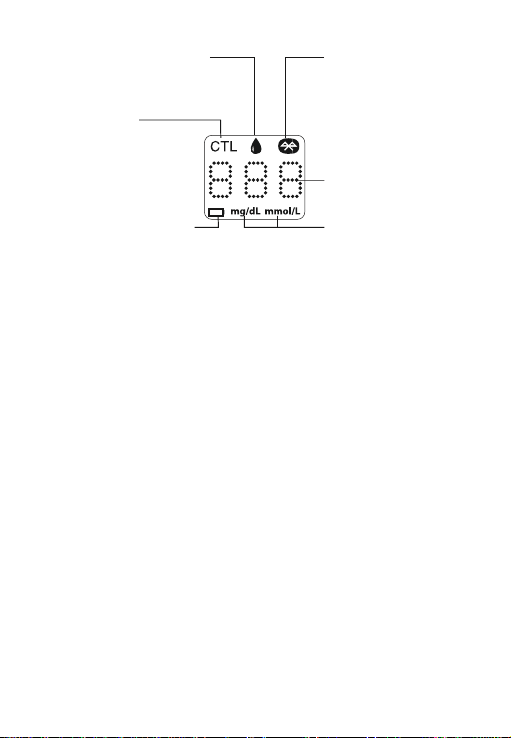
BLUETOOTH SYMBOL
LOW BATTERY SYMBOL
CTL SYMBOL
Appears when doing a
control solution test and
indicates that the result
won’t be stored in the
memory.
BLOOD SAMPLE SYMBOL
Flashes when the meter is ready
to accept the blood sample.
Appears when the meter is
connected with iOS device
through Bluetooth function.
TEST RESULT
DISPLAY
Displays test result.
MEASUREMENT UNIT
Appears with the test result
either in mg/dL or mmol/L.
• iHealth Test Strip
Use only iHealth test strips with the meter. Each test strip can be
used only once, and consists of the following parts:
5
• Lancet • iHealth Control Solution
Absorbent hole
Confirmation window
Test strip handle
Contact bars
Head
Main body
Release button
Cap (for finger testing)
Remove lancet part
l
Lancet cover
Mobile Device Compatibility
6

Works with the following iOS version 5.0 or higher devices:
iPhone 5s iPhone 5c iPhone 5 iPhone 4s iPhone 4 iPhone
3GS iPad Air
iPad mini with Retina display
iPad (4th generation) iPad
mini iPad (3rd generation)
iPad 2
iPod touch (5th generation) iPod
touch (4th generation)
TEST PRINCIPLE
Testing with the iHealth system is based on the measurement of
electrical currents generated by the reaction of glucose with the
reagent of the test strip. The iHealth system measures the current
and converts it to the corresponding blood glucose level. The
strength of the current produced by the reaction depends on the
amount of glucose in the blood sample.
IMPORTANT TEST INFORMATION
Please read the following:
• Severe dehydration and excessive water loss may cause
inaccurate results. If you believe you are suffering from severe
dehydration, consult your healthcare professional immediately.
• Inaccurate results may occur in severely hypotensive
individuals or patients who are in shock. Test results that are
lower than actual values may occur in individuals who are in a
hyperglycemic-hyperosmolar state, with or without ketosis.
7

Critically ill patients should not be tested with blood glucose
meters.
• If your blood glucose results are lower or higher than
usual, and you do not have symptoms of illness, first repeat the
test. If you have symptoms or continue to get results that are
higher or lower than usual, follow the treatment advice of your
healthcare professional.
• If you are experiencing symptoms that are inconsistent
with your blood glucose test, and you have followed all of the
instructions provided in this Owner’s Manual, contact your
healthcare professional immediately.
• Use only fresh whole blood samples to test your blood
glucose.
• Do not use test strips that are expired or appear to be
damaged as they may return inaccurate results.
• The lancing device is for self-use only. Do not share or
re-use lancets. Please refer to the Lancing Device Manual for
the detailed procedure.
For more detailed information, please refer to the resources
identified below:
“FDA Public Health Notification: Use of Fingerstick Devices on
More than One Person Poses Risk for Transmitting Bloodborne
Pathogens: Initial Communication” (2010)
8

http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/uc
m224025.htm
“CDC Clinical Reminder: Use of Fingerstick Devices on More than
One Person Poses Risk for Transmitting Bloodborne Pathogens”
(2010) http://www.cdc.gov/injectionsafety/FingerstickDevicesBGM.html
FIRST TIME SETUP INSTRUCTIONS
• Download the companion app
Prior to first use, download and install the free iHealth
GlucoSmart App from the App Store to your iOS device. Follow
the on-screen instructions to create your iHealth ID.
• Access iHealth Cloud
You can use your iHealth ID to gain access to free and secure
Cloud Services. Go to www.ihealthlabs.com and click on “Sign
In”.
• Charge the battery
Out of the box
Your meter is powered by a built-in, rechargeable battery. Plug
one end of the charging cable into the side of the meter and the
other end into your computer’s USB port. Charge it for two to
four hours before first use. A fully charged battery can typically
take up to 200 tests depending on your daily usage.
Low battery message
After you have used your meter for some time, appears
for three seconds when the battery in your meter is low on
9

power. You must recharge the battery before using it again.
After three seconds, the meter shuts off automatically. The
meter does not take any measurement when the battery is low.
Important: If battery is completely drained, fully charge the
battery and launch the app to sync the meter before using it
again.
• Sync the meter
Prior to first use, follow the steps below to connect the meter to
the app on your iOS device to set your meter’s time and date. By
connecting the two, the date and time of the meter will be
synced with your iOS device.
1. Enable Bluetooth on your iOS device.
10
 Loading...
Loading...