ICU Medical 20677 Users Manual

System Operating Manual
R
for use
for use
with
with
For use with Lists: 12391, 20679-04,
11971-04 (-03 and above ONLY)
and
Back
Prime
Draft Manual- Not to be used
in a Clinical Situation.
HOSPIRA, INC., LAKE FOREST, IL 60045, USA
A
B
430-95520-001 (Rev. 04/05)
Options/
Vol Inf

Change History
Description of
Title
Changes Pages Affected
Change History
430-95520-001
(Rev. 04/05)
Draft Manual- Not to be used
in a Clinical Situation.
First Release All
430-95520-001 (Rev. 04/05)

Contents
Section 1, Descriptive Information . . . . . . . . . . . . . . . 1-1
PRODUCT DESCRIPTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
I
NDICATIONS FOR USE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
user qualification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
C
ONVENTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-2
warnings, cautions, and notes . . . . . . . . . . . . . . . . . . . . . 1-3
P
RECAUTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4
healthcare professionals and patient related . . . . . . . . . . 1-4
concurrent flow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5
epidural administration . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7
battery operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8
sets and accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
backpriming . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
general . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
bolus related . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
artifacts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11
G
UIDENCE ON EMC COMPATIBILITY . . . . . . . . . . . . . . . . . . . . . . 1-13
FCC I
NFORMATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-14
Section 2, Principles of Operation . . . . . . . . . . . . . . . 2-1
FEATURES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
programs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
line programming options . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
plumset capabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
air management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
biomedical . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
options . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
other features . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-2
communication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Section 3, Equipment Description . . . . . . . . . . . . . . . 3-1
OPERATING KEYS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-2
I
NDICATORS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4
D
ISPLAY SYMBOLS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5
430-95520-001 (Rev. 3/05)

Contents
REAR CASE CONTROLS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
A
DMINISTRATION SETS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
P
REPARING THE ADMINISTRATION SET . . . . . . . . . . . . . . . . . . . . 3-7
P
RIMING THE ADMINISTRATION SET . . . . . . . . . . . . . . . . . . . . . . 3-8
L
OADING THE CASSETTE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
P
REPARING THE SECONDARY LINE . . . . . . . . . . . . . . . . . . . . . 3-10
D
ISCONTINUING ELECTRONIC FLOW
C
ONTROL & SETTING GRAVITY FLOW . . . . . . . . . . . . . . . . . . . 3-11
D
ISCONTINUING FLUID ADMINISTRATION . . . . . . . . . . . . . . . . . . 3-12
Section 4, Basic Operation . . . . . . . . . . . . . . . . . . . . .4-1
GETTING STARTED . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
unpacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
tandem carrier instructions . . . . . . . . . . . . . . . . . . . . . . . . 4-2
system self-tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-2
data retention . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
P
OWER ON . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4
S
IMPLE DELIVERY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
T
ITRATION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8
P
IGGYBACK DELIVERY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12
C
ONCURRENT DELIVERY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15
S
TOP AND START WITH ONLY 1 LINE PUMPING . . . . . . . . . . . . . 4-18
S
TOP AND START WITH BOTH LINES PUMPING . . . . . . . . . . . . . 4-19
B
ACKPRIMING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-20
C
LEARING SETTINGS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-21
U
PDATING DRUG LIBRARY . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-23
Section 5, Advanced Programs . . . . . . . . . . . . . . . . . .5-1
DOSE CALC (MCG/KG/MIN ON A) . . . . . . . . . . . . . . . . . . . . . . . . 5-2
D
OSE CALC (MG/MIN ON B) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-9
L
OADING DOSE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-15
M
ULTISTEP PROGRAMMING . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-22
Section 6, Additional Features . . . . . . . . . . . . . . . . . . .6-1
SIMPLE DELIVERY USING DELAYED START . . . . . . . . . . . . . . . . . 6-1
P
IGGYBACK WITH NURSE CALLBACK . . . . . . . . . . . . . . . . . . . . . 6-2
P
OSSIBLE NON-DELIVERY PROGRAMMED . . . . . . . . . . . . . . . . . . 6-4
U
SING THE STANDBY FEATURE . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
S
ELECT OPTION- VOLUMES INFUSED, PRESSURE/POST INFUSION
430-95520-001 (Rev. 3/05)

RATE, AND LIGHTING/CONTRAST . . . . . . . . . . . . . . . . . . . . . . . . 6-6
V
ARIABLE RATE CAP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-10
E
XAMPLES OF AUTOMATIC CALCULATION . . . . . . . . . . . . . . . . . 6-10
at startup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-10
while running (titration) . . . . . . . . . . . . . . . . . . . . . . . . . . 6-10
at kvo . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-11
C
HANGING CCA WHILE INFUSING . . . . . . . . . . . . . . . . . . . . . . 6-12
A
UTO-PROGRAMMING . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-13
Section 7, Alarms and Troubleshooting . . . . . . . . . . . 7-1
WARNING MESSAGES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-1
R
ESPONSE TO ALARMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-2
G
ENERAL ALARMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-3
L
INE A ALARMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-5
L
INE B ALARMS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-7
M
ALFUNCTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-8
G
ENERAL MESSAGES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7-9
Section 8, Cleaning, Maintenance, and Storage . . . . 8-1
cleaning and sanitizing . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-1
battery maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-3
storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-4
service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8-4
Section 9, Specifications . . . . . . . . . . . . . . . . . . . . . . 9-1
PHYSICAL . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-1
E
LECTRICAL . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-1
W
IRELESS LAN DEVICE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-2
VTBI R
E
D
A
O
T
B
O
D
E
T
ANGE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-2
NVIRONMENT . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
ELIVERY RATE RANGE . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
IR-IN-LINE ALARM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-3
CCLUSION ALARM AND LIMITS . . . . . . . . . . . . . . . . . . . . . . . . . 9-4
IME TO DETECT DOWNSTREAM OCCLUSIONS . . . . . . . . . . . . . . 9-5
OLUS VOLUME RELEASED AFTER DOWNSTREAM
CCLUSIONS ARECORRECTED . . . . . . . . . . . . . . . . . . . . . . . . . 9-5
ELIVERY ACCURACY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-6
NTERAL & HIGH VISCOSITY FLUIDS EFFECTS . . . . . . . . . . . . . . 9-6
RUMPET CURVES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
430-95520-001 (Rev. 3/05)

Contents
example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9-7
Section 10, Supplies and Accessories . . . . . . . . . . . .10-1
ADMINISTRATION FLUIDS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
administration fluids . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
enteral and high viscosity fluids . . . . . . . . . . . . . . . . . . . 10-1
containers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-1
A
CCESSORIES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-2
general . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-2
networked application . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-2
Section 11, Warranty . . . . . . . . . . . . . . . . . . . . . . . . .11-1
Section 12, Default Drug Library (DDL) . . . . . . . . . . . 12-1
430-95520-001 (Rev. 3/05)

Plum A+ Infusion System
SECTION 1
Descriptive Information
The Plum A+ Volumetric Infusion System is designed to meet
the fluid delivery requirements of today’s evolving healthcare
environments. The infuser is a cassette based multi-function
infusion system. The Plum A+ allows two lines in and one line
out. The infuser can be used for standard, piggyback, or
concurrent delivery. Programming modes include:
• Standard Infusions
• Multistep Programming
• Loading Dose
• Dose Calculation
The Plum A+ is designed to deliver parenteral, enteral, or
epidural infusions over a broad range of infusion rates from
multiple fluid container types.
The Plum A+ is designed to be used in most areas of patient
care, including, but not limited to:
• General Floor • Labor/Delivery/
Post Partum
• Medical/Surgical • OR/Anesthesia • Hemodialysis
• ICU/CCU • Post Op/Recovery • Oncology
• Pediatrics • Cardiac Cath Lab • Mobile Intensive
• Neonatology • Emergency • Nutritional
• Burn Unit
Care
Product Description
The system includes a pumping module (hereafter called the
infuser) and an assortment of disposable IV sets (hereafter
called a set), optional accessories, and this operator’s manual.
The Plum A+ host device contains a Connectivity Engine
peripheral module that provides wired Ethernet and wireless
430-95520-001 (Rev. 04/05) 1- 1
802.11b local area networking capabilities. This allows the
TM
Hospira Mednet
library downloads and Auto-programming to the infuser.
networked application software for drug
Indications for Use
USER QUALIFICATION
The Plum A+ is intended for use at the direction or under the
supervision of licensed physicians or certified healthcare
professionals who are trained in the use of the infuser and the
administration of parenteral, enteral, and epidural fluids and
drugs and whole blood or red blood cell components. The
training should emphasize preventing related IV complications,
including appropriate precautions to prevent accidental infusion
of air. The epidural route can be used to provide anesthesia or
analgesia.
WARNING
ADMINISTER ONLY ANESTHETICS/ANALGESICS
APPROVED FOR EPIDURAL ADMINISTRATION (AS
INDICATED OR ALLOWED BY THE DRUGS’ FDA
APPROVED LABELING). EPIDURAL ADMINISTRATION OF
DRUGS OTHER THAN THOSE INDICATED FOR EPIDURAL
USE COULD RESULT IN SERIOUS INJURY TO THE PATIENT.
Conventions
This section describes the conventions used throughout this
manual, as follows:
CONVENTION APPLICATION EXAMPLE
Italic Reference to a
section, figure, or
table
Function or mode
specific instructions
1- 2 430-95520-001 (Rev. 04/05)
(See Figure 3-1,
Priming Cassette)
Primary Only:
Attach an empty
container.

Plum A+ Infusion System
CONVENTION APPLICATION EXAMPLE
[BRACKETED
ALL CAPS]
Keys or buttons on
the device are
displayed in
[START]
or
[BRACKETED ALL
CAPS] or with a
graphic.
V
[Italic] Softkey Options
Initial Caps
lowercase
Screen displays and
device labels (as
appropriate)
V
[Choose]
Program
Dose Calculation
Bold Emphasis ...sets are supplied
Sterile and are
for....
WARNINGS, CAUTIONS, AND NOTES
Alert messages used throughout this manual are described
below. Pay particular attention to these messages.
WARNING
A WARNING MESSAGE CONTAINS SPECIAL SAFETY
EMPHASIS AND MUST BE OBSERVED AT ALL TIMES.
FAILURE TO OBSERVE A WARNING MESSAGE IS
POTENTIALLY LIFE THREATENING.
CAUTION: A CAUTION
PROCEDURE
COULD
FAILURE
SERIOUS
OR STATEMENT. IT CONTAINS INFORMATION THAT
PREVENT IRREVERSIBLE PRODUCT DAMAGE OR HARDWARE
. FAILURE TO OBSERVE A CAUTION COULD RESULT IN
PATIENT OR USER INJURY.
USUALLY APPEARS IN FRONT OF A
430-95520-001 (Rev. 04/05) 1- 3

NOTE: A Note highlights information that helps explain a
concept or procedure.
This symbol directs the user to consult accompanying
documents.
When visible on the display, this symbol informs the
user to use CAUTION because the specified drug has
NOT been programmed with specified safety limits.
NOTE: Figures are rendered as graphic representations to
approximate the actual product. Therefore, figures may not
exactly reflect the product.
Precautions
The Plum A+ has been designed and manufactured to be safe,
reliable, and easy to use. This section details precautions and
possible hazards.
For safe operation of the Plum A+, observe the following
precautions and hazards.
HEALTHCARE PROFESSIONALS AND PATIENT RELATED
• In vitro studies have suggested that packed red blood cells
with unusually high hematocrit be diluted with bloodcompatible fluids, such as 0.9% sodium chloride injection, to
decrease hemolysis and increase flow rate.
• Setting the primary rate greater than the secondary rate will
result in a more rapid infusion of any residual secondary drug
remaining in the line and the cassette.
• Consult drug labeling to confirm drug compatibility,
concentration, delivery rates, and volumes are all suitable for
secondary, concurrent and piggyback delivery modes.
• Arrange tubing, cords, and cables to minimize the risk of
patient strangulation or entanglement.
• Before opening the door, close clamp on the primary line or
remove the secondary container from the secondary port to
prevent mixing of primary and secondary fluids.
1- 4 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
• Although unlikely, failure of certain robust mechanical
components such as the anti-free flow mechanism or valve
control springs could cause fluid delivery limited to the
contents of the fluid container. Single fault failure of certain
electronic/motor control components would result in no more
than 5 mL of unexpected fluid delivery.
• A small amount of fluid is expelled from the set (less than
0.05 ml) each time the door is opened or closed with a set
installed. If potent drugs are being used, take appropriate
action to guard against overmedication of the patient.
• Before disconnecting a syringe from the cassette, pull up the
plunger slightly to avoid spilling the fluid. For rigid containers,
close the upper slide clamp, open the cassette door, then
remove and invert the cassette (ports down).
• Air bubbles may form distal to the cassette as result of
normal outgassing of dissolved air in the fluid. This may
occur if chilled solution is in use, if the infuser is mounted
significantly above the patient, or when using certain fluids
known to routinely outgas. In these cases, an air eliminating
filter may be used.
• Repeated opening and closing of the door may defeat the
proximal air-in-line alarm and may cause a distal air-in-line
alarm, requiring repriming.
• The screen displays the VTBI (volume to be infused) in
integers when value is above 99.9. Any fraction of a milliliter
delivered is not displayed, but is retained in memory.
CONCURRENT FLOW
GUIDELINES
When delivering short half-life critical drugs (see Critical Drugs,
this section) using the Plum A+ in the Concurrent mode, the
following delivery rate guidelines should be observed:
• If the critical drug (with half-life less than 6 minutes) is to be
infused at less than 2.0 mL/hr, the other infusion should be no
faster than 5 times the critical drug’s rate. Dopamine, for
example, delivered at 1.5 mL/hr should not be accompanied
by an infusion programmed any faster than 7.5 mL/hr.
430-95520-001 (Rev. 04/05) 1- 5
• If the critical drug (with half-life less than 6 minutes) is to be
infused at 2.0 to 5.0 mL/hr the other infusion should be no
faster than ten times the critical drug’s rate. Dopamine, for
example, delivered at 3.5 ml/hr should not be accompanied
by an infusion programmed any faster than 35 mL/hr.
• If the critical drug (with half-life less than 6 minutes) is to be
infused at 5.1 mL/hr or greater, the other infusion can be
programmed at any desired rate.
NOTE: The total of the primary rate plus the secondary rate
cannot exceed 500 mL/hr.
These guidelines apply only when infusing short half-life critical
drugs in Concurrent mode. Individual patient responses may
vary requiring adjustment of delivery rates.
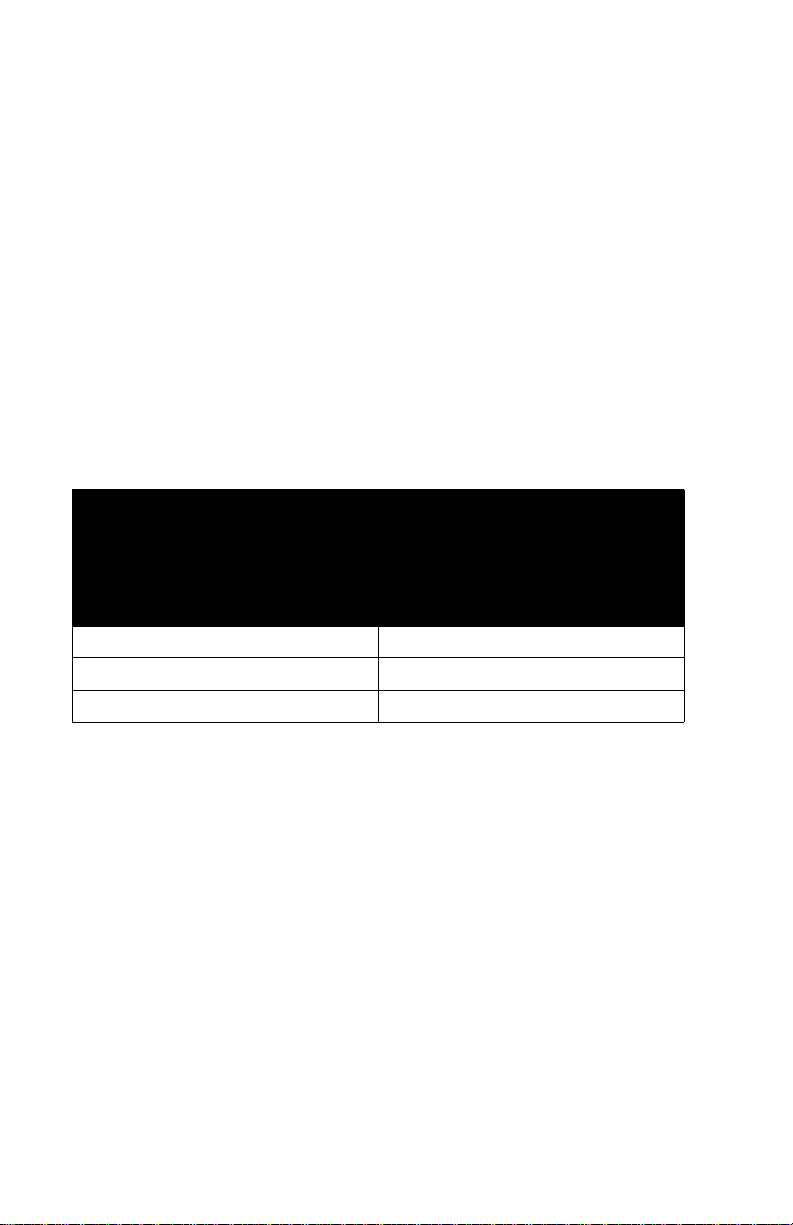
DELIVERY RATE GUIDELINES
SHORT HALF-LIFE (LESS THAN
6 MINUTES) CRITICAL DRUG
INFUSION RATE
0.5 - 1.9 mL/hr 5 Times the Critical Drug Rate
2.0 - 5.0 mL/hr 10 Times the Critical Drug Rate
5.1 or Greater Any Desired Ratio
MAXIMUM RATE OF
ACCOMPANYING INFUSION
1- 6 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
CRITICAL DRUGS
Examples of drugs with a short half-life (approximately 6
minutes or less when given IV) include:
Dobutamine Lidocaine
Dopamine Nitroglycerin
Epinephrine Nitroprusside
Epoprostenol Norepinephrine
Esmolol Oxytocin
Isoproterenol Procainamide
For these drugs, the concurrent flow guidelines should be
followed when the infusion rate of the drug will be 5 mL/hr or
less.
NOTE: This list of critical drugs is not intended to be allinclusive of critical drugs or drugs with a short half-life.
The clinician should become familiar with the
pharmacodynamics of any critical drug before administration.
This information is presented to inform clinicians of a rare
situation that could be misinterpreted if they are unfamiliar with
this phenomenon.
EPIDURAL ADMINISTRATION
• Recommended use of the epidural route is to provide
anesthesia or analgesia for periods up to 96 hours.
• This device can be used to administer only those anesthetics/
analgesics approved for epidural administration (as indicated
or allowed by the drugs’ FDA approved labeling). Epidural
administration of drugs other than those indicated for epidural
use could result in serious injury to the patient.
• For epidural administration, the use of Hospira catheters,
Plum sets without Y-sites, and "epidural" stickers indicating
ongoing epidural administration are recommended.
430-95520-001 (Rev. 04/05) 1- 7

• Administration of drugs via the epidural route should be
limited to personnel familiar with associated techniques and
patient management problems. Proper epidural placement of
the catheter is essential since catheter migration could result
in intravascular or intrathecal administration. Facilities
practicing epidural administration must be equipped with
resuscitative equipment, oxygen, naloxone, and other
resuscitative drugs. Adequate monitoring equipment (e.g.,
Oximetry) is recommended for continuous monitoring of the
patient during epidural administration. Patients must be
observed frequently for side effects in a fully-equipped and
staffed environment for at least 24 hours following completion
of drug administration by the epidural route. DELAYED
RESPIRATORY DEPRESSION FOLLOWING
CONTINUOUS EPIDURAL ADMINISTRATION OF
PRESERVATIVE-FREE MORPHINE SULFATE HAS BEEN
REPORTED.
• The epidural space has 58 openings through which fluid can
exit. Pressure buildup during administration is transient.
However, if a large volume of fluid is administered over a
short time period, the pressure will take longer to return to
normal. If overdelivery occurs during administration, observe
the patient closely for signs of spinal cord compression
(disorientation, headache, transient neuralgias) and drug
overdose.
BATTERY OPERATION
• When the battery is removed from the Plum A+, do not
operate on patients. Use of a properly maintained and
charged battery helps confirm proper operation.
• The battery may not be fully charged upon receipt. Connect
the infuser to AC power for at least six hours.
• Use AC power whenever possible. Connect to AC power
during storage to ensure a fully charged battery for
emergencies. If the quality of the earth grounding source is in
doubt, use battery power.
• If the low-battery alarm sounds, connect the infuser to AC
power immediately.
1- 8 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
SETS AND ACCESSORIES
• Only compatible LifeCare PlumSets can be used with the
Plum A+. See individual set instructions for additional
information.
• Administration sets should be changed per CDC guidelines
or healthcare provider policy. Discard after use.
• LifeCare IV infusion sets with integral nonblood filters are not
for use in the administration of blood, blood products,
emulsions, suspensions, or any medications not totally
soluble in the solution being administered. These
medications may be administered through the lower Yinjection site, below the filter.
• When infusing at low delivery rates (5.0 mL/hr or less) the
use of thick-walled microbore PlumSets is recommended.
This will reduce the amount of the fluid bolus that may be
delivered when a distal line occlusion is released.
• Syringes must be larger than 3 cc. Use syringe adapter (List
11986-48) when using syringes smaller than 10cc. Some
10cc syringes may require use of a syringe adapter. Syringes
larger than 10cc may be attached directly to the secondary
port of the cassette. Use of a syringe adapter may decrease
the occurrence of proximal occlusion alarms.
• Use a 19-gauge or larger needle or catheter at the
venipuncture site for viscous fluids if operating at rates
greater than 500 ml/hr.
See Section 10 for information on sets and accessories.
BACKPRIMING
• Backpriming is not recommended for reconstituting
secondary containers containing dry powders.
• To avoid pressurization when backpriming into a syringe, the
user must confirm there is sufficient empty space to accept
the backprimed fluid.
GENERAL
• Possible explosion hazard exists if used in the presence of
flammable anesthetics.
430-95520-001 (Rev. 04/05) 1- 9

• Do not place Plum A+ in service if it fails the self-test.
• Do not operate the Plum A+ with the case opened.
• Keep the cassette door securely closed while the infuser is
not in use, to avoid cassette door damage.
• Values beyond a fields maximum hard limit will be diplayed
as dashes (-- -- --). The user must clear these fields using the
[CLEAR] key prior to entering new values.
CLEANING
For more information on cleaning the infuser, see Section 8.
• To avoid mechanical or electronic damage, do not immerse
the Plum A+ in any fluids or cleaning solutions.
• Do not spray cleaning solutions toward any opening in the
instrument.
• Certain cleaning and sanitizing solutions may slowly degrade
components made from some plastic materials. Using
abrasive cleaners or cleaning solutions not recommended by
Hospira may result in product damage. Do not use
compounds containing combinations of isopropyl alcohol and
dimethyl benzyl ammonium chloride.
• Never use sharp objects such as fingernails, paper clips, or
needles to clean any part of the infuser.
• Do not sterilize by heat, steam, ethylene oxide (ETO), or
radiation.
• To avoid infuser damage, cleaning solutions should only be
used as directed. The disinfecting properties of cleaning
solutions vary; consult the manufacturer for specific
information.
BOLUS RELATED
Use the following procedure to avoid the administration of a
bolus following a distal occlusion (i.e., a closed distal clamp):
• If a secondary container is in use, clamp proximal tubing
before opening cassette door.
• Open cassette door and remove the cassette.
1- 10 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
• Open the flow regulator briefly to dissipate the pressure
and then close it.
• Eliminate the source of occlusion (closed clamp).
• Reinsert the cassette and close the cassette door.
• Open all clamps and resume infusion.
NOTE: When troubleshooting an occlusion where all clamps
are in the OPEN position, use care to avoid delivery of a bolus
by opening the flow regulator to release any built-up pressure.
Close the clamp between the cassette and the patient before
opening the flow regulator to relieve the pressure. See Section
7, Alarms and Troubleshooting, for more information.
ARTIFACTS
• Nonhazardous, low-level electrical potentials are commonly
observed when fluids are administered using infusion
devices. These potentials are well within accepted safety
standards, but may create artifacts on voltage-sensing
equipment such as ECG, EMG, and EEG machines. These
artifacts vary at a rate that is associated with the infusion
rate. If the monitoring machine is not operating correctly or
has loose or defective connections to its sensing electrodes,
these artifacts may be accentuated so as to simulate actual
physiological signals. To determine if the abnormality in the
monitoring equipment is caused by the infusion device
instead of some other source in the environment, set the
infusion device so that it is temporarily not delivering fluid.
Disappearance of the abnormality indicates that it was
probably caused by the electronic noise generated by the
infusion device. Proper setup and maintenance of the
monitoring equipment should eliminate the artifact. Refer to
the appropriate monitoring equipment system documentation
for setup and maintenance instructions.
• The Plum A+ system is designed to operate normally in the
presence of most encountered electromagnetic interference
(EMI) conditions. In the event of extreme levels of
interference, such as encountered next to an electrosurgical
generator, it is possible that the normal operation of a sensor
430-95520-001 (Rev. 04/05) 1- 11

or microcomputer might be disrupted. Even in this event, the
outcome would likely be a false alarm or detected system
malfunction and would not result in a hazard to patient or
operator.
• This equipment has been tested and found to comply with the
EMC limits for the Medical Device Directive 93/42/EEC (EN
55011 Class B and IEC/EN 60601-1-2:2001). These limits
are designed to provide reasonable protection against
harmful interference in a typical medical installation. The
equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with the
instructions, may cause harmful interference to other devices
in the vicinity. However, there is no guarantee that
interference will not occur in a particular installation. If this
equipment does cause harmful interference with other
devices (radio or television reception), which can be
determined by turning the equipment off and on, the user is
encouraged to try to correct the interference by one or more
of the following measures:
• Reorient or relocate the receiving device
• Increase the separation between the equipment
• Connect the equipment into an outlet on a circuit
different from that to which the other device(s) is
connected
• Consult the manufacturer or field service technician for
help
• Portable and mobile RF communications equipment, such as
cellular telephones, 2-way radios, Bluetooth devices,
microwave ovens, in close proximity to this device may affect
wireless and wired communications with the Infusion pump
and/or the operation of the Infusion pump. Special
precautions need to be exercised regarding EMC, These
include:
• Use of a shielded Ethernet cable (CAT5 STP or better)
for plugging into the RJ45 Ethernet connector. Using
an unshielded Ethernet cable may result in increased
emissions.
1- 12 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
• Maintaining a minimum separation distance of 2 ½ ft
between the Infusion pump system and portable/
mobile RF communications equipment
• List Numbers 12391 and 11971 are compliant to IEC/EN
60601-1-2 (1993)
List Number 20679 is compliant to IEC/EN 60601-1-2 (2001)
and have been tested and found to comply with EMC limits
for the Medical Device Directive 93/42/EEC (EN 55011 Class
B and EN 60601-1-2:2001).
Guidance on EMC Compatibility
• There is a shared responsibility between manufacturers,
customers and users to ensure that Medical Equipment and
Systems are designed and operated as intended. Medical
electrical equipment needs special precautions regarding
electromagnetic compatibility and needs to be installed and
used according to the electromagnetic compatibility
information provided in this manual.
• The device is suitable for use in all establishments, including
domestic establishments. If extended operation during
power mains interruption is needed, use battery power.
• Always manage the electromagnetic environment.
• The guidance included in this manual provides information
needed to:
• Determine the device’s suitability for use in the
intended environment.
• Manage the electromagnetic environment to permit the
device to perform as intended without disturbing other
equipment.
• Separate the device from all other electronic equipment. If
the device must be used near other electrical equipment,
monitor the equipment to ensure there is no electromagnetic
interference.
• Devices should not be used adjacent to or stacked with other
equipment. If the device must be used adjacent to or stacked
430-95520-001 (Rev. 04/05) 1- 13

with other equipment, monitor the devices to verify normal
operation.
• USE ONLY components specifically labeled for use with the
Plum A+ Infusion System to help ensure the device operates
as intended.
• If you suspect external RF sources or other equipment are
influencing device operation, contact the biomedical
engineering department for additional guidelines concerning
electromagnetic immunity.
• Contact the biomedical engineering department for additional
information in the technical service manual concerning
operating devices near RF sources.
FCC Information
U.S. Pat. No. 5,681,285 FCC ID: STJ-20677
IC: 5627A-20677
US FCC (FEDERAL COMMUNICATIONS COMMISSION)
STATEMENT
• This device complies with part 15 of the FCC Rules.
Operation is subject to the following two conditions: (1) This
device may not cause interference, and (2) This device must
accept any interference, including that may cause undesired
operation of this device.
FCC INTERFERENCE STATEMENT
• This equipment has been tested and found to comply with the
limits for a Class B digital device, pursuant to Part 15 of the
FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential
installation. This equipment generates, uses, and can radiate
radio frequency energy. If not installed and used in
accordance with the instructions, it may cause harmful
interference to radio communications. However, there is no
guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference
1- 14 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to
try and correct the interference by one or more of the
following measures:
• Reorient or relocate the receiving antenna.
• Increase the distance between the equipment and the
receiver.
• Connect the equipment to an outlet on a circuit
different from that to which thereceiver is connected.
• Consult the dealer or an experienced radio/TV
technician for help.
• Changes or modifications not expressly approved by Hospira
could void the user's authority to operate the equipment.
RADIO FREQUENCY EXPOSURE STATEMENT
• The Wireless LAN radio device in the Connectivity Engine
peripheral board with this infusion device has been evaluated
and found compliant to the requirements of the following
Radio Frequency exposure standards:
• Federal Communications Commission, OET Bulletin
65 (Edition 97-01), Supplement C (Edition 01-01),
Evaluating Compliance with FCC Guidelines for
Human Exposure to Radio frequency Electromagnetic
Fields, July 2001.
• Industry Canada, Evaluation Procedure for Mobile and
Portable Radio Transmitters with respect to Health
Canada's Safety Code 6 for Exposure of Humans to
Radio Frequency Fields, Radio Standards
Specification RSS-102 Issue 1 (Provisional):
September 1999.
• Federal Communications Commission, OET Bulletin
65 (Edition 97-01), Supplement C (Edition 01-01),
Evaluating Compliance with FCC Guidelines for
Human Exposure to Radio frequency Electromagnetic
Fields, July 2001.
• The radiated output power of this Wireless LAN device is far
below the FCC radio frequency exposure limits. The Wireless
430-95520-001 (Rev. 04/05) 1- 15

LAN device has been evaluated with zero inches separation
of human body from the antenna and found to be compliant
with FCC RF exposure limits.
1- 16 430-95520-001 (Rev. 04/05)

Plum A+ Infusion System
SECTION 2
Principles of Operation
Features
The Plum A+ is a dual-line volumetric infusion system designed
to meet the growing demand for hospital-wide, as well as
alternate site and home healthcare, standardization. With its
primary line, secondary line, and piggyback fluid delivery
capability, the Plum A+ is suited for a wide range of medical/
surgical and critical care applications. Full compatibility with
LifeCare Plum Series administration sets and accessories and
the LifeShield® and CLAVE® needleless protection systems,
makes the Plum A+ a convenient and cost-effective infusion
system.
PROGRAMS
• Standard Programming
• Loading Dose
• Multistep Programming
LINE PROGRAMMING OPTIONS
• Drug List
• Nurse Call Back
• Delayed Start/Standby Setting
• Concurrent delivery
•Titration
• Micro 0.1-99.9 mL/hr (in 0.1 mL increments) flow rate range
for both lines
• Macro 100-999 mL/hr (in 1 mL increments) flow rate range for
both lines
• Automated Secondary drug delivery (Piggyback)
430-95520-001 (Rev. 04/05) 2- 1

P
LUMSET CAPABILITIES
• Anti Free-Flow Protection
• Direct Connection for syringe delivery
AIR MANAGEMENT
•Air Trap
• Air Removal/Backpriming
• Air Detection-Proximal
• Air Detection-Distal
BATTERY
• Battery Gauge
• Less than 8-Hour Battery Recharge Time
• Long battery life (3 hours) for emergency backup and
temporary portable operation
BIOMEDICAL
• Upgradability (Field)
• Variable Rate Cap
• Alarm History
• Nurse Call Relay Connector
OPTIONS
• Volumes Infused (A, B, Total Volume)
• KVO at dose end (1 ml/hr or less depending on delivery rate)
or Continue Rate (CR) to continue at the current rate
• Variable Distal Pressure Settings
OTHER FEATURES
• Nonpulsatile volumetric accuracy
• Microprocessor control
• Large liquid crystal display (LCD) screen
2- 2 430-95520-001 (Rev. 04/05)
Plum A+ Infusion System
• Panel back illumination on mains power
• Lockout switch
• Standard syringe use
• Parenteral and nonparenteral (enteral) fluid delivery
• Blood and blood products delivery
• Wide range of Standard and Specialty administration sets
• Password protected keypad lock
COMMUNICATION
• Networked Communication (Ethernet & Wireless)
• Autoprogramming
• Drug Library Download
Definitions
TERM DEFINITION
Administration
Set
Alarm A condition requiring operator
Alert While entering a program, a visual
Alternate Units Dose Rate units that may be selected.
Alternate Units
Parameters
The sterile, disposable assembly with
flexible tubing that connects to a source
fluid container for input to the Cassette
pumping chamber and to some output
device for administration to the patient.
intervention that invokes both audible
and visible alarm indicators. Confirmation
of the condition usually is required.
warning that informs the clinician of the
violation of a hospital-defined rule set,
e.g., a dose limit for a specific drug.
Alternate Units are any units other than
mL/hr.
Drug Amount, Diluent, Patient Weight (if
applicable), and Dose Rate.
430-95520-001 (Rev. 04/05) 2- 3
TERM DEFINITION
Auto-Program A physician order sent to the infuser.
Auto-Program
(new)
Auto-Program
(titration)
Awake CE The CE is considered awake when the
Backpressure Resistance to fluid flow on the Distal or
Backprime Removal of air or fluid from the proximal
Basic Delivery A single-step delivery specifying rate,
Biomed Mode The name for the non-delivery or service
An auto-program is considered “new” if
either of the following conditions are met:
A) There is no program on the associated
line. B) The drug and concentration in the
auto-program is different from a
currently-programmed drug and
concentration.
An auto-program is considered a
“titration” if the drug and concentration in
the auto-program match the currentlyprogrammed drug and concentration.
Non-promotion titration programs include
some subset of the following: Rate, Dose,
Duration. Promotion titration programs
include some subset of the following:
Rate, Dose, Duration, Patient Weight,
Dosing Units, Drug Amount, Drug Units,
Diluent Amount.
infuser is powered on with battery power
and the battery is above the low battery
threshold or is plugged into AC.
output portion of the Administration Set,
usually expressed as PSIG.
tubing and Cassette air trap by rapid
pumping of fluid from Line A to an output
receptacle on Line B. No fluid delivered
distal to the cassette during a Backprime.
VTBI, and duration only. Drug name may
or may not be specified.
mode of Infuser operation.
2- 4 430-95520-001 (Rev. 04/05)
Plum A+ Infusion System
TERM DEFINITION
Bolus A single uninterrupted discrete volume of
fluid delivered over a discrete period of
time.
Cassette A Hospira standard Plum Macro Cassette
that contains the actual pumping
chamber with inlet and outlet valves. It
also contains access ports to diaphragms
for proximal and distal line pressure
sensing and chambers for air trapping
and air quantity measurement.
Caution Contains information which could
prevent hardware failure, irreversible
damage to equipment, or loss of data.
Channel Distal Line output to the patient.
Cleared Program Same as Cleared Settings, but restricted
to the program delivery settings for an
individual line.
Cleared Settings Programmed delivery settings for both
lines and the general control parameters
are at their default values.
Clinical Care
Area (CCA)
Concentration For Dose Calculation based Line A or B
An area of the hospital for which
authorized hospital staff has allowed use
of up to 149 specific drugs, plus No Drug
Selected. The clinician selects a CCA
after turning on the Infuser. The hospital
may create from one to eighteen CCA’s.
delivery programming of premixed drugs,
the concentration consists of Drug
Amount (in micrograms [mcg], milligrams
[mg], grams, milliequivalents [mEq],
millimols [mmols], or units [USP Units])
and Diluent (Volume in milliliters [mL]).
Abbreviated on screen displays as
“Conc”.
430-95520-001 (Rev. 04/05) 2- 5
TERM DEFINITION
Concurrent
Delivery
Concurrent Mode The Line B control selection mode that
Connectivity
Engine (CE)
Continue Rate
(CR)
Default Drug
Library (DDL)
Deliver Together A Biomed Delivery option setting, which
Delivery Mode The normal operational mode for the
Delivery Rate The delivery rate (as Volume/Time)
Device The Plum A+ IV Infusion Device (Infuser),
Diluent (Volume) The volume of fluid used with the Diluent
Simultaneous delivery of two fluids at
independent flow rates.
allows either Line A or Line B (or both) of
the Mechanism to be programmed for
delivery that completes with KVO or CR.
Line A operation is unaffected by Line B
delivery.
A component of the infuser that controls
ethernet and wireless communication
between the MMU and Infuser processor.
An option used to provide fluid delivery at
the current fluid delivery rate when the
VTBI becomes zero.
A drug list of 99 drug names, default units
and default concentrations, plus ‘No Drug
Selected’. Does not contain Soft or Hard
Limits. The DDL is a subset of Plum A+
Version 11.3 drug list. The Infuser
functions initially with the DDL.
selects default of Piggyback or
Concurrent delivery mode.
Infuser, either Concurrent or Piggyback,
during which fluid delivery may occur.
assigned by the operator or calculated
from other delivery parameters.
not including the disposable
administration sets.
Unit (e.g., 250 mL), which dilutes the drug
to be infused.
2- 6 430-95520-001 (Rev. 04/05)
Plum A+ Infusion System
TERM DEFINITION
Distal Downstream (output) from the Cassette
portion of the Administration Set.
Door The lever-operated, bottom-hinged,
Cassette holder on the front of the
Mechanism.
Dose (Rate) Delivery rate times drug concentration.
Dose Calculation Allows programming Dose Rates in
alternative units of measure. Dose
Calculation can be used in Simple
Delivery, Loading Dose, and Multistep.
Drug Amount The mass or quantity of the drug
premixed with a Diluent to express the
Concentration for the Line being
programmed.
Drug Library Drug names available for use with the
Infuser in the selected CCA, and each
drug’s concentration, units of measure,
and dose limits, as determined by
authorized hospital staff. The Drug
Library is based on hospital-defined best
practices. The Drug Library is customized
and downloaded into the Infuser by
authorized hospital personnel using
Hospira MedNet Networked software.
Duration The time period used to deliver a VTBI at
an existing Delivery Rate or as set by the
operator.
Enteral Delivery via an intestinal route.
Error A condition resulting in an audible
warning and often an accompanying
message, such as an invalid key press,
resulting from an operator attempt to
make an input that is not valid for the
current operational context. Not a
Malfunction.
430-95520-001 (Rev. 04/05) 2- 7
TERM DEFINITION
Filling Head
Height (FHH)
Flow Rate The resulting rate of fluid flow. (See Rate
Fluid Delivery
Begin
Hard Limit The dosing amount (upper and/or lower
Infuser A Plum A+ device, not including the
Initialized
Position
Key Any of the marked locations on the front
KVO (Keep Vein Open) Internally selected
Line A Inlet tubing on the top center of Cassette
The gravity induced Proximal Line
pressure due to fluid height in source
container above the Distal Line output
level.
and Dose.)
The time required, measured from the
initial start of a keypad press, for a digit in
the third decimal place of a scale
measuring grams to change when
pumping water, the delivery tube is fluid
filled, and the end of the delivery tube is
immersed.
amount) that when assigned to a drug
cannot be overridden. Defined by the
hospital for each drug in its Drug Library.
The hard limits for a particular drug may
vary across different CCAs.
disposable administration set.
The plunger is in the Plunger Park
Position, and each valve is in the Valve
Home Position.
panels intended for user input via a
pressing action.
minimal delivery rate, the lesser of 1.0
mL/Hr or actual Rate, intended to provide
sufficient fluid flow to decrease the
potential for clotting at the IV infusion
site.
when viewed after it has been loaded in
Mechanism Door.
2- 8 430-95520-001 (Rev. 04/05)
 Loading...
Loading...