HoMedics Circulator, CB-200-GB Instruction Manual
1
Instruction Manual
Customer Service Number +44 (0) 1732 378 557
CB-200-GB
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 1
2
GB
QUICK START GUIDE
PLEASE NOTE – THIS DEVICE DOES NOT VIBRATE – IT USES ELECTRICAL IMPULSES, NOT VIBRATION!
For detailed operation of your Circulator please refer to the comprehensive instructions within this
manual.
For a full explanation of setting the intensity, refer to page 14
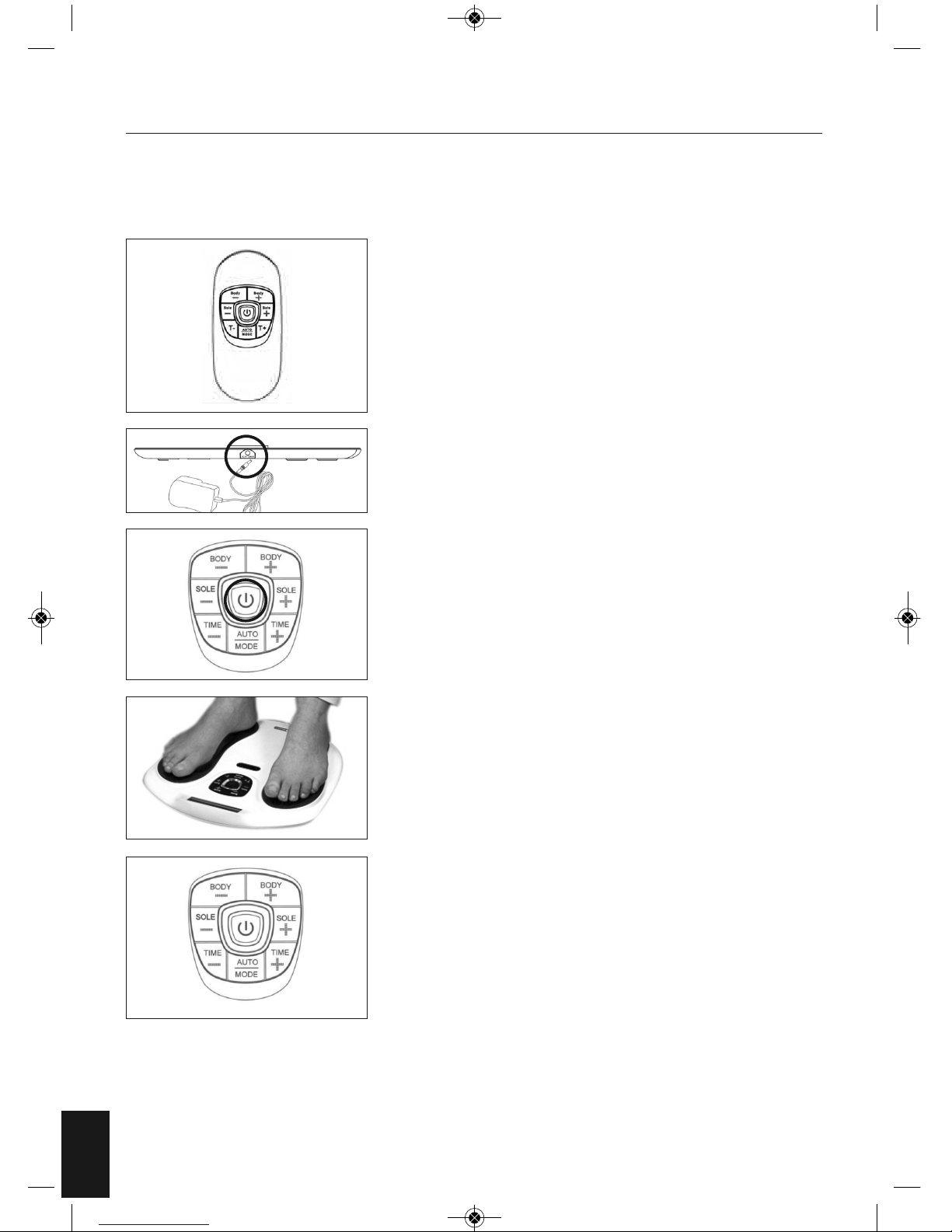
Remove your Circulator from the packaging. Take out the Remote
Control and remove the screw from the back door using a
screwdriver. Then insert 2 piece AAA batteries into the compartment
as per indication. Then screw up the battery door. Please refer to
page 16 for a step-by-step guide on changing the battery on the
remote control.
Connect the DC adapter to a suitable mains outlet and plug the
small DC socket into the device.
Turn on the power the central display will light up orange and go off.
Remove your footwear and socks or stockings. PLACE YOUR BARE
FEET ONTO THE FOOT PADS. YOUR RIGHT FOOT ON THE RIGHT FOOT
PAD AND YOUR LEFT FOOT ON THE LEFT FOOT PAD. BOTH FEET
NEED TO BE ON THE DEVICE FOR IT TO WORK.
Sit in a comfortable chair. Place your bare feet on the left and right
foot plates. Increase the intensity levels for the foot by pressing the
“SOLE+” or to press “SOLE – “ to decrease the intensity. The intensity
level ranges from 0-99, slowly increase the level until you begin to
feel the micro-current stimulation.
1
2
3
4
5
4
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 2
3
GB
IMPORTANT CUSTOMER INFORMATION
PLEASE READ:
Q: How do I use it?
A: Simply place your ‘BARE FEET’ ON THE FOOT PADS. The right foot on the right footpad and the
left foot on the left footpad at the same time. The device will not work unless you have your
soles on the foot pads.
Q: Does it vibrate?
A: No. This device DOES NOT VIBRATE. The Circulator has been specifically designed to send tiny
electrical impulses through the soles of your feet. This action causes your calf muscles to
contract and release forcing the blood back up through the veins in your legs.
Q: I’m not feeling anything in my feet or legs?
A: Please note that the ‘intensity’ level goes all the way up to 99. The aim is not to get to 99, but to
a level that suits you. This level may change on a daily basis.
Q: My feet are very dry and I’m not feeling the electrical impulses
A: Remember to keep hydrated; drink plenty of fluids. Also, if you moisturise your feet, this will
boost the health benefits.
Q: Is it difficult to use?
A: No. Simply place your bare feet onto the footpads, select the intensity setting and it
automatically counts down from 30 minutes.
Q: Am I too old to get any benefit from it?
A: No. The product is suitable for any adult age.
Q: Can it really help me? I’m not very active and sit for most of the day.
A: Yes. As we sit, the blood naturally pools to the lower legs due to gravity, this is a natural action
in the body. If we do not take frequent walks or exercise, the blood will pool and could cause
your legs and feet issues such as swelling and poor blood circulation. The Circulator may
reduce these symptoms.
Q: My legs ache after using the device
A: Either you had it on a SOLE setting that was too high for you (so reduce this setting next time
you use it) or you have used it too many times within a few days. Just give your legs time to
relax and then re-use the device.
WARNING
The product should not be used during pregnancy, by people fitted with a
pacemaker or other implanted medical device, or anyone being treated for Deep
Vein Thrombosis (DVT). Not suitable for persons under 16 years of age. Any
questions, please call our Customer Service Number or consult your health
professional.
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 3
4
GB
IMPORTANT SAFETY INFORMATION
1) Please read these instructions thoroughly before use.
2) Please check that you have all of the component parts as detailed in this user manual.
3) Take all parts out of the plastic bags and examine them to familiarize yourself with the components.
Notes on safety
• The icons and warning signs are indicated here for your safety and correct usage of the product as well as
to prevent injuries and/or damage to properties.
• The icons and meanings are as follows:
D
escription of markings
The icon indicates prohibitions (must not do).
Matters involving certain prohibitions are indicated by text or pictures in or near.
The icon to the left means “Prohibitions to Disassemble”.
The icon indicates something that is compulsory (must be observed).
Matters involving certain compulsory actions are indicated by text or pictures in or near.
The icon to the left refers to “General compulsory action”.
This product should not be used by persons with medical implants, e.g. heart pacemakers, artificial heart, lung
or other electronic life support systems.
This symbol indicates that batteries must not be disposed of in the domestic waste as they contain substances
which can be damaging to the environment and health. Please dispose of batteries in designated collection
points.
This marking indicates that this product should not be disposed with other household wastes throughout the
EU. To prevent possible harm to the environment or human health from uncontrolled waste disposal, recycle it
responsibly to promote the sustainable reuse of material resources. To return your used device, please use the
return and collection systems or contact the retailer where the product was purchased. They can take this
product for environmental safe recycling.
Consult instructions for use.
Date of manufacturers.
Manufacturer’s name.
Batch Code.
Class II equipment
Caution, consult accompanying documents
Type BF applied Part
This symbol means serial number which is on the underside of the device and on the packaging.
This symbol indicates that the unit meets the basic requirements set by the CE Directive 93/42/EEC concerning
medical devices.
Danger
This unit must not be used in combination with the following medical devices:
(1) Internally transplanted electronic medical devices, e.g. pacemakers
(2) Electronic life support equipment, such as respirators
(3) Electronic medical devices attached to the body, such as electrocardiographs
Using this unit with other electronic medical devices may cause erroneous operation of those devices.
0120
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 4
5
GB
W
arning
Persons with the following conditions must consult a doctor before using this unit:
1) acute disease
2) malignant tumor
3
) infectious disease
4) pregnancy
5) cardiac dysfunction
6
) high fever
7
) abnormal blood pressure
8) skin sensory disorders or skin problems
9
) receiving medical treatment, especially those feeling discomfort. May cause an accident or ill health.
Do not use this unit near the heart, above the neck, on the head, around the mouth or on diseased skin.
May cause an accident or ill health.
- Application of electrodes between the neck and diaphragm (chest area) may increase the risk of cardiac fibrillation.
Do not use this unit simultaneously with other therapeutic device or in combination with
ointments including spray type ointments.
May cause discomfort or ill health.
-
Simultaneous connection of a PATIENT to a h.f. surgical EQUIPMENT may result in burns at the site of the
STIMULATOR electrodes and possible damage to the STIMULATOR.
- Operation in close proximity (e.g. 1 m) to a shortwave or microwave therapy EQUIPMENT may produce
instability in the STIMULATOR output.
Do not use this unit for purposes other than treatment indicated in this manual.
May lead to accident, problems, or failure of the unit.
Do not insert the electrode cord plug into any place other than the electrode cord jack of the main unit.
May cause an electric shock or accident.
Do not disassemble or remodel this unit.
No user serviceable parts.
Caution
If the unit is not functioning properly or you feel discomfort, immediately stop using the unit.
If you feel any problems with your body or skin, consult a doctor and follow his/her instructions.
If you want to move the Electrode Pad to another region or your body during treatment, be sure to turn
off the power first.
If not, you may receive a strong electrical shock.
Do not try to attach the Pads to any other person during the treatment.
You may receive strong electrical shock.
Do not start treatment while wearing an electronic device.
The settings and timings of the device may be affected.
Do not use this unit on infants or people not capable of expressing their intentions.
May cause an accident or ill health.
Do not use this unit in places with high humidity such as bathrooms or while taking a bath or shower.
You will receive a strong electrical shock.
Do not use this unit while sleeping.
The main unit may develop trouble, or the pad may move to an unexpected region and cause ill health.
Do not use this unit while driving.
Receiving sudden strong stimulation may lead to traffic accident.
Do not leave the Electrode Pad attached to the skin after treatment.
Prolonged attachment may cause skin irritation or infection.
Be careful not to allow any metal object, such as a belt buckle or necklace to come into contact with the
Electrode Pad during treatment.
You may receive a strong electrical shock.
Do not use cellular phones or other electronic devices near this unit.
Put the Long Life pads only on skin or on the Long Life pads holder to avoid damage of the adhesive
surfaces of the pads.
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 5
6
GB
Important information regarding Electro Magnetic Compatibility
With the increased number of electronic devices such as PC’s and mobile (cellular) telephones, medical
devices in use may be susceptible to electromagnetic interference from other devices. Electromagnetic
interference may result in incorrect operation of the medical device and create a potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the aim to prevent unsafe
product situations, the EN 60601-1-2 standard has been implemented. This standard defines the levels of
immunity to electromagnetic interferences as well as maximum levels of electromagnetic emissions for
medical devices.
This medical device manufactured by Homedics conforms to this EN 60601-1-2 standard for both immunity
and emissions. Nevertheless, special precautions need to be observed:
Do not use mobile (cellular) telephones and other devices, which generate strong electrical or
electromagnetic fields, near the medical device. This may result in incorrect operation of the unit and create a
potentially unsafe situation.
CB-200-GB needs special precautions regarding EMC and needs to be installed and put into service according
to the EMC information provided in the ACCOMPANYING DOCUMENTS.
Portable and mobile RF communications equipment can affect CB-200-GB.
WARNING that the use of accessories, transducers and cables other than those supplied with the exception of
the transducers and cables sold by the manufacturer of the CB-200-GB as replacement parts for internal
components, may result in increased EMISSIONS or decreased IMMUNITY of CB-200-GB.
WARNING the CB-200-GB should not be used adjacent to or stacked with other equipment.
Equipment not suitable for use in the presence of a flammable anaesthetic mixture with air or with oxygen or
nitrous oxide.
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 6
7
GB
WHAT IS ELECTRONIC NERVE STIMULATION?
INTENDED USE: Medical Purpose
This Electronic Nerve Stimulator is intended to be used as a massager to relieve (muscle) pain, increase blood
circulation, relax stiff muscles, reduce swollen feet, ankles and fatigue in the feet and lower legs. The
massaging effect is achieved by electronic stimulation of the nerves through electrode pads placed on the
skin. Various massage regions and treatment programs can be selected.
Suitable Users: Please read “Notes on safety” before using the unit. (This unit should not be used by people
prohibited from doing so in “Notes on safety”.).
Environment: This unit is intended for home use only.
Effectiveness: Massager: relief of (muscle) pain, stiffness and fatigue in the feet and lower legs.
Precautions for use: Please read “Notes on safety” before using the unit.
Electronic Nerve Stimulation is a non-invasive, safe nerve stimulation intended to reduce pain. The Circulator
uses proven neuromuscular electrical stimulation therapy to send micro current pulses through the soles of
your feet. This type of electrical stimulation is clinically proven to be safe and effective and can be carried out
in the comfort of your own home. The Circulator improves muscles function by stimulating nerves increasing
the flow of blood helping to reduce PAIN, SWELLING, TIRED AND ACHING LEGS.
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 7
8
GB
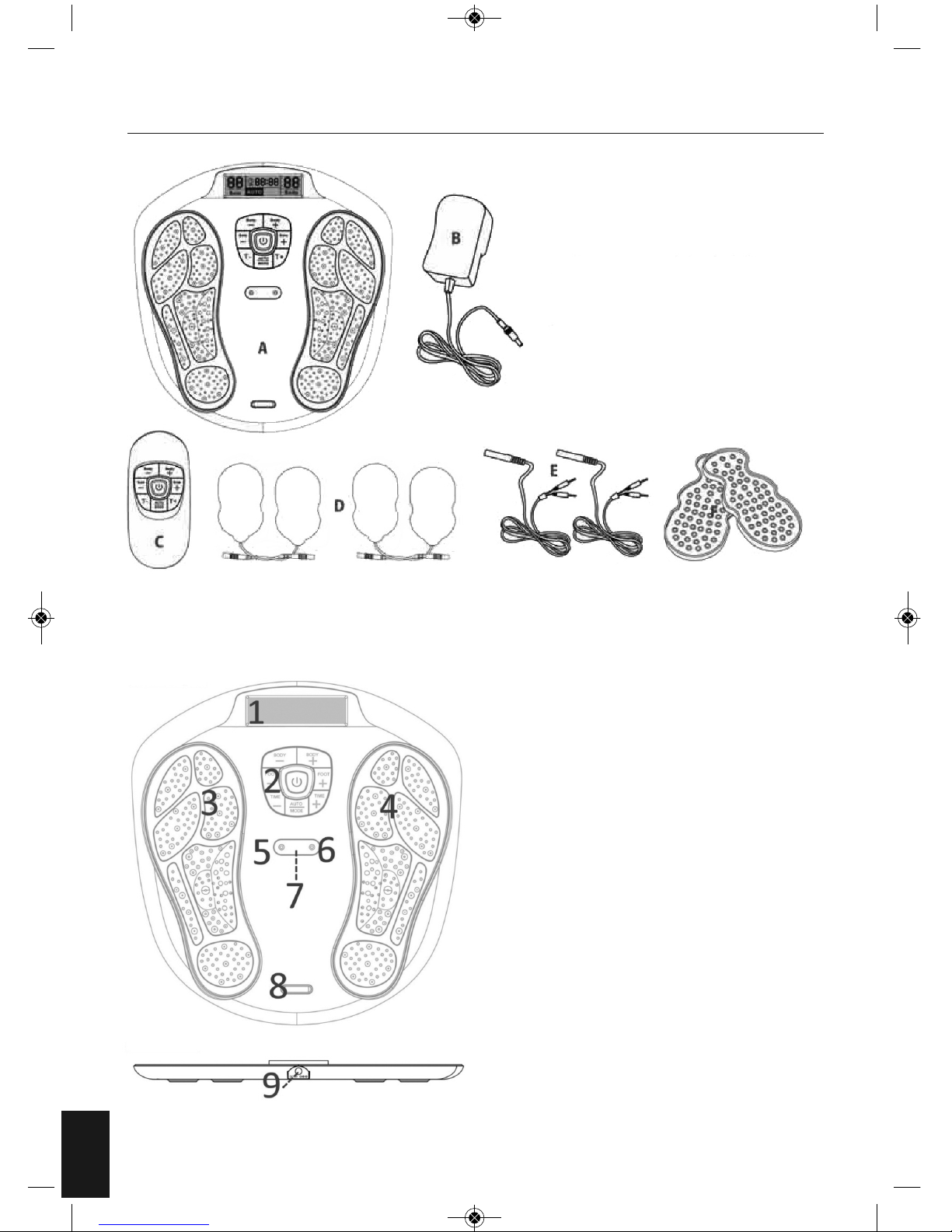
MACHINE OVERVIEW AND PART NAMES
A. Main Unit
B. AC Power Adapter
C. Remote Control
D. Electrode Gel Pad x 4 pcs.
E. Wire cable connecting the
Electrode Gel pads and device
F. Plastic Protector for Gel Pads
1. LCD Display Screen
2. Control Panel
3. Output for the feet – Electrode Area for Left Foot
4. Output for the feet – Electrode Area for Right
Foot
5. Cable Connecting the Electrode Gel Pads and
Device.
6. Cable Connecting the Electrode Gel Pads and
Device.
7. Remote Control Receiver Sensor
8. Silver colour decoration plate
9. Adaptor Jack
Top View
Side View
IB-CB200GB-1213-02_Layout 1 16/12/2013 12:02 Page 8
 Loading...
Loading...