H&C MEDICAL DEVICES CARDIETTE Service Manual

1
CARDIETTE ®
SERVICE MANUAL
A
SSTTA
RTT 110000
R
Rev. 01 Data: 28 May 1999 Code Nr. 66500092
AFTER SALES SERVICE

2
INDEX
Foreword and special remarks page 4
Installation program page 4
Register number page 4
Aim of the manual page 5
Reference standards page 6
Special remarks page 6
1. Technical characteristics page 7
2. Apparatus description page 9
2.1 Case page 9
2.2 Battery page 9
2.3 Mother board page 9
2.4 Keyboard page 11
2.5 Paper sensors board page 11
2.6 Printer mechanical group complete with thermal head page 11
2.7 Mechanical group of the paper driving system page 11
2.8 Protection from defibrillation page 11
3. Inputs and outputs page 12
3.1 Connection to the patient input socket page 12
4. Check of the safety characteristics pag. 13
4.1 Required instruments page 13
4.2 Applied voltage test page 13
4.3 Leakage current test page 14
5. Check of the main characteristics of the electrocardiograph page 16
5.1 Required instruments page 16
5.2 Sensitivity test page 16
5.3 Test of the ECG leads page 16
5.4 Check of the paper driving system page 17
5.5 Check of the response in frequency page 18
5.6 Check of the power supply and battery recharge page 18
5.7 Technical self-test page 19
6. Detection of the damaged circuits and analysis of the page 20
main malfunctions
6.1 Introduction page 20
6.2 Purpose page 20
6.3 The apparatus does not switch on page 21
6.4 The apparatus does not correctly accept the keyboard commands page 23
6.5 Malfunctions during printing process page 24
6.6 Malfunctions during paging and paper presence signalling page 26
6.7 Malfunctions caused by defective paper driving page 28
6.8 Illustration of the power supply voltage page 30
AFTER SALES SERVICE

3
7. Disassembling and reassembling the apparatus page 31
7.1 Foreword page 31
7.2 Opening and closing the apparatus page 31
7.3 Disassembling the mother board page 31
7.4 Disassembling the battery page 32
7.5 Disassembling the keyboard page 33
7.6 Disassembling the paper driving motor group page 33
7.7 Disassembling the printer group page 33
7.8 Disassembling the paper and notch sensors card page 33
7.9 Disassembling the paper lid page 34
7.10 Disassembling and replacing the keyboard plate page 34
7.11 Mains fuses page 34
8 Calibrations page 35
8.1 General information page 35
8.2 Calibrating the sensitivity of paper and notch presence sensors page 36
8.3 Calibrating the paper speed page 36
9. General directions for maintenance page 37
9.1 Foreword page 37
9.2 Messages page 37
9.3 Inspection schedule page 38
9.4 Cleaning the thermal head page 39
10. List of spare parts page 40
10.1 General information page 40
APPENDIX A
Procedures for handling and storing electronic components sensitive
to electrostatic discharges (ESD) page 45
Ilustrated tables page 47
AFTER SALES SERVICE

4
FOREWORD AND SPECIAL REMARKS
This apparatus is a multilingual, class I (first) and CF type portable electrocardiograph with
3/6 printing channels and dual power supply (mains/battery).
The apparatus uses 130 mm wide rolls of thermosensitive paper, provided with grid.
It is supplied in two different models:
START 100 P 110 ÷ 120 Vac 220 ÷ 240 Vac
This model, in addition to recording the ECG, grants the possibility of:
− calculating the main electrocardiographic parameters with either brief or extended
report;
− recording in both manual and automatic mode, with copy possibility in printing mode 3
and/or 6 channels;
− selecting the language (Italian - English - German - French -Spanish - Russian);
− selecting the 50 -55 - 60 Hz filters;
− automatically numbering the pages by either terns or six lines groups, 1 or 2;
− inserting the user name.
START 100 H 110 ÷ 120 Vac 220 ÷ 240 Vac
This model differs from the previous one in that it features the implementation of the
interpreting program instead of the parameters calculation.
INSTALLATION PROGRAM
The access to the programming menus can be achieved in two steps.
The first one takes place by depressing the STOP key within 3 seconds after switching the
apparatus on. If the procedure has been correctly performed, all the leds relevant to the
velocity, amplitude and filters keys will blink.
In such condition the AUTO - STOP - SPEED keys shall be depressed in sequence.
The menu activation is very clearly guided on the print-outs.
For more detailed information, please refer to the “installation instructions”.
REGISTER NUMBER
The label with the apparatus identification data is placed on the movable bottom (Table
T3).
The label is subdivided into three parts:
1 - The upper part gives the seller’s data and the apparatus trademark.
2 - The middle part gives:
The model __________
the characteristics of the power supply in Volt __________
The code number __________ the frequency in Hz. ___________
The serial number __________ the absorption in Amperes ___________
CE mark __________
AFTER SALES SERVICE

5
3 - The lower part gives:
the language (or langages) ___________
the manufacturer’s identification data ___________
NOTE:
Always use the series code and the apparatus code when communicating with the Seller
or with the Assistance Service.
CE MARKING
The CE marking identification label is placed on the movable bottom and defines the type
of apparatus conformity.
CE Marking only
CE
it defines conformity to European Community Directive 89/336 relative to electromagnetic
compatibility.
CE Marking 0470
CE 0470
it defines conformity to European Community Directive 93/42 relative to the safety of
medical devices.
The number 0470 indicates the homology number of the Nemko Certified Body.
AIM OF THE MANUAL
The aim of this manual is the following:
a) to give a description of how the unit operates;
b) to give a description of the procedures necessary to carry out a complete test of the
apparatus;
c) to give a description of the procedures necessary to carry out the safety tests
according to the IEC safety regulations;
d) to identify and isolate blocks of operational breakdowns;
e) to describe the maintenance interventions necessary for a correct and long-lasting
operation of the apparatus;
f) to supply a list of spare parts.
AFTER SALES SERVICE

6
REFERENCE STANDARDS
The safety characteristics of the electromedical class apparatus are in accordance with the
regulations:
EN 60601-1: (1990) General regulations for the safety of electromedical equipments
EN 60601-1: (1990/A1-1993)
EN 60601-1: (1990/A2-1995)
EN 60601-1-2: (1993) Regulations about the electromagnetic compatibility of
electromedical equipments
IEC 601-2-25: (1993) Special safety regulations for electrocardiographs
IEC 62D(CO)6: (1978) Special regulations about the performance of the
electrocardiographs
SPECIAL REMARKS
a) Please remember that, by following the instructions outlined in this manual, the
apparatus and its accessories will be correctly and efficiently maintained and
consequently will be safer and will last longer.
b) Please remember that this service manual is only addressed to technically competent
persons.
c) Please remember that all the instrumentation described or indicated in this service
manual is necessary to correctly carry out tests, calibrations and controls of the safety
characteristics of the apparatus.
d) Please remember that whenever the apparatus is opened for inspection or assistance,
a complete control of the safety characteristics, as described in chapter 5, must be
carried out before the apparatus is returned.
e) Please remember that this apparatus has been designed using CMOS technology.
Most of the electronic components belongs to the ELECTROSTATIC SENSITIVE
DEVICES (ESD).family.
Therefore, it is necessary to follow specific working procedures.
Appendix A describes the special procedures required in the treatment of electrostatic
sensitive devices (ESD.).
The manufacturer refuses all responsibility for any damage undergone by the
apparatus caused by inadequate or non existent working procedure necessary
in treating with ESD devices
NOTE:
Transportation and packaging of the apparatus not in its original packaging or
an incorrectly packaged apparatus, frees the manufacturers of any
responsibility with regard to damages to the apparatus and its accessories thus
resulting in the annulment of the warranty.
f) H&C MEDICAL DEVICES S.p.A. reserves to itself the right to modify at any time,
and without any advance notice, the product or this manual.
g) Before starting the assistance service, please ready the entire content of this
manual.
AFTER SALES SERVICE

7
1. TECHNICAL CHARACTERISTICS
Power supply from mains Class I (first)
110..120 V~ ± 10% 50..60 Hz
220..240 V~ ± 10% 50..60 Hz
Max input 0.28 A at 110 ÷ 120V~ ± 10%
014 A at 220 ÷ 240V~ ± 10%
Mains power supply protection T 0.315 A - 250 V (5x20 mm.) fuse at 110..120 V~
T 0.16 A - 250 V (5 x 20 mm) fuse at 220..240 V~
Built-in power supply Built-in lead rechargeable battery 12V 0.8 Ah
Battery protection T 2 A - 250V (5x20mm) fuse
Applied part CF type
Protection from defibrillation Inside the apparatus
Input dynamics ± 300 mV @ 0 Hz
± 6.4 mV in the passing through band
Input impedance > 100 MΩ on each electrode
Common rejection 95 dB
Frequency response 0.05..150 Hz (-3dB) without filters
Time constant > 3.3 sec
Acquisition 12 bits
1000 samples/sec/printing channel and filters
500 samples /sec/channel during calculation phase
3.125 µV/bit resolution
Leads 12 STANDARD leads, out of which:
8 acquired - 4 reconstructed (III - aVR - aVL - aVF)
Signal memory 10 s for each lead in self-isochronous mode
Sensitivity:
manual 5 - 10 - 20 mm/mV ± 5%
automatic 3 channels printing
10 mm/mV for 0.3 ÷ 3 mV signals
5 mm/mV for signals > 3 mV
20 mm/mV for signals < 0.3 mV
6 channels printing
10 mm/mV for 0.15 ÷ 1.5 mV signals
5 mm/mV for signals > 1.5 mV
20 mm/mV for signals < 0.15 mV
Printing system Thermal printing unit 8 dot/mm
Printing useful height 108 mm
Printing channels 3 - 6
Paper driving speed 5 mm/s ± 10% 25 - 50 mm/s ± 5%
Thermo-sensitive paper DOT-CARD® 130 mm in 25 metres rolls
Filters Mains disturbancies: modified 50 - 55 - 60 Hz notch digital filter
Anti drift : 0.5 Hz, pass high and linear phase digital filter
Keyboard Membrane type, with 8 functional and numerical keys
Led 9 Function indicators
Interpreting Program HES ECG program developed by
(START 100 H) Medizinische Hochschule Hannover - Germany
Parameters calculation Developed by “lstituto di Fisiologia Clinica (C.N.R.)” in Pisa Italy
(START 100 P)
Operating mode Manual (real time acquisition)
Automatic (isochronous)
Usage mode Continuous operation
Autonomy Built-in battery: 30 minutes in the 3 channel mode
10 mm/mV
25 mm/sec.
10 Hz p.v.
Recharging time Built-in battery:
12 h 100%
Caovering protection degree IP20
4 h 80%
AFTER SALES SERVICE

8
Environmental conditions:
operation Ambient temperature: +10°C to +40°C
Relative humidity: 25% to 95% (without condensation)
Atmospheric pressure: 700 hPa to 1060 hPa
Transport and storage Ambient temperature: -10°C to +40°C
Relative humidity: 10% to 95% (without condensation)
Atmospheric pressure :500 hPa to 1060 hPa
Dimensions 247 x 69 x 262 mm. (width x height x depth)
Weight 2.4 Kg
Compliance with standards EN 60601-1: (1990)
EN 60601-1: (1990/A1-1993)
EN 60601-1: (1990/A2-1995)
General standards for the electromedical equipment safety
EN 60601-1-2: (1993)
Standards related to the electromagnetic
compatibility of electromedical equipment
IEC 601-2-25: (1993)
Special safety standards for electrocardiographs
IEC 62D(CO)6: (1978)
Special performance standards for electrocardiographs
NOTE REGARDING THE PROTECTION:
The maximum applicable voltage to inputs and outputs is the maximum value causing no
damage to the apparatus.
AFTER SALES SERVICE

9
2. APPARATUS DESCRIPTION
The apparatus consists of the following main components:
− case, internally painted with conductive and shielding paint, complete with paper lid,
plate, keyboard and serigraphs;
− sealed lead battery;
− main electronic card, hereinafter called “mother board”;
− keyboard card;
− paper sensors card;
− printer mechanical group complete with thermal head;
− mechanical group of the paper driving device
2.1 CASE
The case consists of two parts:
− the upper housing, made of polycarbonate Lexan 940, colour RAL 7035;
− the lower housing, made of ABS, colour RAL 7025.
Both parts are internally painted with conductive and shielding paint. Such lining is highly
conductive and works as a shielding both against the radio disturbances generated by the
apparatus and those externally produced, which could cause malfunctions.
The fastening columns of cards and mechanical components, as well as the PVC
insulating plates, are an integral part of the case.
SAFETY NOTE
The mentioned insulations are strictly required to guarantee the safety features of the
apparatus. If they are removed during an inspection or repair of the apparatus, they must
be exactly restored as foreseen by the design.
2.2 BATTERY
The battery is a lead sealed type accumulator having the following characteristics:
− voltage: 12 V;
− capacity: 0.8 Ah;
− dimensions: 96 x 62 x 25 mm (width - depth - height);
− weight: 350 g;
− recommended make: FIAMM-GS FG20086.
SAFETY NOTE
The battery can only be replaced with the recommended type or an equivalent one, as to
both the electic characteristics and the mechanical ones.
2.3 MOTHER BOARD
The mother board is a multilayer printed circuit card (six layers) in “fine-line” technology for
the assembly of SMD components (Surface Mounting Devices).
It houses most of the apparatus electronic circuits and may be divided in the following
sections, with reference to the electrical block diagram file name: Blok - 100.
AFTER SALES SERVICE

10
2.3.1 POWER SUPPLY FROM MAINS
The power supply from mains is a Class I type, therefore it assures the apparatus
electrical safety only if the protection earthing (yellow/green wire of the power supply
cable) is connected to a system realized in compliance with the laws in force.
The section consists of the following parts:
− separable three-wire mains cable;
− three-pin mains plug with built-in extractable fuses (table T 2);
− protection devices (VDR and PTC) and disturbances filtering devices;
− mains transformer complying with medical standards, code ET 69701082 (table T 6);
− rectifying, filtering and voltage adjustment circuits with current limiter for the recharge of
the built-in battery. Such circuits do not allow operating the apparatus without the
battery;
− connection socket with earthing functions and equipotential connection (table T 2).
The equipotential connection must be carried out according to the instructions and using
the materials listed hereunder.
Required material:
• cylindrical head, M5X10 mm type screw;
• 5 mm internal diameter washer;
• 5 mm internal diameter cable terminal;
• wire with overall resistance lower than 0.1 Ohm and 1.5 cm2 minimum section.
Instructions:
• prepare and solder the wire to the cable terminal;
• insert the washer and the cable terminal in the screw;
• fix and adequately tighten the screw in the equipotential threaded bushing.
SAFETY NOTE
The replacement of the components belonging to the mains power supply section may
only be carried out using identical components to the original ones, homologated by the
manufacturer, or else the safety features of the apparatus will be lost.
2.3.2. POWER SUPPLY TO INTERMEDIATE CIRCUITS
This section consists of a DC/DC converter and a high frequency transformer generating
the following voltages:
• 5 V to supply the control logic;
• 24 V to supply the printer thermal head;
• ± 5 V to supply the insulated part.
2.3.3. INTERMEDIATE CIRCUITS FOR CONTROL AND INTERFACING
This section includes the microprocessor system controlling the operation of the whole
apparatus and the external interfaces of the mother board, such as the keyboard and the
serial interface RS232, the analog inputs and outputs, the remote control and the auxiliary
power supply, if provided.
AFTER SALES SERVICE

11
SAFETY NOTE
The connection to the intermediate circuits, for instance through the serial interface
RS232, may be realized only and exclusively towards other medical apparatus, according
to IEC 601-1-1 standard relating to the medical systems safety. Should any doubt exist
about the compliance to the medical standards of the apparatus connected to the
intermediate circuits (e.g. a Personal Computer), make use of a galvanic de-coupling
device, that may be provided by request.
2.3.4. INSULATED PREAMPLIFIER
This section consists of a 12 channels ECG preamplifier with active control of the
reference electrode, complete with protection from the effects of defibrillation and 12 bit
analog/digital conversion circuit.
The galvanic de-coupling is performed by means of a guard zone on the printed circuit (4
mm minimum insulation) and photo-couplers (2500V rms minimum for 1 minute
insulation).
SAFETY NOTE
We strongly advise against any intervention on the components assuring the patient
insulation, such as the photo-couplers and the insulation transformer of the DC/DC
converter generating the intermediate and insulated power supplies. In case of failure
replace the mother board.
2.4 KEYBOARD
This card houses only the keys and leds required for the operation of the apparatus.
2.5 PAPER SENSORS BOARD
This card houses the sensors signalling the paper presence and the paging black notch.
2.6 PRINTER MECHANICAL GROUP COMPLETE WITH THERMAL HEAD
This group consists of the supporting plate for the printer thermal head and the mechanical
components required for its correct positioning.
A complete sparee group is provided.
2.7 MECHANICAL GROUP OF THE PAPER DRIVING SYSTEM
The group consists of the driving motor complete with support and gears.
A complete spare group is provided.
2.8 PROTECTION FROM DEFIBRILLATION
The apparatus has a built-in protection from the defibrillator discharges.
Therefore this apparatus allows the use of patient cables not protected from defibrillation
and supplied by the manufacturer.
AFTER SALES SERVICE
12
3. INPUTS AND OUTPUTS
START 100 does not allow any connection to other external apparatus.
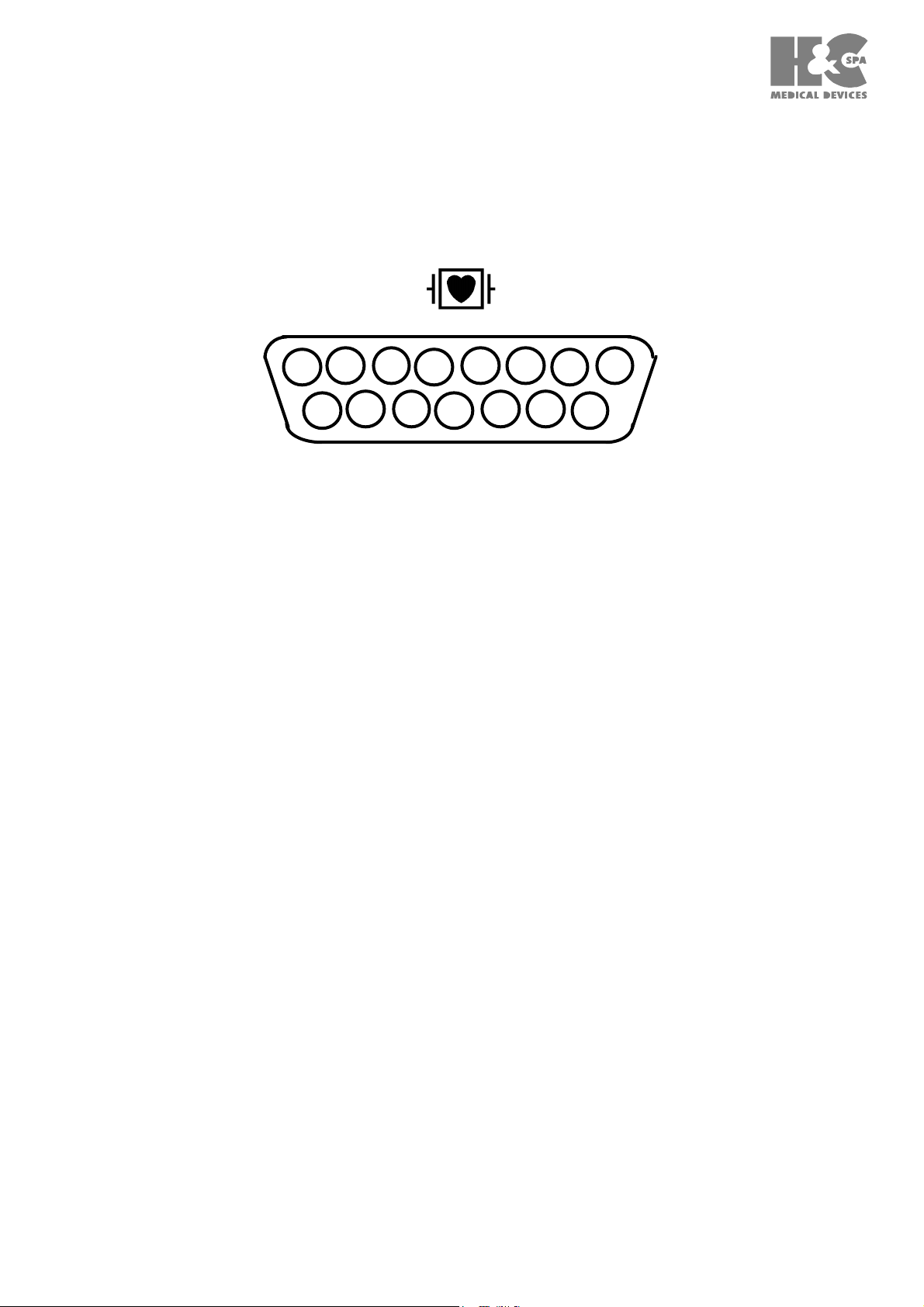
3.1 CONNECTION TO THE PATIENT INPUT SOCKET (table T2)
4
11
3
10
7
8
15 9
Socket seen from the connection side
Pin 1 = IN C2 (C2 electrode)
Pin 2 = IN C3 (C3 electrode)
Pin 3 = IN C4 (C4 electrode)
Pin 4 = IN C5 (C5 electrode)
Pin 5 = IN C6 (C6 electrode)
Pin 6 = IAGND
Pin 7 = NC (not connected)
Pin 8 = NC (not connected)
Pin 9 = IN R (R electrode)
Pin 10 = IN L (L electrode)
Pin 11 = IN F (F electrode)
Pin 12 = IN C1 (C1 electrode)
Pin 13 = NC (not connected)
Pin 14 = IN N (N electrode)
Pin 15 = NC (not connected)
The inputs have the following characteristics:
a) Sensitivity: 1 mV/5 - 10 - 20 mm depending on the selected sensitivity;
b) Input impedance higher than 100 MOhm for each electrode;
c) Input dynamic:+/- 300 mV.at 0 Hz.
+/- 6.4 mV in the passing through band;
d) The inputs are protected from defibrillation.
5
6
1314
12
1
2
AFTER SALES SERVICE

13
4. SAFETY CHARACTERISTICS CONTROL
The safety regulation expects two important tests:
a) The applied voltage test:
it verifies the insulating efficiency of the power supply circuits and those relative to the
patient connections.
b) The leakage current test:
it measures the value of the leakage currents in relation to patient safety.
NOTE:
All safety tests should be performed according to IEC 601-1 regulations(paragraphs 19-
20).
4.1 NECESSARY INSTRUMENTS
a) Insulating strength analyser:
model "U28 M" Elektrotechn. Laboratorium D - 7015 Korntal Germany or its
equivalent;
b) Leakage current analyser:
model “AMPLAID ST 10“ - Amplifon Division S.p.A. Italy, or
model METRON QA 80” Electrical Safety Analyser, or
model “BIO-TEK 601-PRO” Amplisim Division srl - Italy or its equivalents
4.2 APPLIED VOLTAGE TEST
The test should be carried out in a suitable location in accordance with safety regulations
by using the instrument 4.1 a).
This test must be carried out only in those apparatuses in which components with special
insulating characteristics have been substituted:
a) power supply transformers;
b) transformer on the TR1 voltage converter;
c) optoinsulators: FC1...FC7 of the mother board;
d) entire mother board.
4.2.1 INSULATING TEST TOWARDS MAINS POWER SUPPLY
Perform this test with the mains switch inserted (Table T2).
a) Apply the test voltage between the pins with the exclusion of the central pin in the
apparatus mains plug (Table T2) and the equipotential outlet which is connected to the
frame (ground) of the apparatus (Table T2).
b) How the performance test is carried out:
(class I electrocardiograph (first).
Apply for 10 seconds a voltage equal to 0.750 KVac, then raise it to 1.5 KVac and hold it
at this value for 1 minute.
AFTER SALES SERVICE
14
4.2.2 DECOUPLING TRANSFORMER AND OPTOINSULATOR TEST.
(CF type electrocardiograph defined by this symbol )
Perform this test with the apparatus turned off.
a) Apply the test voltage between all the Pins of the patient input outlet connector (Table
T2) and the equipotential outlet (Table T2);
b) Apply for 10 seconds a voltage equal to 1.25KVac then raise it to 2.5KVac and hold it
at this value for 1 minute.
4.2.3 IN BOTH TESTS (4.2.1 - 4.2.2)
Verify that during the test there are neither superficial nor destructive discharges.
Moderate discharges due to the back effect can be ignored, as long as they stop when the
test voltage is temporarily lowered to a lower value, which nevertheless must remain
greater than the reference voltage U (250V), as long as the discharges do not result in test
voltage drops.
4.3 LEAKAGE CURRENT TEST
THIS TEST TO BE MADE AFTER EACH OPENING FOR INSPECTION
AND/OR REPAIR USING TOOL 4.1 B AND IN ANY CASE EVERY TWO
YEAR PERIOD.
Proceed as follows:
4.3.1 Connect the electrocardiograph to the measuring instrument according to the
instructions given in its user’s manual and remember that:
a) The leakage current towards ground, is measured between the mains power supply
circuits and the ground point of the equipotential outlet (Table T2).
b) The leakage current towards the case, is measured between the mains power supply
circuits(Table T2), the equipotential outlet (Table T2) and a metallic sheet not greater
than 20 x 10 cm in size which should be pressed against the case, if composed of
insulating material.
c) The leakage current in the patient, is measured between the mains (Table T2) and the
applied part (Table T2).
The same patient cable can be used for the connection with the applied part.
d) The leakage current in the patient with mains voltage directly on the applied part (first
fault condition), is measured between the equipotential connection (Table T2) and the
applied part (Table T2).
e) The auxiliary current in the patient, is measured individually on each electrode (with
the exclusion of the black one) with respect to all the other electrodes connected
together.
4.3.2 Program the instrument according to the electrocardiograph type (CF) and class I
(first).
4.3.3 Perform the measurement according to the instructions given in the user’s manual of
the instrument itself and verify that the values of the leakage currents measured are
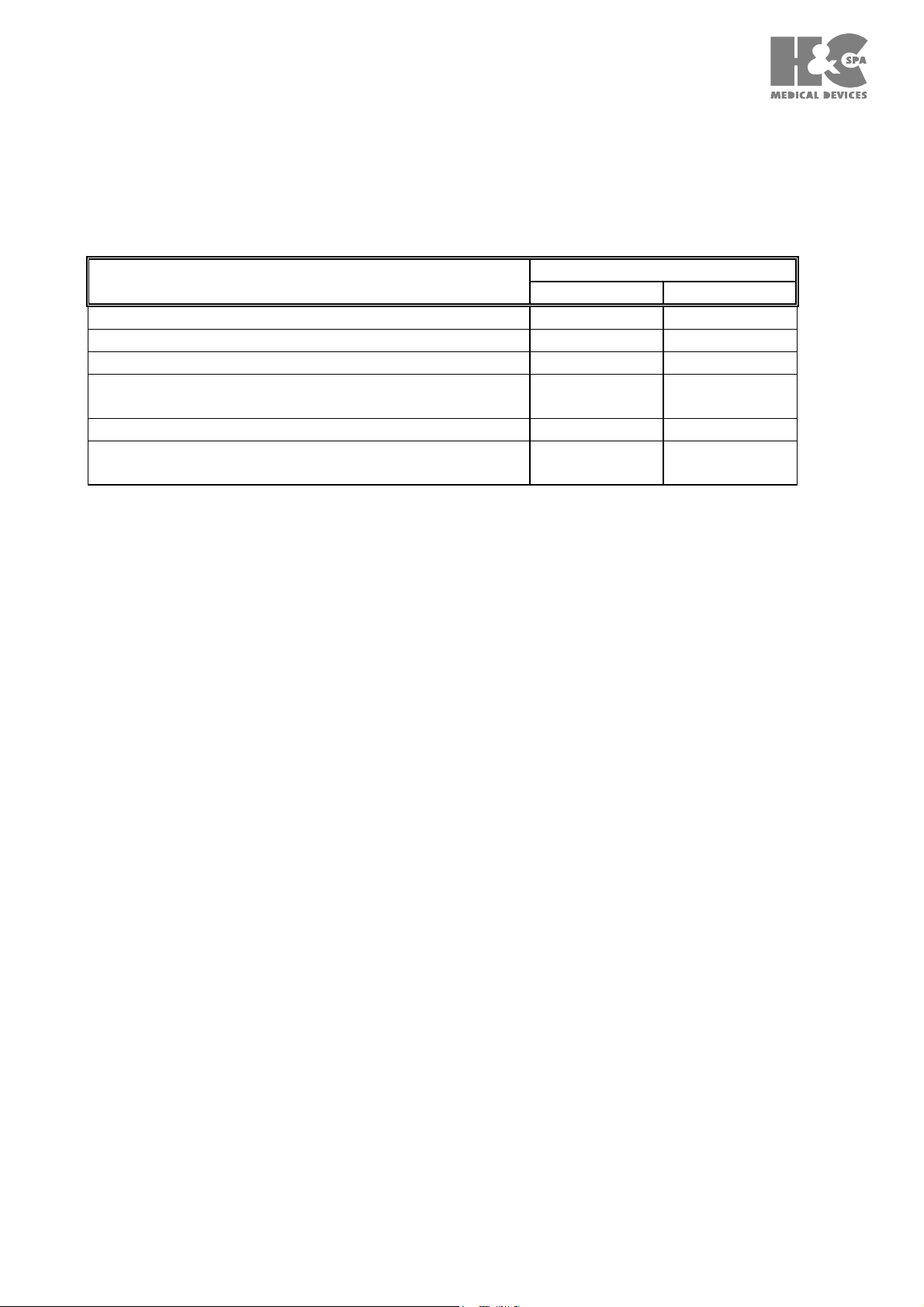
less than or equal to those reported in Table IV.
AFTER SALES SERVICE
15
IEC REGULATI
ONS 60601
-1
(1990)
60601 - 2 - 25 (1993)
Table IV
Permanent admissible values for the leakage currents and for the auxiliary currents in the
patient in mA.
Current path
CF type
N.C. (+) S.F.C.(++)
Leakage current towards ground 0.5 1
Leakage current in the case 0.1 0.5
Leakage current in the patient 0.01 0.05
Leakage current in the patient with
----- 0.05
mains voltage in the applied part
Auxiliary current in the patient 0.01 0.05
(+) N.C. = Usual condition
(++) S.F.C. = First fault condition
AFTER SALES SERVICE
 Loading...
Loading...