HoMedics CB-200-EU Instruction Manual

1
Instruction Manual
CB-200-EU
Circulation Pro
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 1
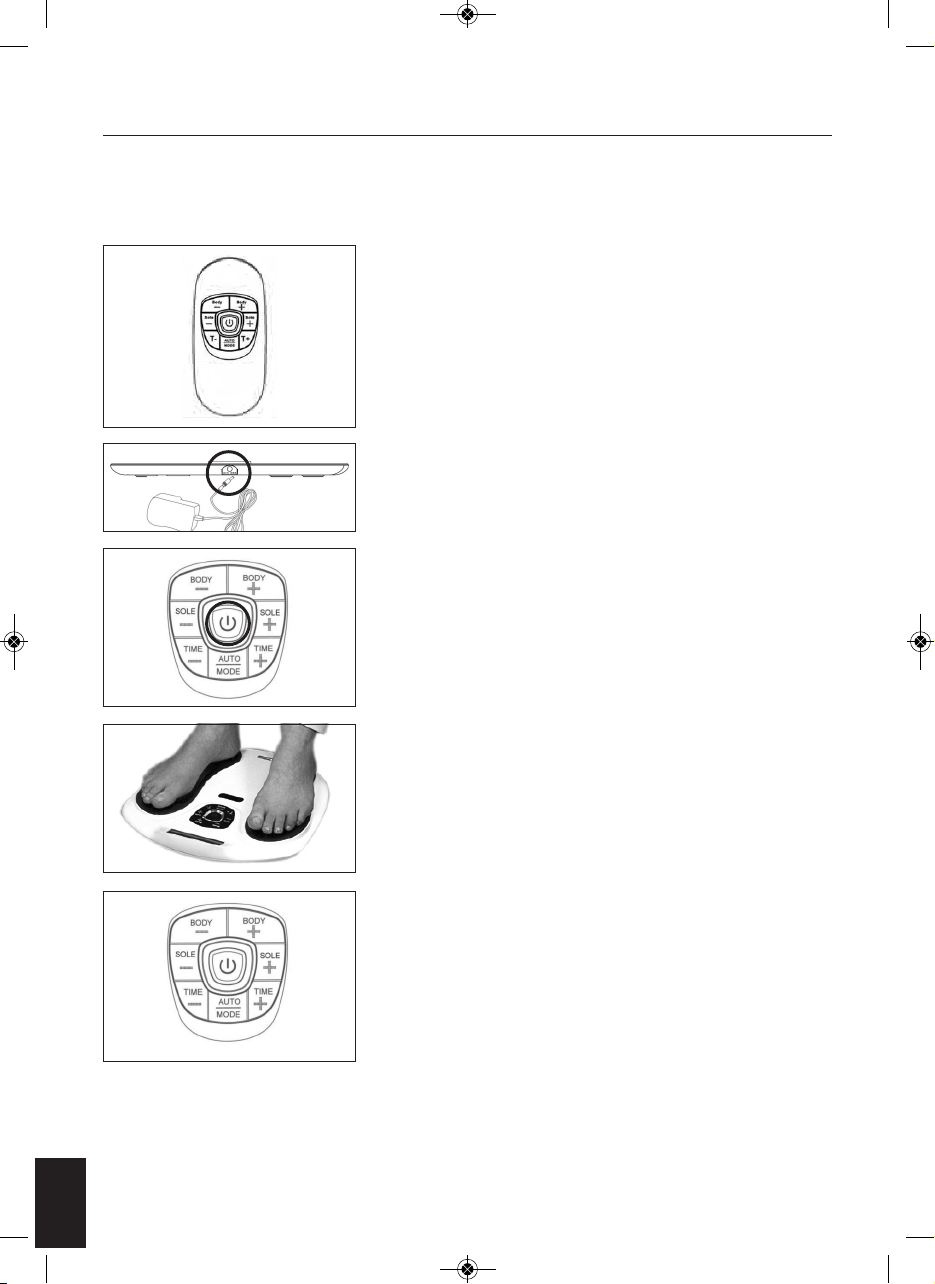
2
GB
QUICK START GUIDE
PLEASE NOTE – THIS DEVICE DOES NOT VIBRATE – IT USES ELECTRICAL IMPULSES, NOT VIBRATION! For
detailed operation of your Circulation Pro please refer to the comprehensive instructions within this
manual.
For a full explanation of setting the intensity, refer to page 14
Remove your Circulation Pro from the packaging. Take out the
Remote Control and remove the screw from the back door using a
screwdriver. Then insert 2 piece AAA batteries into the compartment
as per indication. Then screw up the battery door. Please refer to
page 16 for a step-by-step guide on changing the battery on the
remote control.
Connect the DC adapter to a suitable mains outlet and plug the
small DC socket into the device.
Turn on the power the central display will light up orange and go off.
Remove your footwear and socks or stockings. PLACE YOUR BARE
FEET ONTO THE FOOT PADS. YOUR RIGHT FOOT ON THE RIGHT FOOT
PAD AND YOUR LEFT FOOT ON THE LEFT FOOT PAD. BOTH FEET
NEED TO BE ON THE DEVICE FOR IT TO WORK.
Sit in a comfortable chair. Place your bare feet on the left and right
foot plates. Increase the intensity levels for the foot by pressing the
“SOLE+” or to press “SOLE – “ to decrease the intensity. The intensity
level ranges from 0-99, slowly increase the level until you begin to
feel the micro-current stimulation.
1
2
3
4
5
4
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 2
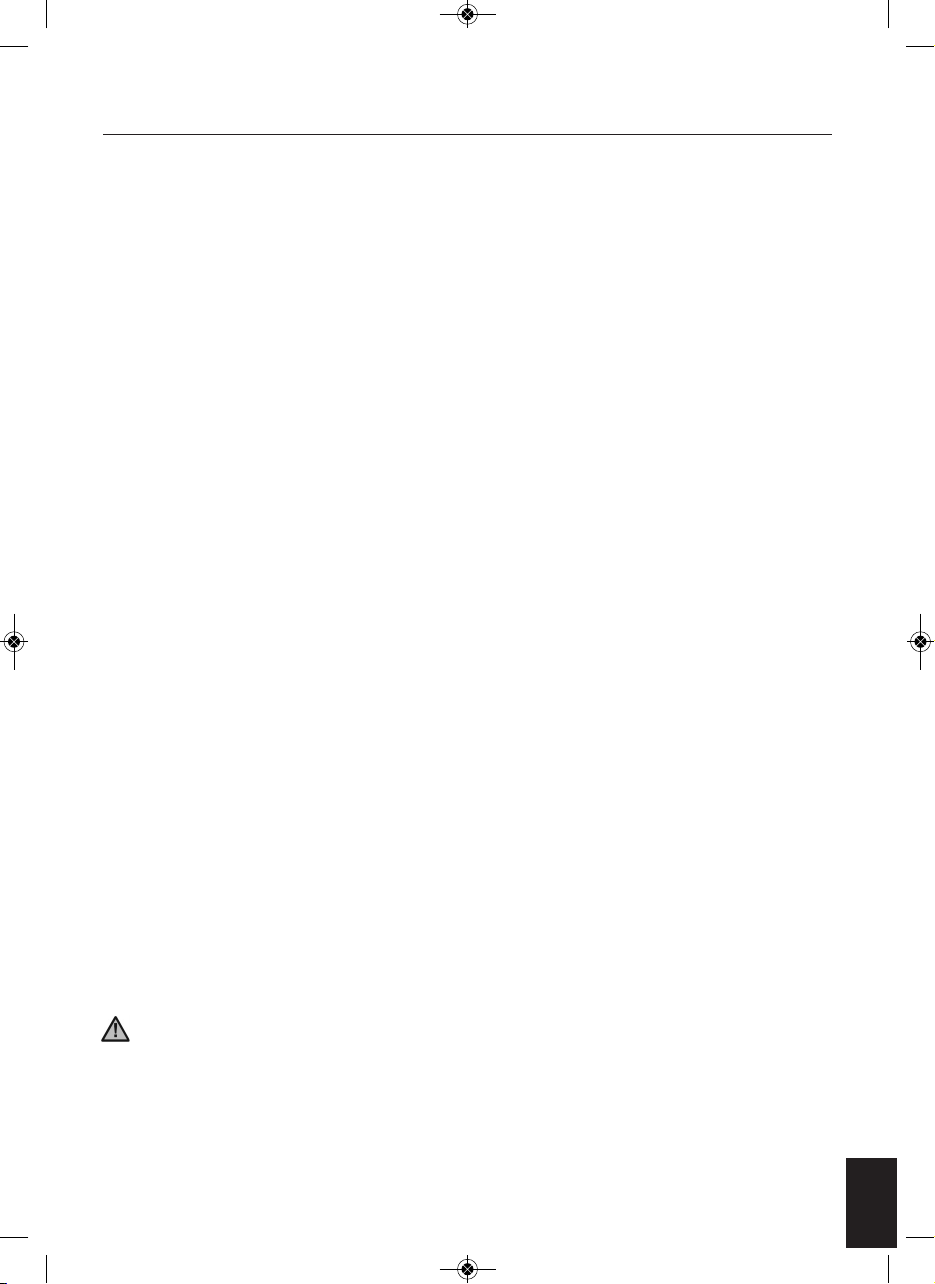
3
GB
IMPORTANT CUSTOMER INFORMATION
PLEASE READ:
Q: How do I use it?
A: Simply place your ‘BARE FEET’ ON THE FOOT PADS. The right foot on the right footpad and the
left foot on the left footpad at the same time. The device will not work unless you have your
soles on the foot pads.
Q: Does it vibrate?
A: No. This device DOES NOT VIBRATE. Circulation Pro has been specifically designed to send tiny
electrical impulses through the soles of your feet. This action causes your calf muscles to
contract and release forcing the blood back up through the veins in your legs.
Q: I’m not feeling anything in my feet or legs?
A: Please note that the ‘intensity’ level goes all the way up to 99. The aim is not to get to 99, but to
a level that suits you. This level may change on a daily basis.
Q: My feet are very dry and I’m not feeling the electrical impulses.
A: Remember to keep hydrated; drink plenty of fluids. Also, if you moisturise your feet, this will
boost the health benefits.
Q: Is it difficult to use?
A: No. Simply place your bare feet onto the footpads, select the intensity setting and it
automatically counts down from 30 minutes.
Q: Am I too old to get any benefit from it?
A: No. The product is suitable for any adult age.
Q: Can it really help me? I’m not very active and sit for most of the day.
A: Yes. As we sit, the blood naturally pools to the lower legs due to gravity, this is a natural action
in the body. If we do not take frequent walks or exercise, the blood will pool and could cause
your legs and feet issues such as swelling and poor blood circulation. Circulation Pro may
reduce these symptoms.
Q: My legs ache after using the device.
A: Either you had it on a SOLE setting that was too high for you (so reduce this setting next time
you use it) or you have used it too many times within a few days. Just give your legs time to
relax and then re-use the device.
WARNING
Should not be used by women in the first trimester of pregnancy, by people fitted
with a pacemaker or other implanted medical device, or anyone being treated for
existing deep vein thrombosis (DVT). Any questions, please call our Customer
Service Number or consult your health professional.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 3
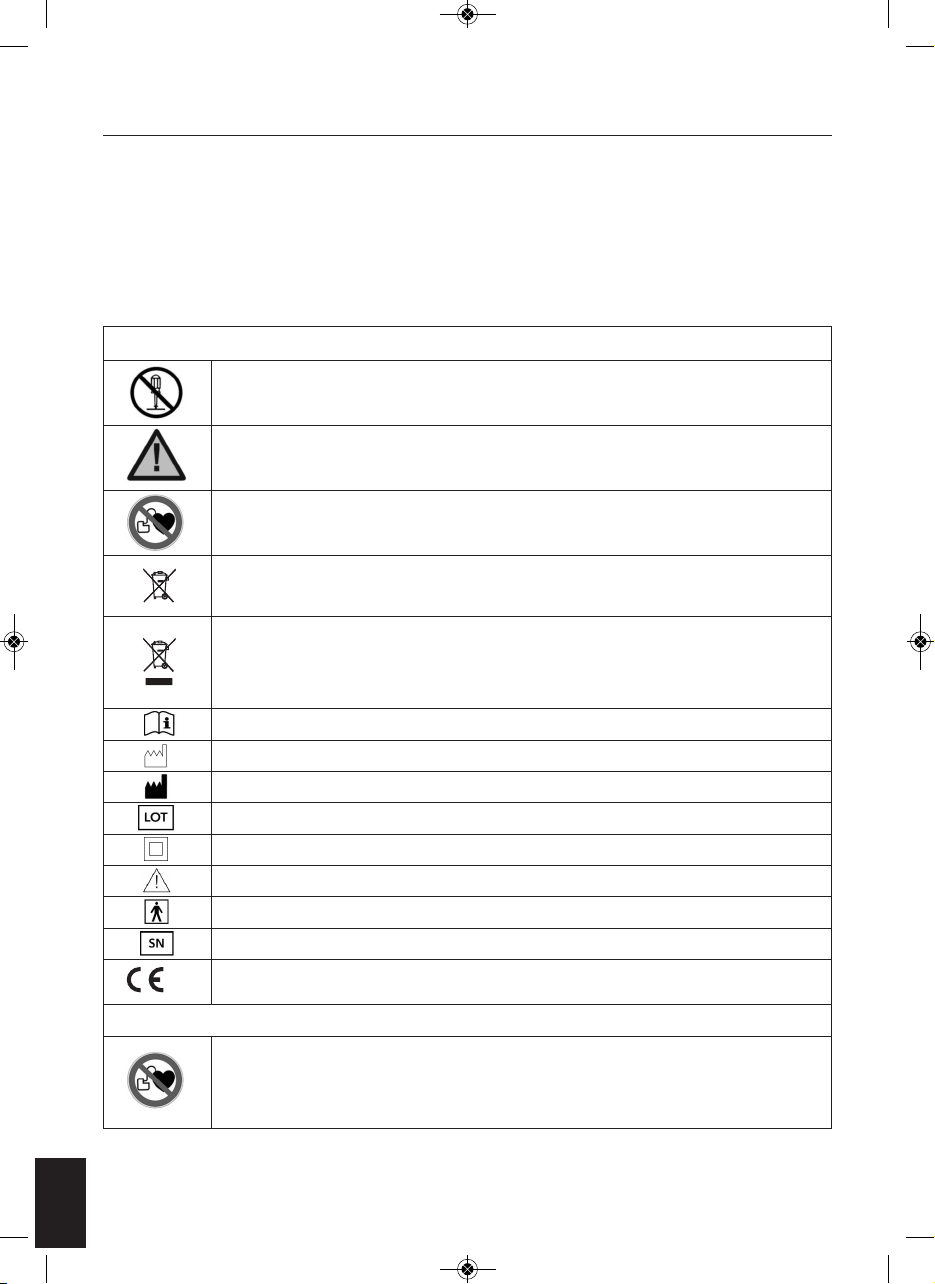
4
GB
IMPORTANT SAFETY INFORMATION
1) Please read these instructions thoroughly before use.
2) Please check that you have all of the component parts as detailed in this user manual.
3) Take all parts out of the plastic bags and examine them to familiarize yourself with the components.
Notes on safety
• The icons and warning signs are indicated here for your safety and correct usage of the product as well as
to prevent injuries and/or damage to properties.
• The icons and meanings are as follows:
D
escription of markings
The icon indicates prohibitions (must not do).
Matters involving certain prohibitions are indicated by text or pictures in or near.
The icon to the left means “Prohibitions to Disassemble”.
The icon indicates something that is compulsory (must be observed).
Matters involving certain compulsory actions are indicated by text or pictures in or near.
The icon to the left refers to “General compulsory action”.
This product should not be used by persons with medical implants, e.g. heart pacemakers, artificial heart, lung
or other electronic life support systems.
This symbol indicates that batteries must not be disposed of in the domestic waste as they contain substances
which can be damaging to the environment and health. Please dispose of batteries in designated collection
points.
This marking indicates that this product should not be disposed with other household wastes throughout the
EU. To prevent possible harm to the environment or human health from uncontrolled waste disposal, recycle it
responsibly to promote the sustainable reuse of material resources. To return your used device, please use the
return and collection systems or contact the retailer where the product was purchased. They can take this
product for environmental safe recycling.
Consult instructions for use.
Date of manufacturers.
Manufacturer’s name.
Batch Code.
Class II equipment
Caution, consult accompanying documents
Type BF applied Part
This symbol means serial number which is on the underside of the device and on the packaging.
This symbol indicates that the unit meets the basic requirements set by the CE Directive 93/42/EEC concerning
medical devices.
Danger
This unit must not be used in combination with the following medical devices:
(1) Internally transplanted electronic medical devices, e.g. pacemakers
(2) Electronic life support equipment, such as respirators
(3) Electronic medical devices attached to the body, such as electrocardiographs
Using this unit with other electronic medical devices may cause erroneous operation of those devices.
0120
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 4
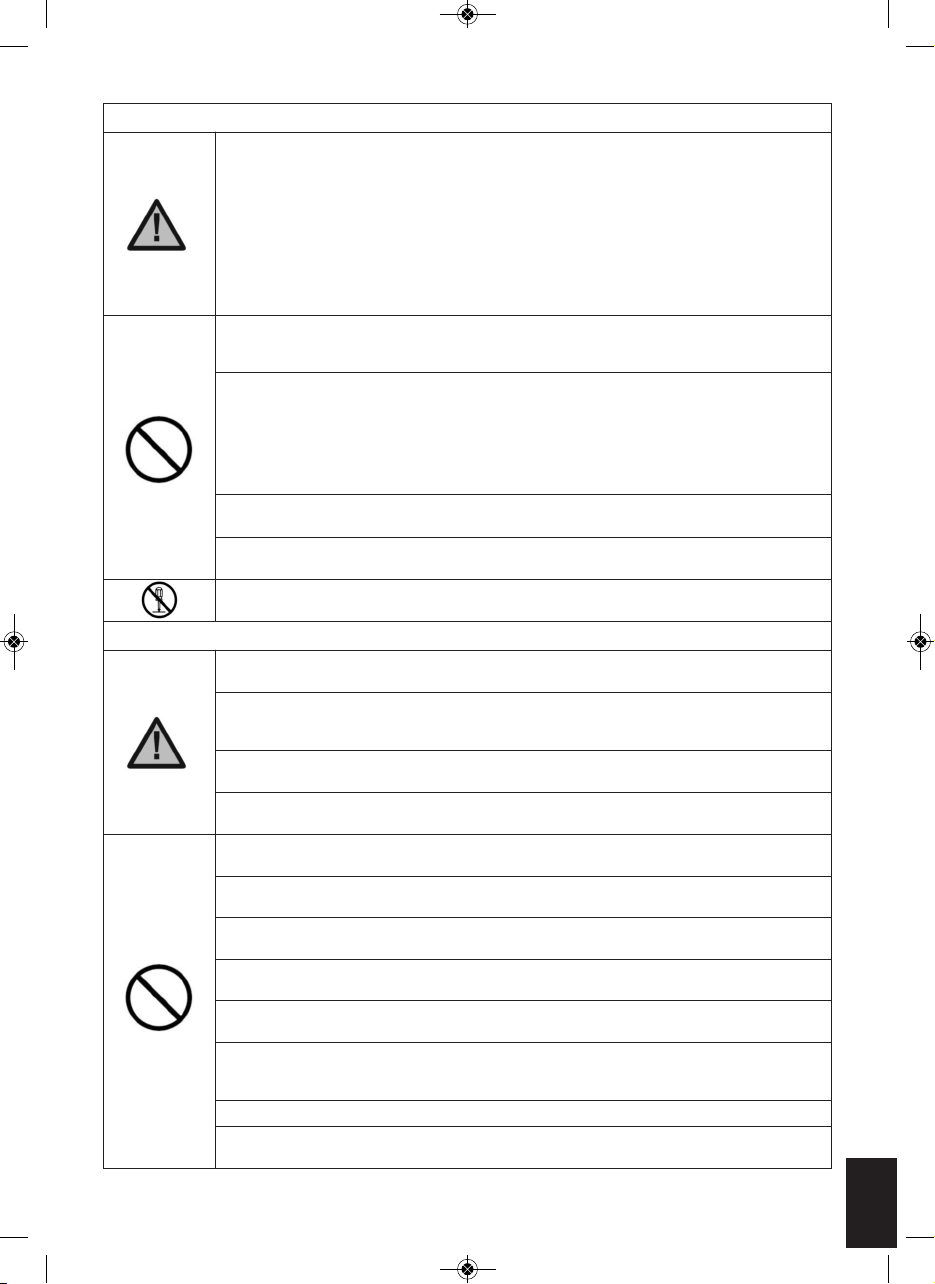
5
GB
W
arning
Persons with the following conditions must consult a doctor before using this unit:
1) acute disease
2) malignant tumor
3
) infectious disease
4) pregnancy
5) cardiac dysfunction
6
) high fever
7
) abnormal blood pressure
8) skin sensory disorders or skin problems
9
) receiving medical treatment, especially those feeling discomfort. May cause an accident or ill health.
Do not use this unit near the heart, above the neck, on the head, around the mouth or on diseased skin.
May cause an accident or ill health.
- Application of electrodes between the neck and diaphragm (chest area) may increase the risk of cardiac fibrillation.
Do not use this unit simultaneously with other therapeutic device or in combination with
ointments including spray type ointments.
May cause discomfort or ill health.
-
Simultaneous connection of a PATIENT to a h.f. surgical EQUIPMENT may result in burns at the site of the
STIMULATOR electrodes and possible damage to the STIMULATOR.
- Operation in close proximity (e.g. 1 m) to a shortwave or microwave therapy EQUIPMENT may produce
instability in the STIMULATOR output.
Do not use this unit for purposes other than treatment indicated in this manual.
May lead to accident, problems, or failure of the unit.
Do not insert the electrode cord plug into any place other than the electrode cord jack of the main unit.
May cause an electric shock or accident.
Do not disassemble or remodel this unit.
No user serviceable parts.
Caution
If the unit is not functioning properly or you feel discomfort, immediately stop using the unit.
If you feel any problems with your body or skin, consult a doctor and follow his/her instructions.
If you want to move the Electrode Pad to another region or your body during treatment, be sure to turn
off the power first.
If not, you may receive a strong electrical shock.
Do not try to attach the Pads to any other person during the treatment.
You may receive strong electrical shock.
Do not start treatment while wearing an electronic device.
The settings and timings of the device may be affected.
Do not use this unit on infants or people not capable of expressing their intentions.
May cause an accident or ill health.
Do not use this unit in places with high humidity such as bathrooms or while taking a bath or shower.
You will receive a strong electrical shock.
Do not use this unit while sleeping.
The main unit may develop trouble, or the pad may move to an unexpected region and cause ill health.
Do not use this unit while driving.
Receiving sudden strong stimulation may lead to traffic accident.
Do not leave the Electrode Pad attached to the skin after treatment.
Pro longed attachment may cause skin irritation or infection.
Be careful not to allow any metal object, such as a belt buckle or necklace to come into contact with the
Electrode Pad during treatment.
You may receive a strong electrical shock.
Do not use cellular phones or other electronic devices near this unit.
Put the Long Life pads only on skin or on the Long Life pads holder to avoid damage of the adhesive
surfaces of the pads.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 5
6
GB
Important information regarding Electro Magnetic Compatibility
With the increased number of electronic devices such as PC’s and mobile (cellular) telephones, medical
devices in use may be susceptible to electromagnetic interference from other devices. Electromagnetic
interference may result in incorrect operation of the medical device and create a potentially unsafe situation.
Medical devices should also not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the aim to prevent unsafe
product situations, the EN 60601-1-2 standard has been implemented. This standard defines the levels of
immunity to electromagnetic interferences as well as maximum levels of electromagnetic emissions for
medical devices.
This medical device manufactured by HoMedics conforms to this EN 60601-1-2 standard for both immunity
and emissions. Nevertheless, special precautions need to be observed:
Do not use mobile (cellular) telephones and other devices, which generate strong electrical or
electromagnetic fields, near the medical device. This may result in incorrect operation of the unit and create a
potentially unsafe situation.
Recommendation is to keep a minimum distance of 7 m. Verify correct operation of the device in case the
distance is shorter.
CB-200-EU needs special precautions regarding EMC and needs to be installed and put into service according
to the EMC information provided in the ACCOMPANYING DOCUMENTS.
Portable and mobile RF communications equipment can affect CB-200-EU.
WARNING: the use of accessories, transducers and cables other than those supplied with the exception of the
transducers and cables sold by the manufacturer of the CB-200-EU as replacement parts for internal
components, may result in increased EMISSIONS or decreased IMMUNITY of CB-200-EU.
WARNING: the CB-200-EU should not be used adjacent to or stacked with other equipment.
Equipment not suitable for use in the presence of a flammable anaesthetic mixture with air or with oxygen or
nitrous oxide.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 6
7
GB
WHAT IS ELECTRONIC NERVE STIMULATION?
INTENDED USE: Medical Purpose
This Electronic Nerve Stimulator is intended to be used as a massager to relieve (muscle) pain, increase blood
circulation, relax stiff muscles, reduce swollen feet, ankles and fatigue. The massaging effect is achieved by
electronic stimulation of the nerves through electrode pads placed on the skin. Various massage regions and
treatment programs can be selected.
Suitable Users: Please read “Notes on safety” before using the unit. (This unit should not be used by people
prohibited from doing so in “Notes on safety”.)
Environment: This unit is intended for home use only.
Effectiveness: Massager: relieve of (muscle) pain, stiffness and fatigue.
Precautions for use: Please read “Notes on safety” before using the unit.
Electronic Nerve Stimulation is a non-invasive, safe nerve stimulation intended to reduce pain. The Circulation
Prouses proven neuromuscular electrical stimulation therapy to send micro current pulses through the soles
of your feet. This type of electrical stimulation is clinically proven to be safe and effective and can be carried
out in the comfort of your own home. The Circulation Pro improves muscles function by stimulating nerves
increasing the flow of blood helping to reduce PAIN, SWELLING, TIRED AND ACHING LEGS.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 7
8
GB
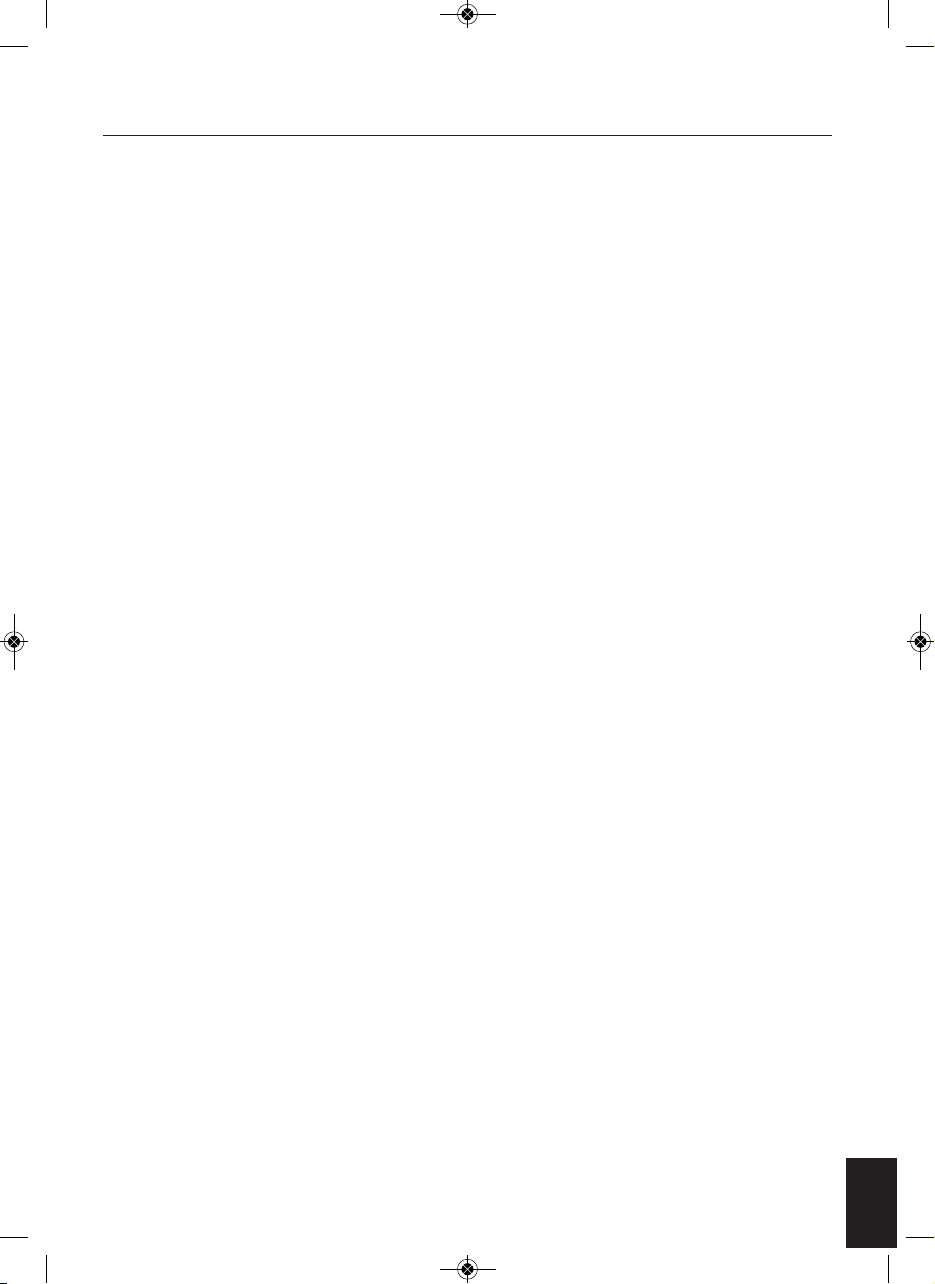
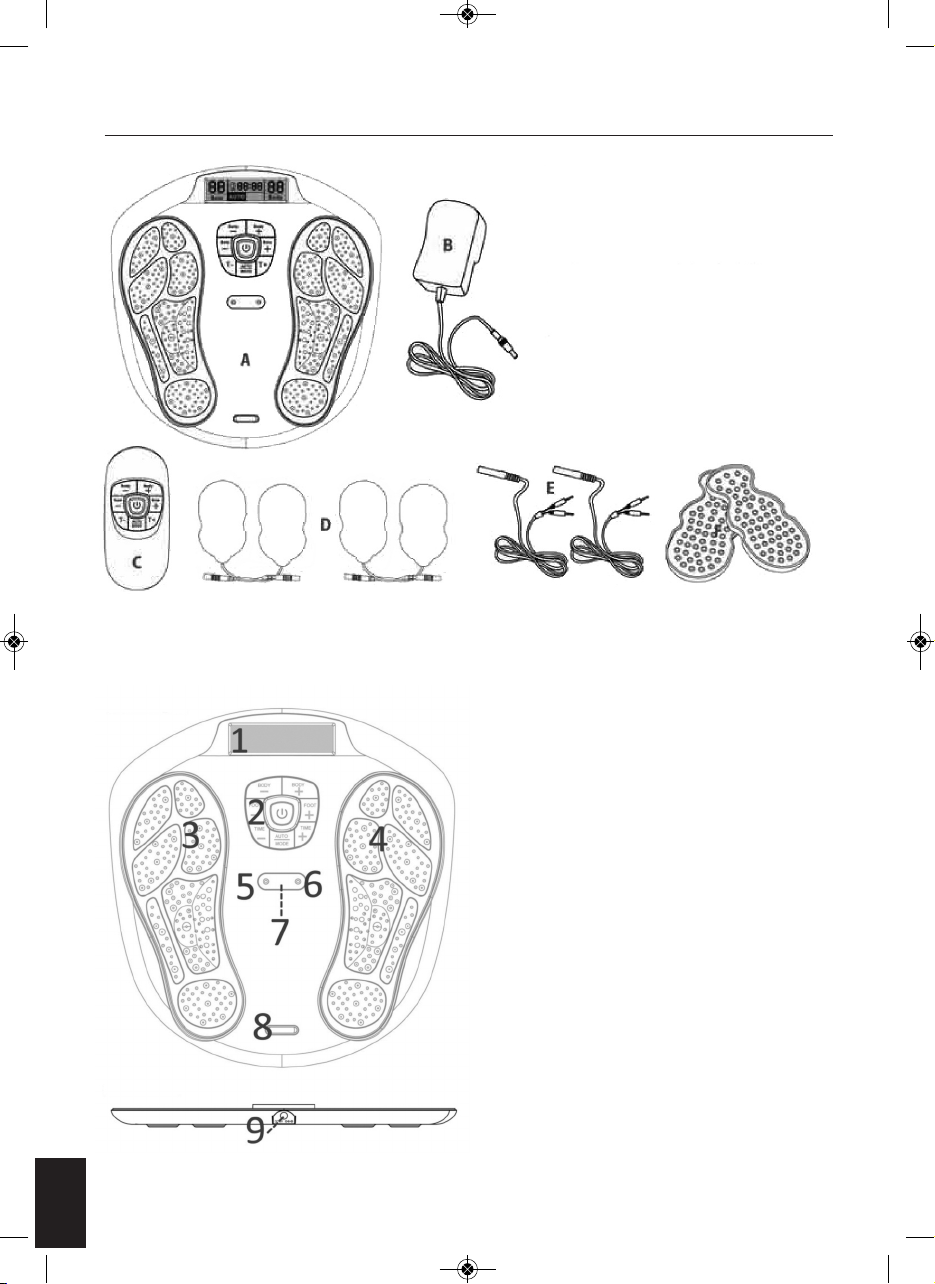
MACHINE OVERVIEW AND PART NAMES
A. Main Unit
B. AC Power Adapter
C. Remote Control
D. Electrode Gel Pad x 4 pcs.
E. Wire cable connecting the
Electrode Gel pads and device
F. Plastic Protector for Gel Pads
1. LCD Display Screen
2. Control Panel
3. Electrode Area for Left Foot
4. Electrode Area for Right Foot
5. Cable Connecting the Electrode Gel Pads and
Device.
6. Cable Connecting the Electrode Gel Pads and
Device.
7. Remote Control Receiver Sensor
8. Silver colour decoration plate
9. Adaptor Jack
Top View
Side View
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 8
9
GB
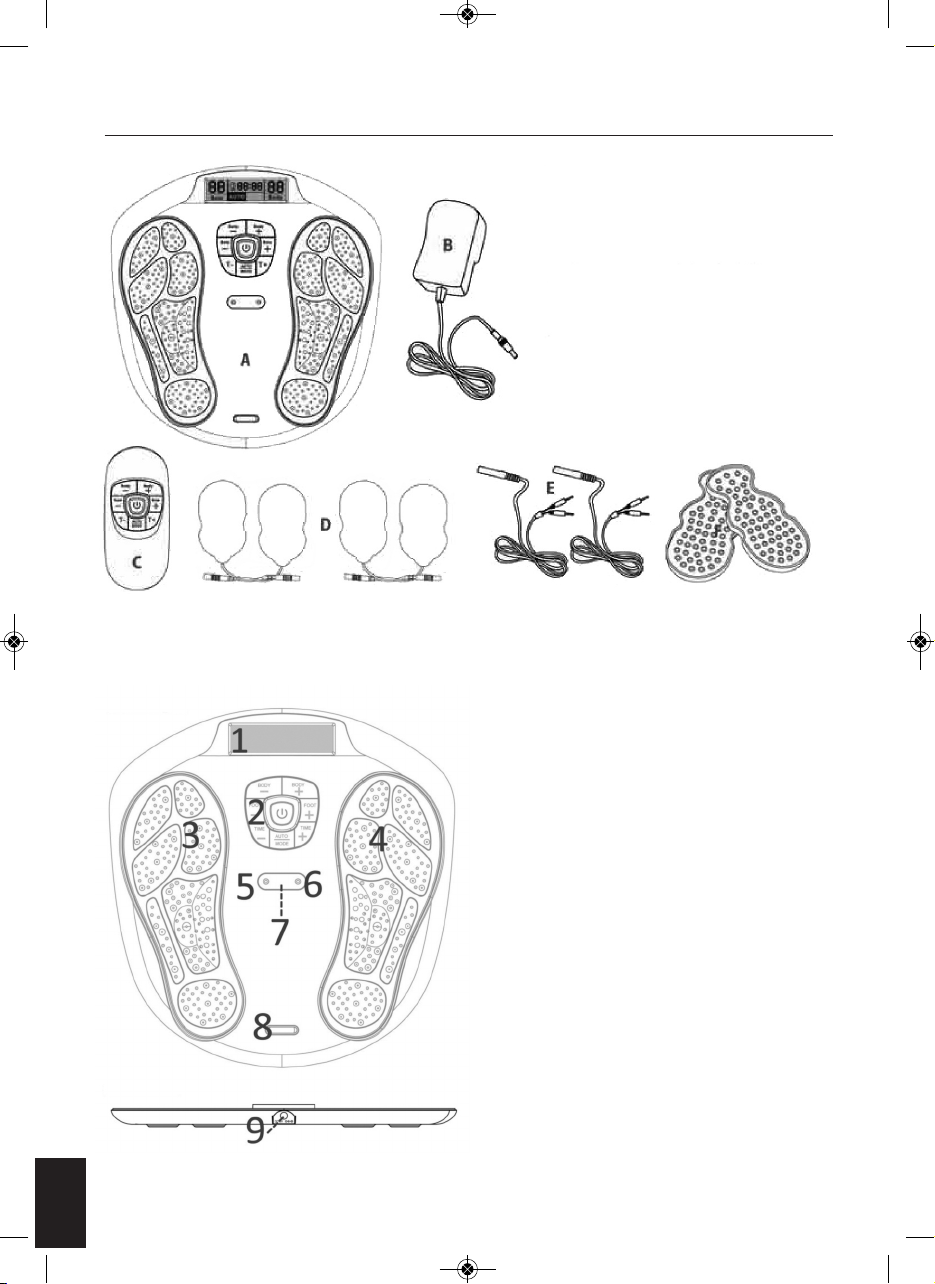
FUNCTION OF CONTROL PANEL
LCD showing the
intensity level for Sole
– maximum 99 levels
LCD showing the
program in AUTO or
in MODE.
LCD showing the timer
LCD showing the
intensity level for body
– maximum 99 levels
On the device the black colour area on the device which
is the electrode area for the sole. (see. the fig. A).
On the gel pad, the black colour
area on the sticky part is the
electrode area for the body, size is
5 cm x 9 cm. (see. the fig. B)
ON/OFF switch button
BODY – Decrease the output intensity of body (Available from 1 - 99 levels)
BODY + Increase the output intensity of body (Available from 1 - 99 levels)
SOLE - Decrease the output intensity of Sole (Available from 1 - 99 levels)
SOLE + Increase the output intensity of sole (Available from 1 - 99 levels)
TIME - Decrease the operation time (available from 1-60 minutes)
TIME + Increase the operation time (available from 1-60 minutes)
AUTO/MODE
Auto - the preset program with 14 pattern in cycle running for Foot and 10 pattern in cycle running
for body
Mode - user can fix the program to the exiting massage pattern on the rest of the time
Electrode area of the unit and the gel pad
Fig. A
Fig. B
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 9

10
GB
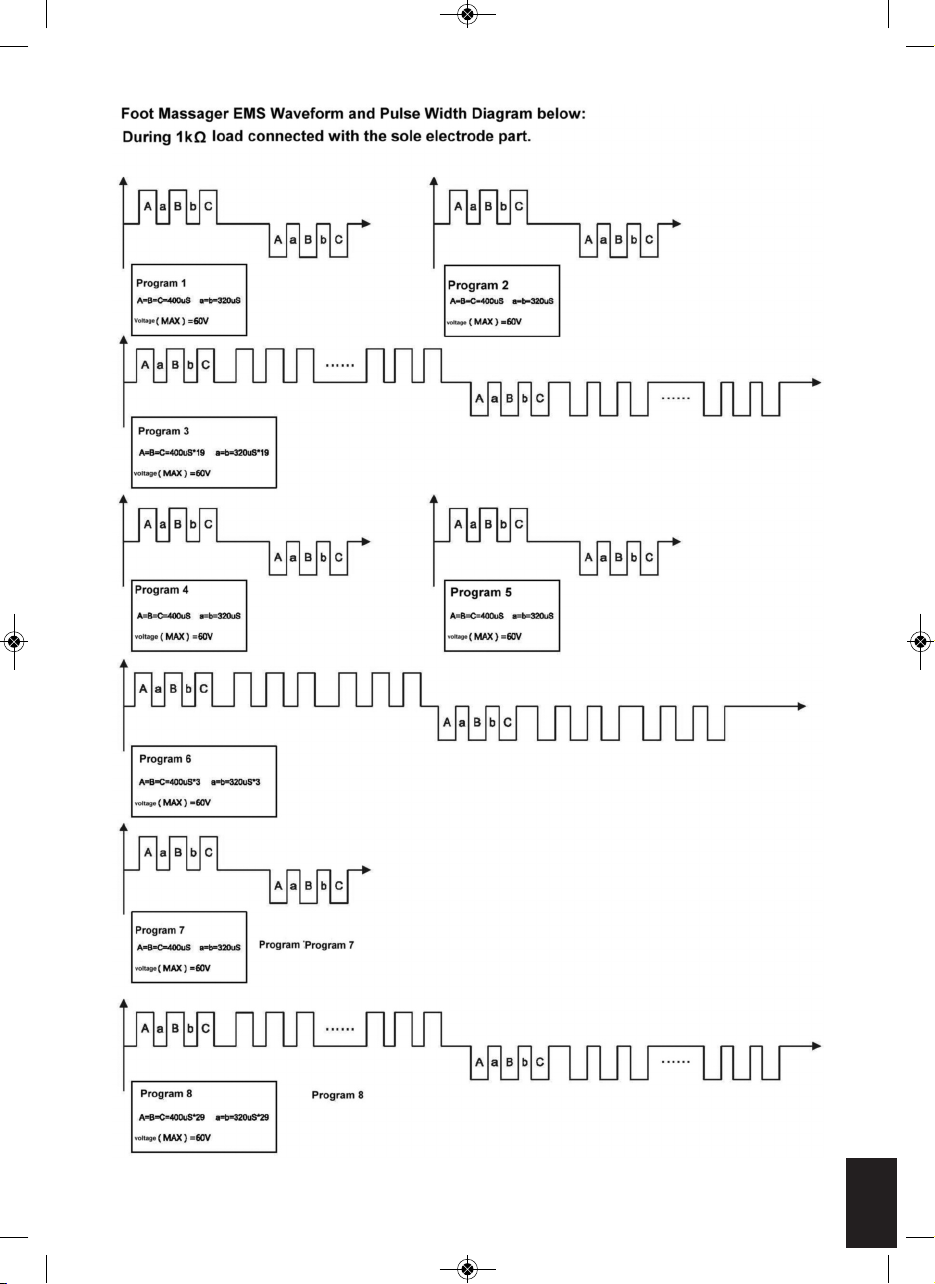
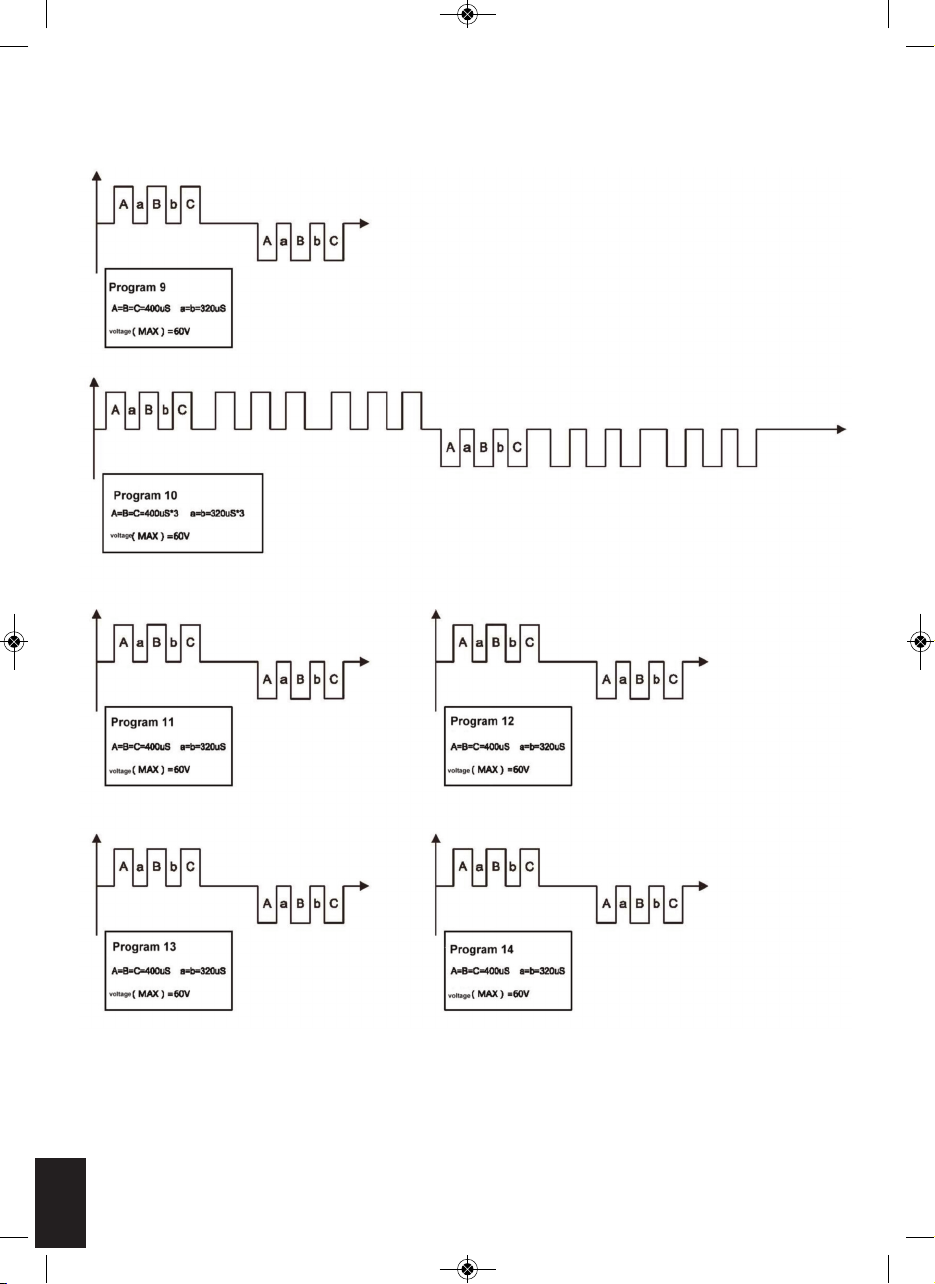
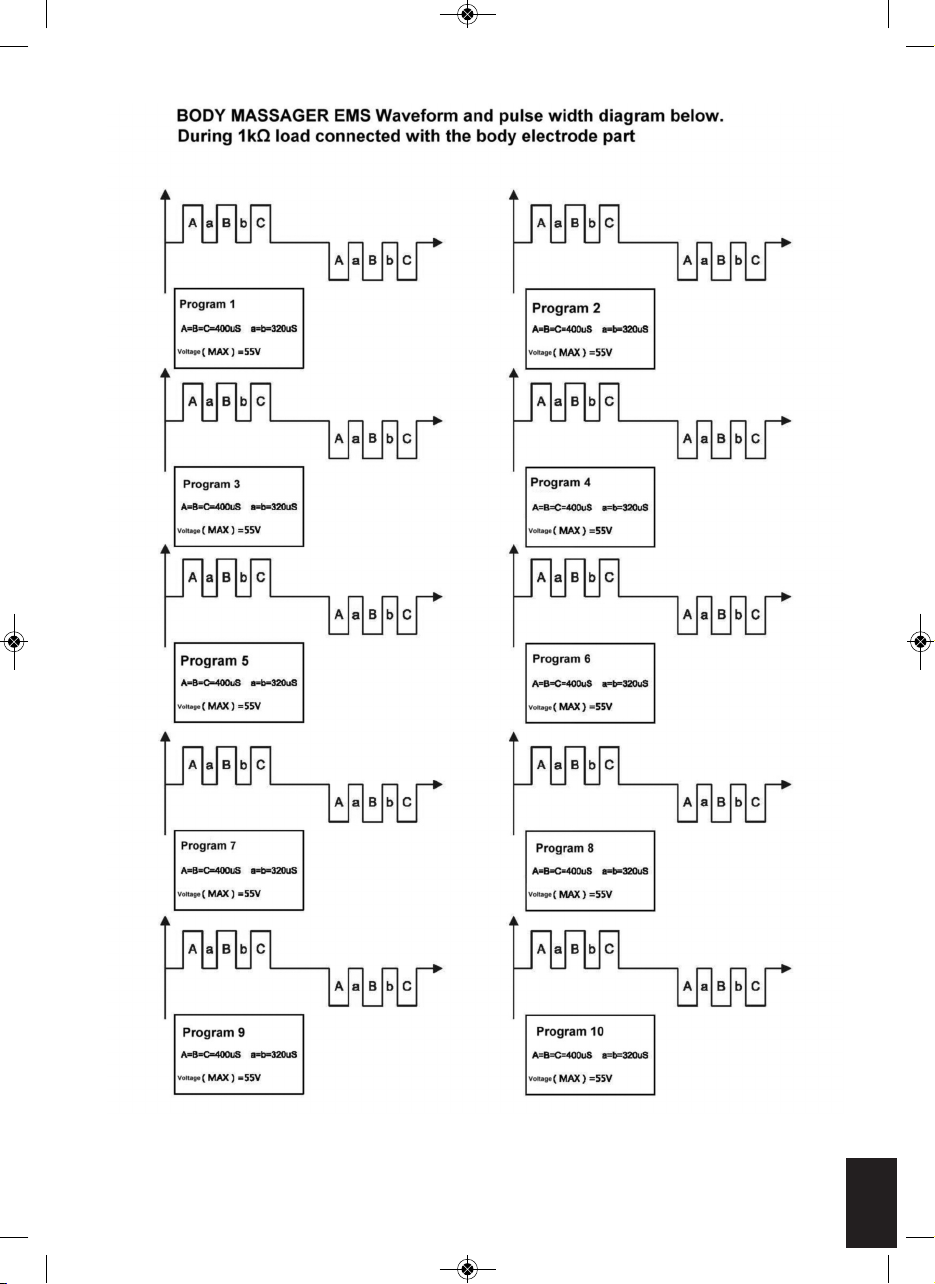
CIRCULATION PRO OUTPUT WAVE FORMS
FOOT ELECTRO THERAPY MASSAGE: We will examine the operation in more detail later in your instructions
but the principle is relatively easy to understand. Place your feet on the electrode areas, turn on Circulation
Prowith the central on/off switch, then increase the intensity for the foot. There are 99 different levels. When
you start to feel the mild electro-therapy will depend on your own nerve sensitivity. Certain individuals will
feel nothing until the intensity is up at a high level, others will feel the stimulation at relatively low levels. This
is completely normal.
BODY TONING: if you choose to tone muscle groups or target pain in other areas of your body the Circulation
pro comes with four gel pads. These can be used to tone arms, hips, thighs, abs or buttocks or target neck
muscle or back pain.
OUTPUT WAVEFORM
***THE OUTPUTS HAVE NO DC COMPONENT
Programme Output
1
Pulse rate 12.2Hz with 8.5 seconds and off time in
900mS, A cycle repeating for 1 minute
2
Pulse rate 16.13Hz with2.8 seconds and off time in
900mS, A cycle repeating for 1 minute
3
Pulse rate 20.0Hz with 8.4 seconds and off time in
900mS, A cycle repeating for 1 minute
4
Pulse rate 16.13Hz with 5.8 seconds and off time in
900mS, A cycle repeating for 1 minute
5
Pulse rate 16.16Hz with 7.0 seconds and off time in
900mS, A cycle repeating for 1 minute
6
Pulse rate 33.33Hz with 2.3 seconds and off time in
900mS, A cycle repeating for 1 minute
7
Pulse rate 12.50Hz with 4.6 seconds and off time in
900mS, A cycle repeating for 1 minute
8
Pulse rate 55.56Hz with 11.5 seconds and off time in
900mS, A cycle repeating for 1 minute
9
Pulse rate 23.32Hz with 5.6 seconds and off time in
900mS, A cycle repeating for 1 minute
10
Pulse rate 20.0Hz with 4.5 seconds and off time in
900mS, A cycle repeating for 1 minute
11
Pulse rate 10Hz with 5.3 seconds and off time in 900mS,
A cycle repeating for 1 minute
12
Pulse rate 16.13Hz with 5.60 seconds and off time in
900mS, A cycle repeating for 1 minute
13
Pulse rate 26.32Hz with 3.5 seconds and off time in
900mS, A cycle repeating for 1 minute
14
Pulse rate 25Hz with 7.0 seconds and off time in 900mS,
A cycle repeating for 1 minute
Programme Output
1
Pulse rate 25.00Hz with 5.8 seconds and off time in
900mS, A cycle repeating for 1 minute
2
Pulse rate 16.67Hz with 11.6 seconds and off time in
900mS, A cycle repeating for 1 minute
3
Pulse rate 12.5hz 9.7 seconds and off time in 900mS, A
cycle repeating for 1 minute
4
Pulse rate 12.50Hz with 4.4 seconds and off time in
900mS, A cycle repeating for 1 minute
5
Pulse rate 25.00Hz with 13 seconds and off time in
900mS, A cycle repeating for 1 minute
6
Pulse rate 16.67Hz with 10.2 seconds and off time in
900mS, A cycle repeating for 1 minute
7
Pulse rate 12.5Hz with 5.6 seconds and off time in
900mS, A cycle repeating for 1 minute
8
Pulse rate 12.5Hz with 18.2 seconds and off time in
900mS, A cycle repeating for 1 minute
9
Pulse rate 16.67Hz with 5.1 seconds and off time in
900mS, A cycle repeating for 1 minute
10
Pulse rate 10Hz with 21.8 seconds and off time in
900mS, A cycle repeating for 1 minute
SOLE MASSAGER (during 1 kΩ load)
The auto mode will cycle through the 14 programs
during the units operation, repeating automatically.
BODY MASSAGER (during 1 kΩ load)
During the operation, the unit will cycle through the
10 programs, repeating automatically.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 10
11
GB
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 11
12
GB
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 12
13
GB
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 13
14
GB
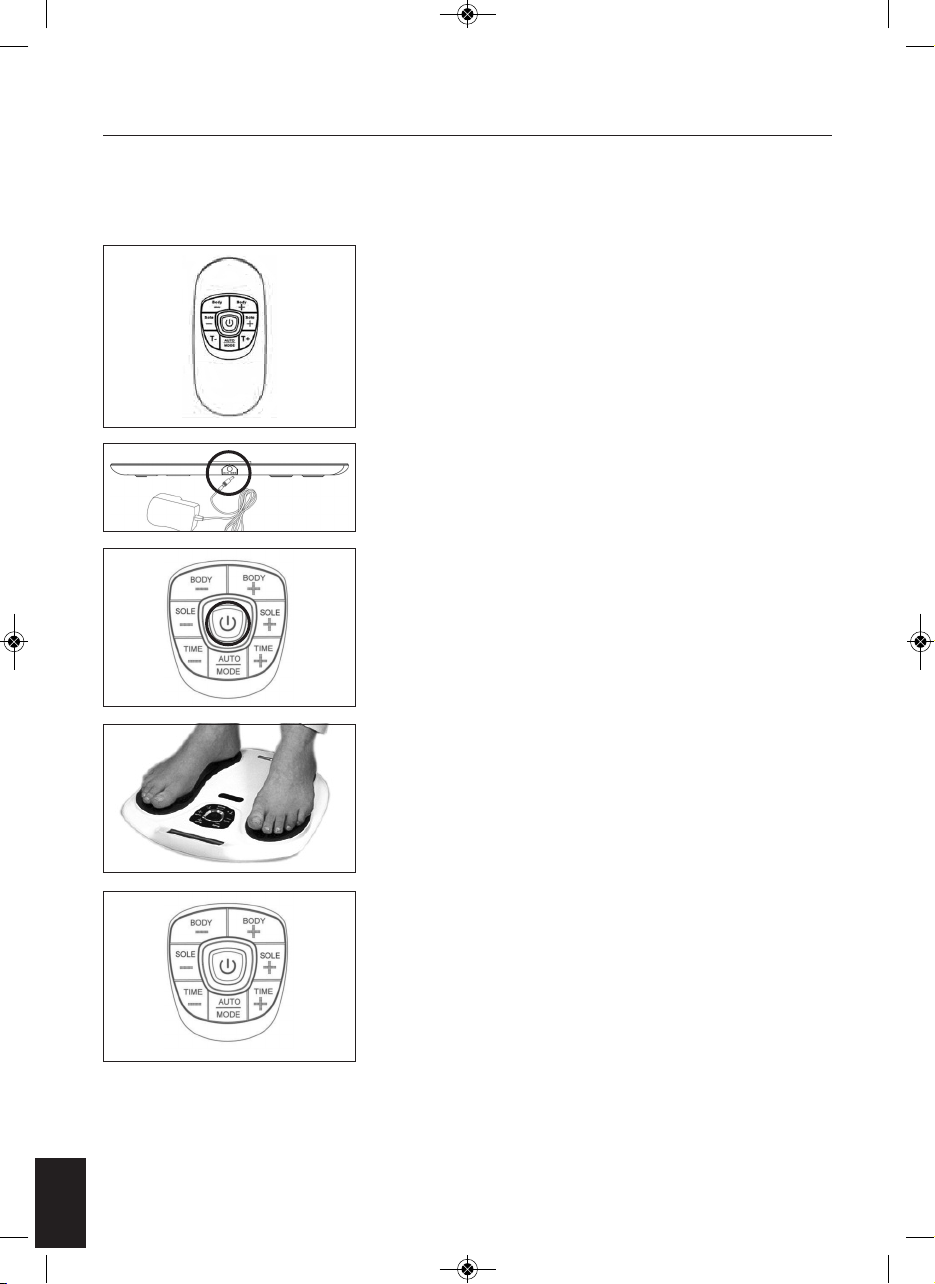
HOW TO OPERATE
For Feet – SOLE
1. Place your bare feet onto Circulation Pro(do not wear socks).
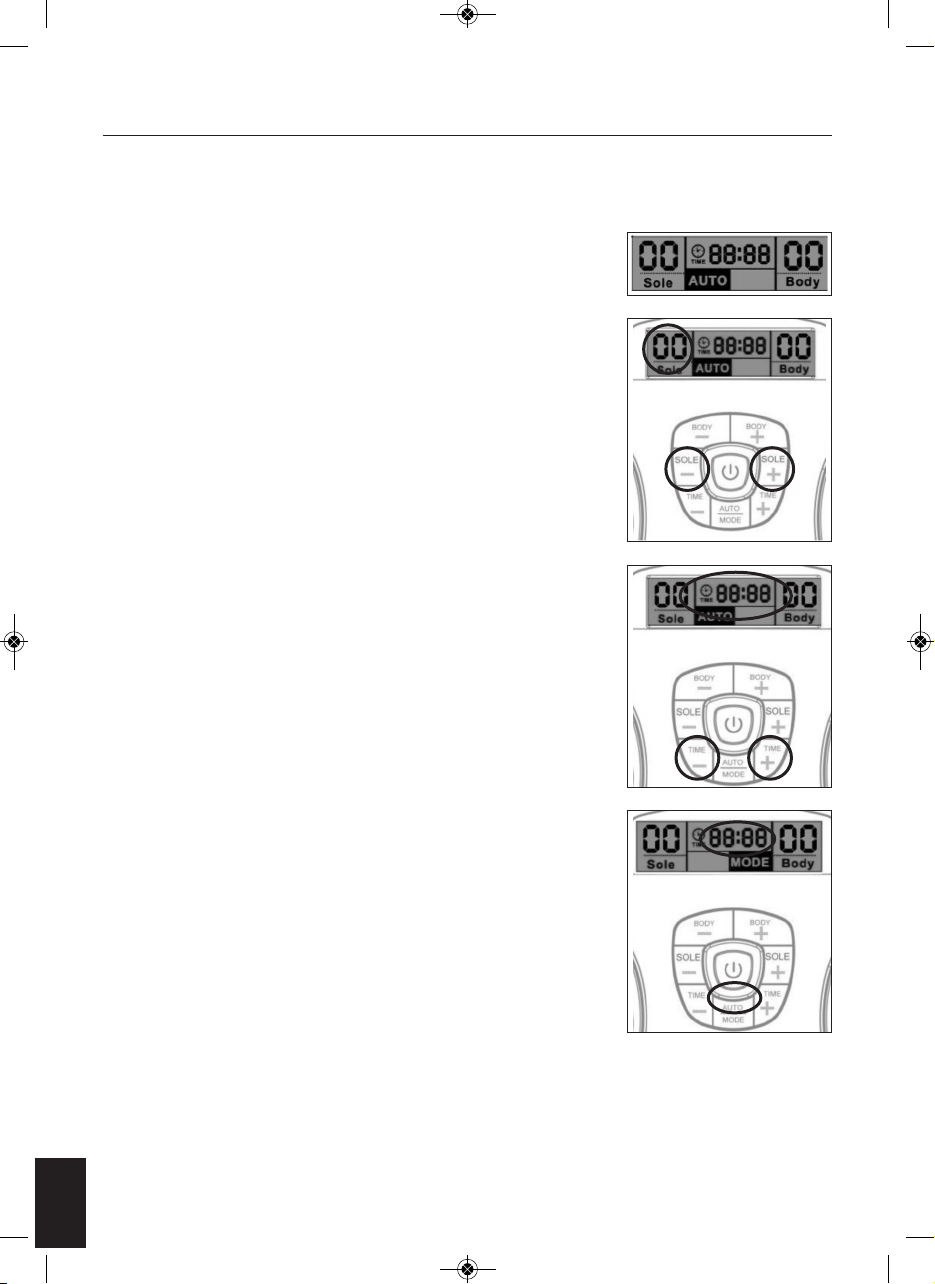
2. Press the on/off button, the LCD screen will light up in Orange. And the
program show AUTO and both Band shown in 00, which is in standby
mode (see Fig. 1).
3. Gently increase intensity setting by pushing the button of “SOLE +”. Or
decrease intensity setting by pushing the button of “SOLE -”. The
intensity level is adjustable between 0 and 99. The LCD display will show
the selected level (see Fig. 2).
4. You can adjust the auto off timer by pressing the “Time –“ or “Time +”.
Timer range from 1-60 minutes. The timer will begin to count down from
the time setting you select (see Fig. 3). To terminate the massage period,
user can turn off the unit anytime by pressing the on/off button once.
5. If you are satisfied with the current massage programme, you can lock
the current massage programme by pressing the Auto/Mode key. The
rest of the massage time will then only run the selected massage
programme (see Fig. 4).
IMPORTANT INFORMATION:
a. The aim is not to get up to level ‘99’.
b Chose an intensity level that is comfortable for you! This level may
vary from day to day.
c. Remember to drink plenty of fluid – if you are dehyrdated, this will
reduce the effectiveness of the device.
Fig. 1
Fig. 2
Fig. 3
Fig. 4
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 14
15
GB
For Body
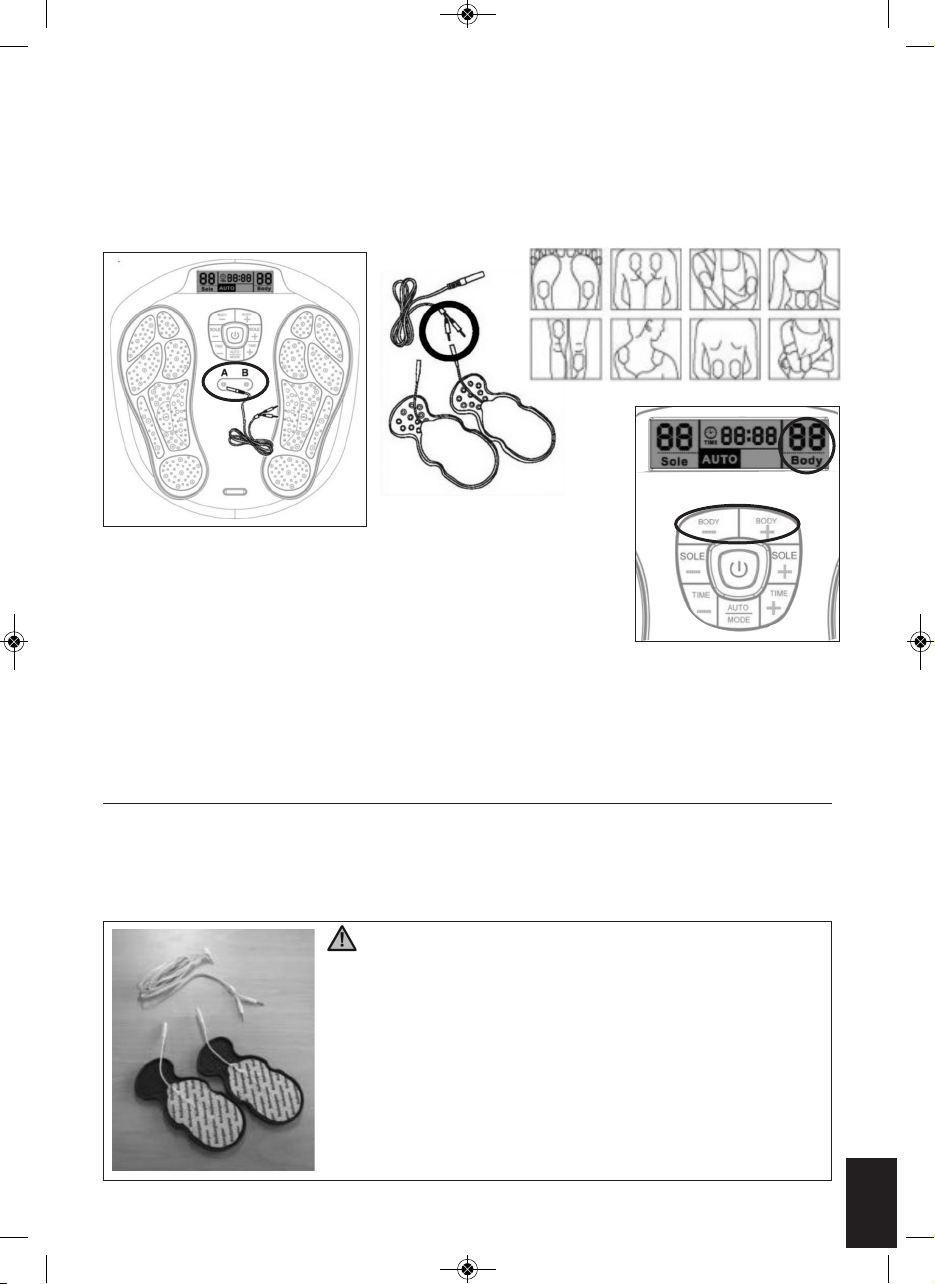
USING THE GEL PADS
Wash and dry skin before use. Connect the output wire to the gel pads. Connect the other end of the output
wire to the output jack on Circulation pro. Remove the protective film from the adhesive pads. Attach the gel
pads to the skin. Press the on/off button to turn on the unit and adjust the stimulating output intensity to the
desired level. (The display will show the mode and level that you selected and start to count down).
1. Plug in the 2 cables into the cable jack on the unit (see Fig. 5).
2. Connect pin of the cable to the gel pad properly (see Fig.6).
3. Remove the protective film on the gel pad, and attached the 4 gel pads to
the area of the body you wish to treat in accordance with warnings.
4. Repeat operation as in foot instructions, adjust the intensity using for body.
5. Gently increase intensity setting by pushing the button of “Body + ”. Or
decrease intensity setting by pushing the button of “Body - ”. The LCD will also show the level which you
have selected (see Fig. 7).
6. To terminate the massage period, user can turn off the unit anytime by pressing the on/off button.
If you want to use 2 gel pads only, then you must connect 1 gel pad to jack A and 1 gel pad to Jack B.
ADDITIONAL ACCESSORIES
Replacement gel pads
For information on how to buy replacement gel pads, please visit www.homedics.co.uk
Care of your gel pads
Never stick two adhesive pads to each other. Keep the adhesive gel pads
clean, never expose them to high temperature or direct sunlight. If the
electrode gel pads are insufficiently adhesive or dirty, wipe with a wet cloth
or change for new ones, replacement parts will be available directly from
HoMedics or your distributor.
Do not clean the electrode gel pads with any chemical.
ALWAYS try and protect the gel pads. Store the gel pads on the gel pad
protector when not in use, as in the illustration.
Fig. 5
Fig. 6
Fig. 7
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 15
16
GB
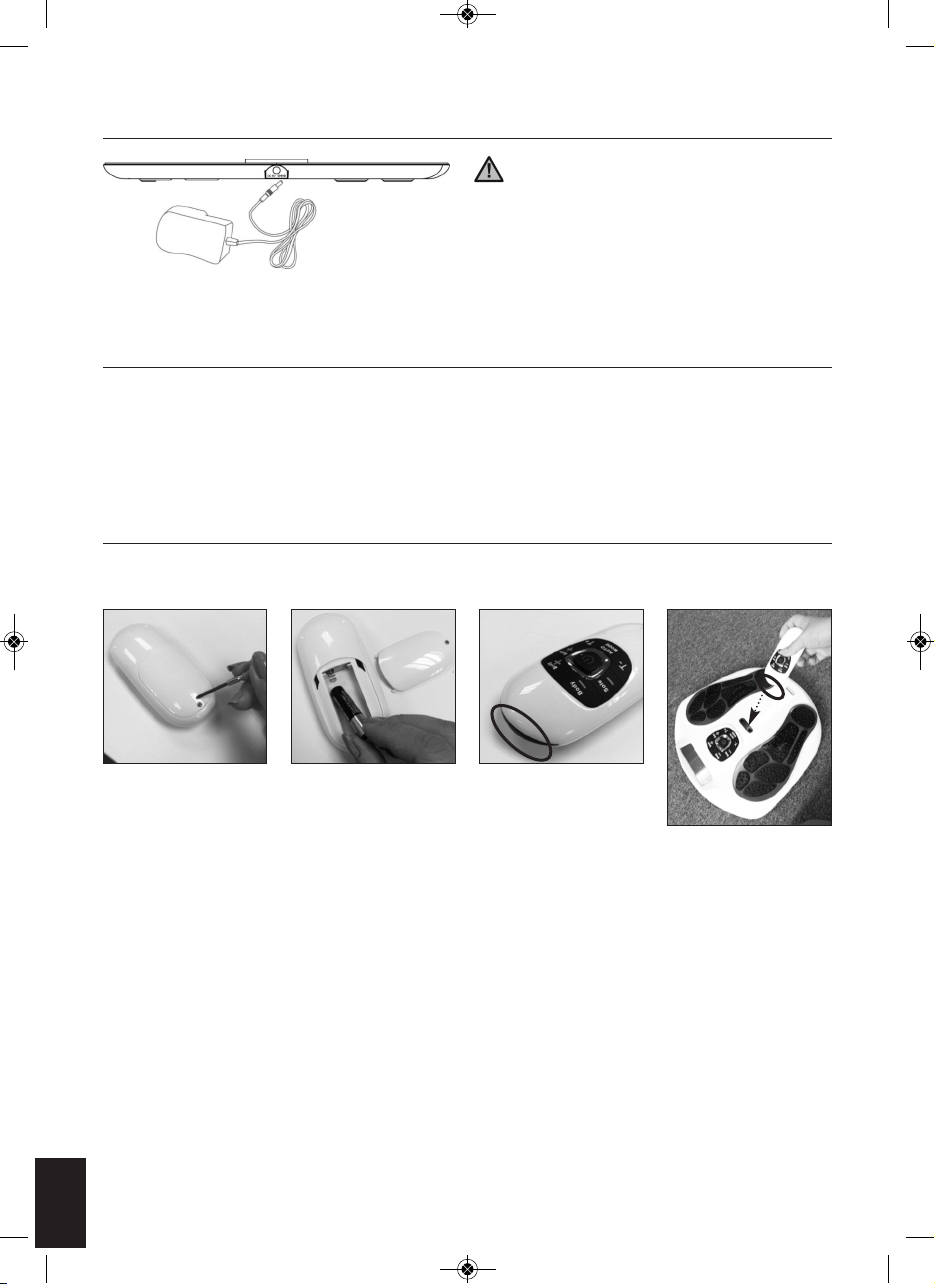
CONNECT WITH THE SUPPLIED AC/DC POWER ADAPTER
Plug the DC plug of the power supply into the
socket on the side of Circulation Pro. (See Fig 8) Plug
in power adapter to a suitable wall socket. (Make sure
that the input voltage of the wall socket is suitable for
the supplied adapter.)
INSTALLING BATTERIES FOR MAIN UNIT
If you want to use the Circulation Prowith battery power instead of the supplied main adapter, the battery
compartment is located on the underside of the unit.
Remove the battery cover from the unit by removing the screw with a screwdriver. Insert the new 4 pieces
1.5V size AA batteries with the + and - marks correctly aligned.
INSTALLING BATTERIES FOR REMOTE CONTROL
Remove the battery cover from the unit by removing the screw with the screwdriver. Insert the new 2 pieces
1.5V size AAA batteries with the + and - marks correctly aligned.
The transmitter of the remote control is at the top of it in black colour (Fig. 10), for
using the remote control, please remember to point the transmitter to the
receiver of the main unit, which is located at between the gel pad jack of the main
unit. (Fig. 11).
Note on batteries:
Do not mix different types of batteries or old batteries with new ones. To prevent the risk of leakage or
explosions, never recharge the batteries, apply heat or take them apart.
When not using batteries, remove them to prevent battery drain. If liquid leaks from the batteries, throw them
away. See page 4 for correct disposal. Thoroughly clean the battery compartment with a dry cloth.
F
ig. 8
Fig. 8 Fig. 9 Fig. 10
Fig. 11
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 16
17
GB
CLEANING
Electrode Gel pad
• When not in use, store the Electrode Gel pads on the plastic pad protectors provided at room temperature.
• Keep the Electrode Gel pads clean and dust free in a dry location, keep away from oily or sticky location.
Otherwise the life of the electrodes varies depending on skin conditions, storage, amount of use, type of
stimulation, and stimulation site. Usage may be extended by carefully cleaning the gel surface with water.
Do not spill liquid on the wire.
• Single patient use only.
• Do not apply to cracked skin. Should a skin rash occur , discontinue use and contact your physician
• Do not use tissue, cloth etc. to wipe the electrode surface.
• Do not use finger nails, brushes etc. against the electrode surface as it might damage.
• Do not clean the pads too often, and don’t use the detergent or hot water to clean the electrode gel pads
Main Device
• Switch off the power and remove the adapter and the electrode gel pad from the unit for storage in correct
way.
• Always keep the main device clean by using a soft cloth to clean the surface of the unit.
• To clean the foot pedals, use a soft, damp, soapy cloth but make sure you squeeze the cloth dry and clean
the foot pedal area.
• If the device is very dirty, use a soft, damp, soapy cloth, but make sure you squeeze the cloth dry before
cleaning the unit.
• Do not spill liquid on the device
• Do not immerse the device in water
• Do not clean with chemicals
• Store in a dry, dust free location in temperature between 10 to 400C and 30% to 90% relative humidity.
Safety Precautions
• Do not open the device or repair it yourself. This will invalidate your warranty and may cause serious harm.
• If the device malfunctions, disconnect it from the power source and contact your selling agent as soon as
possible.
• Use only the accessories supplied by the manufacturer.
• Use the device only for its intended purpose.
• Do not expose the device to extreme heat.
• Do not overload the electrical outlet.
• Do not stand on the machine. Use it when sitting down only.
• Do not spill liquid on the device or its accessories
The warranty is void if the product has been altered, misused or abused. HoMedics will not take any
responsibility.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 17
18
GB
TROUBLESHOOTING & MAINTENANCE
Hygiene
After using the product
Clean the device with a soft, damp cloth, but make sure you squeeze the cloth dry before cleaning the foot
pedal area.
Store the Electrode Gel pads on the plastic pad protectors provided.
Storage
Keep the whole set of product clean and store in a dust free and dry location.
Storage temperature and humidity -10°C to 60°C, 10% to 95% RH
Operating temperature and humidity -5°C to 50°C, 30% to 90% RH
P
roblem
C
ause
R
ectification
Device will not turn on. - Batteries inserted in wrong direction.
- The adapter does not plug well into device
properly.
- Insert batteries in correct direction or check the
b
attery is in full power.
- Check the connection of the adapter jack is in
well connected. And also the DC adapter with
well connecting to the main socket.
Power turns off too
s
oon
- Gel pads not attached correctly to the skin. - Attach Gel pads correctly to the skin
Power turns off while
using massager
- If you are using the batteries operation, then
the batteries might be weak/exhausted
- Fit 4 new identical 1.5V alkaline batteries type
AA
-
Treatment period of 30 minutes is over and
power turns off automatically
-
Restart treatment or turn off the massager.
- If you are using the body massage, the
electrode gel pad may be broken
-Replace electrode gel pad
It is difficult to attach
Gel pad to the skin
- Transparent film not peeled off
- Gel pad applied immediately after washing
- Adhesive surface of Gel pad damaged
- The gel pads got dirty and lost their
adhesive/stickiness
- Peel off film on the adhesive surface of Gel pad
- Sufficiently dry Gel pad
- Replace Gel pad
- Replace Gel pad or clean the gel pad with a
small drop of water onto the sticky side of the
electrode pad and rub into the surface
Adhesive surface of
Gel pad is not sticky
- Use of Gel pad whilst perspiring
- Gel pad washed too long and/or too frequently
- Gel pads stored under high temperature, high
humidity, direct sunlight
- Leave Gel pad in freezer for overnight
It is difficult to feel
stimulation
- You sole is too dry, not enough moisture
- Your sole is not placing on the foot pedal
properly
- Gel pads not attached correctly to the skin
- Gel pads overlap each other
- Electrode cord not connected correctly
- Applied intensity too weak
- Put some water on your sole to moisturise your
sole
- Ensure both of your soles are placed on each
pedal properly.
- Attach Gel pad firmly to the skin
- Reattach Long Life pads with no overlap
- Connect electrode cord correctly
- Increase the intensity by pressing the + button.
The skin turns red or
the skin feels irritated
- Adhesive surface of Gel pads dirty or dry
- Adhesive surface of Gel pads damaged
- Wash adhesive surface of Gel pads softly with
your fingertips for about 3 seconds under slow
running water
- Replace Gel pads
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 18

19
GB
TECHNICAL SPECIFICATIONS
Product Name Circulation pro
Model CB-200-EU
P
ower supply
6V DC or 4x1.5V alkaline batteries type AA* for the main unit
2x1.5V alkaline batteries type AAA* for the remote control
S
upplier of Adapter
G
olden Profit Electronics Ltd.
M
odel no. of Adapter
G
PE038-060050-3
A
dapter Input
A
C 100-240V~50-60Hz 0.1A
Adapter Output DC 6V 500mA 3.0W
Battery life >350 minutes
Frequency generation Approx. 10 Hz to 55.56 Hz
Power consumption 1.05 W
Maximum Output Voltage U <54.8V (during 1 kΩ load)
Maximum Output Current I < 910 μA (during 1 kΩ load)
Operating temperature and humidity -5°C to 50°C, 30%to 90% RH
Storage temperature and humidity -10°C to 60°C, 10% to 95% RH
Main unit dimensions 338(L) x 324 (W) x 48(H) mm
Weight Approx. 950 g
Package Contents
Quantity Parts
1 Circulation pro
1 AC/DC Adapter
1 Remote Control
2 Cable Wire for Electrode Gel pads
4 Electrode Gel pads
2 Plastic Gel pads protector
1 Instruction manual
Accessories :
• Only use original accessories.
Check that the contents of the delivery are complete.
* batteries not included.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 19
20
GB
IMPORTANT INFORMATION
Electro Magnetic Compatibility (EMC)
1. CIRCULATION PRO needs special precautions regarding EMC and needs to be installed and put into service
according to the EMC information provided in the ACCOMPANYING DOCUMENTS;
2. Portable and mobile RF communications equipment can affect CIRCULATION PRO.
3. Warning: the use of accessories, transducers and cables other than those specified with the exception of
transducers and cables sold by the manufacturer of the CIRCULATION PRO as replacement parts for internal
components, may result in increased EMISSIONS or decreased IMMUNITY of the CIRCULATION PRO.
4. Warning: the CIRCULATION PRO should not be used adjacent to or stacked with other equipment.
Guidance and manufacturer’s declaration – electromagnetic emissions
The CIRCULATION PRO is intended for use in the electromagnetic environment specified below. The
customer or the user of the CIRCULATION PRO should assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
The CIRCULATION PRO uses RF energy only for its
internal function. Therefore, its RF emissions are very
low and are not likely to cause any interference in
nearby electronic equipment.
RF emissions
CISPR 11
Class B
The CIRCULATION PRO is suitable for use in all
establishments, including domestic establishments
and those directly connected to the public low-voltage
power supply network that supplies buildings used for
domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Complies
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 20
21
GB
Guidance and manufacturer’s declaration – Electromagnetic Immunity
The CIRCULATION PRO is intended for use in the electromagnetic environment specified below. The
customer or the user of the CIRCULATION PRO should assure that it is used in such an environment.
Immunity test
IEC 60601 test
level
Compliance level Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floors should be wood, concrete or ceramic
tile. If the floor is covered with synthetic
material, the relative humidity should be at
least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines
±1kV for
input/output lines
±2 kV for power
supply lines
±1kV for
input/output lines
Mains power quality should be that of a
typical commercial or hospital environment.
Surge IEC
61000-4-5
±1 kV line(s) and
neutral
±1 kV line(s) and
neutral
Mains power quality should be that of a
typical commercial or hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines IEC
61000-4-11
<5% U
T
(>95% dip in UT)
for 0.5 cycle
<5% U
T
(>95% dip in UT)
for 0.5 cycle
Mains power quality should be that of a
typical commercial or hospital environment.
If a dips or an interruption of mains power
occurs, the current of the CIRCULATION PRO
may be dropped off from normal level, it may
be necessary to use uninterruptible power
supply or a battery.
40% U
T
(60% dip in UT)
for 5 cycles
40% UT)
(60% dip in UT)
for 5 cycles
70% U
T
(30% dip in UT)
for 25 cycles
70% U
T
(30% dip in UT)
for 25 cycles
<5% U
T
(>95% dip in UT)
for 5 sec
<5% U
T
(>95% dip in UT)
for 5 sec
Power frequency
(50Hz) magnetic
field IEC61000-4-8
3A/m Not applicable Not applicable
NOTE: UTis the a.c. mains voltage prior to application of the test level.
5.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 21
22
GB
6.
Gu
i
d
a
n
c
e
a
n
d
m
a
n
u
fa
c
t
u
r
e
’
s
d
e
c
l
a
r
a
t
i
on
–
e
l
e
c
t
r
om
a
g
n
e
t
i
c
i
m
m
u
n
i
t
y
T
h
e
C
I
R
C
UL
ATI
ON
PR
O
is
i
n
t
en
d
e
d
f
o
r
u
s
e
in
t
h
e
el
e
ct
r
o
m
a
g
n
e
t
ic
en
v
ir
o
n
me
n
t
s
pec
if
ie
d
be
l
o
w
.
T
h
e
c
u
s
t
o
mer
o
r
t
h
e
u
s
e
r
s
h
o
u
l
d
a
s
s
u
r
e
t
h
a
t
it
is
u
s
ed
in
s
u
c
h
a
n
en
v
ir
o
n
me
n
t
.
I
m
m
u
n
i
t
y
t
e
s
t
I
EC
6
0
6
0
1
t
e
s
t
l
e
v
e
l
Com
p
l
i
a
n
c
e
l
e
v
e
l
El
e
c
t
r
om
a
g
n
e
t
i
c
e
n
vi
r
on
m
e
n
t
-
g
u
i
d
a
n
c
e
Cond
ucted
RF
I
EC
61000-4-6
Rad
i
ate
d
RF
I
EC
61000-4-3
3 V
/ms
150 k
Hz
to 80 MHz
3 V
/m
26 MHz
to 2.5 GHz
10 V/m
26 MHz
to 2.5 GHz
3 V
/ms
3 V/m
10 V
/m
P
or
t
abl
e
a
nd
mobi
l
e RF
comm
unic
at
i
ons
eq
uipm
ent
shoul
d
be used
no c
l
oser to an
y
pa
r
t
of
t
he ELECTR
O F
LEX, i
nc
l
ud
ing
c
abl
e
s,
t
ha
n t
he r
ecomme
nd
ed
sepa
rat
ion d
ist
a
nce
c
al
c
ul
ate
d
f
r
om t
he
e
q
uat
ion appl
ic
abl
e
to
t
he f
r
eq
ue
nc
y
of
t
he t
ransmit
ter
.
Re
comme
n
de
d
se
p
a
r
ati
on
di
sta
n
ce
d
= 1,2
√
—
P
d
= 1,2
√
—
P
80 MHz
to 800 MHz
d
= 2,3
√
—
P
800 MHz
to 2.5 GHz
w
he
r
e P
i
s t
he max
imum out
put
po
wer
rat
ing
of
t
he t
ransm
it
te
r in w
at
t
s (
W)
accor
d
i
ng
to t
he t
ransm
it
ter
manuf
a
ct
ur
er a
nd
d
is t
he r
ecomm
e
nd
ed
sepa
rat
ion d
ist
ance i
n met
r
es (m).
Fiel
d
st
r
eng
t
hs f
r
om f
i
x
ed
RF
t
ransmit
ters, a
s
d
eter
mined
b
y
an el
ect
r
oma
gnet
ic
site
sur
v
ey
, a shoul
d
be l
ess t
han t
he compl
iance
l
ev
el
in eac
h f
r
eq
uenc
y
rang
e b
.
Inter
f
er
ence may
occ
ur in t
he vi
c
inity
of
eq
uipment
mar
k
ed
w
it
h t
he f
ol
l
o
w
ing
sy
mbol
:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and
land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted
theoretically with accuracy.
a To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which the CIRCULATION PRO is
used exceeds the applicable RF compliance level above, the CIRCULATION PRO should be observed to
verify normal operation. If abnormal performance is observed, additional measures may be necessary,
such as reorienting or relocating the CIRCULATION PRO.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 22

23
GB
Recommended separation distances between portable and mobile RF communications equipment
and the Circulation Pro
The CIRCULATION PRO is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the CIRCULATION PRO can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the CIRCULATION PRO as recommended below, according
to the maximum output power of the communications equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d = 1,2
√
—
P
80 MHz to 800 MHz
d = 1,2
√
—
P
800 MHz to 2,5 GHz
d = 2,3
√
—
P
0,01 0.12 0.12 0.23
0,1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance
d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P
is the maximum output power rating of the transmitter in watts (W) according to the transmitter
manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
7.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 23
24
F
GUIDE DE RAPIDE
RAPPEL - CET APPAREIL NE VIBRE PAS - IL UTILISE DES IMPULSIONS ÉLECTRIQUES ET NON DES VIBRATIONS !
Pour en savoir plus sur votre Circulation pro, veuillez consulter les instructions complètes de ce manuel.
Pour obtenir une explication complète sur le réglage de l'intensité, veuillez consulter la page 36
Déballez votre Circulation pro. Déballez la Télécommande et retirez
la vis de la plaque arrière avec un tournevis. Puis insérez 2 piles AAA
dans le compartiment conformément aux instructions. Puis vissez le
couvercle du compartiment des piles. Veuillez consulter la page 38
pour voir le guide étape par étape de changement des piles de la
télécommande.
Branchez l'adaptateur secteur à une prise de courant appropriée et
connectez le petit connecteur CC à l'appareil.
Mettez en marche, l'écran central s'allume en orange et s'éteint.
Retirez vos chaussures et chaussettes ou bas. PLACEZ VOS PIEDS
NUS SUR LES PLATEAUX. VOTRE PIED DROIT SUR LE PLATEAU DE
DROITE ET VOTRE PIED GAUCHE SUR LE PLATEAU DE GAUCHE. LES
DEUX PIEDS DOIVENT ÊTRE BIEN POSÉS SUR L'APPAREIL POUR
PROFITER DE SES BIENFAITS.
Asseyez-vous sur un siège confortable. Placez vos pieds nus sur les
reposes-pieds de gauche et de droite. Augmentez les niveaux
d'intensité pour les pieds en appuyant sur « SOLE+ » (PLANTE+) ou
sur « SOLE- » (PLATE -) pour réduire l'intensité. L'intensité va de 0 à
99, augmentez progressivement le niveau jusqu'à ce que vous
commenciez à ressentir la stimulation du micro-courant.
1
2
3
4
5
4
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 24
25
F
RENSEIGNEMENTS IMPORTANTS À L'INTENTION DU CLIENT
VEUILLEZ LIRE :
Q : Comment puis-je l'utiliser ?
R : Il vous suffit de placer vos « PIEDS NUS » SUR LES PLATEAUX. Le pied droit sur le plateau de
droite et le pied gauche sur le plateau de gauche en même temps. L'appareil ne fonctionnera
pas tant que vos pieds ne seront pas bien posés sur les plateaux.
Q : Est-ce qu'il vibre ?
R : Non. Cet appareil NE VIBRE PAS. Le Circulation Pro a été spécialement conçu pour envoyer de
légères impulsions électriques à travers la plante des pieds. Cette action stimule les muscles
des mollets et améliore la circulation sanguine.
Q : Je ne sens rien au niveau des pieds ou des jambes ?
R : Veuillez noter que le niveau d'« intensité » va jusqu'à 99. L'objectif n'est pas d'atteindre les
99 mais un niveau qui vous convient. Ce niveau peut varier en fonction de la journée.
Q : Mes pieds sont très secs et je ne ressens aucune impulsion électrique.
R : Surtout, veillez à toujours rester hydraté ; buvez abondamment. Les bienfaits pour la santé
offerts par l'appareil sont optimisés si vos pieds sont bien hydratés.
Q : Est-il difficile à utiliser ?
R : Non. Il vous suffit de poser vos pieds sur les reposes-pieds et de sélectionner le réglage de
l'intensité pour que le compte à rebours de 30 minutes se déclenche automatiquement.
Q : Suis-je trop âgé(e) pour l'utiliser ?
R : Non. Le produit convient à tout âge.
Q : Peut-il vraiment m'aider ? Je ne suis pas très actif (ve) et reste assis (s) une
grande partie de la journée.
R : Oui. En position assise, le sang stagne naturellement au niveau des jambes à cause de la
gravité, ce qui est tout à fait normal. Le manque d'exercice contribue au ralentissement de la
circulation du sang causant des problèmes comme des gonflements et une mauvaise
circulation sanguine. Le Circulation Pro peut atténuer ces symptômes.
Q : Mes jambes me font mal après l'utilisation de ce produit.
R : Soit votre réglage SOLE était trop élevé (il faudra donc le réduire la prochaine fois) soit vous
l'avez trop utilisé dans la journée. Laissez à vos jambes le temps de se reposer avant de vous
resservir de l'appareil.
AVERTISSEMENT
Cet appareil ne devrait pas être utilisé par des femmes étant dans leur premier
trimestre de grossesse, par des personnes portant un stimulateur cardiaque ou
d'autres implants médicaux ou par toute personne prenant un traitement contre
les thromboses veineuses profondes (TVP). Si vous avez des questions, veuillez
appeler notre Numéro du Service clientèle ou consulter votre médecin traitant.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 25
26
F
RENSEIGNEMENTS IMPORTANTS SUR LA SÉCURITÉ
1) Veuillez bien lire ces instructions avant d'utiliser le produit.
2) Veuillez vérifier que vous disposez de toutes les pièces énoncées dans ce manuel de l'utilisateur.
3) Déballez toutes les pièces et examinez-les pour vous familiariser avec les composants.
Remarques sur la sécurité
• Les icônes et les signes d'avertissement sont indiqués ici pour votre sécurité et l'utilisation correcte du
produit ainsi que pour éviter les blessures et/ou dégâts matériels.
• Les icônes et significations sont les suivantes :
D
escriptions des marquages
L'icône indique des interdictions (ne pas faire).
Les points impliquant certaines interdictions sont décrits par du texte ou des images incluses ou à proximité.
L'icône de gauche signifie « Interdictions de démonter ».
L
'icône indique une chose obligatoire (doit être suivi).
Les points impliquant certaines actions obligatoires sont indiqués par du texte ou des images incluses ou à
proximité.
L'icône de gauche se réfère à une « Action générale obligatoire ».
Ce produit ne devrait pas être utilisé par des personnes portant des implants, ex. stimulateurs cardiaques, cœur
artificiel, systèmes d'assistance respiratoire ou électroniques.
Ce symbole indique que les piles ne doivent pas être jetées avec les ordures ménagères car elles contiennent
des substances nocives pour l'environnement et la santé. Veuillez jeter les piles dans des points de collecte
spécialisés.
Ce marquage indique que ce produit ne devrait pas être jeté avec les ordures ménagères dans l'UE. Afin d'éviter
toute mise aux rebuts inappropriée et nuisible pour l'environnement ou la santé, il faut recycler le produit de
manière responsable afin de contribuer à la réutilisation durable des ressources matérielles. Pour renvoyer votre
appareil arrivé en fin de vie, veuillez utiliser des systèmes de retour et de collecte appropriés ou contacter votre
revendeur. Il pourra confier ce produit à un centre de recyclage approprié.
Veuillez consulter le mode d'emploi.
Date de fabrication.
Nom du fabricant.
Code de lot.
Équipement de catégorie II
Attention, veuillez consulter les documents joints
Pièce appliquée de type B
Ce symbole signifie le numéro de série placé sous l'appareil et sur l'emballage.
Ce symbole indique que l'unité se conforme aux exigences de base énoncées par la Directive CE 93/42/CEE sur
les appareils médicaux.
Danger
Cette unité ne doit pas être utilisée avec les appareils médicaux suivants :
(1) Implants électroniques médicaux, ex. stimulateurs cardiaques
(2) Équipement d'assistance médicale électronique, comme des respirateurs
(3) Appareils médicaux électroniques fixés sur le corps, comme des électrocardiographes
L'utilisation de cet appareil pourrait perturber le fonctionnement de ces appareils médicaux.
0120
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 26
27
F
A
vertissement
L
es personnes souffrant des maladies suivantes doivent consulter un médecin avant d'utiliser cette unité :
1) maladie aigüe
2
) tumeur maligne
3) maladie infectieuse
4) grossesse
5) problème cardiaque
6) fièvre élevée
7
) pression sanguine anormale
8) troubles sensoriels cutanés ou problèmes de peau
9
) traitement médical en cours, en particulier en cas de gêne ressentie.
N'utilisez pas cette unité à proximité du cœur, au-dessus du cou, autour de la bouche ou sur une peau à problèmes.
Pourrait entraîner un accident ou des problèmes de santé.
- L'application des électrodes entre le cou et le diaphragme (poitrine) pourrait accroître le risque de fibrillation cardiaque.
N'utilisez pas cette unité en même temps qu'un autre appareil thérapeutique ou en combinaison avec
des onguents y compris des onguents pulvérisés.
Pourrait entraîner une gêne ou des problèmes de santé.
- La connexion simultanée d'un PATIENT déjà relié à un ÉQUIPEMENT chirurgical HF pourrait causer de graves brûlures
s
ur le site où les électrodes du STIMULATEUR sont placées et potentiellement endommager le STIMULATEUR.
- L'utilisation à proximité (ex. 1 m) d'un ÉQUIPEMENT thérapeutique à ondes courtes ou microondes pourrait
déstabiliser la sortie du STIMULATEUR.
N'utilisez pas cette unité à des fins différentes que celles inhérentes au traitement indiqué dans ce manuel.
Pourrait causer des accidents, des problèmes ou une défaillance de l'unité.
N'insérez pas le connecteur de l'électrode dans un autre port que celui de l'unité principale.
Pourrait entraîner une électrocution ou un accident.
Ne démontez ou de remodelez pas cette unité.
Aucune pièce ne peut être remise en état par l’utilisateur.
Attention
Si l'unité ne fonctionne pas bien ou si vous ressentez une gêne, veuillez immédiatement cesser d'utiliser l'unité.
Si vous ressentez des gênes au niveau de la peau ou du corps, veuillez consulter un médecin et suivre ses conseils.
Si vous souhaitez placer l'électrode à un autre endroit ou sur votre corps pendant le traitement, veuillez
d'abord arrêter l'appareil.
Sinon, vous pourriez vous électrocuter.
N'essayez pas de placer les Électrodes sur une autre personne pendant le traitement.
Vous pourriez vous électrocuter.
Ne démarrez pas le traitement si vous portez un dispositif électronique.
Les réglages et la synchronisation du dispositif pourraient être altérés.
N'utilisez pas cette unité sur des nourrissons ou personnes incapables de s'exprimer.
Pourrait entraîner un accident ou des problèmes de santé.
N'utilisez pas cette unité dans des endroits humides comme des salles de bain ou lorsque vous prenez un bain
ou une douche.
Vous pourriez vous électrocuter.
N'utilisez pas cette unité pendant que vous dormez.
L'unité principale pourrait dysfonctionner, ou l'électrode pourrait se décaler et toucher une zone dangereuse et
entraîner des problèmes de santé.
N'utilisez pas cette unité en conduisant.
Une impulsion soudaine pourrait entraîner un accident de la route.
Ne laissez pas l'électrode sur votre peau après le traitement.
Une fixation prolongée pourrait provoquer des irritations et des infections cutanées.
Veuillez éviter tout contact entre des objets métalliques, comme une boucle de ceinture ou un collier et
l'électrode pendant le traitement.
Vous pourriez vous électrocuter.
N'utilisez pas de téléphones mobiles ou autres appareils électroniques à proximité de cette unité.
Placez les électrodes Longue durée uniquement sur votre peau ou sur les supports pour électrodes Longue
durée afin de ne pas endommager les surfaces adhésives des électrodes.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 27
28
F
Informations importantes sur la compatibilité électromagnétique
Les appareils électroniques, comme des ordinateurs et des téléphones mobiles et des appareils médicaux
pourraient créer des interférences électromagnétiques. Une utilisation incorrecte d'un appareil médical
pourrait entraîner la génération d'interférences électromagnétiques et créer une situation potentiellement
dangereuse. Les appareils médicaux ne devraient pas non plus interférer avec d'autres appareils.
La norme EN 60601-1-2 a été mise en place pour réglementer les exigences en termes de Compatibilité
électromagnétique (EMC) dans le but d'éviter toute situation dangereuse. Cette norme définit les niveaux
d'interférences électromagnétiques ainsi que les niveaux maximum d'émissions électromagnétiques des
appareils médicaux.
Cet appareil médical fabriqué par HoMedics se conforme à la norme EN 60601-1-2 pour l'immunité et les
émissions. Néanmoins, des précautions particulières doivent être prises :
Ne pas utiliser de téléphones mobiles et d'autres appareils, générant de forts champs électriques ou
électromagnétiques près de l'appareil médical. Une utilisation incorrecte de l'unité pourrait générer des
interférences et créer une situation potentiellement dangereuse.
Il est recommandé de garder une distance minimum de 7 m. Vérifiez le bon fonctionnement de l'appareil si
la distance est plus courte.
Le CB-200-EU nécessite la prise de précautions particulières quant à l'EMC et doit être installé et mis en
service conformément aux informations sur l'EMC données en ANNEXE.
Un équipement de communications FR portable et mobile peut affecter le CB-200-EU.
AVERTISSEMENT: l'utilisation d'accessoires, transducteurs et câbles autres que ceux fournis, sauf les
transducteurs et câbles vendus par le fabricant du CB-200-EU comme pièces de rechange, pourrait
augmenter les ÉMISSIONS ou réduire l'IMMUNITÉ du CB-200-EU.
AVERTISSEMENT: le CB-200-EU ne devrait pas être utilisé près de ou au-dessus d'un autre appareil.
L'équipement ne convient pas à une utilisation en présence d'un mélange anesthésique inflammable avec
l'air, l'oxygène ou l'oxyde nitreux.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 28
29
F
QU'ESTCE QUE LA STIMULATION NERVEUSE ÉLECTRONIQUE ?
USAGE PRÉVU : Usage médical
Ce stimulateur nerveux électronique a été conçu pour masser afin de réduire les courbatures (musculaires),
stimuler la circulation sanguine, détendre les muscles fatigués, réduire les gonflements des pieds, des
chevilles et la fatigue. L'effet massant est généré par une stimulation électronique des nerfs par le biais
d'électrodes placées sur la peau. Diverses zones de massage et programmes de traitement peuvent être
sélectionnés.
Utilisateurs ciblés : Veuillez lire les « Remarques de sécurité » avant d'utiliser l'unité. (Cette unité ne devrait
pas être utilisée par les personnes non autorisées indiquées dans les « Remarques sur la sécurité »).
Environnement : Cette unité est exclusivement réservée à l'usage domestique.
Efficacité : Appareil de massage : soulage les douleurs, courbatures et fatigue (muscles).
Précautions d’utilisation : Veuillez lire les « Remarques de sécurité » avant d'utiliser l'unité.
La stimulation nerveuse électronique n'est pas invasive et soulage les douleurs musculaires en toute sécurité.
Le Circulation Pro utilise une thérapie de stimulation électrique testée et approuvée pour envoyer des
impulsions de micro courant à travers la plante des pieds. Ce type de stimulation électrique sûre et efficace a
été cliniquement prouvée et peut être utilisée dans le confort de son canapé. Le Circulation Pro améliore
l'activité musculaire en stimulant les nerfs augmentant le flux sanguin et aidant à réduire les DOULEURS,
GONFLEMENTS, FATIGUE et MAUX DE JAMBES.
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 29
30
F
VUE D'ENSEMBLE DE LA MACHINE ET NOM DES PIÈCES
A. Unité principale
B. Adaptateur secteur
C. Télécommande
D. Électrode gel x 4 pcs.
E. Câble électrique connectant les
électrodes gels et l'appareil
F. Protection en plastique pour
électrodes gel
1. Écran LCD
2. Panneau de commande
3. Zone d'électrode pour pied gauche
4. Zone d'électrode pour pied droit
5. Câble connectant les électrodes et l'appareil.
6. Câble connectant les électrodes et l'appareil.
7. Capteur du récepteur de la télécommande
8. Plaque décorative argentée
9. Connecteur d'adaptateur
Vue du dessus
Vue du dessous
IB-CB200EU-0613-01_Layout 1 26/06/2013 16:06 Page 30
 Loading...
Loading...