Horiba ABX Pentra 60 C+ User manual

ABX Pentra 60 C
+
HAN 616A
Explore the future
User Manual
P/n: RAB092IEN


Instrument User Manual Update
RAM227AEN
ABX Pentra 60 C+
User Manual Update for Electrical Standard Certification
Please, take note of the modifications on next pages. Please, cross out the
appropriate sections in the user manual prior to inserting this addendum
at the beginning of the user manual.
FORM 0860 - rev 1
Date: 28/7/08

Contents RAM227AEN
Contents
Contents ................................................................. 2
Update chart ........................................................... 3
Updates .................................................................. 4
Manufacturer ..................................................................4
Logo modification ..........................................................4
Copyright .......................................................................4
Revisions .......................................................................4
Warnings .......................................................................4
Graphic and symbols .......................................................5
Pentra 60 C+ back side ...................................................5
Waste levels ...................................................................6
NE flag ..........................................................................6
Verification after a reagent replacement ..........................6
2/6
Update chart RAM227AEN
Update chart
Tab.1-1: Concerned sections of the Pentra 60 C+ user manual:
Section Page Paragraph Item change
Front page I Manufacturer
Front page I Manufacturer
Introduction II Copyright
Introduction III Revisions
Introduction IV 1.1. Warnings
Introduction VIII 1.6. Graphics and Symbols
Description &
Technology
Specimen Run &
Results
Specimen Run &
Results
Maintenance &
Troubleshooting
2.3 1.2. Pentra 60 C+ back side
3-2 1.1. Waste levels
3-34 NE flag
5.2 1.1. Reagent replacement
Telephone and fax number addition.
See “1. Manufacturer, page 4”
Modification of Manufacturer logo.
See “2. Logo modification, page 4”
Modification of Copyright.
See “3. Copyright, page 4”
Modification of paper documents updates.
See “4. Revisions, page 4”
Peripheral devices compliance sentence modification.
See “5. Warnings, page 4”
Modification of Manufacturer logo and addition of new
RoHS logos.
See “6. Graphic and symbols, page 5”
New Serial Number Label.
See “7. Pentra 60 C+ back side, page 5”
Addition of precautions about waste.
See “8. Waste levels, page 6”
Ne flag information.
See “9. NE flag, page 6”
Addition of verification after reagent replacement.
See “10. Verification after a reagent replacement, page 6”
3/6
Updates RAM227AEN
Updates
1. Manufacturer
Telephone and fax numbers:
◆ Phone: + 33 (0)4.67.14.15.16
◆ Fax: + 33 (0)4.67.14.15.17
2. Logo modification
New Manufacturer logo.
3. Copyright
Copyright 2008 HORIBA ABX SAS
4. Revisions
Index Technical note Revision Section Date
A RAH774AA V1.50 All 26/06/200
B RAH792AA V1.61 All 20/02/2001
C RAH873AA V2.2 ALL 14/01/2002
D RAH986AA V2.2.9 All 24/09/2003
E RAN179A V2.3.0 (RAM146AEN) 1, 3 16/11/2005
RAB092FEN /
RAB092GEN RAN321A
RAB092HEN RAN321B V2.4.X (RAM215BEN) 3, 4, 5 30/11/2007
RAB092IEN / Electrical standard certification (RAM227AEN) Intro, 2, 3, 5 21/05/2008
This document applies to the latest software listed and higher versions.
When a subsequent software version changes the information in this document, a new electronic issue (CD-ROM
and/or online help) will be released and supplied by HORIBA ABX.
To update a paper document, please contact your local HORIBA ABX representative.
Instrument default settings (RAM200AEN)
User manual update (RAM205AEN)
Platelet interferences (RAM210BEN)
V2.4.0 (RAM215AEN)
4
Intro, 1, 2, 3
2
3, 4, 5
10/10/2006
08/06/2007
5. Warnings
4/6
◆ All peripheral devices should comply with relevant standards.

Updates RAM227AEN
6. Graphic and symbols
Manufacturer
Packaging recycling mark
Notice of environment-friendly use period
7. Pentra 60 C+ back side
Diluent input
Waste output
Serial number label
Serial number label
Barcode reader
(not used on Pentra 60C+)
Printer
(not used on Pentra 60C
RS 232 output
Main supply cable
+
)
5/6
Updates RAM227AEN
8. Waste levels
At the beginning of each day, before startup, check if the waste container needs to be
emptied.
During the operation, do not remove the reagent tubes and the waste liquid tube under any
condition.
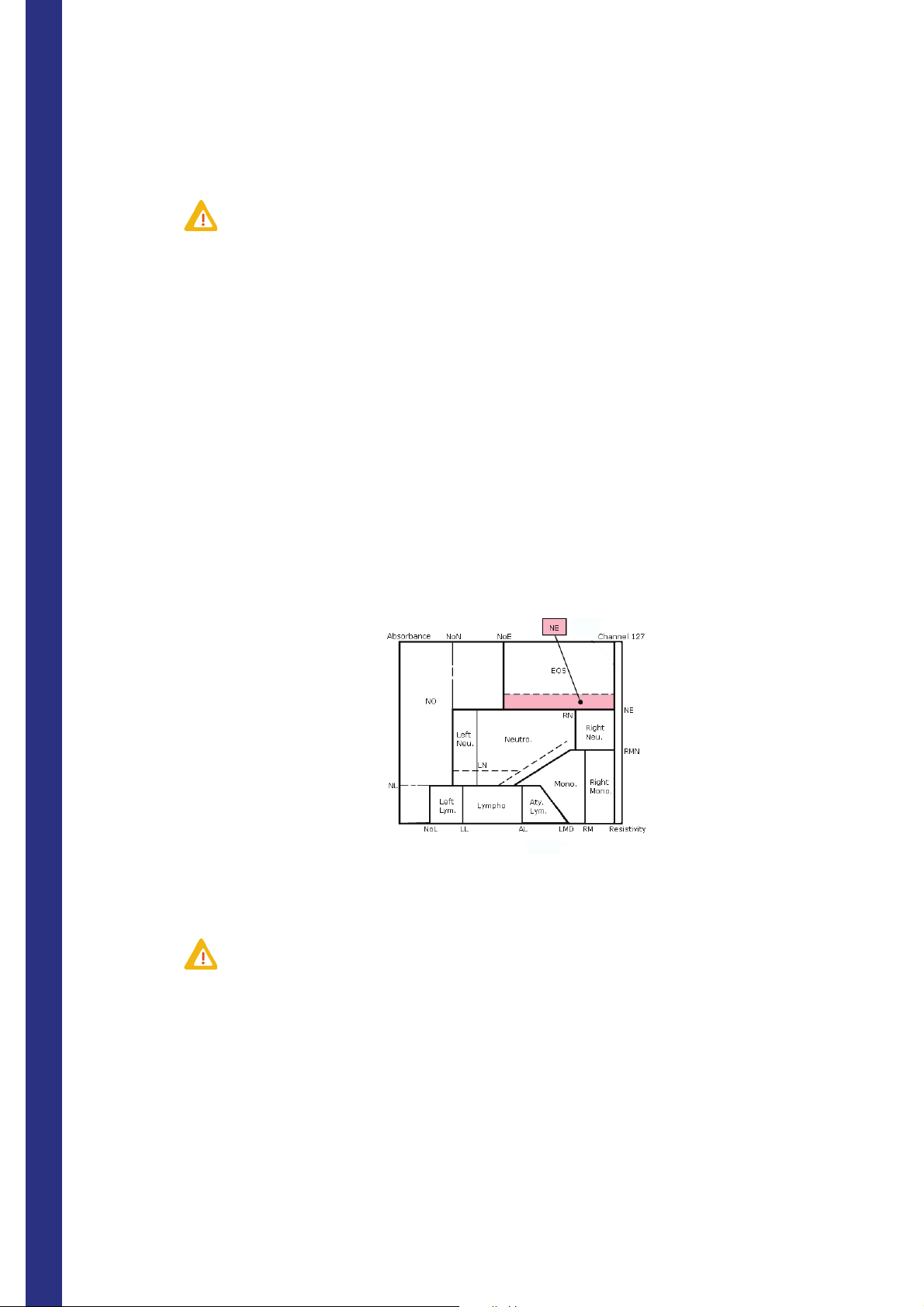
9. NE flag
Meaning: Neutro/Eosino.
Presence of a significantly large population of cells located in the separation area between neutrophils and
eosinophils because of a superimposition of the 2 populations. This flag occurs when the number of particles
counted in this area is higher than the limit setup in NE# or when the number of particules counted regarding the
total number of WBC is above the NE% limit.
Standard values for NE: % 1.1 / # 60
Suspected abnormalities:
◆ Young eosinophils
◆ Giant hypersegmented neutrophils
◆ Eosinophils with low intracytoplasmic material
◆ Immature cells
◆ Neutrophils with cytotoxic granulations
This flag is associated with an (!) on LIC % and LIC # and replaces NEU %, NEU #, EOS %, EOS # by ----.
6/6
10. Verification after a reagent replacement
Make sure that a blank cycle and Control run will be carried out after the change of a
reagent in the course of day.

Instrument User Manual Update
RAM215BEN
ABX Pentra 60 C+
Update for instrument version V2.4.X
Please, take note of the modifications on next pages. Please, cross out the
appropriate sections in the user manual prior to inserting this addendum
at the beginning of the user manual.
FORM 0860 - rev 1
Date: 30/11/07
Update for instrument version V2.4.X RAM215BEN
Update for instrument version V2.4.X
Tab.1-1: Concerned sections of the manual
Section Page Paragraph Item change
Specimen run &
Results
Specimen run &
Results
Maintenance &
Troubleshouting
Instrument
configuration
Index History:
From RAM215AEN to RAM215BEN: V2.4.0 replaced by V2.4.X and modification of conditions on platelets.
3-24 7.4. Flags
3-45 7.5.3. Plt messages
5-2 1.1. Reagent replacement
4-2 1. Control
Addition of paragraph “Suspicion flags”
See “1. Suspicion flag, page 2”
Addition of message condition in “PLT Aggregate (1)”
See “2. Plt messages, page 3”
Addition of ABX Diluent 10L
See “3. ABX Diluent 10L, page 3”
Addition of Export QC
See “4. Export QC, page 3”
1. Suspicion flag
1.1. PLT
Conditions Triggered flag Consequences
If PLT < 120x103/mm3 (in CBC mode only)
If PLT < 120x103/mm3 + PDW > 20 (in CBC and DIFF mode)
(!) on PLT and on
PDW, MPV, PCT
1.2. WBC
◆ If a flag LMNE+ or LMNE- or BASO+ is triggered, then a suspicion flag «!» on WBC parameter is generated too.
See “7.4.7. WBC balance”.
◆ If a suspicion flag «!» on WBC occurs, then «!» is also generated on BAS#, BAS%, LYM#, LYM%, MON#, MON%,
EOS#, EOS%, NEU#, NEU%, ALY#, ALY%, LIC#, LIC% parameter results.
◆ A suspicion flag «!» is generated on the BAS#, BAS%, LYM#, LYM%, MON#, MON%, NEU#, NEU% and EOS#, EOS%
parameter results if Hgb > 17,5 g/dl or invalid «---».
PLT result needs to be confirmed according to Good Laboratory Working
Practices
2/5

Update for instrument version V2.4.X RAM215BEN
2. Plt messages
"H": high extreme limit
"L": low extreme limit
Message Triggering conditions
Thrombocytosis PLT > PLT H
Thrombocytopenia PLT < PLT L
Microcytosis
Schizocyte
Small Cell
PLT Aggregate (1)
Erythroblasts(2)
Erythroblasts
PLT Aggregate
Macroplatelets MPV > 11
See triggering conditions for these flags in paragraph ”7.4.5.Flags on RBC histogram“.
PLT < 150x103/mm3 + WBC Reject or
NO + PDW > 20 or
NO + MPV > 10 or
NO + PLT < 150x10
NO + WBC Reject or
(L1 or LL1) + PDW > 20 or
(L1 or LL1) + MPV > 10 or
(L1 or LL1) + PLT < 150x10
PDW > 20 + PLT < 120x103/mm3 (in CBC mode only)*
LL or
WBC reject + L1 or
WBC reject + LL1
If conditions 1 and 2 are not satisfied
and if L1 or LL1 or WBC reject
3
/mm3 or
3
/mm
3
* New condition
3. ABX Diluent 10L
A 10 liters container of ABX Diluent is available under the part number: 0901010.
Software version v2.4.X introduces 10L or 20L volumes management (barcode identification, capacity, level, expiration date, etc.).
4. Export QC
Software version v2.4.X allows backup of QC results and statistics on floppy disk.
This backup file is registered on a «.csv» format in order to be reused on a spreadsheet type software.
4.1. Saved QC data
◆ Only one «.csv» file is generated for each control lot number.
◆ Each «.csv» file has a unique name and is generated in the current language and units. File name is saved on
the following model : «xxx_AAAAMMJJ.csv» where xxx is the lot# and AAAAMMJJ the file creation date.
4.2. Limitations
RUO (for Research Use Only) parameters (PCT, PDW, ALY, LIC) are not saved on the Export QC file.
3/5

Update for instrument version V2.4.X RAM215BEN
4.3. Export QC file content
Each file includes the following fields:
Header
Instrument name / Lab ID
Instrument serial number
Lot number
File creation date time
Start period for lot use
End period for lot use
Expiration date
Comment
User lot comment
Unit used
CBC data (Minotrol)
3 parts for Low, Normal and High levels
Results detailed by parameters
Statistics results
lot target values
DIFF data (Difftrol)
3 parts for Low, Normal and High levels
Results detailed by parameters
Statistics results
lot target values
See “Tab.1-1: DIF example” in the following page.
4.4. Procedure to export QC data
◆ Insert a Windows formatted Floppy disk into the drive.
◆ Open the «QC & Calibration» Tab (See “Fig.1-1: «QC & Calibration» tab, page 4”).
◆ Select the lot to export (Fig.1-1-A).
◆ Click the «Export QC» button (Fig.1-1-B).
A
4/5
B
Fig.1-1: «QC & Calibration» tab
Update for instrument version V2.4.X RAM215BEN
◆ Accept when the following message is displayed «Do you confirm QC export?»
◆The use of Export QC function will copy all levels results of the selected lot on the floppy
disk at the same time.
◆Lot barcode identification must not be modified to allow QC exportation on floppy disk.
◆Statistical calculation results displayed in .csv file on the floppy disc are based on the
raw values determined by the instrument, and not by the rounded values of control runs
displayed in this .csv file.
5/5

LIC#
LIC#
ALY#
ALY#
BAS#
BAS#
EOS#
EOS#
NEU#
NEU#
MON#
MON#
LYM #
LYM #
LIC%
LIC%
ALY%
ALY%
BAS%
BAS%
EOS%
EOS%
NEU%
NEU%
MON%
MON%
LYM %
LYM %
PDW
PDW
PCT
PCT
MPV
MPV
PLT
PLT
RDW
RDW
MCHC
MCHC
MCH
MCH
MCV
MCV
HCT
HCT
HGB
HGB
RBC
RBC
WBC
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
LIC#
alarm
LIC#
ALY#
alarm
ALY#
BAS#
alarm
BAS#
EOS#
alarm
EOS#
NEU#
alarm
NEU#
MON#
alarm
MON#
LYM #
alarm
LYM #
LIC%
alarm
LIC%
ALY%
alarm
ALY%
BAS%
alarm
BAS%
EOS%
alarm
EOS%
NEU%
alarm
NEU%
MON%
alarm
MON%
LYM %
alarm
LYM %
PDW
alarm
PDW
PCT
alarm
PCT
MPV
alarm
MPV
PLT
alarm
PLT
RDW
alarm
RDW
MCHC
alarm
MCHC
MCH
alarm
MCH
MCV
alarm
MCV
HCT
alarm
HCT
HGB
alarm
HGB
RBC
alarm
RBC
WBC
alarm
WBC RBC HGB HCT MCV MCH MCHC RDW PLT MPV PCT PDW LYM% MON% NEU% EOS% BAS% ALY% LIC% LYM# MON# NEU# EOS# BAS# ALY# LIC#
WBC
LIC#
LIC#
ALY#
ALY#
BAS#
BAS#
EOS#
EOS#
NEU#
NEU#
MON#
MON#
LYM #
LYM #
LIC%
LIC%
ALY%
ALY%
BAS%
BAS%
EOS%
EOS%
NEU%
NEU%
MON%
MON%
LYM %
LYM %
PDW
PDW
PCT
PCT
MPV
MPV
PLT
PLT
RDW
RDW
MCHC
MCHC
MCH
MCH
MCV
MCV
HCT
HCT
HGB
HGB
RBC
RBC
WBC
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
LIC#
alarm
LIC#
ALY#
alarm
ALY#
BAS#
alarm
BAS#
EOS#
alarm
EOS#
NEU#
alarm
NEU#
MON#
alarm
MON#
LYM #
alarm
LYM #
LIC%
alarm
LIC%
ALY%
alarm
ALY%
BAS%
alarm
BAS%
EOS%
alarm
EOS%
NEU%
alarm
NEU%
MON%
alarm
MON%
LYM %
alarm
LYM %
PDW
alarm
PDW
PCT
alarm
PCT
MPV
alarm
MPV
PLT
alarm
PLT
RDW
alarm
RDW
MCHC
alarm
MCHC
MCH
alarm
MCH
MCV
alarm
MCV
HCT
alarm
HCT
HGB
alarm
HGB
RBC
alarm
RBC
WBC
alarm
WBC RBC HGB HCT MCV MCH MCHC RDW PLT MPV PCT PDW LYM% MON% NEU% EOS% BAS% ALY% LIC% LYM# MON# NEU# EOS# BAS# ALY# LIC#
WBC
LIC#
LIC#
ALY#
ALY#
BAS#
BAS#
EOS#
EOS#
NEU#
NEU#
MON#
MON#
LYM #
LYM #
LIC%
LIC%
ALY%
ALY%
BAS%
BAS%
EOS%
EOS%
NEU%
NEU%
MON%
MON%
LYM %
LYM %
PDW
PDW
PCT
PCT
MPV
MPV
PLT
PLT
RDW
RDW
MCHC
MCHC
MCH
MCH
MCV
MCV
HCT
HCT
HGB
HGB
RBC
RBC
WBC
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
alarm
LIC#
alarm
LIC#
ALY#
alarm
ALY#
BAS#
alarm
BAS#
EOS#
alarm
EOS#
NEU#
alarm
NEU#
MON#
alarm
MON#
LYM #
alarm
LYM #
LIC%
alarm
LIC%
ALY%
alarm
ALY%
BAS%
alarm
BAS%
EOS%
alarm
EOS%
NEU%
alarm
NEU%
MON%
alarm
MON%
LYM %
alarm
LYM %
PDW
alarm
PDW
PCT
alarm
PCT
MPV
alarm
MPV
PLT
alarm
PLT
RDW
alarm
RDW
MCHC
alarm
MCHC
MCH
alarm
MCH
MCV
alarm
MCV
HCT
alarm
HCT
HGB
alarm
HGB
RBC
alarm
RBC
WBC
alarm
WBC RBC HGB HCT MCV MCH MCHC RDW PLT MPV PCT PDW LYM% NEU% EOS% BAS% ALY% LIC% LYM# MON# NEU# EOS# BAS# ALY# LIC#
WBC
04/24/2007 13:17 2.5 2.48 6.9 22.7 H 92 H 27.7 30.2 L 12.5 46 L 4.8 L 24.1 0.9 57.7 14.0 3.3 0.61 0.02 1.46 0.35 0.08
04/24/2007 13:24 2.6 2.50 6.7 22.9 H 91 H 26.7 29.2 L 12.8 56 4.4 L 25.0 0.7 58.1 12.9 3.3 0.64 0.02 1.49 0.33 0.08
04/24/2007 13:26 2.5 2.49 6.8 22.8 H 92 H 27.4 29.9 L 13.0 71 4.3 L 24.8 0.5 55.9 15.6 3.2 0.63 0.01 1.41 0.39 0.08
Lower limits 2.0 2.40 6.3 18.4 75 24.6 30.7 10.0 46 6.7 19.0 --- 48.0 3.0 1.5 0.43 --- 1.12 0.04 0.03
Target values 2.4 2.52 6.7 19.9 79 26.6 33.7 14.0 66 8.7 27.0 1.5 58.0 10.0 3.5 0.65 0.04 1.39 0.24 0.08
Upper limits 2.8 2.64 7.1 21.4 83 28.6 36.7 18.0 86 10.7 35.0 3.0 68.0 17.0 5.5 0.87 0 .08 1.66 0.44 0.13
Mean 2.5 2.49 6.8 22.8 92 27.3 29.8 12.8 57 4.5 24.6 0.7 57.2 14.2 3.3 0.63 0.02 1.45 0.36 0.08
Mean/target diff. 0.1 0.03 0.1 2.9 13 0.7 3.9 1.2 9 4.2 2.4 0.8 0.8 4.2 0.2 0.02 0.02 0.06 0.12 0.00
Standard deviation 0.02 0.01 0.1 0.07 0.07 0.49 0.52 0.27 12.72 0.27 0.47 0.2 1.17 1.36 0.06 0.01 0.01 0.04 0.03 0.00
Variation Coeff. 0.65 0.37 1.46 0.29 0.08 1.81 1.74 2.08 22.17 H 5.92 1.92 28.57 2.05 9.58 1.77 2.26 28.67 2.62 8.97 2.29
DIF Normal
Run date WBC
04/24/2007 13:10 8.0 4.44 L 13.2 L 44.5 H 100 H 29.7 29.7 L 11.4 229 4.1 L 25.9 0.6 64.8 5.5 3.2 H 2.08 0.05 5.21 0.44 0.26
04/24/2007 13:12 7.3 4.35 L 13.4 L 43.7 H 100 H 30.9 30.8 L 11.8 218 4.1 L 27.3 0.6 63.4 5.3 3.4 H 2.00 0.04 4.65 0.39 0.25
04/24/2007 13:13 7.2 4.41 L 13.4 L 44.3 H 101 H 30.5 30.3 L 11.3 219 4.1 L 25.2 0.6 65.6 5.3 3.3 H 1.81 0.04 4.70 0.38 0.24
Lower limits 6.3 4.54 13.5 38.8 83 27.9 31.3 8.5 205 6.2 20.5 --- 53.0 --- 0.0 1.45 --- 3.82 --- 0.04
Target values 7.3 4.69 14.0 40.8 87 29.9 34.3 12.5 235 8.2 28.5 1.5 63.0 3.5 0.3 2.08 0.11 4.60 0.26 0.26
Upper limits 8.3 4.84 14.5 42.8 91 31.9 37.3 16.5 265 10.2 36.5 3.0 73.0 7.0 0.5 2.71 0.22 5.38 0.52 0.48
Mean 7.5 4.40 13.4 44.1 100 30.4 30.3 11.5 222 4.1 26.1 0.6 64.6 5.4 3.3 1.96 0.05 4.85 0.40 0.25
Mean/target diff. 0.2 0.29 0.6 3.3 13 0.5 4.0 1.0 13 4.1 2.4 0.9 1.6 1.9 3.0 0.12 0.06 0.25 0.14 0.01
Standard deviation 0.46 0.05 0.13 0.44 0.2 0.61 0.56 0.25 6.04 --- 1.07 --- 1.11 0.12 0.1 0.14 0.00 0.31 0.03 0.01
Variation Coeff. 6.18 1.09 1.01 0.99 0.2 2.0 1.86 2.19 2.72 --- 4.09 --- 1.72 2.15 3.03 7.24 6.18 6.4 8.38 4.24
DIF High
Run date WBC
04/24/2007 13:38 28.1 H 5.14 16.6 53.6 H 104 32.3 31.0 L 10.3 450 3.8 L 13.8 1.1 75.4 5.5 4.2 3.88 0.31 21.21 H 1.55 1.18
04/24/2007 13:39 28.2 H 5.15 16.7 53.7 H 104 32.4 31.0 L 10.7 459 3.7 L 13.7 1.1 76.0 5.0 4.2 3.86 0.31 21.41 H 1.41 1.18
04/24/2007 13:41 27.9 H 5.11 16.6 53.2 H 104 32.5 31.2 L 10.5 453 3.8 L 13.5 1.3 75.5 5.5 4.2 3.77 0.36 21.06 H 1.53 1.17
Lower limits 15.5 4.95 16.0 45.4 79 29.7 31.7 7.8 450 5.3 5.5 MON% 65.5 1.0 1.0 1.09 --- 11.56 0.17 0.13
Target values 17.7 5.15 16.6 47.9 93 32.2 34.7 11.8 500 7.3 13.5 --- 75.5 5.5 4.0 2.69 0.27 13.36 0.97 0.71
Upper limits 19.9 5.35 17.2 50.4 107 34.7 37.7 15.8 550 9.3 21.5 1.5 85.5 10.0 7.0 4.29 0.54 15.16 1.77 1.29
Mean 28.1 5.13 16.6 53.5 104 32.4 31.1 10.5 454 3.8 13.7 1.2 75.6 5.3 4.2 3.84 0.33 21.23 1.50 1.18
Mean/target diff. 10.4 0.02 0.0 5.6 11 0.2 3.6 1.3 46 3.5 0.2 0.3 0.1 0.2 0.2 1.15 0.06 7.87 0.53 0.47
Standard deviation 0.15 0.02 0.03 0.25 0.05 0.11 0.12 0.17 4.46 0.04 0.15 0.12 0.32 0.29 --- 0.06 0.03 0.18 0.08 0.01
Tab.1-1: DIF example
Instrument name/Lab ID Pentra60C+/ABX Parc Euro
Instrument serial number 454548887
Lot Number PX017
File creation date time 04/24/2007 13:43
Start period for lot use
End period for lot use
Expiration Date 2/6/07
Comment Calculation results displayed on this floppy are based on the raw values determined by the instrument and not by the rounded value
User lot comment
Unit used STD
DIF Difftrol
DIF Low
Run date WBC
Variation Coeff. 0.53 0.43 0.17 0.47 0.04 0.34 0.38 1.62 0.98 0.98 1.12 9.9 0.43 5.41 --- 1.61 9.34 0.83 5.12 0.53

Instrument User Manual Update
RAM210BEN
ABX Pentra 60/60 C+
Platelet interferences
Please, take note of the modifications on next pages. Please, cross out the
appropriate sections in the user manual prior to inserting this addendum
at the beginning of the user manual.
FORM 0860 - rev 1
Date: 6/4/07

Platelet interferences RAM210BEN
Platelet interferences
1. ABX Pentra 60
Tab.1-1: Concerned sections of the manual
Section Paragraph Item change
Specifications 5.3. Known interfering substances
1.1. Plt (Platelets)
◆ Elevated lipids and/or cholesterol: may interfere with correct platelet counting.
From patients undergoing parenteral treatment with intralipids brought, it is noted an over-estimate of the
platelet counting which can mask a thrombopenia in DIFF mode. In this case, sample re-run should be done in
CBC mode.
Modification of information about interference in the platelet results
See “1.1. Plt (Platelets), page 2”
◆ Elevated bilirubine : may interfere with correct platelet counting.
From patients with severe hepatic disorder, liver transplant…It is noted an over-estimate of the platelet counting which can mask a thrombopenia.
2. ABX Pentra 60 C+
Tab.1-2: Concerned sections of the manual
Section Paragraph Item change
Specifications 5.3. Known interfering substances
2.1. Plt (Platelets)
◆ Elevated lipids and/or cholesterol: may interfere with correct platelet counting.
From patients undergoing parenteral treatment with intralipids brought, it is noted an over-estimate of the
platelet counting which can mask a thrombopenia in DIFF mode. In this case, sample re-run should be done in
CBC mode.
◆ Elevated bilirubine : may interfere with correct platelet counting.
From patients with severe hepatic disorder, liver transplant…It is noted an over-estimate of the platelet counting which can mask a thrombopenia.
Modification of information about interference in the platelet results
See “2.1. Plt (Platelets), page 2”
2/2

Instrument User Manual Update
RAM205AEN
ABX Pentra 60 C+
User manual update
Please, take note of the modifications on next pages. Please, cross out the
appropriate sections in the user manual prior to inserting this addendum
at the beginning of the user manual.
FORM 0860 - rev 1
Date: 10/10/06
User manual update RAM205AEN
User manual update
Tab.1-1: Concerned sections of the manual
Section Page Paragraph Item change
Introduction II Notice of liability
Introduction III Revisions
Introduction VI 1.4. Biological risks
Introduction VIII 1.6. Graphics and symbols
Introduction IX 2.1. Environment
Introduction X 2.6. Environment protection
Specifications 1-6 2.1. Power requirements
Specifications 1-8 2. Physical specifications
Specifications 1-15 4.1. Reagent specifications
Specifications 1-17
Specifications 1-19
Specifications 1-20
Description &
Technology
Specimen run
& Results
Specimen run
& Results
Specimen run
& Results
Specimen run
& Results
5.3. Known interfering
substances
5.3. Known interfering
substances
5.3. Known interfering
substances
2-11 2.3. Hgb measurement
3. Calibration verification
3-5
(control blood sampling)
3-14 6. Running specimen
7.4.4. Flags on WBC/BASO
3-39
histogram
3-43 7.4.7. WBC balance
Paragraph correction
See “1. Notice of liability, page 3”
Addition of internet link to Declaration of conformity
See “2. Declaration of conformity, page 3”
Recommendations addition
See “3. Biological risks, page 3”
WEEE Directive symbol addition
See “4. Graphics and symbols, page 3”
“Safety requirements” Standard evolution
See “5. Environment, page 3”
WEEE Directive addition
See “6. Environment protection, page 3”
Heat output addition
See “7. Power requirements, page 3”
Addition of “2.9. Compatible tube list” paragraph
See “8. Compatible tube list, page 4”
A.F.S.S.A.P.S reference deletion and Reagent leaflets update
See “9. Reagent specifications, page 6”
Modification of known interferences due to Chemotherapy
See “10.1. White Blood Cells (Leukocytes), page 6”
Modification of interference in the platelet result information
See “10.2. Plt (Platelets), page 6”
Addition of interferences in the basophil count information
See “10.3. BAS# (Basophil cell count absolute), BAS% (Basophil
percentage), page 6”
Addition of measuring principle with Lysebio
See “11. Hgb measurement, page 7”
Addition of calibration general information See “12. Calibration
verification (control blood sampling), page 7”
Addition of recommendations on the analysis mode selection
See “13. Running specimen, page 8”
Addition of recommendations on CBC mode: L1 flag
See “14. Flags on WBC/BASO histogram, page 8”
Addition of CBC mode limitations
See “15. WBC balance, page 8”
2/8
User manual update RAM205AEN
1. Notice of liability
The Information in this manual is distributed on an "As Is" basis, without warranty. While every precaution has
been taken in the preparation of this manual, HORIBA ABX will not assume any liability to any persons or entities
with respect to loss or damage, caused or alleged to be caused directly or indirectly by not following the instructions contained in this manual, or by using the computer software and hardware products described herein in a
manner inconsistent with our product labeling.
2. Declaration of conformity
Latest version of the CE declaration of conformity for this instrument is available on www.horiba-abx.com.
3. Biological risks
Consider all instrument accessible surfaces as potentially contaminated with blood.
Use protective gloves to operate instrument.
4. Graphics and symbols
This product should be disposed of and recycled at the end of the
useful life in accordance with the WEEE Directive (2002/96/CE)
5. Environment
The instrument is designed for safety from voltages surges according to INSTALLATION CATEGORY II and POLLUTION
DEGREE 2 (IEC 61010-1).
6. Environment protection
◆ European Legislation:
In accordance with the European Directive (2002/96/CE, known also as W.E.E.E) instruments having the above
symbol, and sold into a European country by HORIBA ABX or an authorised representative must disposed of and
recycled correctly at the end of its useful life.
Due to the local changing regulations in each country, please contact your local representative for detailed and
upto date information on how to appropriately dispose of the instrument.
7. Power requirements
◆ Heat output....... ......... Max 1440 Kjoules/h (1365 BTU/h)
3/8
User manual update RAM205AEN
8. Compatible tube list
8.1. Compatible tube list for Tube holder
Tube lists given in the tables below are not exhaustive. If the tubes in use in your laboratory does not match with this lists, please contact your HORIBA ABX service representative.
On microsampling tubes, the 100µl volume can only be use in the following conditions:
- The tube must be held always in vertical position
- Blood mixing must be obtained by slight tapping on the tube
Do not rotate the tube for mixing, otherwise the blood will be spread on the tube side, and
the minimum required level will be lost.
◆ Legend:
- MAN: Manufacturer
- BC: Barcode
◆ Tube holder position:
- GBG214AS Standard tube holder positions: Position 1, position 2, position 3, position 4
- GBG215AS Optional tube holder positions: Position 1, position 3, position 5, position 6
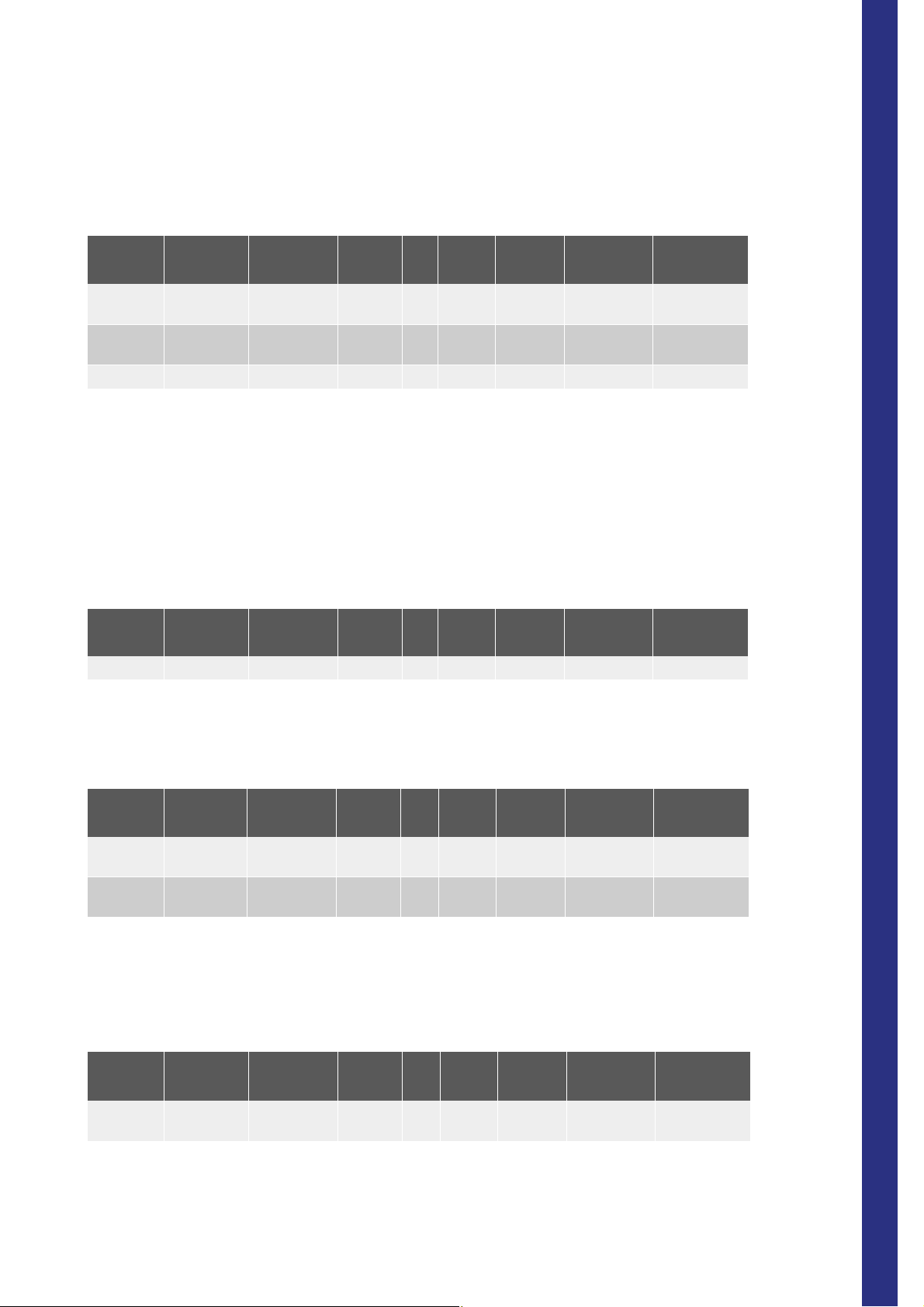
8.2. Tube holder position 1
Tab.1-2: Compatible tube position 1
Manufact Model Part number Additive Vol Vacuum Stickers
Becton D Vacutainer 368452 K3-EDTA 5ml MAN+BC With cap
Becton D Vacutainer 367651 K3-EDTA 5ml 2ml MAN+BC With cap Hemogard
Becton D Vacutainer 367856 K3-EDTA 5ml 3ml MAN+BC With cap Hemogard
Becton D Vacutainer 367652 K3-EDTA 5ml 3ml MAN+BC With cap Hemogard
Becton D Vacutainer 367654 K3-EDTA 5ml 4,5ml MAN+BC With cap Hemogard
Terumo Venoject II VP-053SDK K3-EDTA 5ml 3ml MAN With cap UltraSeal
Terumo Venoject VT-050STK K3-EDTA 5ml 5ml MAN With cap
Terumo Venoject VT-053STK K3-EDTA 5ml 3ml MAN With cap
CML ABX 3004002 TH5C0C K3-EDTA 5ml 4ml MAN+BC With cap
Greiner Vacuette 454087 K3-EDTA 5ml 2ml MAN+BC With cap Hemogard
Greiner Vacuette 454086 K3-EDTA 5ml 3ml MAN+BC With cap Hemogard
Greiner Vacuette 454036 K3-EDTA 5ml 4ml MAN+BC With cap Hemogard
Greiner Vacuette 454223 K3-EDTA 5ml 4,5ml MAN+BC With cap Hemogard
LDM Paris EDTAKE 5ml 4,5ml MAN+BC With cap Hemogard
Pirecing
condition
Type of cap
Rubber with
groove
Rubber with
groove
Rubber with
groove
Rubber strongly
not advisable
4/8
User manual update RAM205AEN
8.3. Tube holder position 2
Tab.1-3: Compatible tube position 2
Manufact Model Part number Additive Vol Vacuum Stickers
Becton D Vacutainer 6385 K3-EDTA 3ml MAN *Without cap
Terumo Venoject VT-03STK K3-EDTA 3ml 3ml MAN With cap
Greiner Minicollect** 450403 K3-EDTA 1ml With cap With valve
*Because of the cap thickness and the lack of space between the holder and the top of the tube, the tube holder
may not open correctly.
**Requires an additional adjustment procedure: in the Menu «Service\Mechanical Systems\Sampling needle Ad-
justment», adjust the «Level» on «Position 2» to «8.0» instead of «3.0» (See Section 5: Maintenance &
Troubleshooting, 2.3.4. Sampling needle adjustment)
Pirecing
condition
Type of cap
Rubber strongly
not advisable
Rubber strongly
not advisable
8.4. Tube holder position 3
Tab.1-4: Compatible tube position 3
Manufact Model Part number Additive Vol Vacuum Stickers
Comar R&D Systems TX2B 18533IF 5ml 2,2ml Without cap With thread
Pirecing
condition
Type of cap
8.5. Tube holder position 4
Tab.1-5: Compatible tube position 4
Manufact Model Part number Additive Vo l Vacuum Stickers
Sarstedt 901091 0,5ml
KABE ABX 3001001 0777008RED 0,5ml
*The tube accepts a small sticker (not supplied by the manufacturer).
*Out of
format
*Out of
format
8.6. Tube holder position 5
Tab.1-6: Compatible tube position 5
Manufact Model Part number Additive Vol Vacuum Stickers
Becton D Microtainer 365975 0,5ml
*The tube accepts a small sticker (not supplied by the manufacturer).
**Cap fitted with an adaptor (require another probe adjustment).
*Out of
format
Pirecing
condition
Without cap Unlostable
With cap Unlostable
Pirecing
condition
Without cap **Microgard
Type of cap
Type of cap
5/8

User manual update RAM205AEN
8.7. Tube holder position 6
Tab.1-7: Compatible tube position 6
Manufact Model Part number Additive Vol Vacuum Stickers
Becton D Microtainer 365973 0,5ml
*The tube accepts a small sticker (not supplied by the manufacturer).
*Out of
format
Pirecing
condition
Without cap
Type of cap
9. Reagent specifications
The HORIBA ABX reagents specified for this instrument have been approved in accordance
with the European Directive 98/79/CE (Annex III) for in-vitro medical devices.
◆ The CD ROM RAX055 delivered with your instrument provided Reagents, Controls and Calibrators leaflets/msds.
Latest versions of these documents are available on www.horiba-abx.com/documentation.
10. Known interfering substances
10.1. White Blood Cells (Leukocytes)
Chemotherapy - Cytotoxic and immunosuppressive drugs may increase the fragility of the leukocyte membranes
which may cause low WBC counts. In these particular cases, CBC mode must not be used as WBC balance alarm
(See Section 3: Specimen run & Results, 7.4.7. WBC balance) is disabled. It is recommended to run these samples
in DIFF mode.
10.2. Plt (Platelets)
Interference in the Platelet result may occur for samples from Patients undergoing parenteral treatment with
injection of lipid emulsion.
10.3. BAS# (Basophil cell count absolute), BAS% (Basophil percentage)
◆ Over evaluation in the Basophil count:
Excessive number of leukocytes (leukocytosis) can cause artificial rise in the number of counted basophils due
to the shifting of the leukocytes population in the zone of the basophil ones.
Monocytes and Blasts show large granules and may shift on the basophil counting area. This may interfere with
an accurate count.
An abnormally of leukocytes (leukopenia) may increase too the basophil results. The elements present in the
zone of basophil are brought back on a small total quantity of leukocytes, which increases the statistical error
and may cause variabilities in the percentage.
The weakness of leukocyte cells shown in certain diseases (Chronic Lymphocytic Leukomia) or during anti-cancer
treatment (chemotherapy) can be translated on the basophilic channel by under evaluation of the leukocytes
because of their destruction and thus cause a statistical increase in the basophil ones.
6/8
User manual update RAM205AEN
◆ Under evaluation in the Basophil count:
During leukomia, basophils may lose their cytochemical characters and react abnormally with the reagent. The
destruction of the basophil cytoplasms prevents their differentiation with the other leukocytes.
The basophils with very small sizes (following treatments) may interfere with leukocyte counts, as cell sizes
cannot be distinguished.
The abnormal basophils (degranulation following allergies) may interfere with leukocyte counts, because cell
sizes cannot be distinguished and because they may lose their characteristic intracytoplasmic material.
11. Hgb measurement
◆ Lysebio
Reagent for erythrocyte lysis and cyanide-free determination of hemoglobin.
By addition of agent of lysis, hemoglobin is released. All the heme iron is oxidized and stabilized. Oxidation
resulting complexes are quantified by spectrophotometry at a wavelength of 550nm.
12. Calibration verification (control blood sampling)
The calibration on HORIBA ABX instruments is an exceptional procedure, which must be
carried out particularly in case of certain technical interventions (installation, maintenance, service intervention). The calibration should not be carried out to compensate a
drift on a results due for example to a clogging of the instrument.
Before carrying out a calibration, it is essential to make sure that the instrument is in perfect condition of operation, and to follow the steps below:
1- Carry out a Concentrated cleaning procedure (refer to Section 5: Maintenance & Troubleshooting).
2- Perform two blank cycles to check the cleanliness of the instrument (if the blank measurement is not correct,
please contact your HORIBA ABX representative).
3- Check the repeatability of the instrument by running 6 times a normal human blood without taking account of
the first result (if the repeatability is not correct, please contact your HORIBA ABX representative).
4- Calculate the CV obtained out on the other 5 results. The values of CV must be lower than: WBC: 2%, RBC: 2%,
HGB: 1%, HCT: 1%, PLA: 5%.
5- Run a control blood and check that the values are within the acceptable limits. If not, run a new control blood.
If the instrument is clean (blank cycles in conformity with the values given in the manual), the repeatability is
correct (acceptable CV values) and the values of control are not within the acceptable limits, then it is possible
to carry out the calibration:
6- Run at least 4 times Calibrator without taking the values of the first result into account.
7- Calibrate the instrument on the average of the last 3 results according to indications of the manual. Take care
to respect the minimum and maximum calibration coefficient values given in the manual. Run 3 times again
Calibrator to check the values.
8- Confirm the calibration while passing a control blood, the values have to be returned within acceptable limits.
9- After about thirty analyses of the day, check that values of MCV, MCH and MCHC are in conformity with the usual
values of the laboratory.
7/8

User manual update RAM205AEN
13. Running specimen
◆ Recommendations on the analysis mode selection (CBC or DIFF)
When selecting CBC analysis, there is no control mode on WBC erroneous countings that may be caused by specific treatments on patients (See Section 1: Specifications, 5.3. Known interfering substances) and WBC balance
(See Section 3: Specimen run & Results, 7.4.7. WBC balance).
14. Flags on WBC/BASO histogram
CBC and DIFF mode
In certain cases, the L1 flag will not be triggered off because of the poor sensitivity of this
flag (large platelet aggregates and/or erythroblasts that are beyond the electronic treshold).
This happens in CBC mode only. Two additional flags LL and LL1 are available in DIFF mode
and provide more reliability in anomaly detection. This mode should be recommended.
15. WBC balance
CBC mode Limitations
The WBC balance flag will indicate an instrument defect or it can also highlight a known
interference (See Section 1: Specifications, 5.3. Known interfering substances).
In the case of pathology whose treatments weaken the leukocytic membranes, the agent
of lysis of WBC channel can damaged the cells and give a lower leukocytes counting.
The LMNE+ flag will then be triggered off and a suspicion will be integrated to the WBC
results. We thus recommend not to disable WBC balance flag and to work in DIFF mode for
all the samples which can present this possible interference. Selecting the CBC mode will
disable this control mode. It is thus recommended to use this mode for patient not presenting this type of interference.
8/8

Instrument User Manual Update
RAM200AEN
ABX Pentra 60 C+
Instrument default settings
Please, take note of the modifications on next pages. Please, cross out the
appropriate sections in the user manual prior to inserting this addendum
at the beginning of the user manual.
FORM 0860 - rev 1
Date: 10/10/06

Instrument default settings RAM200AEN
Instrument default settings
Tab.1-1: Concerned sections of the manual
Section Page Paragraph Item change
Instrument
Configuration
Type parametering
Addition of Instrument Default parametering charts
1. General information
Type parametering:
- The following values are the software default values for pathological limits & thresholds (See “2.Pathological
limits and thresholds, page 2”), Alarm levels (“3.Alarms levels, page 5”), Matrix thresholds (See “4.Matrix thresholds, page 8”), classified by types (standard, man, woman, Child 1,2,3).
Other modifications
- Default values for XB limits ( See “6.XB limits, page 11”).
- Default values for Quality Control coefficients (“5.QC Variation Coefficients, page 11” ).
:
2. Pathological limits and thresholds
From the menu: Settings \ Type Parametering
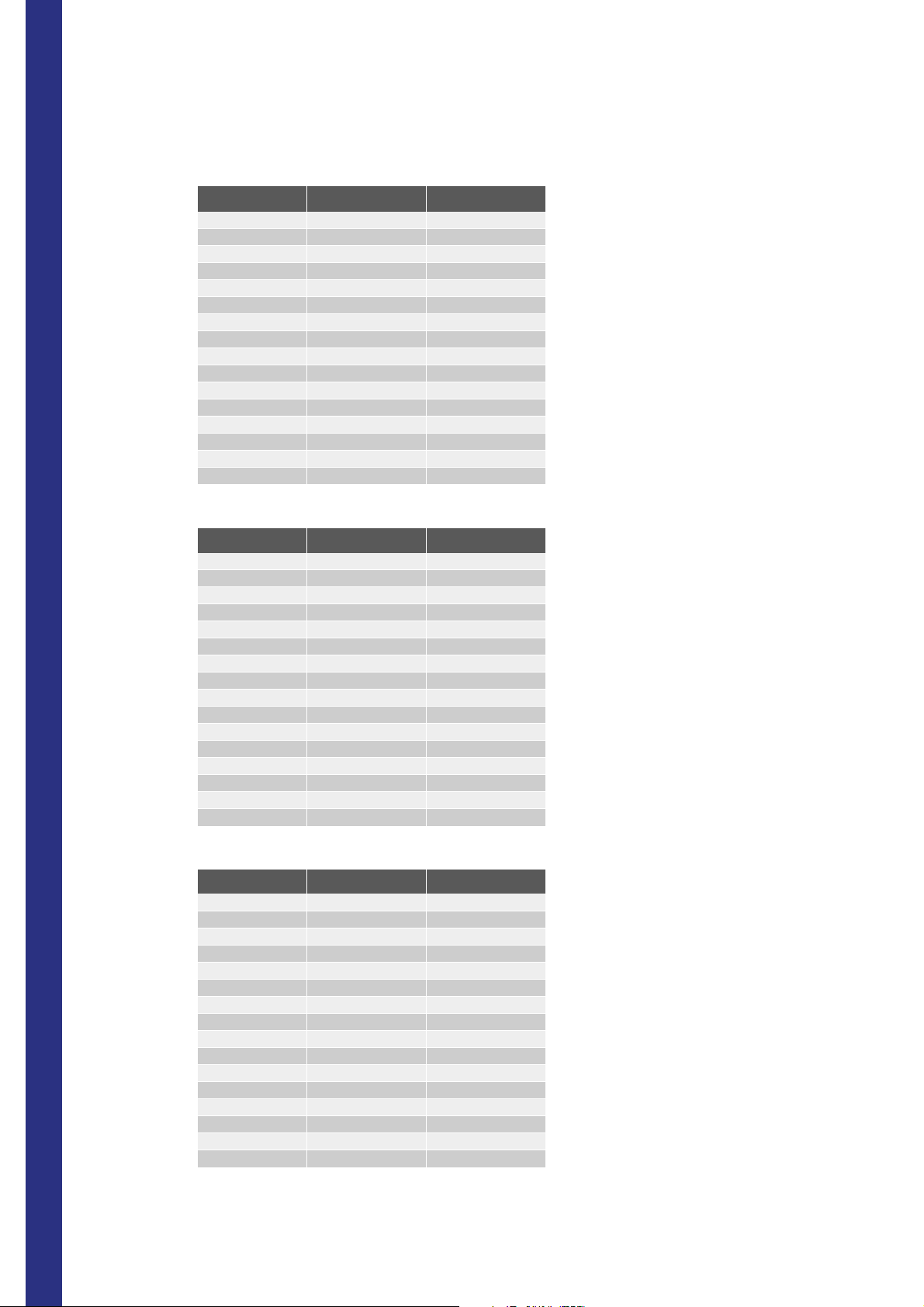
Tab.1-2: STANDARD
Panic L Normal l Normal h Panic H
WBC 3.00 4.00 10.00 13.00
RBC 3.50 3.80 6.50 6.50
HGB 9.50 11.5 17.0 18.0
HCT 34.0 37.0 54.0 54.0
MCV 70 80 100 110
MCH 25.0 27.0 32.0 34.0
MCHC 32.0 32.0 36.0 36.0
RDW 10.0 11.0 16.0 17.0
PLT 100 150 500 550
MPV 6 6 11 12
PCT 0.00 0.15 0.50 1.00
PDW 7 11 18 20
NEU% 0 0 99.9 99.9
LYM % 0 0 99.9 99.9
MON% 0 0 99.9 99.9
EOS% 0 0 99.9 99.9
BAS% 0 0 99.9 99.9
NEU 1.70 2.00 7.50 8.0
LYM 1.00 1.00 4.00 5.00
MON 0.00 0.20 1.00 1.50
EOS 0.00 0.00 0.50 0.70
BAS 0.00 0.00 0.20 0.25
LYA% 0 0 2.5 2.5
CGI% 0 0 3.0 3.0
LYA# 0 0 0.25 0.25
GCI# 0 0 0.30 0.30
2/11

Instrument default settings RAM200AEN
Tab.1-3: MAN
Panic L Normal l Normal h Panic H
WBC 3.00 4.00 10.00 13.00
RBC 3.50 4.50 6.50 6.50
HGB 11.0 13.0 17.0 18.0
HCT 37.0 40.0 54.0 54.0
MCV 70 80 100 110
MCH 25.0 27.0 32.0 34.0
MCHC 32.0 32.0 36.0 36.0
RDW 10.0 11.0 16.0 17.0
PLT 100 150 500 550
MPV 6 6 11 12
PCT 0.00 0.15 0.50 1.00
PDW 7 11 18 20
NEU% 0 0 99.9 99.9
LYM % 0 0 99.9 99.9
MON% 0 0 99.9 99.9
EOS% 0 0 99.9 99.9
BAS% 0 0 99.9 99.9
NEU 1.70 2.00 7.50 8.0
LYM 1.00 1.00 4.00 5.00
MON 0.00 0.20 1.00 1.50
EOS 0.00 0.00 0.50 0.70
BAS 0.00 0.00 0.20 0.25
LYA% 0 0 2.5 2.5
CGI% 0 0 3.0 3.0
LYA# 0 0 0.25 0.25
GCI# 0 0 0.30 0.30
Tab.1-4: WOMAN
Panic L Normal l Normal h Panic H
WBC 3.00 4.00 10.00 13.00
RBC 3.50 3.80 5.80 6.00
HGB 9.50 11.5 16.0 17.0
HCT 34.0 37.0 47.0 50.0
MCV 70 80 100 110
MCH 25.0 27.0 32.0 34.0
MCHC 32.0 32.0 36.0 36.0
RDW 10.0 11.0 16.0 17.0
PLT 100 150 500 550
MPV 6 6 11 12
PCT 0.00 0.15 0.50 1.00
PDW 7 11 18 20
NEU% 0 0 99.9 99.9
LYM % 0 0 99.9 99.9
MON% 0 0 99.9 99.9
EOS% 0 0 99.9 99.9
BAS% 0 0 99.9 99.9
NEU 1.70 2.00 7.50 8.0
LYM 1.00 1.00 4.00 5.00
MON 0.00 0.20 1.00 1.50
EOS 0.00 0.00 0.50 0.70
BAS 0.00 0.00 0.20 0.25
LYA% 0 0 2.5 2.5
CGI% 0 0 3.0 3.0
LYA# 0 0 0.25 0.25
GCI# 0 0 0.30 0.30
3/11

Instrument default settings RAM200AEN
Tab.1-5: CHILD 1 (1 day old to 1 month old)
Panic L Normal l Normal h Panic H
WBC 10.0 10.0 26.0 30.0
RBC 4.00 4.00 6.00 6.00
HGB 13.5 13.5 19.5 19.5
HCT 44.0 44.0 64.0 64.0
MCV 98 100 112 114
MCH 30.0 30.0 38.0 38.0
MCHC 32.0 32.0 36.0 36.0
RDW 10.0 11.0 16.0 17.0
PLT 150 200 400 450
MPV 6 6 11 12
PCT 0.00 0.15 0.50 1.00
PDW 7 11 18 20
NEU% 0 0 99.9 99.9
LYM % 0 0 99.9 99.9
MON% 0 0 99.9 99.9
EOS% 0 0 99.9 99.9
BAS% 0 0 99.9 99.9
NEU 6.00 6.00 26.0 26.0
LYM 2.00 2.00 11.0 11.0
MON 0.40 0.40 3.10 3.10
EOS 0.00 0.00 0.85 0.85
BAS 0.00 0.00 0.65 0.65
LYA% 0 0 2.5 2.5
CGI% 0 0 3.0 3.0
LYA# 0 0 0.35 0.35
GCI# 0 0 0.35 0.35
Tab.1-6: CHILD 2 (1 month old to 2 year old)
Panic L Normal l Normal h Panic H
WBC 10.0 10.0 26.0 30.0
RBC 4.00 4.00 6.00 6.00
HGB 13.5 13.5 19.5 19.5
HCT 44.0 44.0 64.0 64.0
MCV 98 100 112 114
MCH 30.0 30.0 38.0 38.0
MCHC 32.0 32.0 36.0 36.0
RDW 10.0 11.0 16.0 17.0
PLT 150 200 400 450
MPV 6 6 11 12
PCT 0.00 0.15 0.50 1.00
PDW 7 11 18 20
NEU% 0 0 99.9 99.9
LYM % 0 0 99.9 99.9
MON% 0 0 99.9 99.9
EOS% 0 0 99.9 99.9
BAS% 0 0 99.9 99.9
NEU 6.00 6.00 26.0 26.0
LYM 2.00 2.00 11.0 11.0
MON 0.40 0.40 3.10 3.10
EOS 0.00 0.00 0.85 0.85
BAS 0.00 0.00 0.65 0.65
LYA% 0 0 2.5 2.5
CGI% 0 0 3.0 3.0
LYA# 0 0 0.35 0.35
GCI# 0 0 0.35 0.35
4/11

Instrument default settings RAM200AEN
Tab.1-7: CHILD 3 (2 years old to 14 years old)
Panic L Normal l Normal h Panic H
WBC 4.50 4.50 13.5 15.0
RBC 4.00 4.00 5.40 5.40
HGB 11.0 11.5 14.5 15.0
HCT 37.0 37.0 45.0 45.0
MCV 75 77 91 93
MCH 24.0 24.0 30.0 30.0
MCHC 32.0 32.0 36.0 36.0
RDW 10.0 11.0 16.0 17.0
PLT 150 200 400 450
MPV 6 6 11 12
PCT 0.00 0.15 0.50 1.00
PDW 7 11 18 20
NEU% 0 0 99.9 99.9
LYM % 0 0 99.9 99.9
MON% 0 0 99.9 99.9
EOS% 0 0 99.9 99.9
BAS% 0 0 99.9 99.9
NEU 1.80 1.80 8.00 8.00
LYM 1.50 1.50 6.50 6.50
MON 0.00 0.00 0.8 0.8
EOS 0.00 0.00 0.60 0.60
BAS 0.00 0.00 0.20 0.30
LYA% 0 0 2.5 2.5
CGI% 0 0 3.0 3.0
LYA# 0 0 0.25 0.25
GCI# 0 0 0.30 0.30
3. Alarms levels
From the menu: Settings \ Type parametering
Tab.1-8: STANDARD
Level % Level #
NO 100 80
LL 100 80
LL1 5 55
NL 3 120
MN 15 120
RM 0.7 999
LN 2.5 999
RN 1.1 999
NE 1.1 30
L1 3 200
LMNE Reject 50
MIC 5
MAC 45
MACp 11
HGB 3 60
5/11
Instrument default settings RAM200AEN
Tab.1-9: MAN
Level % Level #
NO 100 80
LL 100 80
LL1 5 55
NL 3 120
MN 15 120
RM 0.7 999
LN 2.5 999
RN 1.1 999
NE 1.1 30
L1 3 200
LMNE Reject 50
MIC 5
MAC 45
MACp 11
HGB 3 60
Tab.1-10: WOMAN
Level % Level #
NO 100 80
LL 100 80
LL1 5 55
NL 3 120
MN 15 120
RM 0.7 999
LN 2.5 999
RN 1.1 999
NE 1.1 30
L1 3 200
LMNE Reject 50
MIC 5
MAC 45
MACp 11
HGB 3 60
Tab.1-11: CHILD 1 (1 day old to 1 month old)
Level % Level #
NO 100 80
LL 100 80
LL1 5 55
NL 3 120
MN 15 120
RM 0.7 999
LN 2.5 999
RN 1.1 999
NE 1.1 30
L1 3 200
6/11
LMNE Reject 50
MIC 5
MAC 45
MACp 11
HGB 3 60
 Loading...
Loading...