HoMedics PRO Instruction Manual

IB-CB200CA VER: 001
Made in China
©2013 HoMedics, LLC. and its affiliated companies, all rights reserved.
HoMedics is a registered trademark of HoMedics, LLC. and its affiliated
companies. All rights reserved.
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:04 PM Page 1
1
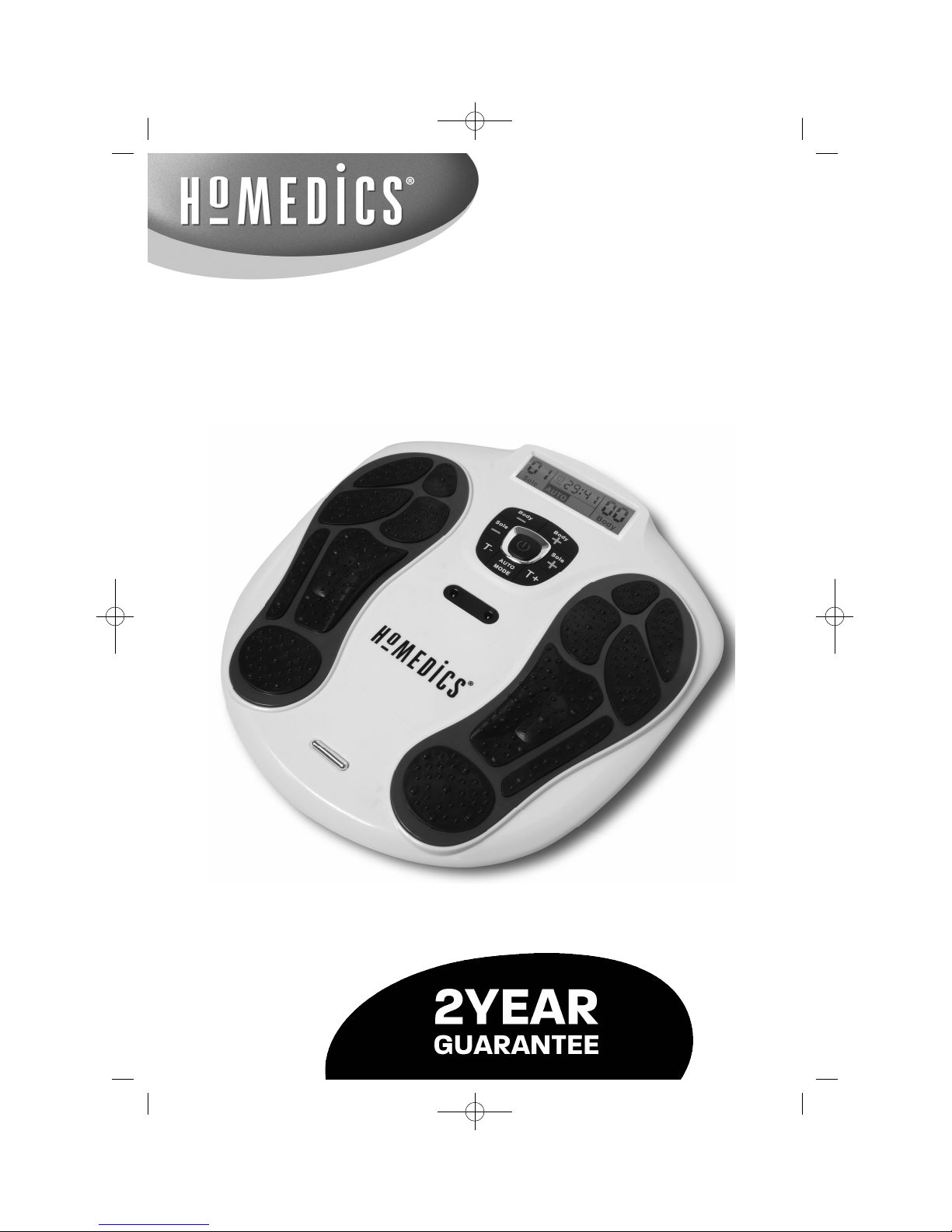
Instruction Manual
CB-200-CA
Circulation Pro
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 1
2
E
QUICK START GUIDE
PLEASE NOTE – THIS DEVICE DOES NOT VIBRATE – IT USES ELECTRICAL IMPULSES, NOT VIBRATION!
For detailed operation of your Circulation Pro please refer to the comprehensive instructions within
this manual.
For a full explanation of setting the intensity, refer to page 13
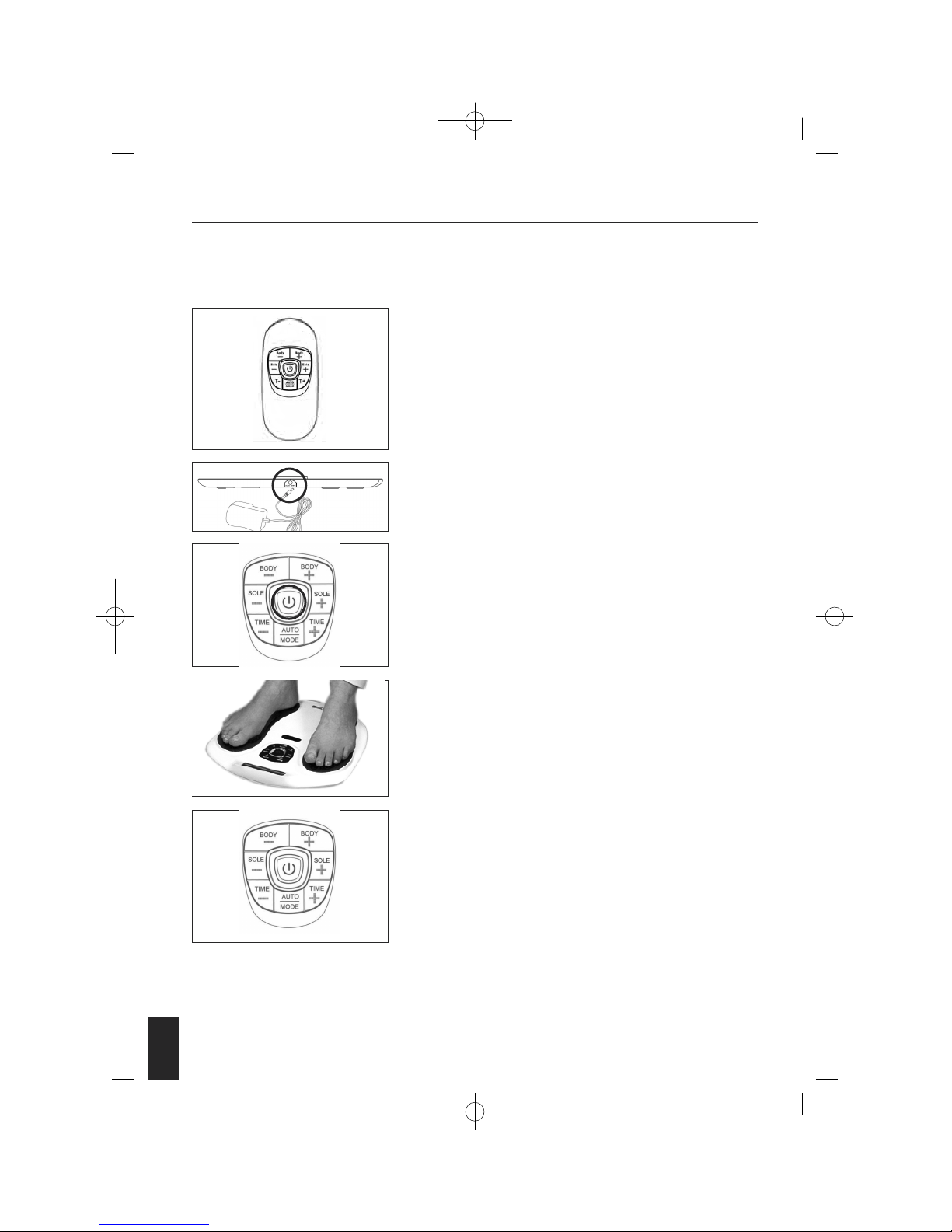
Remove your Circulation Pro from the packaging. Take out the
Remote Control and remove the screw from the back door using the
screwdriver provided. Then insert 2 AAA batteries into the
compartment as per indication. Then screw the battery door closed.
Please refer to page 15 for a step-by-step guide on changing the
battery on the remote control.
Connect the DC adapter to a suitable power outlet and plug the
small DC socket into the device.
Turn on the power. The central display will light up orange and
go off.
Remove your footwear and socks or stockings. PLACE YOUR BARE
FEET ONTO THE FOOT PADS. YOUR RIGHT FOOT ON THE RIGHT FOOT
PAD AND YOUR LEFT FOOT ON THE LEFT FOOT PAD. BOTH FEET
NEED TO BE ON THE DEVICE FOR IT TO WORK.
Sit in a comfortable chair. Place your bare feet on the left and right
foot plates. Increase the intensity levels for the foot by pressing the
“SOLE+” or press “SOLE – “ to decrease the intensity. The intensity
level ranges from 0-99, slowly increase the level until you begin to
feel the micro-current stimulation.
1
2
3
4
5
4
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 2
3
E
IMPORTANT SAFETY INFORMATION
1) Please read these instructions thoroughly before use.
2) Please check that you have all of the component parts as detailed in this user manual.
3) Take all parts out of the plastic bags and examine them to familiarize yourself with the components.
Notes on safety
Danger
This unit must not be used in combination with the following medical devices:
(1) Internally transplanted electronic medical devices, e.g. pacemakers
(2) Electronic life support equipment, such as respirators
(3) Electronic medical devices attached to the body, such as electrocardiographs
Using this unit with other electronic medical devices may cause erroneous operation of those devices.
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 3

4
E
Warning
Persons with the following conditions must consult a doctor before using this unit:
1) acute disease
2) malignant tumor
3) infectious disease
4) pregnancy
5) cardiac dysfunction
6) high fever
7) abnormal blood pressure
8) skin sensory disorders or skin problems
9) receiving medical treatment, especially those feeling discomfort. May cause an accident or ill health.
Do not use this unit near the heart, above the neck, on the head, around the mouth or on diseased skin.
May cause an accident or ill health.
- Application of electrodes between the neck and diaphragm (chest area) may increase the risk of cardiac fibrillation.
Do not use this unit simultaneously with other therapeutic device or in combination with
ointments including spray type ointments.
May cause discomfort or ill health.
- Operation in close proximity (e.g. 1 m) to a shortwave or microwave therapy EQUIPMENT may produce
instability in the STIMULATOR output.
Do not use this unit for purposes other than treatment indicated in this manual.
May lead to accident, problems, or failure of the unit.
Do not insert the electrode cord plug into any place other than the electrode cord jack of the main unit.
May cause an electric shock or accident.
Do not disassemble or remodel this unit.
No user serviceable parts.
Caution
If the unit is not functioning properly or you feel discomfort, immediately stop using the unit.
If you feel any problems with your body or skin, consult a doctor and follow his/her instructions.
If you want to move the Electrode Pad to another region or your body during treatment, be sure to turn off the power first.
If not, you may receive a strong electrical shock.
Do not try to attach the Pads to any other person during the treatment.
You may receive strong electrical shock.
Do not start treatment while wearing an electronic device.
The settings and timings of the device may be affected.
Do not use this unit on infants or people not capable of expressing their intentions.
May cause an accident or ill health.
Do not use this unit in places with high humidity such as bathrooms or while taking a bath or shower.
You will receive a strong electrical shock.
Do not use this unit while sleeping.
The main unit may develop trouble, or the pad may move to an unexpected region and cause ill health.
Do not use this unit while driving.
Receiving sudden strong stimulation may lead to traffic accident.
Do not leave the Electrode Pad attached to the skin after treatment.
Prolonged attachment may cause skin irritation or infection.
Be careful not to allow any metal object, such as a belt buckle or necklace to come into contact with the Electrode Pad during treatment.
You may receive a strong electrical shock.
Do not use cellular phones or other electronic devices near this unit.
Put the Long Life pads only on skin or on the Long Life pads holder to avoid damage of the adhesive
surfaces of the pads.
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 4

5
E
Important information regarding Electro Magnetic Compatibility
With the increased number of electronic devices such as PC’s and mobile (cellular) telephones, medical
devices in use may be susceptible to electromagnetic interference from other devices. Electromagnetic
interference may result in incorrect operation of the medical device and create a potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the aim to prevent unsafe
product situations, the IEC 60601-1-2 standard has been implemented. This standard defines the levels of
immunity to electromagnetic interferences as well as maximum levels of electromagnetic emissions for
medical devices.
This medical device manufactured by Homedics conforms to this IEC 60601-1-2 standard for both immunity
and emissions. Nevertheless, special precautions need to be observed:
Do not use mobile (cellular) telephones and other devices, which generate strong electrical or
electromagnetic fields, near the medical device. This may result in incorrect operation of the unit and create a
potentially unsafe situation.
Recommendation is to keep a minimum distance of 7 m. Verify correct operation of the device in case the
distance is shorter.
CB-200-CA needs special precautions regarding EMC and needs to be installed and put into service according
to the EMC information provided in the ACCOMPANYING DOCUMENTS.
Portable and mobile RF communications equipment can affect CB-200-CA.
WARNING that the use of accessories, transducers and cables other than those supplied with the exception of
the transducers and cables sold by the manufacturer of the CB-200-CA as replacement parts for internal
components, may result in increased EMISSIONS or decreased IMMUNITY of CB-200-CA.
WARNING the CB-200-CA should not be used adjacent to or stacked with other equipment.
Equipment not suitable for use in the presence of a flammable anaesthetic mixture with air or with oxygen or
nitrous oxide.
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 5
6
E
WHAT IS ELECTRONIC NERVE STIMULATION?
INTENDED USE: Medical Purpose
This Electronic Nerve Stimulator is intended to be used as a massager to relieve (muscle) pain, increase blood
circulation, relax stiff muscles, reduce swollen feet, ankles and fatigue. The massaging effect is achieved by
electronic stimulation of the nerves through electrode pads placed on the skin. Various massage regions and
treatment programs can be selected.
Suitable Users: Please read “Notes on safety” before using the unit. (This unit should not be used by people
prohibited from doing so in “Notes on safety”.).
Environment: This unit is intended for home use only.
Effectiveness: Massager: relieve of (muscle) pain, stiffness and fatigue.
Precautions for use: Please read “Notes on safety” before using the unit.
Electronic Nerve Stimulation is a non-invasive, safe nerve stimulation intended to reduce pain. The Circulation
Pro uses proven neuromuscular electrical stimulation therapy to send micro current pulses through the soles
of your feet. This type of electrical stimulation is clinically proven to be safe and effective and can be used in
the comfort of your own home. The Circulation Pro improves muscles function by stimulating nerves and
increasing the flow of blood helping to reduce PAIN, SWELLING, TIRED AND ACHING LEGS.
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 6
7
E
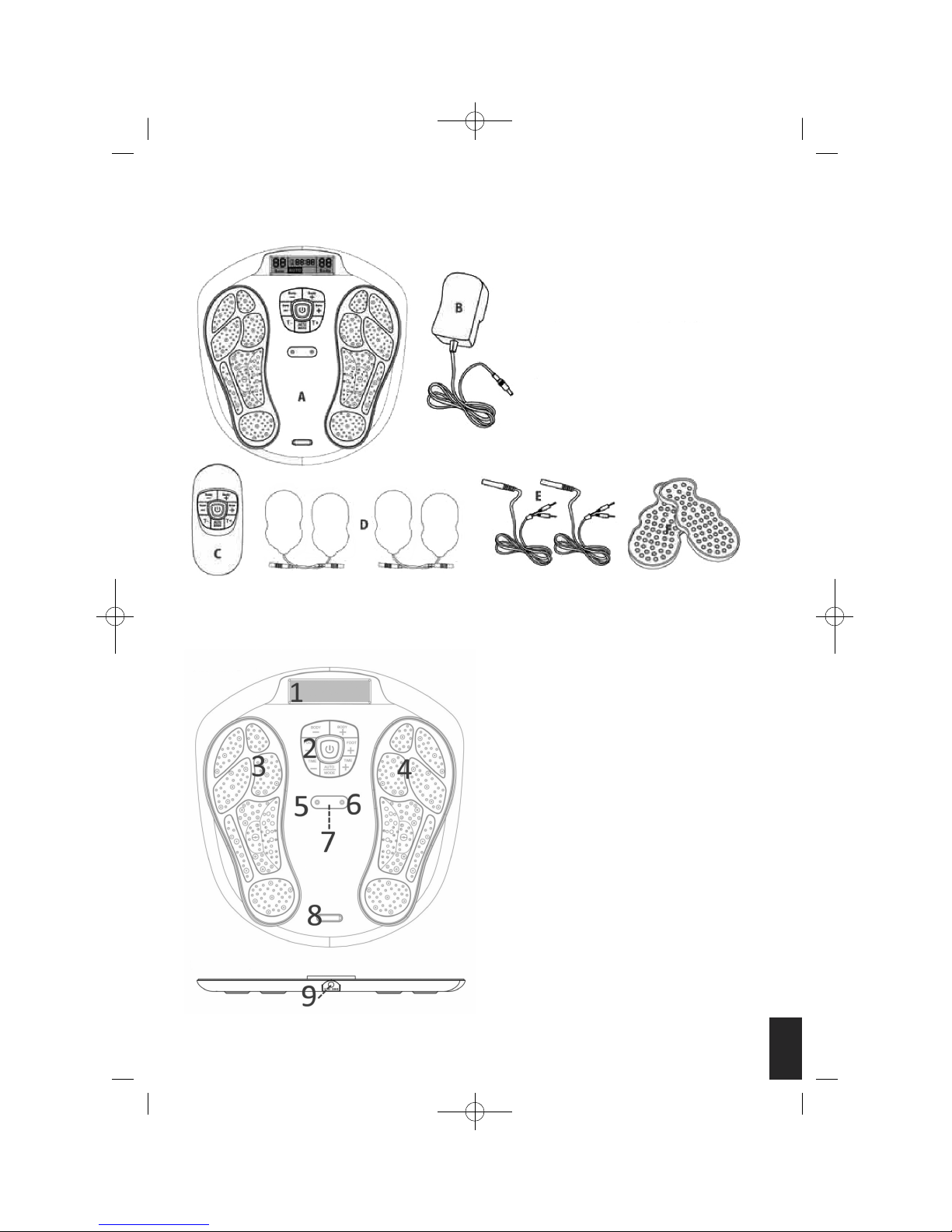
MACHINE OVERVIEW AND PART NAMES
A. Main Unit
B. AC Power Adapter
C. Remote Control
D. Electrode Gel Pad x 4 pcs.
E. Wire cable connecting the
Electrode Gel pads and device
F. Plastic Protector for Gel Pads
1. LCD Display Screen
2. Control Panel
3. Output for the feet – Electrode Area for Left Foot
4. Output for the feet – Electrode Area for Right
Foot
5. Cable Connecting the Electrode Gel Pads and
Device.
6. Cable Connecting the Electrode Gel Pads and
Device.
7. Remote Control Receiver Sensor
8. Silver colour decoration plate
9. Adaptor Jack
Top View
Side View
13-0095 CB200-CA_IM_E_vE.qxd:Layout 1 7/2/13 2:05 PM Page 7
 Loading...
Loading...