Page 1

user manual
Hoefer SE900
Second Dimension Electrophoresis System
um SE900-IM/Re v.B0/06-12
Page 2

Page Finder
Important Information ................................................................ii
Waste Electrical And Electronic Equipment (WEEE) .................... vi
Function and Description ...........................................................1
Specifications ...........................................................................2
Unpacking and Inventory ............................................................3
Operating Instructions ..............................................................10
Using the Multiple Gel Caster ...................................................11
IPG Strip Equilibration ............................................................14
Page Separation .....................................................................16
Care and Maintenance .............................................................19
Technical Service and Repair ....................................................19
Troubleshooting .......................................................................20
Ordering Information ...............................................................23
Appendix A: Theory and Recipes ...............................................24
Solutions ................................................................................25
Gel Recipes ............................................................................28
Appendix B: References ...........................................................30
pi
•
Page 3

Important Information – English
• If this equipment is used in a manner not specied by Hoefer, Inc. the
protection provided by the equipment may be impaired.
• This instrument is designed for indoor laboratory use only.
• Only accessories and parts approved or supplied by Hoefer, Inc. may
be used for operating, maintaining, and servicing this product.
• Only use a power supply that is CE marked or safety certied by a
nationally recognized testing laboratory.
• The safety lid must be in place before connecting the power supply
leads to a power supply.
• Turn all power supply controls o and disconnect the power leads
before removing the safety lid.
• Circulate only water or 50/50 water/ethylene glycol through the
heat exchanger if so equipped. Do not connect the heat exchanger
to a water tap or any coolant source where the water pressure is
unregulated.
• Never introduce antifreeze or any organic solvent into any part of the
instrument. Organic solvents will cause irreparable damage to the
unit!
• Do not operate with buer temperatures above the maximum
specied technical specications. Overheating will cause irreparable
damage to the unit!
Duležité Informace – Czech
• Pokud by toto zařízení je použito způsobem, který není podle Hoefer,
Inc. ochrana poskytovaná na základě zařízení může být narušena.
• Tento nástroj je určen pro vnitřní použití v laboratoři pouze.
• Pouze příslušenství a části schválen, nebo poskytnutých Hoefer, Inc.
mohou být použity pro provoz, údržbu, a údržbě tohoto výrobku.
• zdroj napájení používají jen že je opatřen označením CE osvědčena
nebo bezpečnost vnitrostátně uznanými zkušebními laboratoř.
• Bezpečnosti lid musí být zavedena před připojením napájecí zdroj
napájení vede k.
• Turn veškeré napájení kontroly vypnuto a odpojit před odběrem
energie vede bezpečnostní víko.
• Rozeslat pouze voda nebo 50/50 voda/ethylenglykolu
prostřednictvím výměník tepla je li to vybavena. Nemají připojení
výměník tepla s vodními setřepná nebo jakékoli chladicí kapaliny
zdroje, kde tlak vody je neregulo.
• Nikdy zavést prostředek proti zamrznutí nebo jakákoli organická
rozpouštědla do jakékoli části z tohoto nástroje. Rozpustidlům
způsobí nenapravitelné poškození jednotka!
• Nejsou provozována s pufru teplotách nad maximální stanovenou
technickými specikacemi. Přehřátí způsobí nenapravitelné poškození
jednotka!
Vigtig Information – Danish
• Hvis dette udstyr bruges i en måde ikke speciceret ved Hoefer,
Inc. den beskyttelse, som er blevet forsynet af udstyret kan måske
svækkes.
• Dette instrument er designet for indendørs laboratoriumbrug bare.
• Bare tilbehør og del godkendede eller forsynede ved Hoefer, Inc. kan
måske bruges for drive, funktionsfejl, og betjening dette produkt.
• bruger Bare en strømforsyning, der er CE markerede eller sikkerhed,
som er blevet attesteret af en, som nationalt er blevet anerkendt
prøve laboratorium.
• Sikkerhedlåget må være på plads før forbinding strømforsyningsblyet
til en strømforsyning.
• Drejer alle strømforsyningskontroller af og afbryder kraftblyet før
erning sikkerhedlåget.
• Cirkulerer bare vand eller 50/50 vand/ethylene glykol gennem
varmeveksleren i så fald udrustet. Forbind ikke varmeveksleren
til en vandhane eller nogen kølemiddelkilde hvor vandtrykket er
unregulated.
• Introducerer Aldrig antifreeze eller noget organisk opløsningsmiddel
ind i nogen del af instrumentet. Organiske opløsningsmidler vil
forårsage uboelig skade til enheden!
• Driver ikke med stødpudetemperaturer over maksimummet
specicerede tekniske specications. Overheding vil forårsage uboelig
skade til enheden!
Belangrijke Informatie – Dutch
• Indien deze uitrusting in een manier wordt gebruikt die niet door
Hoefer, Inc. is gespeciceerd de bescherming die door de uitrusting is
verzorgd kan worden geschaad.
• Dit instrument is voor binnenlaboratoriumgebruik enkel ontworpen.
• Enkel onderdelen en delen keurden goed of leverden door Hoefer,
Inc. kan voor het bedienen worden gebruikt, handhavend en
onderhouden van dit product.
• gebruik Enkel een netvoeding die CE is markeerde of veiligheid
die door een is gecerticeerd die nationaal is herkend testene
laboratorium.
• Het veiligheidsdeksel moet in plaats voor het verbinden van de
netvoeding leidt tot een netvoeding zijn.
• Doe alle netvoedingscontroles Uit en koppel los de machtleiding voor
het verwijderen van het veiligheidsdeksel.
• Circuleer enkel water of 50/50 water/ethyleenglycol door de
hitte exchanger zo ja uitrust. Verbind de hitte exchanger naar
een waterkraan of koelmiddelbron niet waar de waterdruk niet
geregulariseerd is.
• Stel Nooit antivriesmiddel of organische oplosmiddelen in deel van
het instrument voor. Organische oplosmiddelen zullen onherstelbare
schade aan de eenheid veroorzaken!
• Bedien niet met buertemperaturen boven het maximum
speciceerde technische specicaties. Oververhittend zal
onherstelbare schade aan de eenheid veroorzaken!
Tärkeää Tietoa – Finnish
• Jos tätä varusteita käytetään tavassa ei määritetty Hoefer, Inc. suojelu
ehkäisty varusteille saattaa olla avuton.
• Tämä väline suunnitellaan sisälaboratoriokäytölle vain.
• Vain lisävarusteet ja osat hyväksyivät tai toimitti Hoefer, Inc. oheen ää
voi käyttää käyttämiselle, valvoalle, ja servicing tämä tuote.
• Vain käyttää käyttöjännitettä joka on CE merkitsi tai turvallisuus
joka on todistanut aidoksi ohi joka on kansallisesti tunnustettnut
testaaminen laboratoriota.
• Turvallisuuskansi täytyy olla paikallaan ennen yhdistäminen
•
pii
Page 4

käyttöjännitelyijyjä käyttöjännitteeseen.
• Kiertää kaikki käyttöjännitevalvonnat ja irrottaa valtalyijyt ennen
poistaminen turvallisuuskantta.
• Kiertää vain vesi tai 50/50 vesi/ethyleneä glycol siinä tapauksessa
varustetun lämmönvaihtimen läpi. Älä yhdistä lämmönvaihdinta
vesinapautukseen eikä jäähdytysnestelähteeseen, missä vesipaine on
unregulated.
• Pakkasneste eikä orgaaninen liuotin välineen osassa ei esitele
Koskaan. Orgaaniset liuottimet aiheuttavat korvaamattoman
vahingon yksikköön!
• Ei käytä puskuria yllä olevia lämpötiloja enintään määritetyillä
teknisillä täsmennyksillä. Ylikuumeneminen aiheuttaa
korvaamattoman vahingon yksikköön!
Information Importante – French
• Si cet équipement est utilisé dans une manière pas spécié par
Hoefer, Inc. la protection fourni par l’équipement pourrait être
diminuée.
• Cet instrument est conçu pour l’usage de laboratoire intérieur
seulement.
• Seulement les accessoires et les parties ont approuvé ou ont fourni
par Hoefer, Inc. pourrait être utilisé pour fonctionner, maintenir, et
entretenir ce produit.
• utilise Seulement une alimentation qui est CET a marqué ou la
sécurité certié par un nationalement reconnu essayant le laboratoire.
• Le couvercle de sécurité doit être à sa place avant connecter
l’alimentation mene à une alimentation.
• Tourner tous contrôles d’alimentation de et débrancher les avances
de pouvoir avant enlever le couvercle de sécurité.
• Circuler seulement de l’eau ou 50/50 glycol d’eau/éthylène par
l’exchanger de chaleur si si équipé. Ne pas connecter l’exchanger de
chaleur à un robinet d’eau ou à la source d’agent de refroidissement
où la pression d’eau est non régulée.
• Ne Jamais introduire d’antigel ou du dissolvant organique dans
n’importe quelle partie de l’instrument. Les dissolvants organiques
causeront des dommages irréparables à l’unité!
• Ne pas fonctionner avec les températures de tampon au-dessus du
maximum a spécié des spécications techniques. La surchaue
causera des dommages irréparables à l’unité !
Wichtige Informationen – German
• Wenn diese Ausrüstung gewissermaßen nicht angegeben durch
Hoefer, Inc. verwendet wird, kann der durch die Ausrüstung zur
Verfügung gestellte Schutz verschlechtert werden.
• Dieses Instrument wird für den Innenlaborgebrauch nur dafür
entworfen.
• Nur Zusätze und Teile genehmigten oder lieferten durch Hoefer, Inc.
kann für das Funktionieren, das Aufrechterhalten, und die Wartung
dieses Produktes verwendet werden.
• Verwenden Sie nur eine Energieversorgung, die CE gekennzeichnet
oder durch ein national anerkanntes Probelaboratorium bescheinigte
Sicherheit ist.
• Der Sicherheitsdeckel muss im Platz vor dem Anschließen der
Energieversorgung sein führt zu einer Energieversorgung.
• Alle Energieversorgungssteuerungen abdrehen und die Macht
trennen führt vor dem Entfernen des Sicherheitsdeckels.
• Nur Wasser oder 50/50 Glykol des Wassers/Äthylens durch den
Wärmeaustauscher, wenn so ausgestattet, in Umlauf setzen.
Verbinden Sie den Wärmeaustauscher mit einem Wasserklaps oder
jeder Kühlmittel-Quelle nicht, wo der Wasserdruck ungeregelt wird.
• Führen Sie nie Frostschutzmittel oder jedes organische Lösungsmittel
in jeden Teil des Instrumentes ein. Organische Lösungsmittel werden
nicht wiedergutzumachenden Schaden der Einheit verursachen!
• Mit Puertemperaturen über angegebenen technischen
Spezizierungen des Maximums nicht funktionieren. Die Überhitzung
wird nicht wiedergutzumachenden Schaden der Einheit verursachen!
Informazioni Importanti – Italian
• Se quest’apparecchiatura è usata in un modo specicato da Hoefer,
Inc. la protezione fornito dall’apparecchiatura potrebbe essere
indebolita.
• Questo strumento è disegnato per l’uso di laboratorio interno solo.
• Solo gli accessori e le parti hanno approvato o hanno fornito da
Hoefer, Inc. potrebbe essere usato per operare, per mantenere, e per
revisionare questo prodotto.
• usa Solo un alimentatore che è CE ha marcato o la sicurezza certicato
da un nazionalmente riconosciuto testando il laboratorio.
• Il coperchio di sicurezza deve essere nel luogo prima di collegare i
piombi di alimentatore a un alimentatore.
• Spegne tutto i controlli di alimentatore e disinserisce i piombi di
potere prima di togliere il coperchio di sicurezza.
• Circola solo l’acqua o 50/50 glicole di acqua/etilene attraverso
lo scambiatore di calore se così equipaggiato. Non collegare lo
scambiatore di calore a un rubinetto di acqua o qualunque fonte di
refrigerante dove la pressione di acqua è sregolata.
• Non introduce mai l’antigelo o qualunque solvente organico in
qualunque parte dello strumento. I solventi organici causeranno il
danno irreparabile all’unità!
• Non opera con le temperature di tampone al di sopra del massimo
ha specicato le descrizioni tecniche. Il surriscaldamento causerà il
danno irreparabile all’unità!
Viktig Informasjon – Norwegian
• Hvis dette utstyret blir brukt i en måte ikke spesisert ved Hoefer, Inc.
beskyttelsen som ha blitt git av utstyret kan bli svekket.
• Dette instrumentet er utformet for innendørs laboratoriumbruk bare.
• Bare tilbehør og deler godkjente eller forsynte ved Hoefer, Inc. kan bli
brukt for drive, vedlikeholde, og betjene dette produktet.
• bruker Bare en kraftforsyning som er CE merket eller sikkerhet
som ha blitt sertisert av et som nasjonalt ha blitt anerkjent prøver
laboratorium.
• Sikkerheten lokket må være på plass før forbinding kraftforsyningene
blyene til en kraftforsyning.
• Vender all kraftforsyningsstyring av og frakopler kreftene blyene før
erning sikkerheten lokket.
• Sirkulerer bare vann eller 50/50 vann/ethylene glykol gjennom
oppvarmingen veksleren i så fall utstyrer. Ikke forbind oppvarmingen
veksleren til en vanntapp eller noe kjølemiddelkilde hvor vannet
•
piii
Page 5

trykket er unregulated.
• Introduserer Aldri antifreeze eller noe organisk løsemiddel inn i noe
del av instrumentet. Organiske løsemiddler vil forårsake irreparabel
skade på enheten !
• Driver med buertemperaturer over maksimum ikke spesiserte
teknisk spesikasjoner. Å overoppheting vil forårsake irreparabel
skade på enheten !
Wazne Informacje – Polish
• Jeżeli ten sprzęt jest wykorzystywany w sposób nie określone przez
Hoefer, Inc. do ochrony przewidzianej przez urządzenie może zostać
obniżony.
• Instrument ten jest przeznaczony do użytku w laboratoriach kryty
tylko.
• Tylko akcesoriów i części zatwierdzone lub dostarczone przez Hoefer,
Inc. mogą być wykorzystane do eksploatacji, utrzymania i obsługi
tego produktu.
• korzystać jedynie zasilacza że jest noszące oznakowanie CE lub
bezpieczeństwa uwierzytelnione przez uznane na poziomie krajowym
laboratorium badawcze.
• Bezpieczeństwo lid musi być w miejsce przed podłączeniem zasilania
prowadzi do zasilania.
• Zaś wszystkie źródła zasilania urządzenia sterujące o i odłączyć moc
prowadzi przed odbiorem bezpieczeństwa lid.
• Krążą tylko wody lub wody 50/50/ethylene glycol wymiennik ciepła
poprzez jeśli tak wyposażone. Nie należy połączyć wymiennik ciepła
woda z kranu lub jakimkolwiek chłodziwo źródła, jeżeli ciśnienie wody
jest nieuregulowanych.
• Nigdy nie wprowadzać rozpuszczalnika organicznego przeciw
zamarzaniu lub jakichkolwiek na dowolną część dokumentu.
Rozpuszczalniki organiczne spowoduje nieodwracalne szkody dla
jednostki!
• Nie działają w buforze temperatury powyżej maksymalnego
określone specykacje techniczne. Przegrzania spowoduje
nieodwracalne szkody dla jednostki!
Informações Importantes –
Portuguese
• Se este equipamento é usado numa maneira não especicada por
Hoefer, Inc. que a protecção fornecida pelo equipamento pode ser
comprometida.
• Este instrumento é projectado para uso de interior de laboratório só.
• Só acessórios e partes aprovaram ou forneceu por Hoefer, Inc. pode
ser usada para operar, manter, e servicing este produto.
• Só usa um estoque de poder que é CE marcou ou segurança
registrada por um nacionalmente reconhecido testando laboratório.
• A tampa de segurança deve estar em lugar antes de ligar o estoque
de poder leva a um estoque de poder.
• Desliga todos controlos de estoque de poder e desconecta os
chumbos de poder antes de retirar a tampa de segurança.
• Circulam só água ou 50/50 glicol de água/ethylene pelo exchanger de
calor se for assim equiparam. Não ligue o exchanger de calor a uma
torneira de água nem qualquer fonte de refrigerante onde a pressão
de água é não regulado.
• Nunca introduz anticongelante nem qualquer orgânico solvente em
qualquer parte do instrumento. Orgânico solvente causará agressão
irreparável à unidade!
• Não opera com temperaturas de buer acima do máximo especicou
especicações técnicas. Superaquecer causará agressão irreparável à
unidade!
Información Importante – Spanish
• Si este equipo es utilizado en una manera no especicado por Hoefer,
Inc. la protección proporcionado por el equipo puede ser dañada.
• Este instrumento es diseñado para el uso interior del laboratorio sólo.
• Sólo accesorios y partes aprobaron o suministraron por Hoefer, Inc.
puede ser utilizado para operar, para mantener, y para atender a este
producto.
• Sólo utiliza una alimentación que es CE marcó o la seguridad
certicada por un nacionalmente reconocido probando el laboratorio.
• La tapa de la seguridad debe estar en el lugar antes de conectar la
alimentación lleva a una alimentación.
• Apaga todos controles de alimentación y desconecta los plomos del
poder antes de quitar la tapa de la seguridad.
• Circula sólo agua o 50/50 glicol de agua/etileno por el intercambiador
de calor si ése es el caso equiparon. No conecte el intercambiador
de calor a un toque de la agua ni cualquier fuente del líquido
refrigerante donde la presión del agua está libre.
• Nunca introduce anticongelante ni algún solvente orgánico en
cualquier parte del instrumento. Los solventes orgánicos causarán
daño irreparable a la unidad!
• No opera con temperaturas de búfer encima del máximo especicó
especicaciones técnicas. Recalentar causará daño irreparable a la
unidad!
Viktig Information – Swedish
• om denna utrustning används i ett sätt som inte har speciceras av
Hoefer, Inc. skyddet tillhandahöll vid utrustningen kan skadas.
• Detta instrument formges för inomhuslaboratorium användning bara.
• Bara medhjälpare och delar godkände eller levererade vid Hoefer, Inc.
kan användas för fungera, underhålla, och servicing denna produkt.
• använder bara en kraft tillgång som är CE markerade eller säkerhet
intygade vid en nationellt erkänd testande laboratorium.
• Säkerheten locket måste vara på platsen före koppla kraften
tillgången blyen till en kraft tillgång.
• Vänder sig alla kraft tillgång kontroller av och kopplar bort kraften
blyen före ytta säkerheten locket.
• Cirkulerar bara vatten eller 50/50 vatten/ethylene glycol genom
värmen exchanger i så utrustad fall. Inte kopplar värmen exchanger
till en vatten kran eller något kylmedel källa där vattnet trycket är
unregulated.
• Inför aldrig kylvätska eller något organiska lösningsmedel in i någon
del av instrumentet. Organiskt lösningsmedel ska orsaka irreparable
skada till enheten!
• Använd inte med buert temperaturer över det högsta angivna
tekniska specikationerna. Överhettning skulle orsaka irreparabla
skador på enheten!
•
piv
Page 6

English
Waste Electrical And Electronic Equipment (WEEE)
This symbol indicates that the waste of electrical and electronic
equipment must not be disposed as unsorted municipal waste
and must be collected separately. Please contact an authorized
representative of the manufacturer for information concerning the
decommissioning of your equipment.
French
German
Italian
Ce symbole indique que les déchets relatifs à l’équipement
électrique et électronique ne doivent pas être jetés comme les
ordures ménagères non-triées et doivent être collectés séparément.
Contactez un représentant agréé du fabricant pour obtenir des
informations sur la mise au rebut de votre équipement.
Dieses Symbol kennzeichnet elektrische und elektronische Geräte,
die nicht mit dem gewöhnlichen, unsortierten Hausmüll entsorgt
werden dürfen, sondern separat behandelt werden müssen. Bitte
nehmen Sie Kontakt mit einem autorisierten Beauftragten des
Herstellers auf, um Informationen hinsichtlich der Entsorgung Ihres
Gerätes zu erhalten.
Questo simbolo indica che i rifiuti derivanti da apparecchiature
elettriche ed elettroniche non devono essere smaltiti come
rifiuti municipali indifferenziati e devono invece essere raccolti
separatamente. Per informazioni relative alle modalità di
smantellamento delle apparecchiature fuori uso, contattare un
rappresentante autorizzato del fabbricante.
Spanish
Swedish
Este símbolo indica que el equipo eléctrico y electrónico no debe
tirarse con los desechos domésticos y debe tratarse por separado.
Contacte con el representante local del fabricante para obtener más
información sobre la forma de desechar el equipo.
Denna symbol anger att elektriska och elektroniska utrustningar
inte får avyttras som osorterat hushållsavfall och måste samlas in
separat. Var god kontakta en auktoriserad tillverkarrepresentant för
information angående avyttring av utrustningen.
•
pv
Page 7

Fig 1. The Hoefer SE900.
Function and Description
The Hoefer® SE900 vertical slab gel electrophoresis unit is intended
for the second dimension of 2D electrophoresis. It is designed for gel
systems which use a single buffer within the gel tank such as those
described by Laemmli. Both buffers used in the anodic and cathodic
chambers must be the same. Up to 6 second dimension gel separations
can be performed simultaneously.
The first dimension separation of 2-D protein electrophoresis should
be performed on Immobilized pH Gradient Gels, referred to as IPG
strips in this manual. The Hoefer IEF100 can be used to generate first
dimension separations in which the proteins are separated by pI. The
focused strips are transferred to the SE900 second-dimension slab gel
for size separation.
The SE900 is offered as a self cast gel system. The SE900 glass
cassettes are 28 cm wide and 21 cm in length producing gels 25 cm
wide × up to 20 cm tall, and 1 mm in thickness. The SE900 will also
hold glass plates and gels from other vendors.
The SE900 is the separation tank as a stand alone unit. The
SE900-1.0 comprises the separation tank, a multiple gel caster, and
six glass cassettes.
•
p1
Page 8

Specifications
This declaration of conformity is only
valid for the instrument when it is:
• used in laboratory locations,
• used as delivered from Hoefer, Inc.
except for alterations described in the
user manual, and
• connected to other CE-labeled instruments or products recommended or
approved by Hoefer, Inc.
Gel plate size (w × h): 28 × 21 cm
Gel size: 25 × 20 cm
Maximum watt: 100 W
Maximum volt: 600 V
Maximum ampere: 1000 mA
Maximum temperature: 45 °C
Environmental Indoor use: 4 – 40 °C
operating conditions: Humidity: up to 80%
Altitude: up to 2000 m
Installation category: II
Pollution degree: 2
Maximum recirculation
water pressure: 12 psi
Dimensions (w × h × d): 43 × 43 × 20 cm
17 × 17 × 8 inch
Weight: 22.3 lbs, 10.1 kg
Input rating: 100 – 240 V
50 – 60 Hz 2A
Product certifications: EN61010-1:2001, CE
•
p2
Page 9
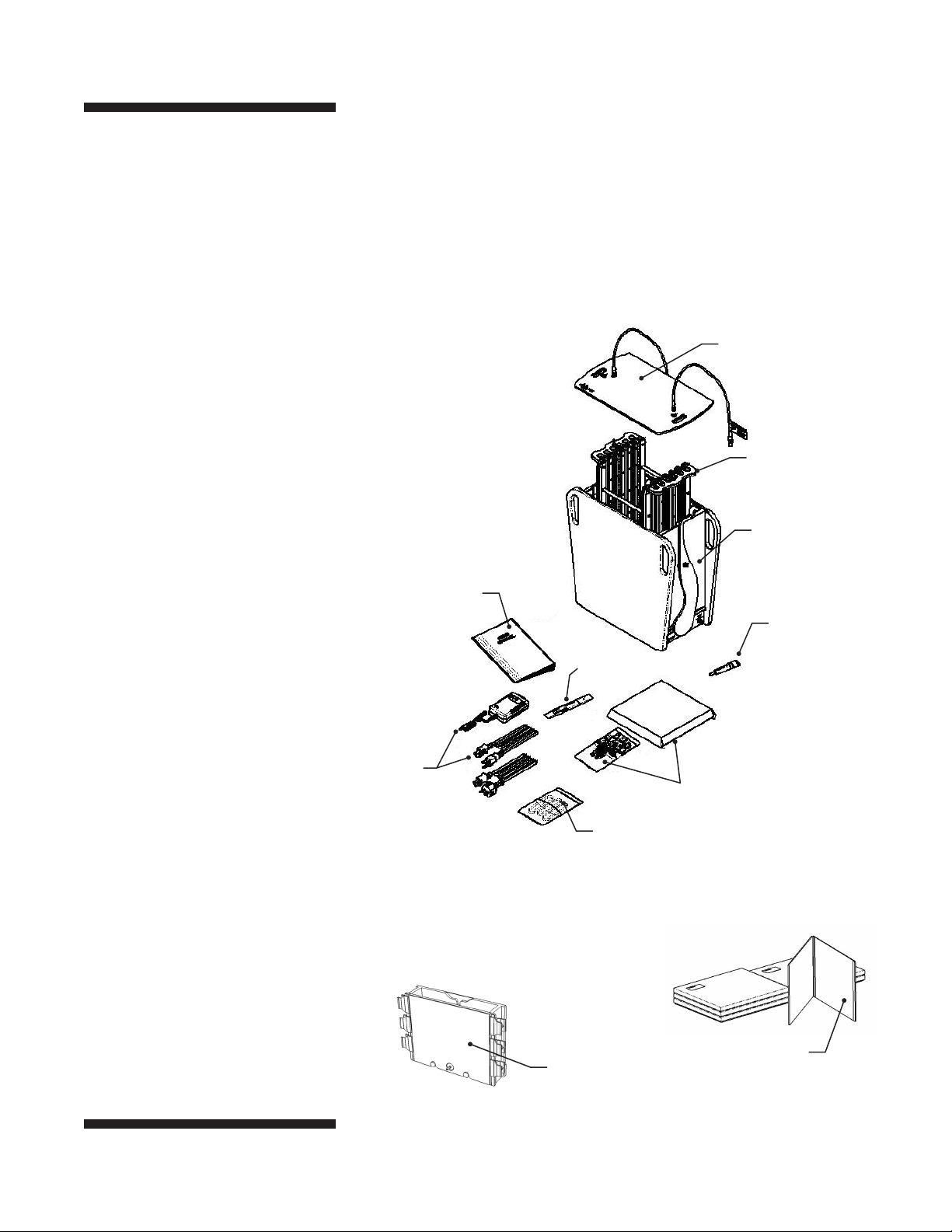
Fig 2. The SE900. The separation tank,
PAGE rack, and safety lid.
Unpacking and Inventory
Unwrap all packages carefully and compare the contents with the
packing list, making sure all the items have arrived. If any part is
missing, contact your local sales office. Inspect all components for
damage that may have occurred while the unit was in transit. If
any part appears damaged, contact the carrier immediately. Be sure
to keep all packing material for damage claims or to use should it
become necessary to return the unit.
Safety Lid (SE9056)
PAGE Rack (SE9054)
Separation Tank
User Manual
(SE900-IM)
Wonder Wedge
(SE1514)
Power Pack
with 115 V Cord, and
230 V (EU&UK) Cords
Drain Filters (SE917)
The SE900-1.0. The system including the SE900 above,
multiple gel caster, (6) glass cassettes.
Gel Seal (SE6070)
Tubing Kit, Recirculation Bath
(SE908)
Multiple Gel Caster
(SE915)
Glass Cassette
(SE9102-1-1.0)
•
p3
Page 10

Caution! Never use water/alcohol mixtures
or commercial antifreeze as the coolant in
the recirculating water bath. This will cause
irreparable damage to the separation tank.
Note: The separation tank is chemically
resistant to common electrophoretic buffers,
but not to organic solvents or strong acids
and alkali.
Temperatures above 45 °C may cause
acrylic to warp.
Note: There is no need for a magnetic stir
bar. The pumps circulate the buffer evenly.
Separation Tank
The Separation tank is transparent to allow visualization of the tracking dyes during electrophoresis.
The chamber base contains a ceramic cooling surface which helps
to efficiently cool the buffer within the tank. Care should be taken
not to drop ANYTHING directly on the ceramic plate. A pump
system forces buffer across the cooling surface and circulates the
buffer through the center region of the tank, maintaining a constant
temperature in the gel cassettes. Cooling ports can be attached to a
temperature regulated recirculating water bath for active cooling of
the separation tank.
The recirculating water bath should have a maximum output pressure
of 12 psi. Use ONLY water or up to a 50% mixture of ethylene glycol
in water in the recirculating bath.
Never connect to an unregulated source of water such as a tap water.
Fig 3. Separation tank.
Note: A light layer of gel seal has been
applied inside the guide channels in the
separation tank to allow the PAGE rack
to slide into the tank easily. Do not wash
off. Reapply as needed, see Instructions
page 9, Gel Seal.
PAGE Rack Channel
•
p4
Page 11

Fig 4. Key aligns with a mating
feature in the PAGE rack to ensure
proper orientation.
Safety Interlocks
Safety interlocks are mounted on the sides of the tank. One is red and
the other is black to help with orienting the gels correctly within the
gel tank. For safety reasons, the top of the safety interlock secures the
lid during electrophoresis, and the bottom prevents access to the drain
port. This prevents electrical hazard of draining electrified buffer. A
key at the bottom of the separation tank (Fig 4) aligns with a mating
feature in the PAGE rack (Fig 6) to ensure proper orientation.
A drain port is included at the bottom behind the black safety interlock. The drain is protected by a removable filter that will block parts
of gels from clogging the drain port. This filter should be removed
and rinsed off periodically.
•
p5
Page 12

Fig 5. PAGE rack.
PAGE Rack
The rack serves three purposes:
1. It divides the tank into anodic and cathodic chambers and supports
and seals the gel cassettes vertically inside the separation tank with
rubber gaskets.
2. It holds the platinum wire electrodes that conduct the current
during electrophoresis.
3. Features in the base create the circulation pattern to maintain
constant temperature, and provide efficient cooling if the SE900 is
connected to a recirculating water bath.
The PAGE rack locates in four channels in the clear sides of the tank.
The PAGE rack has only one proper orientation. A cutout in the base
(Fig 6) aligns with a key in the separation tank. If the PAGE rack is
inserted incorrectly, the buffer circulation will not function properly,
and the safety lid will not fit.
The electrodes terminate at banana plugs that connect to the safety
lid (Fig 7).
Fig 6. Orientation feature.
Fig 7. Banana plug.
Slots with rubber seals accept the gel cassettes and hold them vertically in the separation tank. If one of the six positions is empty, the
rubber seals eliminate electrical leaks without the need for blank
cassettes or space fillers.
•
p6
Page 13

Safety Lid
The safety lid holds the high voltage leads that connect to an external
power supply (not supplied). The lid is held in place by the safety
interlocks during electrophoresis (Fig 8).
Caution! Always install the safety lid
before use!
Fig 8. Safety lid.
The color-coded high voltage leads have safe 4 mm plugs that interface directly with Hoefer power supplies. Adapters may be required
to connect the SE900 to other power supplies. Check the connection
before using the SE900.
Glass Cassettes
The glass plates are 28 cm wide × 21 cm in length. The cassettes have
1 mm thick glass spacers glued into place and are hinged for easy
assembly (Fig 9). Six glass cassettes are included with each SE900-1.0.
Additional cassettes can be ordered separately as SE9102-1-1.0.
It is important that the cassettes be positioned with the hinge side
down in the separation tank during electrophoresis.
Fig 9. SE9102-1-1.0 glass cassette.
•
p7
Page 14

Fig 10. Multiple gel caster.
Multiple Gel Caster
The multiple gel caster is included with the SE900-1.0 (Fig 10), and
can be ordered separately as the SE915.
The multiple gel caster is used to cast up to seven 1 mm thick gels
at the same time. Separator sheets are placed between glass cassettes
before casting to keep the cassettes from sticking to each other after
polymerization.
When casting single percentage gels by hand, pour the gel solution
down the channel in the back wall of the caster (Fig 11). The cassettes
will fill from the bottom producing better quality gels, and reducing
the chances of trapping air bubbles.
When casting gradient gels, the gel solution should be slowly pumped
in through the port at the bottom. The triangular plug at the bottom
should be removed. The triangular region forms a space for the gradient gel to spread out and enter the glass cassettes uniformly.
When using butanol overlays, try to minimize contact of butanol with
the plastic multicaster. Prolonged contact with butanol may craze the
plastic of the multicaster.
Fig 11. Multicaster filling line.
Space Saver
The space saver is used to fill space within the multiple gel caster, and
reduce the amount of gel solution. One space saver is equivalent in
thickness to one 1.0 mm glass cassette.
When casting six 1.0 mm thick gels, use one space saver at the back
of the multiple gel caster.
One space saver is included with the multiple gel caster. Additional
space savers (SE912) can be ordered separately if less than 6 gels are
routinely cast.
•
p8
Page 15

Separator Sheets
The sheets have a protective film on both sides which should be
removed before use.
Wonder Wedge
The Wonder Wedge is helpful to pry apart the glass plates after
electrophoresis.
Gel Seal
Gel seal is used in the separation tank to lubricate the channels for
the PAGE rack, and help it slide into place. If the PAGE rack starts to
stick as it is inserted into the tank, apply a thin layer of gel seal to the
inside of the four channels with a gloved finger.
Gel seal is also used on the gasket in the multiple gel caster.
Plate Rack
The plate rack (SE914) may be ordered separately. It is very convenient to hold the glass cassettes during assembly of the multiple gel
caster, and when applying IPG strips to the top of the second dimension gels.
Fig 12. Plate rack, SE914.
•
p9
Page 16

Operating Instructions
Separation Tank Setup
Note: Do not remove the layer of gel seal
from inside the white channels that position
the PAGE rack.
Note: Do not run the pumps dry.
Fig 13. Panel located on lower right side
of the SE900. The power supply plugs into
the power supply inlet on the right. The
ON/OFF toggle switch is on the left.
1 Position the SE900 with the black safety interlock on the left, as
shown in Fig 20 on page 17.
2 Place the separation tank near a sink for easy buffer draining
and disposal.
3 Before using for the first time, disassemble the unit and wash with a
dilute solution of a laboratory detergent. Rinse thoroughly first with
water and then with distilled water.
Glass plates should be washed well and dried completely before
casting gels.
4 Plug one end of the power pack into the power entry on the lower
right side of the SE900. Plug the other end into an appropriate
grounded outlet.
The circulating pumps are turned on with a toggle switch on the lower
right side of the SE900 (Fig 13).
5 Connect the cooling ports to an external recirculating water bath, if
active cooling is desired.
•
p10
Page 17

Note: If desired, labels printed on filter paper
can be included in the cassette. Sheets of
#1 Whatman filter paper can be used to
print gel id numbers, cut into small pieces
and positioned in the bottom corners of the
cassette. When gel solutions are added the
numbers will be polymerized into the gel
matrix allowing for easier identification of
second dimension gels.
Caution! Glass edges may be sharp so handle
glass cassettes with care.
Using the Multiple Gel Caster
Casting Homogenous Gels
1 Make sure the caster and the glass cassettes are clean and dry.
2 Close each of the glass cassettes making sure the edges align flush.
3 Disassemble the clean multiple gel caster, and lay the back of the
caster flat on the bench.
4 The two faceplate screws should be loosened, but can remain in place.
5 The triangular gasket should be put in place.
6 Place the space saver in the back of the caster.
7 Place a separator sheet over the space saver.
8 Place a glass cassette on top of the first separator sheet.
9 Place a separator sheet over the glass cassette.
10 Continue to stack alternating glass cassettes and separator sheets for
each gel being cast.
11 Complete the assembly by adding enough separator sheets to fill the
caster just above flush.
12 Lightly grease the gasket in the faceplate with gel seal to assure a leak
free casting. Replace the gasket in the faceplate without bunching or
stretching the gasket.
13 Complete the assembly with a separator sheet.
14 Slide the faceplate into place under the two screws and tighten
the screws.
15 Attach the 6 red clamps, 3 per side, along the sides of the caster.
16 Make sure the cap is on the bottom port of the caster before pouring
gels by hand.
17 Stand the assembled caster upright on a level surface.
Fig 14. Multicaster assembly.
Face Plate
Glass Cassette
Separator Sheet
Space Saver
Multiple Gel Caster
Red Clamp
•
p11
Page 18

Caution! Acrylamide is a neurotoxin. Extreme
care should be used when handling and
preparing acrylamide solutions.
Fig 15. Pour the solution in a slow
continuous stream.
Prepare the Acrylamide Solution
1 See Appendix A for SDS-PAGE recipes. For casting six SE9102-1-1.0
gels, 400 ml is needed.
2 De-aerate the gel solution for 5 minutes. Add the (initiator) 10% w/v
APS and the (catalyst) 10% v/v TEMED* just prior to casting the gels.
Pour the gel solution into the channel in the back of the caster. The
solution will flow down the channel and fill the casssettes from the
bottom (Fig 15).
* The TEMED is diluted to 10% for better distribution during mixing of
the gel solutions.
3 Pour the solution as a slow, continuous stream. Try to minimize the
introduction of air bubbles into the flow.
4 Fill solution to 0.5–1 cm below the top of the glass plate to allow
room for the IPG strip and an agarose seal.
5 Overlay each gel with a thin layer of water-saturated n-butanol or
diluted gel buffer to get the best surface on the top of the gel. Slowly
apply the overlay near the gel surface from one side, taking care to
prevent mixing. Allow the overlay to flow across the surface unaided
(Fig 16).
6 Allow the gel to polymerize approximately one hour.
7 Remove the overlay by rinsing the top of the gel several times with
distilled water. Use diluted gel buffer as a final rinse.
8 Optional-storage: The second dimension gels can be temporarily stored
in the caster.
The second dimension gels can also be removed from the caster and
stored 1–2 days submerged in gel buffer before use. There is a risk
that with prolonged storage, the gels abosrb water, and the cassette
partially opens, leading to skewed dye fronts.
Fig 16. Applying overlay.
Note: A stacking gel can be used if desired,
but is not necessary.
Note: It is necessary to remove any butanol
overlays before storing the gels in the caster.
Leaving butanol in the caster can lead to
whitening and brittleness of the acrylic.
•
p12
Page 19

Note: It is a good idea to practice with
water and the peristaltic pump to empirically
determine the volumes and the flow rates to
cast good gradient gels.
Note: Avoid getting air bubbles into the
pump tubing, Air bubbles will interfere with
proper gradient formation.
Note: The displacement solution is required.
The gradient will not form correctly without
displacing the acrylamide up inside the
cassettes. It also helps keep acrylamide from
polymerizing in the tubing and in the bottom
of the caster, simplifying clean up. The
displacement solution should not enter the
bottom of the cassettes.
Gradient Gels
Linear gradient gels can be cast using an optional accessory, the
Hoefer SG500 Gradient Maker. The SG500 mixes low percentage
and high percentage gel solutions that are pumped into the port at the
bottom of the multiple gel caster using a peristaltic pump.
Pouring a Linear Gradient Gel
1 Assemble the multiple gel caster as described on page 11, with the
following two exceptions:
• Do not insert the triangular rubber gasket at the bottom of
the gel caster.
• Remove the cap from the bottom inlet port.
2 Attach one end of laboratory grade tubing to the SG500 outlet port.
Insert the tubing through a peristaltic pump, and attach the other end
to the inlet port of the SE915 multiple gel caster faceplate.
3 Calculate the volume of monomer solution needed. Divide the total
volume in half and prepare this volume of both the higher- and lowerpercentage acrylamide solutions.
4 Optional: Adjust the higher-percentage acrylamide solution to 15%
(w/v) sucrose or up to 25% (v/v) glycerol to improve layering.
5 Pour the higher percentage, or heavy, acrylamide solution into the
SG500 chamber farthest from the outlet. Open the stopcock just long
enough to displace air between the chambers and then close.
6 Pour the lower percentage, or lighter, acrylamide solution into the
mixing chamber, the chamber with the SG500 outlet.
7 Place a stir bar into the mixing chamber. Place the gradient maker
onto a magnetic stirrer, and begin stirring at a rate that mixes well but
does not introduce bubbles into the solution.
8 Turn on the peristaltic pump to a low setting, and open the stopcock
between the two SG500 chambers.
9 The gel solution should slowly layer in the triangular region at the
bottom of the multiple gel caster, and fill the cassettes evenly from the
bottom to the top.
10 Once almost all solution has exited the gradient maker pause the
pump temporarily, and fill the gradient maker with ~200 mls of the
displacement solution. Restart the pump and pump the solution
through the tubing, forcing the acrylamide solutions up into the gel
cassettes until the desired height is reached. Stop the pump.
11 Overlay each gel with 1 ml of water-saturated n-butanol or diluted
gel buffer to get the best top gel surface. Slowly deliver the overlay
solution on one side, minimizing mixing, and allow the overlay to flow
across the top surface unaided.
12 Allow the gels to polymerize for a minimum of one hour. After polym-
erization, pour off the overlay and rinse the gel surface several times
with distilled water.
13 Prepare the stacking gel monomer solution. Pour the stacking gel on
top of the resolving gel. Allow a minimum of one hour for the stacking
gel to polymerize.
•
p13
Page 20

Note: The Plate Rack, SE914, is a
convenient accessory for temporarily holding
the gel cassettes when disassembling the
multiple gel caster.
Disassembling the Multiple Gel Caster
1 Place the caster down on its back in a tray or sink.
2 Remove the red clamps and screws securing the faceplate to
the caster.
3 Slide off the faceplate.
4 Remove gel cassettes and filler sheets. Rinse outside of the gel
cassettes to remove excess polymerized acrylamide.
5 Clean the multiple gel caster components with laboratory detergent.
Rinse and let air dry.
IPG Strip Equilibration
Before IPG strips are placed on top of the second dimension gel, the
buffer in the strips needs to be replaced with an appropriate buffer
for PAGE.
Equilibrate IPG strips in appropriate equilibration buffers. Typically,
a two step equilibration (first with DTT and a second with iodoacetamide) gives better results than a single DTT equilibration step. The
following procedure is recommended.
Equilibration Procedure
1 Thaw two aliquots of the equilibration solution.
2 Add 10 mg/ml DTT to one solution.
3 Place the IPG strips in the rehydration/equilibration tray, or a
glass tube.
4 Add 6.5 ml of solution to each slot containing an IPG strip.
5 Place on rocker for 10–15 minutes.
Following equilibration, discard the first equilibration solution in an
appropriate manner.
6 Add 25 mg/ml iodoacetamide (IAA) to the second aliquot of
equilibration solution.
7 Add 6.5 ml of solution to each slot containing an IPG strip.
8 Place on rocker for 10–15 minutes.
Following equilibration, discard the second equilibration solution in an
appropriate manner.
Following equilibration, the IPG strips are placed on the top of the second
dimension gel, and sealed into place with the agarose overlay.
•
p14
Page 21

Fig 17. Applying IPG strip to second
dimension gel.
Sealing the IPG to the Second Dimension Gel
1 Remove residual liquid from the top of the second dimension gel by
tipping the cassette and blotting one corner with a lint-free tissue.
2 Apply the equilibrated IPG strip to the gel surface (Fig 17). A thin
plastic strip is useful to gently seat the IPG directly against the top of
the second dimension gel. Do not press the strip into the gel or the
results may be distorted. Avoid trapping air bubbles between the strip
and the second dimension gel.
3 Seal the IPG strip in place by applying hot, molten agarose over the
IPG strip (Fig 18). See Appendix A for suggested recipe. Avoid introducing air bubbles while applying the overlay.
4 Allow the agarose to solidify before placing gels into the
separation tank.
Fig 18. Adding agarose overlay.
•
p15
Page 22

Caution! Do not run the circulation pumps
without buffer in the separation tank.
Caution! Glass cassettes will not easily
slide into a dry tank. Fill with buffer first
and use water or buffer to help lubricate
the glass cassettes and rubber flaps.
Page Separation
1 Remove the safety lid.
2 Fill the tank with 12 liters of 1X electrophoresis buffer. This can be
prepared in the separation tank by adding 10X stock and water. Gently
raise and lower the PAGE rack to aid in mixing in buffer.
3 Turn on the circulation pumps. The toggle switch is located on the
lower right side of the SE900.
4 Fully insert the PAGE rack to the bottom of the separation tank,
making sure the keying features are aligned.
5 Insert gels into the slots in the PAGE rack. There is one specific orien-
tation for the gel cassettes. The rubber hinge should be on the bottom
of the separation tank. The sealed IPG strip should be on the left, the
side with the black safety interlock, and the black high voltage lead in
the safety lid (Fig 18).
6 Check that both ends of the gel are exposed to the buffer. The glass
plates should be centered (Fig 19).
Fig 19. Gel orientation.
Good positioning, centered between flaps.
Bad positioning, flap seals closed at end of gel, current cannot get to gel.
•
p16
Page 23

Fig 20. Gel orientation.
Fig 21. Slide safety lid into place.
Note: The black high voltage lead should be
on the same side as the IPG strip.
Black High Voltage Lead
Black Safety Interlock
–
Proper Fill Level
IPG Strip
Rubber Hinge
7 After all gels are placed in the tank, check the level of buffer in the
separation tank. The proper level is about halfway up the spacer on the
top edge of the gel cassettte. The buffer level also must be below the
maximum fill line.
8 Attach the safety lid to the separation tank in the proper orientation
by moving and fitting the top of the safety interlocks through the slots
on the lid, and connecting the high voltage leads to the banana plugs.
The safety interlock will secure the lid in place (Fig 21).
9 Connect the high voltage leads to a power supply capable of delivering
at least 300 V, 500 mA, 90 W, such as the Hoefer PS300-B. Adapters
may be needed if using other power supplies.
10 Set the power supply to the desired values and start the run.
Note: For day runs set the power supply for 80 mA/gel. When running
a full tank of 6 gels set the power supply for 480 mA. Runs should
be complete in about 6 hours. The voltage and wattage should be set
to their maximums, 600 V and 100 W respectively, so as not to limit
the current.
For overnight runs (18 hours), set the power supply for 80 V constant.
The current and wattage should be set not to exceed 500 mA and
100 W.
11 The run is complete when the tracking dye has reached the opposite
side of the gels.
12 Turn off the power supply, the circulation pumps, and the external
recirculating water bath, if being used.
Red High Voltage Lead
Red Safety Interlock
+
•
p17
Page 24

Note: DO NOT use the buffer circulation
pumps to help the tank drain faster.
Fig 22. Drain port.
After Electrophoresis
1 Unplug the leads to the power supply.
2 Remove the lid by moving the safety interlocks while lifting the lid.
3 Carefully lift out each gel. The cassettes are slippery.
Do not use the PAGE rack as a gel carrying rack as the plates may
slide out of the rubber gaskets when tipped or moved.
4 Place the glass cassette on a clean, flat surface.
5 Use the Wonder Wedge to gently open the nonhinged side of the
gel cassette.
6 Use the Wonder Wedge to gently cut the gel away from the side
spacers.
7 Carefully remove the gel from the cassette and place in desired
staining solutions.
8 Attach a length of tubing long enough to reach a sink to the drain
connector (Fig 22). Insert the connector into the drain port on the
lower left side of the SE900, behind the black safety interlock.
9 Allow the tank to drain by gravity. The tank will drain best by having
the drain line at least 18 inches (50 cm) below the tank.
10 Once drained, re-insert empty PAGE rack. Fill the tank with deionied
water, turn on pumps and circulate for 5–10 minutes.
11 Turn off pumps and drain again.
12 Remove the PAGE rack and allow the components to air dry.
13 Remove and rinse any residual acrylamide from the drain filter at the
bottom of the separation tank. Replace the drain filter so that it is
below flush.
•
p18
Page 25

Care and Maintenance
• Handle the PAGE rack with care to prevent damage to the banana
plugs and electrode wires.
• Do not drop items onto the ceramic plate at the bottom of the
separation tank.
• If necessary, clean the tank with mild detergent and rinse with
distilled water. Allow to air dry.
• Clean glass plates and spacers with a dilute solution of a laboratory
cleanser such as RBS-35.TM
• Rinse thoroughly with tap and distilled water. Handle the plates
with care to prevent chipping and do not pull on or stress the
rubber hinge.
• Do not autoclave any components of the system.
• Do not heat any part above 45 °C.
• Do not use organic solvents, abrasives, strong cleaning solutions, or
strong acids or bases to clean the chambers.
If the PAGE rack starts to stick as it is inserted into the tank,
apply a thin layer of gel seal to the inside of the four channels with
a gloved finger.
Technical Service and Repair
Hoefer, Inc. offers complete technical support for all of our
products. If you have any questions about how to use this product,
or would like to arrange to repair it, please call or fax your local
Hoefer, Inc. representative.
•
p19
Page 26

Troubleshooting
Problem Solution
Casting Issues:
Gel caster leaks If the stack is too tall, the front plate may not seat firmly against the gasket.
Remove filler plates or cassettes until the gasket seals.
Apply a light film of gel seal to the foam gasket each time the unit is used.
Check the foam gasket for cracks or nicks and replace if necessary.
Caster face plate not properly aligned. Check that the faceplate is evenly
positioned on caster.
Poor or Incomplete gel polymerization Use only recent stock of the highest quality reagents.
APS reagents or solutions are old and lose their activity when exposed to
moisture. Make up fresh APS daily. If the dry ammonium persulfate does not
crackle when water is added to it, replace the product with fresh stock. Store
reagent tightly closed and in a dessicator to prevent absorption of moisture.
Remove oxygen from the gel environment. Degas the monomer solution
5 minutes before pouring and then overlay the gel surface with water
saturated n-butanol.
Allow gel solutions to come to room temperature before casting (a minimum
of 20 °C, especially for low % T gels).
Increase both APS and TEMED by 30–50%.
Solutions with extreme pH values (especially acidic) may not polymerize.
Check pH of gel buffers.
Poor polymerization at the spacers. Make sure to clean the plates well at
the spacer edges, that the sides are free of dirt or grease, and that the gel is
fully polymerized before removing from the caster.
Gel is too soft, too brittle or white Adjust crosslinker concentration. Crosslinker should be at 2.6% C for
Vertical protein streaks standard SDS gels where %C = (g bis × 100) ÷ (g monomer + g bis)
Gel contains swirls Indicates convection currents during polymerization, usually from
polymerizing too fast.
If gel polymerized in <10 min, too much catalyst. Reduce concentration of
ammonium persulfate and TEMED by 25%.
If gel polymerized in >50 min, not enough catalyst. Increase concentration
of ammonium persulfate and TEMED by 50%.
Make sure gel solutions are near room temperature when casting.
Gels cast simultaneously are different sizes Wait one minute before overlaying each gel so that the solution “settles.”
Use the same amount of overlay on each separation gel. Add the overlay
evenly across the gel surface.
•
p20
Page 27

Problem Solution
Gradient gels-uneven layering Add sucrose (15% final concentration) or glycerol (25% final concentration)
to the high-percent monomer solution.
Solutions pumped in too fast. Add a small amount of bromophenol blue
to the heavy solution to track the gradient formation. Allow for solutions to
underlay without excessive mixing.
Caution! Excessive amounts of bromophenol blue inhibit polymerization.
Electrophoresis Run Problems
Dye front curves up (smiles) at the edges This indicates current leakage at the spacers. Make sure to clean the plates
well at the spacer edges, that the sides are free of dirt or grease, and that
the gel is fully polymerized before removing from the caster.
Gels have been run with hinge side toward the top of the tank. The hinge
also seals the lower edge of the gel from electrical leaks. It is best to place
cassettes hinge side down to get the straightest dye fronts.
Inadequate buffer at the top. Make sure the buffer is filled ½ way through
the top spacer to make the most even electrical field across the gel and
prevent the gel from drying out.
Too much buffer at the top of the gel. Make sure the buffer is filled ½ way
through the top spacer to make the most even electrical field across the gel
and prevent the current from bypassing over the gel at the top.
Check buffer recirculation is on and operating properly.
Use a refrigerated water bath to maintain even buffer temperature within
the tank.
Decrease the current or voltage setting.
Dye front curves down (frowns) at the edges Poor polymerization at the spacers. Make sure to clean the plates well at
the spacer edges, that the sides are free of dirt or grease, and that the gel is
fully polymerized before removing from the caster.
Power Supply detects current leak Cracked or broken alumina plate in the base of the separation tank. Contact
Hoefer or your distributor.
Poor draining of tank Drain fitting has become clogged with debris. Gently back-flush water
through the drain line.
Unusually long run times Too much buffer at the top of the gel. Make sure the buffer is filled ½ way
through the top spacer to make the most even electrical field across the gel
and prevent the current from bypassing over the gel at the top.
Poor reagent quality. Acrylamide solutions can build up acrylic acid over
time. Do not keep stock acrylamide solutions longer than ~3 months and
store acrylamide at 4 °C.
Buffer incorrectly prepared. Use the basic form of Tris. Do not adjust the pH
of the buffer after preparation.
Check power settings and adjust if necessary.
•
p21
Page 28

Problem Solution
Instrument Problems:
No voltage or current at start of the run Instrument not properly attached to power supply. Make sure high voltage
leads are connected into correct +/- terminals and is secure. In some cases
adapters may be required.
Broken electrode. Check continuity of wire with a volt meter.
Lid not secured properly onto banana plugs. Reposition and check lid is fit
securely in place.
Break in HV lead line. Check continuity of wire with a volt meter.
2D Results Problems:
Vertical streaking of sample down from the IPG Strip not properly placed on gel surface. Avoid gouging separating
top of the gel towards the bottom of the gel gel while loading strips.
Perform iodoacetamide treatment.
Make sure IPG strip uniformly contacts the gel surface along the entire
length of the strip.
Overloading of protein.
Horizontal streaking of proteins Poor sample preparation.
Inadequate focusing.
Poor contact between the IPG strip and the second dimension gel.
Spots are skewed or distorted Gels run too fast-uneven migration.
Overlay the resolving gel with water-saturated n-butanol before polymeriza-
tion begins to avoid forming an uneven gel surface.
Uneven gel polymerization or gradient formation. See casting problems for
more support.
IPG strip not properly placed on gel surface. Avoid gouging separating gel
while loading strips.
Sample entry into the second dimension gel is run at too high a power
setting. Run gels at recommended run conditions.
Bubbles present in second dimension gel will distort the spot migration.
No proteins visible on second dimension Quantity loaded too little for detection method. Increase protein load
gel after staining or try a more sensitive staining method.
Too little sample applied to first dimension gel. Check protein concentration
of sample.
Equilibration steps too short or too long. Perform each equilibration step
for 10–15 minutes.
Blank regions in the second dimension gel Tris, salts, and SDS can cause alterations in the focusing position of proteins
in the first dimension. Reduce or eliminate these compounds from the
first dimension.
Bubbles between the strip and the second dimension gel.
•
p22
Page 29

Ordering Information
Product Quantity Order Code
Large Format PAGE 1 SE900
Large Format PAGE Complete 1 SE900-1.0
Tubing Kit for Recirculating Chiller 1 SE908
Multicaster 1 SE915
Space Saver Plate, Multiple Gel Caster 1 SE912
Glass Hinge Cassette 25 × 20, 1 mm 1 SE9102-1-1.0
Plate Rack 1 SE914
Drain Filter 3 SE917
Gel Seal 1 SE6070
Related Products
Isoelectric Focusing Unit 1 IEF100
Recirculating Chiller 1 RCB20
PS300B Power Supply 1 PS300B
Acrylamide 1 kg GR141-1
Agarose 500 g GR140-500
Ammonium Persulfate 10 g GR152-10
Brilliant Blue G 25 g GR134-25
Brilliant Blue R 25 g GR135-25
Bromophenol Blue 10 g GR120-10
Dithiothreitol (DTT) 5 g GR122-5
Glycerol 1 L GR124-1
Glycine 1 kg GR125-1
N,N'-Methylene-bis-Acrylamide 100 g GR142-100
Sodium Dodecyl Sulfate 500 g GR126-500
TEMED 25 g GR151-25
Tris 1 kg GR132-1
Tris-Glycine-SDS Buffer, 10X 1 L GR149-1
Urea 1 kg GR143-1
•
p23
Page 30

Appendix A: Theory and Recipes
Laemmli System Gels
The Laemmli system is the most common electrophoresis protocol for
SDS-denatured proteins.
The leading ion in this discontinuous buffer system is chloride and the
trailing ion is glycine.
Accordingly, the resolving gel (and the optional stacking gel) contain
Tris-Cl buffers (of different concentration and pH), and the electrophoresis buffer contains Tris-glycine. All buffers contain 0.1% SDS.
Polyacrylamide gel composition is indicated by two
different percentages:
% T = total acrylamide = [g (acryl + bis)] × 100
100
% C = crosslinker = g (bis) × 100
g (acryl + bis)
The total percent of acrylamide (% T) in the resolving gel, which
can range from 5 to 20%, determines the pore size. Commonly, the
amount of crosslinker used (% C) is 2.6%.
•
p24
Page 31

Caution! Acrylamide is a neurotoxin. Extreme
care should be used when handling and
preparing acrylamide solutions.
Solutions
1. Acrylamide Stock Solution
(30.8% T 2.6% C Bis, 1000 ml)
Acrylamide (FW 71.08) 30% w/v 300 g
Bis*N,N' Methylenebisacrylamide (FW 154.2) 0.8% w/v 8 g
Deionized H2O to 1000 ml.
Store at 4 °C away from light.
2. 1.5 M TrisCl, pH 8.8
(4X Resolving gel buffer, 2 liter)
Tris (FW 121.1) 1.5 M 363.3 g
Disolve into ~1.5 liters deionized H2O.
4 N HCl (4N hydrochloric acid) to pH 8.8.
Deionized H2O to 2000 ml.
Store at 4 °C.
3. 10% w/v SDS Solution
(100 ml)
Sodium dodecylsulfate (SDS) (FW 288.4) 0.35 M 10 g
Deionized H2O to 100 ml.
Store at room temperature.
4. 10% w/v APS
(Initiator, 1 ml)
Ammonium persulfate (APS) (FW 228.2) 0.44 mM 5 g
Deionized H2O to 5 ml.
Prepare just prior to use.
Fresh APS “crackles” when water is added. If yours does not, replace it with
fresh stock.
5. 10% v/v TEMED
(Catalyst, 5 ml)
N, N, N’, N’-Tetramethylethylenediamine (TEMED) (FW116.2 ) ~0.65 M 0.5 ml
Deionized H2O to 5 ml.
Prepare just prior to use.
Store in a dark glass bottle at room temperature away from light.
TEMED is flammable and should be dispensed in a fume hood.
•
p25
Page 32

6. 0.375 M Tris-Cl, 0.1% SDS, pH 8.8
(Resolving gel overlay, 100 ml)
1.5 M Tris-Cl, pH 8.8 (Solution #2) 0.375 M 25 ml
10% SDS (Solution #4) 3.5 mM 1 ml
Deionized H2O to 100 ml.
Store at room temperature.
— OR —
Water-saturated n-butanol
Shake n-butanol and deionized H2O in a separatory funnel. Remove the
aqueous (lower) phase. Repeat this procedure several times. Use the
upper phase.
Store at room temperature.
7. 10X Electrophoresis Buffer
0.25 M Tris, 1.92 M Glycine, 1.0% SDS
(10X Electrophoresis buffer, 5.0 liters)
Add powders slowly to ~4 liters deionized water while stirring.
Tris (FW 121.1) 0.25 M 151.2 g
Glycine (FW 75.07) 1.92 M 720.6 g
SDS (FW 288.4) 35 mM 50.0 g
Deionized H2O to 5.0 liters.
The pH of this buffer is approximately 8.3. Do not adjust pH.
Store room temperature for up to 2 months.
8. 1X Electrophoresis Buffer
0.025 M Tris, 0.192 M Glycine, 0.1% SDS
(1X Electrophoresis buffer, 12.0 liters)
10X Electrophoresis Buffer (Solution #7) 1200 ml
Deionized H2O 10.8 l
This can be prepared in the separation tank. Add 10X stock, water then the
PAGE rack. Gently raise and lower the PAGE rack to aid in mixing then allow
the recirculation system to mix the buffer well before use.
•
p26
Page 33

9. 1% Agarose in 1X Electrophoresis Buffer
(100 ml)
In a 500 ml flask add:
Agarose 1 g
Caution! SDS may cause the solution to boil
over so exercise caution when heating and
prevent boiling over.
10X Electrophoresis Buffer (Solution #8) 10 ml
Deionized H2O 90 ml
Bromophenol Blue 3 mg
Gently swirl to suspend agarose.
Heat at low power in a microwave oven until agarose is fully dissolved.
Divide into ~1.5 ml plastic screw top tubes.
Store in aliquots at 4 °C.
10. Gradient Gel Casting Displacement Solution
(50% Glycerol in 0.375 mM TrisCl 8.8, 0.1% SDS, 200 ml)
Glycerol 100 ml
1.5M TrisCl ph 8.8 (Solution #2) 50 ml
Deionized H2O 50 ml
Bromophenol Blue 3 mg
Mix well.
11. SDS Equilibration Buffer Solution
This solution is used after IEF, and before second dimension PAGE. The IPG
strips are immersed in excess solution to raise the pH of the strip buffer so
that it is suitable for PAGE, and to coat the proteins in SDS uniformly so
that they migrate properly in the second dimension gel.
Note: IPG strips should be equilibrated
just prior to second dimensin PAGE.
Do not equilibrate the IPG strips before
storing at -20 °C.
Prepares 200 ml
(6 M urea, 75 mM Tris-HCl pH 8.8, 29.3% glycerol, 2% SDS, 0.002%
bromophenol blue)
Final Concentration Amount
Urea (FW 60.06) 6 M 72.1 g
1.5M Tris-HCl, pH 8.8 stock solution 75 mM 10.0 ml
Glycerol (87% w/w) 29.3% (v/v) 69 ml
SDS (FW 288.38) 2% (w/v) 4.0 g
Bromophenol blue 0.002% (w/v) 4 mg
Deionized H2O to 200 ml
Aliquot into 30 ml aliquots and store frozen at -20 °C or below.
24 cm IPG’s require 5 –10 ml per strip per equilibration step. Shorter strips
can use proportionately less volume per equilibrations step.
•
p27
Page 34

Gel Recipes
The Laemmli gel recipes are for 400 ml of a single concentration solution (enough for six 1.0 mm, 25 × 20 cm gels). Tabulated are volumes
for relatively large pore gels (10% T range) as well as smaller pore
gels (12.5–20% T range). 5% and 7.5% gels are difficult to handle in
large format gels but can be blended into gradient gels for better resolution and easier handling. The recipes given here are for guidance
when casting gradient gels. Use ½ total required volume each solution
when casting gradient gels. Using the solutions given in Appendix A,
all gels are crosslinked with 2.6% C.
Laemmli Gel (per 400 ml gel solution)
Resolving Gel Solution
400 ml
10.0% 12.5% 15.0% 17.5% 20%
Acrylamide Stock (Soln. #1) 133.3 166.7 200.0 233.3 266.7
1.5 M TrisCl, pH 8.8 (Soln. #2) 100.0 100.0 100.0 100.0 100.0
10% SDS (Soln. #3) 4.0 4.0 4.0 4.0 4.0
Deionized H2O 158.0 124.8 91.5 58.3 25.0
10% APS (Soln. #4) 4.0 4.0 4.0 4.0 4.0
10% TEMED (Soln. #5) 0.68 0.55 0.45 0.40 0.34
Final Volume 400.0 400.0 400.0 400.0 400.0
•
p28
Page 35

Laemmli Gel (per 200 ml each gel solution)
Gradient Gels Solution
200 ml LIGHT
5.0% 7.5% 10.0% 12.5% 15.0% 17.5%
Acrylamide Stock (Soln. #1) 33.3 50.0 66.7 83.3 100.0 116.7
1.5 M TrisCl, 50.0 50.0 50.0 50.0 50.0 50.0
pH 8.8 (Soln. #2)
10% SDS (Soln. #3) 2.0 2.0 2.0 2.0 2.0 2.0
Deionized H2O 112.2 95.5 79.0 62.4 45.8 29.1
10% APS (Soln. #4) 2.0 2.0 2.0 2.0 2.0 2.0
10% TEMED (Soln. #5) 0.50 0.46 0.34 0.27 0.23 0.20
Final Volume 200.0 200.0 200.0 200.0 200.0 200.0
200 ml HEAVY
7.5% 10.0% 12.5% 15.0% 17.5% 20%
Acrylamide Stock (Soln. #1) 50.0 66.7 83.3 100.0 116.7 133.3
1.5 M TrisCl, 50.0 50.0 50.0 50.0 50.0 50.0
pH 8.8 (Soln. #2)
10% SDS (Soln. #3) 2.0 2.0 2.0 2.0 2.0 2.0
Deionized H2O 83.0 66.5 49.9 33.3 16.6 0.0
10% APS (Soln. #4) 2.0 2.0 2.0 2.0 2.0 2.0
10% TEMED (Soln. #5) 0.46 0.34 0.27 0.23 0.20 0.17
Glycerol 12.50 12.50 12.50 12.50 12.50 12.50
Final Volume 200.0 200.0 200.0 200.0 200.0 200.0
•
p29
Page 36

Appendix B: References
Denaturing Gel Systems
Laemmli, U. K. Cleavage of structural proteins during the assembly of
the head of bacteriophage T. Nature. 227, 680–685 (1970).
Two-dimensional Electrophoresis
Anderson, Leigh and Anderson, Norman G., High resolution two-
dimensional electrophoresis of human plasma proteins. Proc.
Natl. Acad. Sci. USA. 74:5421–5425 (1977). Anderson, L. TwoDimensional Electrophoresis, Operation of the ISO-DALT® System,
Second Edition. Large Scale Biology Press (1991).
O’Farrell, P. H., High resolution two-dimensional electrophoresis of
proteins. J. Biol. Chem. May 25; 250(10):4007– 4021 (1975).
Bjellqvist, B., et al., Isoelectric focusing in immobilized pH gradients:
principle, methodology and some applications. J. Biochem. Biophys.
Methods 6, 317–339 (1982).
Görg, A, et al., The current state of two-dimensional electrophoresis
with immobilized pH gradients. Electrophoresis 9, 531–546 (1988).
Görg, A. Two-dimensional electrophoresis with immobilized pH
gradients: current state. Biochem. Soc. Trans. 21, 130–132 (1993).
•
p30
Page 37

Hoefer, Inc.
84 October Hill Road
Holliston, MA 01746
Toll Free: 1-800-227-4750
Phone: 1-508-893-8999
Fax: 1-508-429-5732
E-mail: support@hoeferinc.com
Web: www.hoeferinc.com
Hoefer is a registered trademark of
Hoefer, Inc.
ISO-DALT is a trademark of Large Scale
Biology Corporation.
© 2012 Hoefer, Inc. -–All rights
reserved.
Printed in the USA.
 Loading...
Loading...