Huntleigh Healthcare Sonicaid FM800, Sonicaid FM820, Sonicaid FM830 User Manual
SonicaidFM800
Reference Manual
© Huntleigh Healthcare Ltd 2005
All rights reserved
329000-5
September 2007

Sonicaid FM800 Series Reference Manual
The Sonicaid™ FM800 is in conformity with the Medical Device
Directive (93/42/EEC) and has been subject to the conformity
assurance procedures laid down in the Council Directive.
2

Sonicaid FM800 Series Reference Manual
Contents
Contents .............................................................................................................................. 3
Standards compliance......................................................................................................... 7
Indications for use ............................................................................................................... 8
System Installation .............................................................................................................. 9
Calibration ........................................................................................................................... 9
Multiple Portable Socket Outlets ....................................................................................... 10
Electromagnetic compatibility............................................................................................ 11
Trademarks ....................................................................................................................... 12
Sensors ............................................................................................................................. 12
Addresses ......................................................................................................................... 13
1 Introduction..................................................................................................................... 14
1.1 The FM800 series ............................................................................................. 14
1.2 Main unit: front panel ........................................................................................ 16
1.3 Connection sockets for transducers ................................................................. 17
1.4 Main unit: rear connectors ................................................................................ 18
1.5 Connector module ............................................................................................ 19
1.6 Event marker connection .................................................................................. 20
1.7 The FM800 display ........................................................................................... 20
1.8 Transducers and cables ................................................................................... 21
1.9 FM800 trolley or wall mounting......................................................................... 22
2 FM800 Controls and Display...................................................................................... 23
2.1 Controls and on/off indicator............................................................................. 23
2.2 FM800 main screen .......................................................................................... 24
2.3 Software control buttons ................................................................................... 25
2.4 Audio controls ................................................................................................... 26
2.5 Printer controls.................................................................................................. 27
2.6 Loading printer paper........................................................................................ 28
3 Setup.......................................................................................................................... 29
3.1 Overview ........................................................................................................... 29
3.2 Current alarms .................................................................................................. 29
3.3 Default alarms................................................................................................... 30
3.4 System settings................................................................................................. 30
3.5 Analysis............................................................................................................. 30
3.6 Time and date ................................................................................................... 30
3.7 Patient details ................................................................................................... 31
3.8 Timer................................................................................................................. 32
3.9 Actogram settings ............................................................................................. 33
3

Sonicaid FM800 Series Reference Manual
4 System Settings and Default Alarms ......................................................................... 34
4.1 Overview ........................................................................................................... 34
4.2 Changing the default alarms............................................................................. 35
4.3 Printer setup...................................................................................................... 35
4.4 Audio/graphic settings ...................................................................................... 37
4.5 International settings......................................................................................... 38
4.6 Serial interface .................................................................................................. 39
5 Monitoring Fetal Parameters...................................................................................... 40
5.1 Preliminary ........................................................................................................ 40
5.2 Audio signal ...................................................................................................... 40
5.3 Ultrasound monitoring....................................................................................... 41
5.4 The FHR confidence indicator .......................................................................... 43
5.5 False recording of low baseline FHR................................................................ 43
5.6 Ultrasound for twins .......................................................................................... 44
5.7 Fetal ECG (using a scalp electrode)................................................................. 45
5.8 Accidentally recording the wrong signal ........................................................... 50
6 Monitoring Maternal Parameters ............................................................................... 51
6.1 Contractions (using Toco transducer) .............................................................. 51
6.2 Contractions (using IUP transducer) ................................................................ 52
6.3 Maternal ECG ................................................................................................... 52
6.4 Maternal Blood Pressure .................................................................................. 56
6.5 Maternal Oximetry ............................................................................................ 58
6.6 Maternal temperature ....................................................................................... 61
7 Events and Alarms ..................................................................................................... 62
7.1 What is meant by an alarm? ............................................................................. 62
7.2 What do you see and hear?.............................................................................. 62
7.3 Responding to alarms ....................................................................................... 64
7.4 Controlling alarms ............................................................................................. 65
7.5 Recording fetal movements .............................................................................. 65
7.6 Actogram........................................................................................................... 66
7.7 Recording clinical events (EasiNotes) .............................................................. 68
8 Setting Alarm Thresholds........................................................................................... 69
8.1 Default alarm thresholds................................................................................... 69
8.2 FHR thresholds (Ultrasound and FECG) .......................................................... 70
8.3 Maternal blood pressure thresholds ................................................................. 73
8.4 MECG thresholds ............................................................................................. 73
8.5 Maternal oximetry threshold ............................................................................. 74
8.6 Temperature thresholds.................................................................................... 74
9 SonicaidTrend Intrapartum Analysis.......................................................................... 75
9.1 Introduction ....................................................................................................... 75
9.2 SonicaidTrend analysis..................................................................................... 76
9.3 Using SonicaidTrend analysis .......................................................................... 77
9.4 SonicaidTrend analysis results......................................................................... 78
9.5 Viewing trend data ............................................................................................ 78
4

Sonicaid FM800 Series Reference Manual
10 SonicaidCare Antepartum Analysis ........................................................................... 79
10.1 Intended use ..................................................................................................... 79
10.2 Overview........................................................................................................... 79
10.3 The Dawes/Redman criteria ............................................................................. 80
10.4 Care analysis .................................................................................................... 80
10.5 Using SonicaidCare analysis ............................................................................ 81
10.6 SonicaidCare analysis report............................................................................ 82
11 Using FM800 with a PC System ................................................................................ 85
11.1 Using FM800 with FetalCare or System8002................................................... 85
11.2 Using FM800 with Sonicaid Axis ...................................................................... 86
11.3 Using FM800 with Sonicaid Centrale, Philips TraceVue™ or GMT Argus ...... 86
12 Telemetry ................................................................................................................... 88
12.1 Connecting the telemetry unit ........................................................................... 88
12.2 Using the telemetry unit .................................................................................... 88
13 Troubleshooting ......................................................................................................... 89
13.1 FHR................................................................................................................... 89
13.2 Oximetry............................................................................................................ 89
13.3 Fetal event marker............................................................................................ 89
13.4 Maternal blood pressure error codes................................................................ 90
13.5 Printing.............................................................................................................. 90
13.6 What to do next?............................................................................................... 91
14 Cleaning and Maintenance ........................................................................................ 92
14.1 The FM800 main unit........................................................................................ 92
14.2 Transducers: NBP cuff, maternal oximetry sensor, temperature probe ........... 92
14.3 Transducers and leads: Ultrasound, FECG, MECG, internal Toco and external
Toco 93
14.4 User maintenance............................................................................................. 94
14.5 Technical maintenance..................................................................................... 95
14.6 Corrective maintenance.................................................................................... 96
14.7 Servicing ........................................................................................................... 96
14.8 Accessories, consumables and spares ............................................................ 97
15 Specifications ............................................................................................................. 99
15.1 Physical and environmental.............................................................................. 99
15.2 AC supply voltage and fuse values .................................................................. 99
15.3 Transducers.................................................................................................... 100
15.4 Controls........................................................................................................... 104
15.5 Printer ............................................................................................................. 104
15.6 Connections.................................................................................................... 105
15.7 Display ............................................................................................................ 106
15.8 Safety.............................................................................................................. 107
15.9 Ultrasound safety considerations ................................................................... 108
16 Appendix 1: Service and Warranty .......................................................................... 110
17 Appendix 2: External Connections........................................................................... 111
5

Sonicaid FM800 Series Reference Manual
18 Appendix 3: Transducer Problems .......................................................................... 113
19 Appendix 4: Getting Started with T800 .................................................................... 115
6
Sonicaid FM800 Series Reference Manual
Standards compliance
SonicaidFM800 complies with:
EN60601-1, 1990 Medical Electrical Equipment Part 1
General Requirements for Safety
EN60601-1-1, 1993 Safety Requirements for Medical
[collateral standard] Electrical Systems
Patient safety
WARNING: DO NOT TOUCH LIVE PARTS OF ANY EQUIPMENT (eg COM
PORT CONNECTOR PINS ON A PC) AND THE PATIENT AT THE SAME TIME.
CE Mark
Denotes conformity with the European Council
Directive 93/42/EEC concerning medical devices.
Classification symbol
Indicates Type CF applied part.
7

Sonicaid FM800 Series Reference Manual
Indications for use
Huntleigh Healthcare Ltd Sonicaid FM800 series fetal monitors are indicated for use in
monitoring fetal and maternal vital signs during the intrapartum and antepartum periods.
Sonicaid FM820 provides comprehensive fetal monitoring facilities, offering twin
ultrasound fetal heart rate, separate fetal and maternal ECG channels, external and
internal uterine activity monitoring and maternally sensed fetal movements.
Sonicaid FM830 provides additional maternal monitoring with facilities for simultaneous
monitoring of maternal pulse oximetry, blood pressure and temperature without the need
for additional stand-alone devices.
Notes
US Federal Law restricts this device to sale on or by the order of a physician.
Sonicaid Care analysis and Sonicaid Trend analysis are not approved for sale in the USA
and Canada.
Warning: do not use the maternal oxi metry sensors during magnetic
resonance imaging (MRI) scan ning. Induced current could cause bur ns .
The oximeter may affect the MRI image, and the MRI unit may affect the
accuracy of oximetry measurements.
8

Sonicaid FM800 Series Reference Manual
System Installation
These requirements must be met when you connect an FM800 fetal monitor to any of the
following pieces of equipment:
● a central review and archiving system
● a PC
● a VGA monitor:
1 Non-medical equipment must comply with the relevant IEC or ISO safety standard.
For Information Technology equipment, this standard is IEC950/EN60950.
2 Medical equipment must comply with IEC601-1/EN60601-1.
3 The configured system must comply with the system standard IEC601-1-1/EN60601-1-1.
4 If non-medical equipment (eg the PC or printer) with enclosure leakage currents greater
than those allowed by IEC601-1/EN60601-1 is to be used in the patient environment
(within 1.5m of the patient), you must bring the enclosure leakage currents within the
limits laid down by IEC601-1/EN60601-1. This may be done by using an isolating
transformer such as the one supplied by Huntleigh Healthcare
Ltd.
5 Anybody who connects additional equipment to signal input or signal output parts of
the system is configuring a medical system, and is therefore responsible for ensuring
that the system complies with IEC601-1-1/EN60601-1-1. If you are
in any doubt whether your system does comply, consult the technical service
department of your local Huntleigh Healthcare Ltd representative.
The connection of extra equipment to the patient or FM800 could lead to the summation
of leakage currents. In such circumstances the user must ensure that
safe leakage currents are not exceeded.
Calibration
The NBP module should be calibrated every 12 months. See Section 14.5. Apart from
this, there is no special procedure for calibrating FM800.
9

Sonicaid FM800 Series Reference Manual
Multiple Portable Socket Outlets
(including isolation transformers)
Note: an isolation transformer is a particular kind of multiple socket outlet.
It is not recommended to power a medical system from a multiple portable socket
outlet which is not supplied from an isolation transformer (IEC601-1-1/EN60601-1-1
Amendment 1).
If such an outlet is in use, it should comply with the requirements of Annexe EEE.2 of
IEC601-1-1/EN60601-1-1 Amendment 1.
WARNINGS
1 Do not exceed the power rating for the multiple portable socket o utlet.
2 Do not place multiple portable so ck et -outlets on the floor. This is to
protect against mechanical damage and the ingress of liquids.
3 Multiple portable socket-outlets supplied with the system must not
be used for powering equipment which does not form part of the
system. This is to prevent increased leakage currents, and overload
of the multiple portable socket outlet.
4 If the system has been specified for use with an isolation transformer,
do not connect any non-medical electrica l equipment which forms part
of the system directly to the wall outlet. This is to prevent excessive
leakage currents.
5 Non-medical electrical equipment situated in the patient environment
(within 1.5 metres of the patie nt) must be powered via an isolati on
transformer, to limit leakage current.
For more information on the connection and use of isolation transformers, consult the
user manual for the medical system you have purchased.
10

Sonicaid FM800 Series Reference Manual
Electromagnetic compatibility
Make sure the environment in which FM800 is installed is not subject to strong sources of
electromagnetic interference (eg radio transmitters, mobile phones).
This equipment generates and uses radio frequency energy. If not installed and used
properly, in strict accordance with the manufacturer's instructions, it may cause or be
subject to interference. Type-tested in a fully configured system, it has been found to
comply with IEC601-1-2/EN60601-1-2, the standard intended to provide reasonable
protection against such interference. Whether the equipment causes interference may be
determined by turning the equipment off and on. If it does cause or is affected by
interference, one or more of the following measures may correct the interference:
● Reorienting the equipment
● Relocating the equipment with respect to the source of interference
● Moving the equipment away from the device with which it is interfering
● Plugging the equipment into a different outlet so that the devices are on different
branch circuits
Adding accessories or components to a system, or modifying a medical device or system,
may degrade the immunity performance. Consult qualified personnel before making
changes to the system configuration.
11

Sonicaid FM800 Series Reference Manual
Trademarks
Sonicaid™ is a registered trademark of Huntleigh Healthcare Ltd.
™
Tem
pHearts
TraceVue
Safelinc
is a registered trademark of YSI Corporation.
™
is a registered trademark of Philips.
™
is a registered trademark of Tyco.
Sensors
Care and disposal
Re-usable probes and sensors: store and maintain in accordance with the instructions
supplied by the manufacturer. Probes and sensors which do not work, or which are no
longer required, should be disposed of in accordance with local regulations.
Single-use probes and sensors: dispose of these after use in accordance with local
regulations.
Oximeter sensors
The use of original oximetry sensors supplied by the manufacturer is strongly
recommended.
12

Sonicaid FM800 Series Reference Manual
Addresses
UK
Huntleigh Healthcare Ltd
35 Portmanmoor Road, Cardiff.
CF24 5HN. UK.
Tel: +44 (0) 2920 485885
Fax: +44 (0) 2920492520
E-mail: sales@huntleigh-diagnostics.co.uk
Web page www.huntleigh-healthcare.com
13

Sonicaid FM800 Series Reference Manual
1 Introduction
1.1 The FM800 series
FM800 series monitors are designed for antepartum and intrapartum monitoring. There
are four monitors in the series:
FM820
FM830
This reference manual covers the whole FM800 range and may describe some facilities
not available in the FM800 you have bought. Note that FM820 can be upgraded to FM830.
Standard monitor
Comprehensive monitoring using twin ultrasound and separate ECG
channels. Gives great flexibility in monitoring multiple pregnancies.
For example:
Twin ultrasound and maternal ECG
Ultrasound, fetal ECG and maternal ECG
Twin ultrasound plus fetal ECG
FM820 also provides external and internal monitoring of uterine activity,
and maternally sensed fetal movements
Standard monitor plus
Maternal pulse oximetry
Maternal non-invasive blood pressure
Maternal temperature
14

Sonicaid FM800 Series Reference Manual
FM820: standard features
The following features are available on all monitors in the FM800 range:
● Alarms
● Annotation
● Audio
● Display autodim
● Direct FECG via scalp electrode
● Fetal event marker
● Interface to Rimkus telemetry
● Interface to Sonicaid Axis
● Interface to Sonicaid System 8002 / Fetalcare
● Interface to Sonicaid Centrale and other CMS packages
● IUP: internal uterine activity
● Maternal Heart Rate via ECG (MECG lead supplied as option)
● Toco: external uterine activity
● Ultrasound 1: 1.5MHz
● Ultrasound 2: 2.0MHz
● Thermal printer
● SonicaidCare (antepartum) analysis*
● SonicaidTrend (intrapartum) analysis*
* Not approved for sale is USA and Canada.
15

Sonicaid FM800 Series Reference Manual
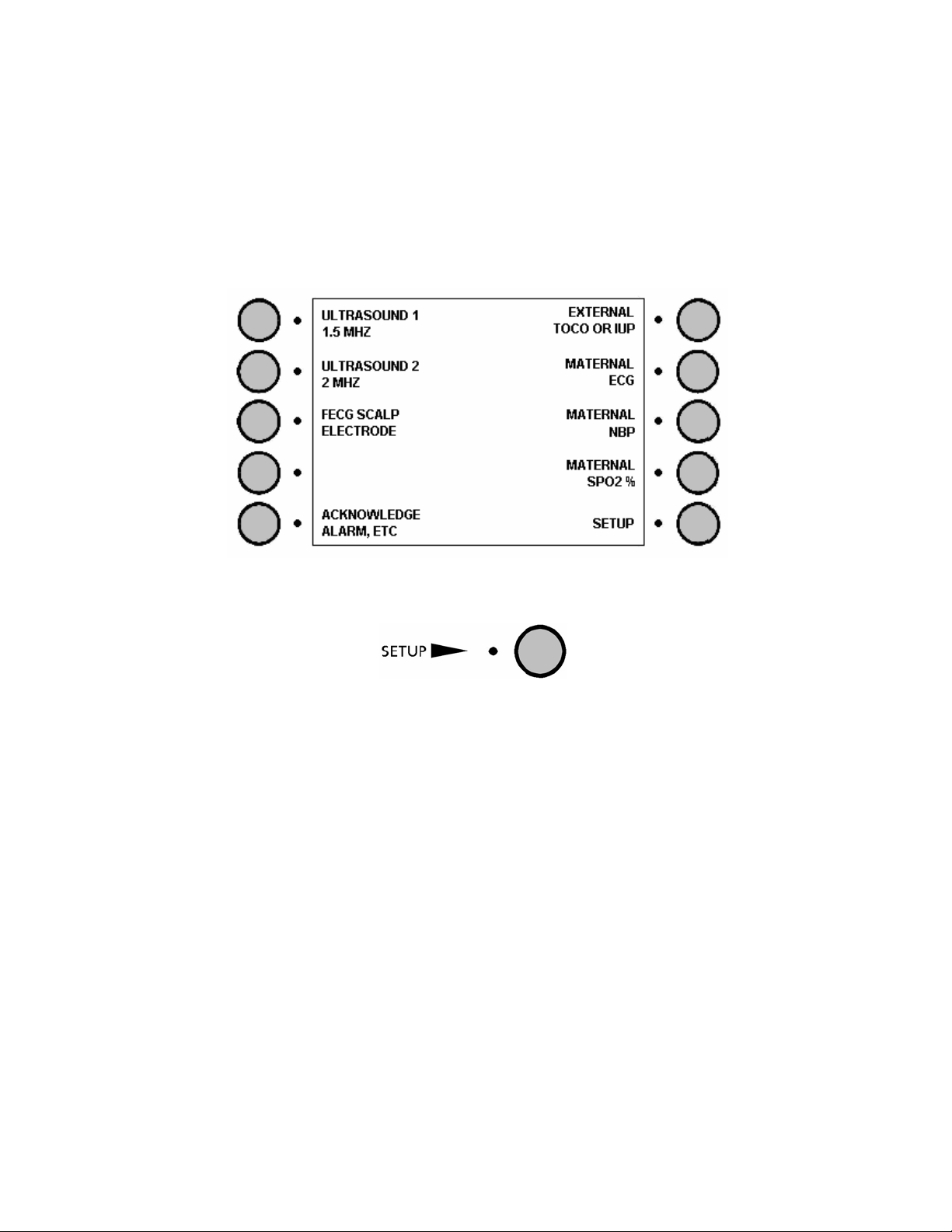
1.2 Main unit: front panel
1 2 1
3 4 5 6
1 Software control buttons
2 Display: see chapter 2
3 Connector module for oximetry, blood pressure and temperature
4 Connection sockets for transducers
5 Printer drawer
6 Controls and on/off indicators: see chapter 2.
16
Sonicaid FM800 Series Reference Manual
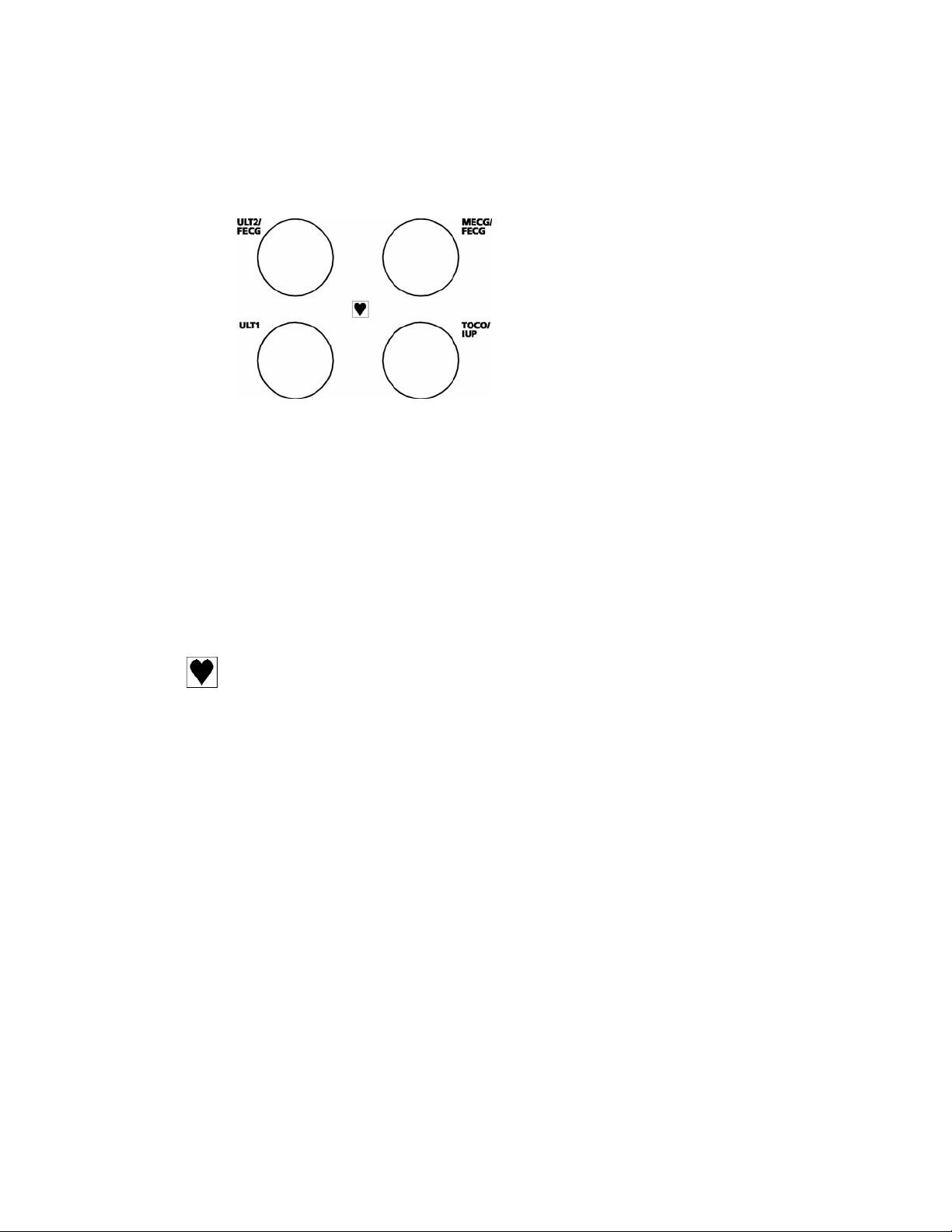
1.3 Connection sockets for transducers
ULT1
ULT2/FECG
MECG
TOCO/IUP
Classification symbol
This symbol indicates Type CF applied part.
1.5 MHz Ultrasound transducer, yellow
2.0 MHz Ultrasound transducer, blue
OR
Fetal ECG electrode, blue (connected via leg plate)
Maternal ECG transducer, blue
Toco contractions transducer, pink
OR
IUP catheter-transducer, pink
17
Sonicaid FM800 Series Reference Manual
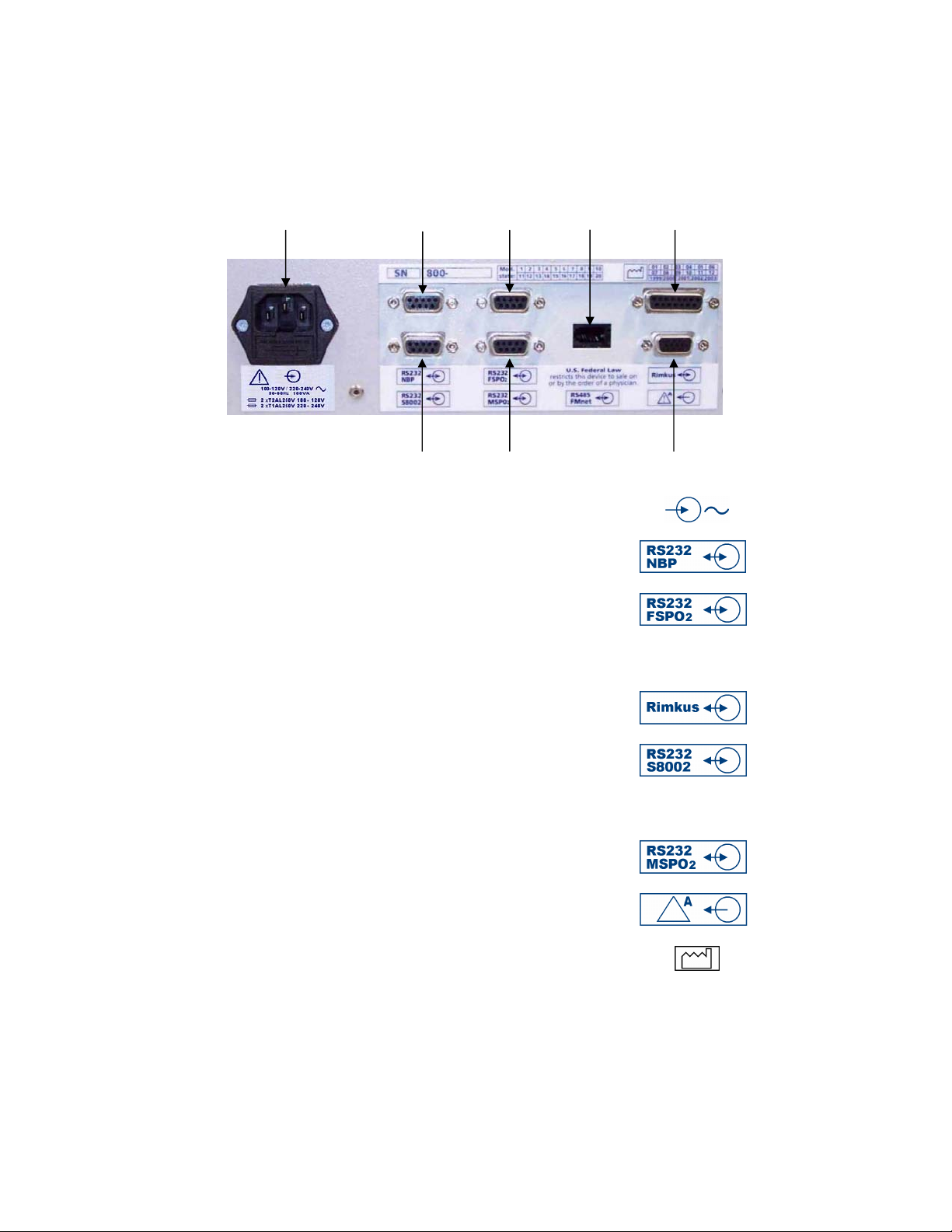
1.4 Main unit: rear connectors
1 2 3 4 5
6 7 8
1 Input socket for the AC mains supply
2 RS232 connector for external NBP,
9-way D-type socket (reserved for future use)
3 RS232 connector for external FspO2,
9-way D-type socket (reserved for future use)
4* RS485 Interface for Axis (1.5kV DC isolation) (not used)
5* Connector for Rimkus Telemetry**
15-way D-type socket
6 RS232 connector for Sonicaid Centrale, FetalCare, Philips
™
TraceVue
, GMT Argus** 9-way D-type socket
NB this connector will also accept an RS232 to RS422
™
adaptor lead for connection to Philips TraceVue
RS232 connector for external MSpO
7
9-way D-type socket (reserved for future use)
2
8 VGA connector, 15-way compact D-type socket
See notes on page 9
This symbol means Date of Manufacture
* For pin connections, see Appendix 2.
** Not approved for use with Sonicaid FM800 in the USA or Canada.
18
Sonicaid FM800 Series Reference Manual
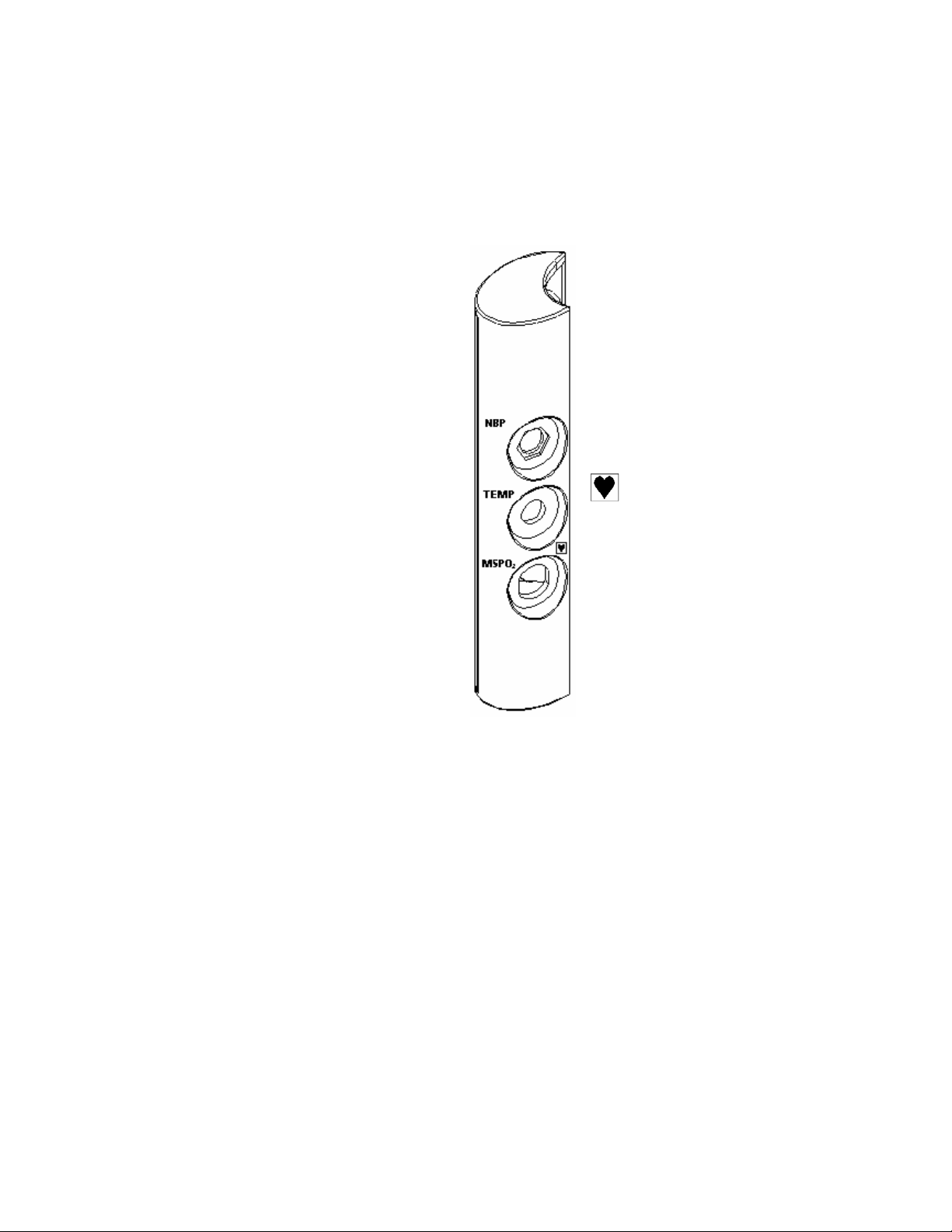
1.5 Connector module
FM830 offers additional features via a connector module.
NBP
TEMP
MSpO
Maternal blood pressure
Maternal temperature
2
Maternal pulse oximetry
Type CF patient
applied part
FM830
The connector module has connectors for maternal blood pressure, maternal oximetry
maternal temperature. The fetal oximetry connector is blanked off.
19
and
Sonicaid FM800 Series Reference Manual
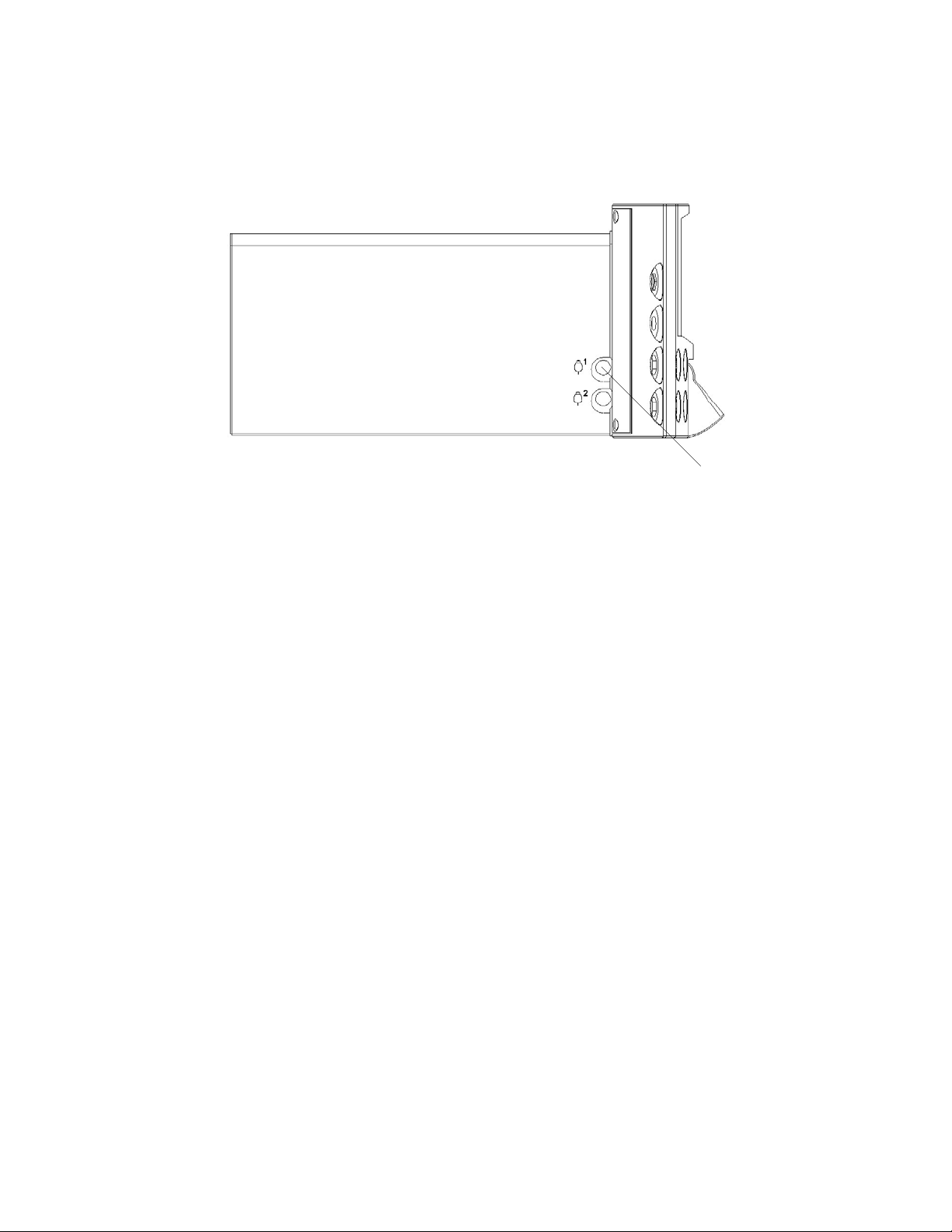
1.6 Event marker connection
1.7 The FM800 display
Event
marker
connector
The FM800’s high-performance display combines superior visual performance with
environmental ruggedness, making it suitable for a wide range of environments.
The principal benefits of the FM800 display are:
● High brightness and contrast
● Wide viewing angle: >160°
● Display auto-dims when FM800 is used in subdued lighting
● Extremely rugged and durable
● Reliable, long operating life
Note: as with other light-emitting displays, displaying fixed patterns on the screen can
cause a limited degree of burn-in. Some slight variation in luminance, as a result of this,
is perfectly normal.
20

Sonicaid FM800 Series Reference Manual
1.8 Transducers and cables
Supplied with all units
Ultrasound transducers Yellow, 1.5MHz (primary)
Blue, 2.0MHz (secondary)
Fetal ECG lead Blue
Toco transducer Pink
Transducer belts 3
Transducer buckles 3
FECG lead leg belts/electrode pads 2
Fetal movement event marker 1
Mains lead 1
Supplied with FM830
NBP air line 1
NBP adult cuff 1
Maternal SpO
Maternal SpO
Maternal temperature probe 1
TempHearts
Supplied as options (with any mon i t or in the FM800 range)
IUP lead Pink
IUP single-use transducers
Maternal ECG lead Blue
NBP small adult cuff
NBP large adult cuff
Trolley
Wall-mounting kit
Accessories supplied with all monitors in the FM800 range
Ultrasound gel 8oz
Printer paper 2 packs
Reference Manual 1
Getting Started card 1
Mother’s guide to FM800 card 1
patient lead
2
re-useable probe
2
™
(for maternal temperature) 1
1
1
21
Sonicaid FM800 Series Reference Manual
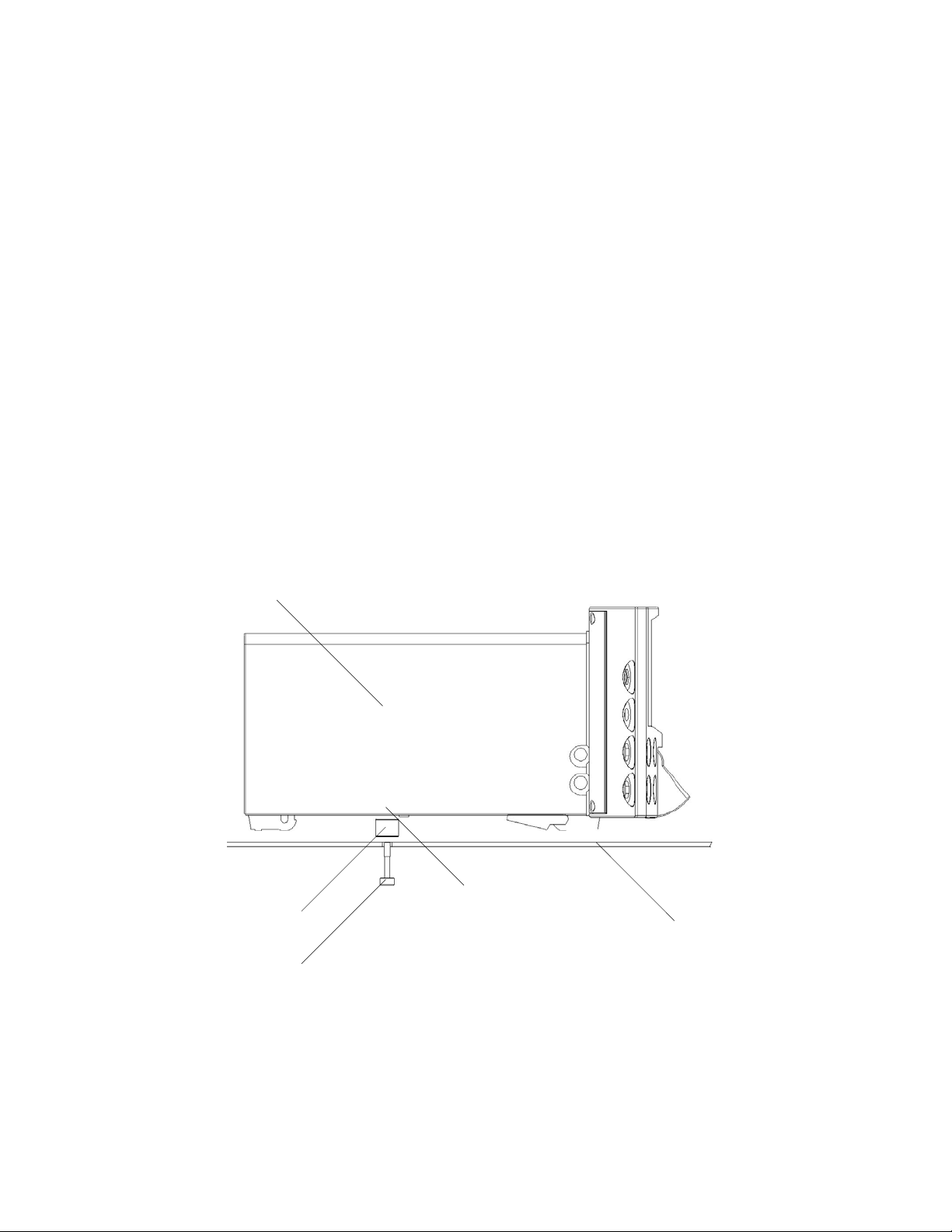
1.9 FM800 trolley or wall mounting
FM800 can be mounted on a trolley or wall mounting. A purpose-designed trolley is
available. If FM800 is used with a trolley or wall mounting, then it must be attached to the
trolley or wall mounting with the securing screw. Otherwise there is a danger of its falling
off the trolley or wall mounting accidentally.
To assemble the trolley or wall mounting, follow the instructions supplied by the
manufacturer.
To attach FM800 to the trolley or wall mounting:
1 Position FM800 on the trolley or wall mounting top so that the securing screw is in line
with the threaded boss in the centre of the FM800 base.
2 Reach under the trolley or wall mounting top, and locate the securing screw.
3 Gently push up and secure the screw tightly.
WARNING: if you use FM800 on a trolley, make sure the trolley brakes
are applied at all times, except when the trolley is being moved.
1
1 FM800 unit 2 Spacer 3 Securing screw 4 Threaded boss
5 Top of trolley or wall mounting unit
2
3
4
22
5
Sonicaid FM800 Series Reference Manual
2 FM800 Controls and Display
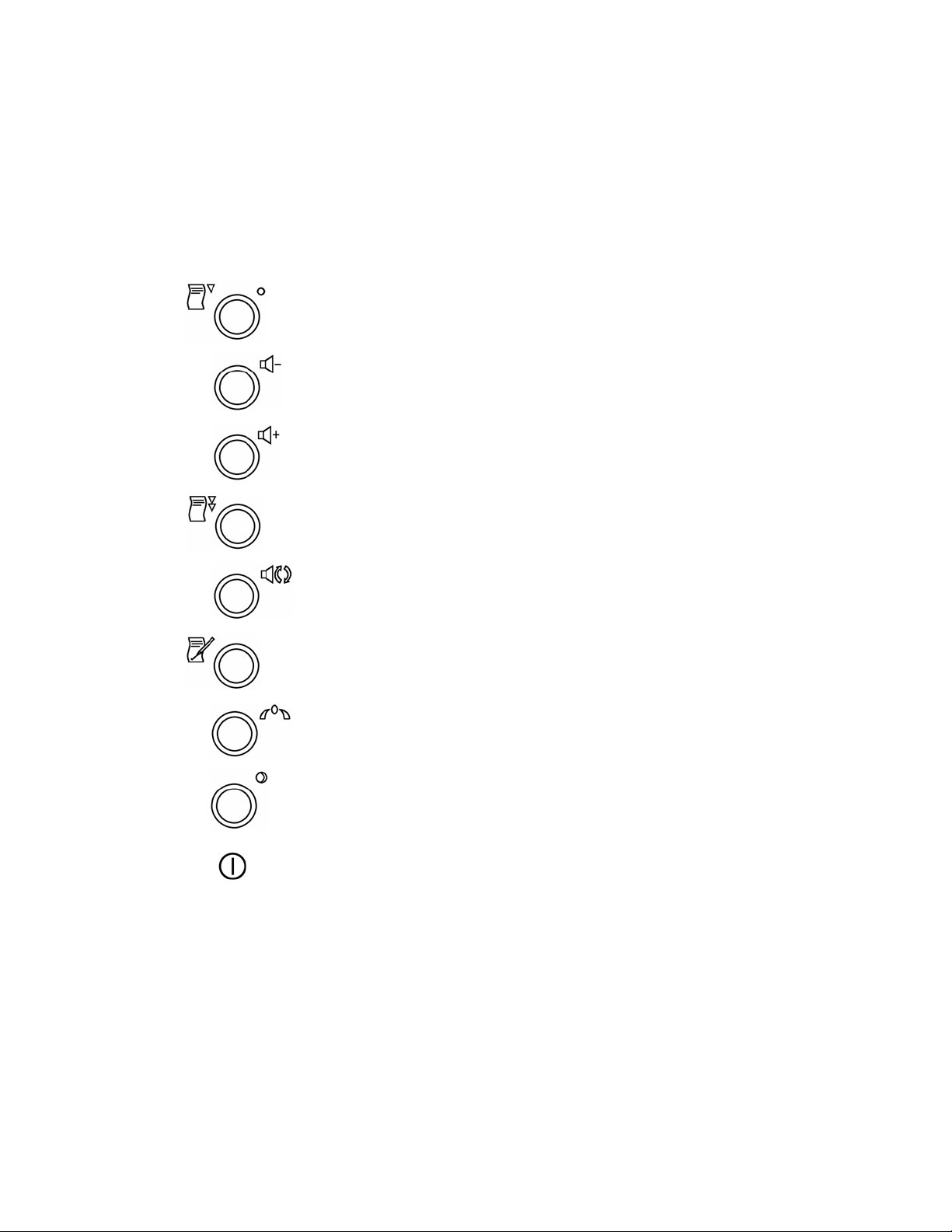
2.1 Controls and on/off indicator
Printer on/off switch and indicator.
Shows amber light when printer is switched on.
Audio volume down.
Audio volume up.
Printer fast forward.
Audio channel select. See Sections 5.2 and 6.3.
EasiNotes annotation. See Section 7.6.
Toco zero.
Power on/off switch and indicator.
Shows green light when FM800 is switched on.
Power on/off symbol.
Switching on
To switch on, press the Power on/off switch.
If FM800 beeps rapidly and continuously, it has failed its power-up self-test routine.
Contact your local Huntleigh Healthcare Ltd representative.
23
Sonicaid FM800 Series Reference Manual
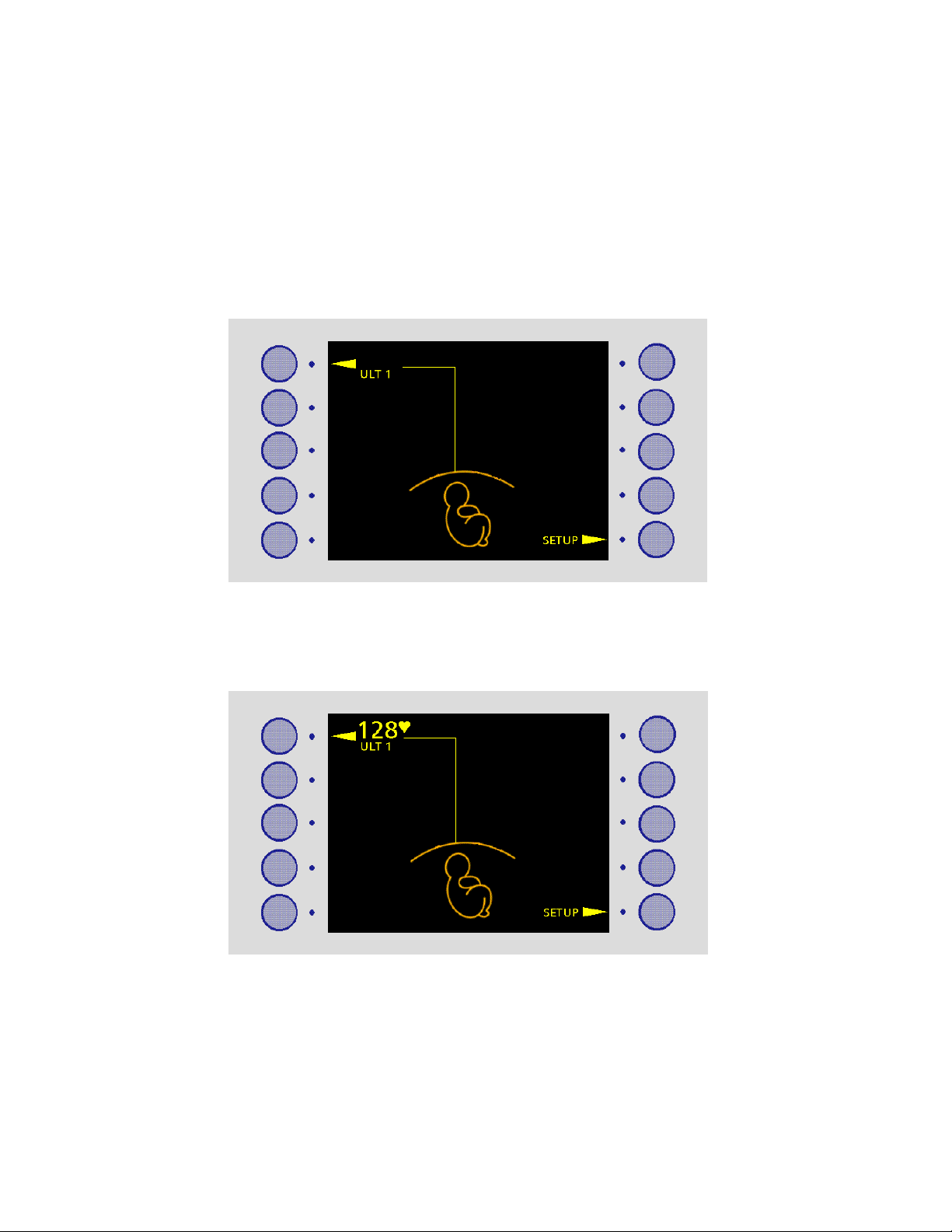
2.2 FM800 main screen
When FM800 is monitoring, fetal parameters are displayed down the left-hand side of the
display, maternal parameters down the right-hand side. Each parameter is always
displayed in the same position on the display. See diagram below.
Buttons are not active until a transducer is connected. A triangular pointer indicates that a
button is active.
For example:
Pressing a button takes you into a setup screen for that parameter. This setup screen
allows you to set alarms and thresholds.
The setup button in the main screen is for general setup procedures (setting the time and
date, altering the default thresholds, and so on).
Stop beep or make it quieter
FM800 beeps when you press a button or key. The loudness of the beep depends on the
loudness of the fetal or maternal alarm, whichever is louder. So to make FM800 beep
more quietly or loudly, change the volume of the fetal and/or maternal alarms. See
Section 8.2.
To stop FM800 beeping when you press a button or key:
> SETUP > SYSTEM SETTINGS > access code (2755) > AUDIO/GRAPHIC > KEY
PRESS
24
Sonicaid FM800 Series Reference Manual
2.3 Software control buttons
When you switch FM800 on, the SETUP button is active. Other buttons are inactive.
When you connect any transducer (apart from NBP), the button for that parameter
becomes active, and the parameter name is displayed.
For example, when you connect Ultrasound 1, the display shows:
When the FM800 detects the fetal heart rate, it displays the rate and a confidence
indicator (the heart symbol). See Section 5.4.
25
Sonicaid FM800 Series Reference Manual
2.4 Audio controls
FM800 can give you an audio signal for one channel at a time (Ultrasound, FECG or
MECG). The default channel is ULT1 (1.5MHz Ultrasound). So if you switch on FM800
with transducers connected for all the audio channels, and then select audio, you will get
an audio signal for ULT1. If you are in any doubt, the FM800 display shows you which
channel currently has the audio signal.
For audio, see also Sections 5.2 and 6.3.
Note: in FM800s with firmware version 1.4.0 or later, if you connect an audio-capable
transducer when audio is already turned on, the audio signal automatically switches to this
most recently connected transducer.
The audio controls on FM800 allow you to:
● change the audio volume
● change the audio channel
Volume control
To change the audio volume, press Audio Volume Up or Audio Volume Down.
Audio Volume Down
Changing the audio channel
To change the audio channel, press the Audio Channel Select button on the front panel
of FM800 until the channel you want is selected.
Audio Volume Up
Audio Channel Select
26
Sonicaid FM800 Series Reference Manual
2.5 Printer controls
There are hardware controls for printer on/off, printer fast forward, and EasiNotes:
Printer On/Off switch and indicator.
Shows amber light when printer is switched on.
Printer Fast Forward.
EasiNotes annotation. See Section 7.6.
Printer setup
Printer setup is controlled by the software buttons ( > SETUP > SYSTEM SETTINGS
> access code (2755) > PRINTER). The options are:
Twin FHR
Print header
Hospital name
Paper speed
FHR vertical scale
FHR graticule
Paper out buffer
See Section 4.3.
EasiNotes setup
> SETUP
> SYSTEM SETTINGS
> access code (2755)
> EasiNotes.
See Section 7.6.
27
Sonicaid FM800 Series Reference Manual
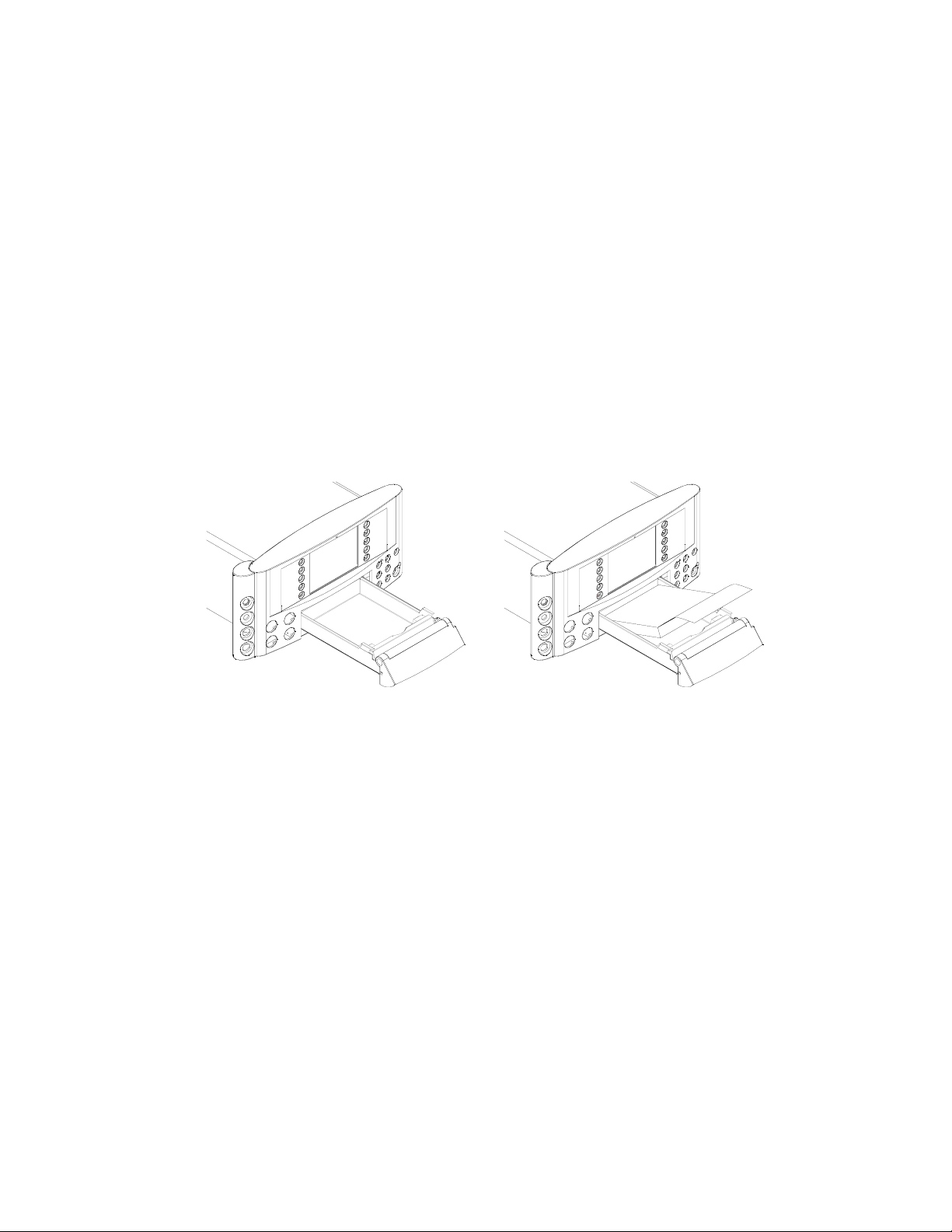
2.6 Loading printer paper
Note: the printer uses a thermal paper pack (part number 8400-8003), with no pre-printed
graticule. If the paper pack shows a procedure for loading paper, this should be ignored,
since it is relevant to Sonicaid Team, and not to the FM800.
1 Pull the FM800 paper drawer as far out as it will come (diagram A).
2 Remove the paper from its plastic wrapping. Make sure the words ‘THIS SIDE UP’ are
visible, and that the arrow points to the back of the paper drawer.
3 Lift the first fold of paper towards you.
4 Place the paper pack centrally in the paper tray, with the first sheet centrally
positioned over the roller (diagram B).
5 With a thumb on either side of the printer drawer, push the drawer in firmly until it
clicks into place.
diagram A
diagram B
28

Sonicaid FM800 Series Reference Manual
3 Setup
3.1 Overview
The Setup procedures are much simpler if you understand the differences between Setup,
Current Alarms, Default Alarms and System Settings.
Setup User preferences available directly from the Setup screen.
Current Alarms Allows you to set alarms before connecting transducers. Settings
remain in force until you switch off FM800. When you switch on
again, the FM800 reverts to the default alarms.
You can set alarms for:
FHR, Maternal temperature, MECG, NBP, MSpO
You can also set alarms from the main screen by pressing the key
beside the parameter whose alarm you want to change.
Default Alarms Default alarm settings protected by the access code (2755):
Changes you make using Default Alarms remain in force when you
switch FM800 off and on again.
2
System Settings User preferences protected by the access code (2755):
Changes you make using System Settings remain in force when you
switch FM800 off and on again.
3.2 Current alarms
This option allows you to alter the alarms for a monitoring session before connecting
transducers. The changes you make using this option do not remain in force when you
switch off the FM800.
See Chapter 8.
29

Sonicaid FM800 Series Reference Manual
3.3 Default alarms
This option allows you to alter the default alarms for the FM800. The changes you make
using this option do remain in force when you switch off the FM800.
See Chapter 8.
3.4 System settings
See Chapter 4.
3.5 Analysis
Use SETUP also if you want to choose SonicaidCare* (antepartum) or SonicaidTrend*
(intrapartum) analysis:
SonicaidTrend analysis See Chapter 9.
SonicaidCare analysis See Chapter 10.
* not approved for sale in USA and Canada
3.6 Time and date
To reset the date or time in FM800:
1 > SETUP > TIME AND DATE.
2 > SETUP beside the time or date.
3 Use + and – to make the necessary changes.
4 > EXIT.
To change the date format, see Section 4.5, International Settings.
30
 Loading...
Loading...