OPERATING INSTRUCTIONS ELEVATOR-2
______________________________________________________________________________________________________________
0125
__________________________________________________________________________________________________
English Edition
07/98 -1 von 18 - 0627 7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

CONTENTS Page
Technical Safety Procedures
- Regulations 3
Product Safety
- Electrical safety 4
- Mechanical safety 4
- Danger of Injury 4
- X-Ray Protection. 5
- Explosion protection 6
- Interference suppression 6
- Classification of product 6
- EC Conformity 6
- Environment condition 6
- Disposal of equipment 6
Design Features
- Conception 7
General
- Brief Description 8
- Range of Application 8
Installation
- Floor Space Required 9
- Room Height 9
- Power 9
- Mains 9
Operating Elements
- Arrangement 10
- Meaning of Symbols/Function 10
- Adjustement of the exposure position/exposure 12
- Optional accessories 14
Maintenance
- Important note 16
- Operator`s service and maintenance 16
- Periodic maintenance 16
- Cleaning 16
- Disinfection 17
- The Council Directive 93/42EEC on Medical Devices 17
Location of Name Plate
- Labeling 18
0627 7326 - 2 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Important Note:
To ensure proper operation of this product it is essential that the service personnel is familiar with the "Operating Instructions" which should be studied carefully
before use.
Special attention is to be given to the chapter "Safety Notes"
The equipment must be used in accordance with the safety procedures described below, and must not be used for purposes other than those for which it
was designed. The equipment may only be used by persons having recognized
qualification, including adequate training in radiation protection, authorizing them to
perform the examination or treatment carried out.
It is the responsibility of the user to ensure that the government regulations are
observed in the installation and operation of the equipment.
Technical safety note:
Regulations
If legal regulations govern the operation of the above equipment, it is the responsibility of the operator to observe them.
For the safety of patients, operators and others, as well as the efficient functioning of the equipment it is necessary to have periodic service inspections at 12month intervals according to the maintenance schedule. Please apply to your
service organisation for inspection and maintenance.
Inspections intervals must by all means meet the requirements of the respective
legislation or government regulations.
Changes and additions to the product must comply with the relevant legislation
as well as with the accepted standards of good manufacturing practice.
As manufacturer of electromedical systems, we assume responsibility for
the safety of the equipment only if maintenance, repairs and changes are carried
out exclusively by us or third parties expressly authorised by us to do so, and if
defective parts relating to the safety of the equipment are replaced by genuine
spare parts.
We recommend that the service personnel is being asked to issue a certificate
specifying the kind and extend of things or work ranges. Also the certificate
should show the date of repair, the name of the service company and the signature of the technician.
07/98 -3 von 18 - 0627 7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Before operating the equipment, the operator must check all devices concerning
the safe and efficient functioning.
If the user of this equipment likes to combine the unit with other units, components or assemblies and this can not be made clear from the technical data, he
must question us as manufacturer or another expert to make sure that the safety
of the patients and operator is given by the planned combination.
Product Safety
Electrical safety
Only trained service personnel are permitted to remove covers and panels from
the x-ray equipment .
In the Federal Republic of Germany, the electrical installation of rooms used for
medical purposes must conform to the provisions of the VDE Standard 0107. In all
other countries, the provisions of the applicable local laws and regulations have
priority.
The unit is only prepared for solid installation with an all poled separation from the
power (ICE 601, Kap. 57.1).
Mechanical safety
It is the responsibility of the operator to ensure safety of patient while the unit is
in operation by visual check, proper patient positioning, and use of devices that
are provided.
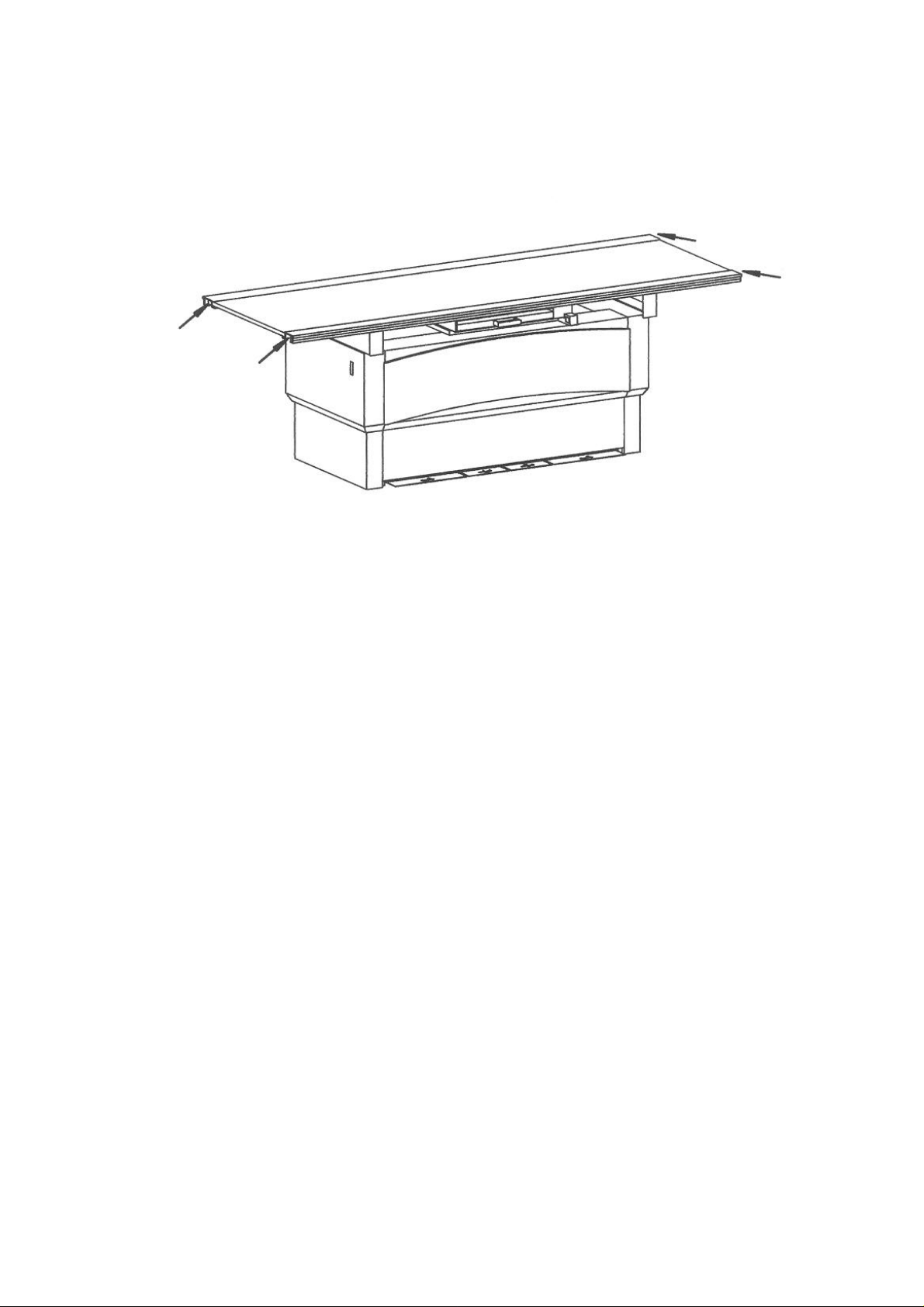
Danger of injury
The solid black arrows and dotted lines in the illustration show areas which present
potential Danger of Injury to operating personnal and patient from the equipment
motion.
See next page.
0627 7326 - 4 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru
X-Ray Protection.
The unit has no controls with which radiation could be triggerd.
Exposure is triggerd only from the radiation-protected location of the generator.
The general radiation-protection measures must be observed.
In addition, we recommend the following:
1 Set the tube current as low as possible .
2 Limit the radiation field to the maximum possible extent.
3 Keep as fare away as possible.
4 Provide radiation protection for the patient.
07/98 -5 von 18 - 0627 7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Explosion Protection
This equipment is not designed for use in areas where explosion hazard can take
place.
Only skin cleansing agents may be used which form non-explosive mixtures with
air.
Interference Suppression
The equipment complies with the EMC-requirements of the guideline 89/336 EWG
of
* Special board International Electronic Commission (IEC). This unit complies to
EN 55011 and the reference value is according EN 55011 Group 1 Class B the in-
ternational electrotechnical committee (IEC).
Classification of product
The equipment complies to the protection degree of Class 1 and
for protection against electric shock Type B.
EC Conformity
The ELEVATOR-2 to which this declaration relates fulfills the essential requirements for safety of medical electrical equipment and follows the provisions of
Medical Device Directive 93/42 EEC part 11 para. 5 according the procedure in
annex VII.
The CE-Mark is only applicable for the product without X-ray components and
Bucky.
The declaration of EC-conformity can be sent to you by request:
Write to:
Hans Pausch
Röntgengerätebau
c/o Quality Assurance Sys. Mgr.
Postfach 28 60
D-91016 Erlangen
Fax #: ..49 9131 99 24 22
Environment Condition
Surrounding temperature range 10° C to 40° C
Humidity 20% to 80%
Atmospheric pressure 700 hPa to 1100 hPa
Disposal of equipment
Legal waste disposal regulations may apply to the disposal of this product. To avoid
causing damage to the environment and personal injury, we recommend that you
contact your Customer Services representative before permanently removing this
product from service.
0627 7326 - 6 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

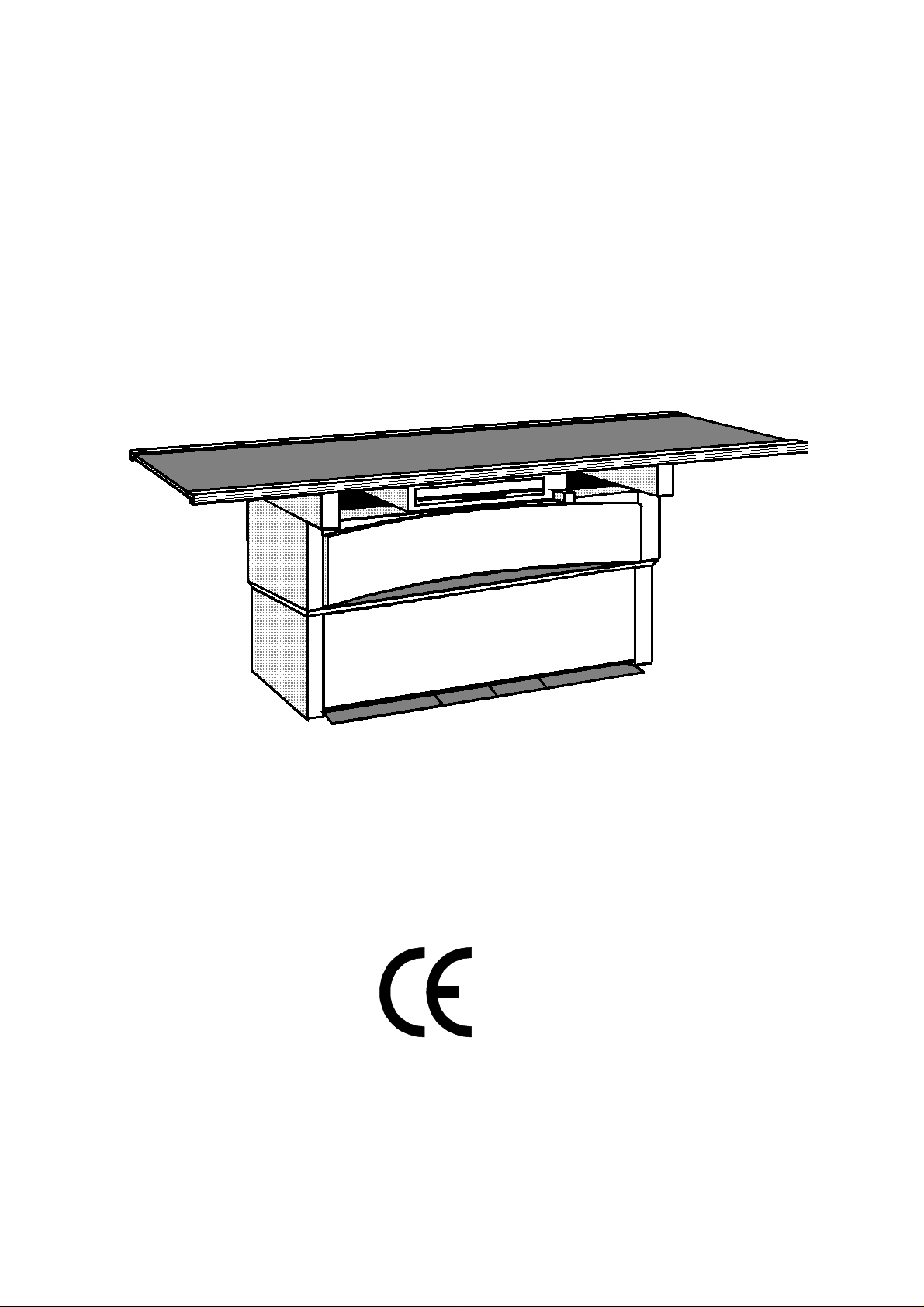
Design Features
Constructional Conception
A - Table Top, floating, manually moveable, scratchproof, height-adjustable
B - Profile Rail with trim cover, smooth, holds accessories
C - Table Frame
D - Bucky Unit, moveable
E - Telescope Section of Table Base
F - Table Base, solid, vibration-free
G - Switch Pedals
H - Safety switch
07/98 -7 von 18 - 0627 7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

General
Description /Area of Application
The solid, vibration-free table base has a telescopic design. The unique advantage of lowering the table top to a level of 54 cm allows
- handicapped and injured patients to climb onto the table without difficulties;
- an easy and gentle transfer of patients from mobile stretchers onto the table by adjusting
the table height.
- the transfer of handicapped patients from the wheelchair onto the table.
The additional possibility of raising the table top up to a level of 85 cm optimises
- the adaptation of the unit to heights preferred by the operating personnel;
- the operating and working conditions.
The large and 220 cm long, floating table top is manually moveable and locks electromagnetically. For fast and easy positioning of the patient, it allows spacious travel -60 cms
to the left, 50 cms to the right, and 12 cms transversely. Especially for patient comfort and
easy cleaning, the table top offers a scratch-proof surface and is trim-covered, smooth
profile rails are on both sides that accept accessories. The brakes as well as the raising
and lowering mechanism of the table top are operated by means of large and easily accessible switch pedals. The safety switch on the Bucky unit prevents an unintentional
movement of the unit. All pedal switches, combined in a foot switch rail along the table
base front, have large and catchy symbols.
The unit takes up Bucky trays as supplied by all leading manufacturers. The carriages
manually moveable along the entire table. It is electro-magnetically locked. The shortest
possible film-to-skin distance of 70 mm guarantees images of superior geometric proportion.
Area of Application
The electromotive and telescopic height adjustment of the table top from 52.5 cm to 85
cm above floor level makes the "ELEVATOR-2 " an ideally suitable unit in hospital emergency rooms as well as in private medical offices. The wide range of action of the table
top and uncomplicated operation of the "ELEVATOR-2"increase patient comfort and facilitate all utine Bucky grid exposures - from head to foot.
Prerequisition
For safe and efficient operation of this product the personnel must be familiar with the operating instructions. The chapter on „Safety procedures“ deserves special attention.
0627 7326 - 8 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Installation Requirements
Floor Space/Room Height/Power/Mains/Prerequisition
Floor space
The unit is designed for stationary operation. The approximate floor space requires dimensions of 330 cm by 110 cm.
Height of room
The required room height depends on the type of tube stand used. Refer to the installation data of the manufacturer. The "ELEVATOR-2" has a maximum working height of 85
cm above floor level.
Power
The system is equipped for single-phase alternating current with fixed installation. Two versions are available, depending on order. The unit is only prepared for solid installation with
an all poled separation from the power (ICE 601, Kap. 57.1).
Without transformer, the system corresponds to nominal ratings as follows:
Nominal voltage : 230 V (115/208 V) A.C. 1L/N/PE
Rated connection current : 3,3 A (6,6/3,6 A)
Rated frequency : 50/60 Hz
Rated current : 5A (10/6,25 A)
Rated power consumption : 0.7 kVA
Mains
The mains connection requires a 30 mA circuit breaker to be installed by the customer. The
electrical installation must meet the relevant legislation, e.g. VDE 0107, IEC/SC 62A.
AL-equivalent
The weakening equivalent of the table top (patient pos. table top) is < 0,7 mm.
According to:
DIN EN 60601-1-1-3 mit 100 kv und HWS 3,7 mm AL
and FDA 21 CFR § 1020.30 (n) mit 100 kv und HWS 2,7 mm AL.
07/98 -9 von 18 - 0627 7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Arrangement
1 Switch for Bucky carriage brakes and Safety Interlock
2 Bucky Carriage
3 Foot Switch Pedal for table top up
4 Foot Switch Pedal for table top down
5 Foot Switch Pedal for unlocks the brakes for the floating table top
Meaning of Symbols/Function
Switch Pedal 4 for motorized lowering the table top down to
52.5 cm above floor level. The table moves downward as
long as the safety interlock 1 and the pedal 4 are depressed.
The lowering speed is with smooth start and finish. Automatic shutdown of the downward movement takes place in
the end position and in exposure position.
The exposure position depends on the system design, and
is pressed by our customer service according to the customer's request.
Radiation can be triggered in exposure position only.
To continue downward movement beyond the exposure position, release switch pedal and safety interlock switch. Then
press again.
0627 7326 - 10 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Switch Pedal 3 for motorized raising the table top up to 85 cm
above floor level, the table raises as long as the safety interlock switch 1 and the switch pedal 3 are depressed. The
raising speed is with smooth start and finish. Automatic shutdown of the upward movement takes place in the end position
and exposure position.
To continue upward movement beyond the exposure position,
release switch pedal and safety interlock switch. Press again.
Switch Pedal 5 unlocks the brakes for the floating table top. As
long as the pedal is depressed, the table top can be manually moved in longitudinal and in transverse direction. Release
of the pedal locks the table top in its new working position.
Switch 1 unlocks the Bucky carriage brakes. As long as the
button is pushed, the carriage can be moved in longitudinal direction. Release of the push-button locks the carriage in its
new working position.
Note
For information on the operation of the Bucky carriage resp.
the Bucky tray, the X-ray generator, tube stand, tube radiation
field control, read instructions of the respective manufacturer.
The "ELEVATOR-2" is ready for operation as soon as the generator is turned on. Concerning the start-up of the generator,
refer to the manufacturer's instructions.
Emergency-stop
An emergency stop switch has been installed in the examination room, the red switch button must be pushed immediately in case of danger for patients, personnel or equipment.
Do not reoperate the equipment unless the danger has been
definitely eliminated.To resume operation, turn the emergencystop switch clockwise.
07/98 -11 von 18 - 0627
7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

SETTING EXPOSURE POSITION/EXPOSURE
Patient Positioning/ Centering of Bucky, Object and Tube Unit.
Patient Positioning
Press the foot switch 5 to unlock the table top brakes.
Move the table top manually floating to the rear stop.
Release the foot switch.
Press the foot switch 3 and the safety switch 1 simultaneously. The table top is lowered by the motor in a telescopic
way.
Release the switch when comfortable working height for patient access or tranfer is reached.
Note
When reaching the preset exposure position of the table top,
the downward movement is interrupted automatically. To continue movement, release the foot switch. Press again.
Press the foot switch 3 and the safety switch 1. The table top
is lifted up by the motor to exposure position. Release the
switch after the automatic stop.
Centering of Bucky
Press the switch 1 and keep it pressed. Push the Bucky carriage to suitable exposure position. Release the switch.
Center the tube over the Bucky carriage, re. operating instructions of tube stand.
0627 7326 - 12 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Step on pedal 1 to unlock the table top brakes.
Manually shift the floating table top, until the exposure object
is in the central beam of the tube unit (light field!).
Release the pedal. The table top is electrically locked in its
new working position.
Optimally collimate the X-ray field (light field) with the beam
limiter of the tube assembly. Re. operating instructions of
tube unit.
Note
Do not forget radiation protection devices for the patient (lead
rubber apron, gonad protection, etc.).
Set the film-focus distance (FFD), re. operating instructions
of tube stand.
Turn on the light field of the X-ray tube used, re. Operating
instructions of tube unit.
Exposure preparation
Insert cassette. Choose SID (FFD). Set exposure dates on
control desk. Control readiness for exposure. Command patient to „hold your breath“.
Note
Do not forget radiation protection devices for the patient (lead
rubber apron, gonad protection, etc.)
Lateral Exposure
For lateral exposures with the cassette holder lateral (cf. page
with accessories), rotate the tube stand to a 90° angle. Then
rotate the tube to a 90° angle (indication of angle!). Proceed
as described above.
07/98 -13 von 18 - 0627
7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Accessories
Compression Belt / Head Supports / Hip Clamps / Table Mattres
Compression Belt
Fastening and application:
Slide support frame B into profile rail at wall side or
into Bucky profile rail. Turn knob screw to clamp in position.
Slide tightener A into front profile rail ( operator side ).
Turn hand screw C to clamp in working position opposite of B
Press ratchet mechanism F. Unroll belt and stretch accross patient.
Guide belt through complementary frame and once
around frame bar. Fix belt bracket D into slot of shaft
G. Turn ratchet mechanism E to tighten belt.
Untightening:
Press locking lever F
0627 7326 - 14 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Lateral cassette holder
The lateral cassette holder permits lateral exposures if
the tube unit is mounted to a tube swivelling device.
The lateral cassette holder is slipped in one of the profile
rails.
Grip screw (A): secures the holder at the table top
Grip screw (B): locks the holder setting
Grip screw (C): fixes the lateral position of the cassette
clamps.
Head Supports
The head supports slide into profile rails of the table or
Bucky. The supports can be clamped in any position
desired. The patient`s head is fixed to the appropriate
exposure position by cushioned plates on adjustable
bars.
Handscrew A: Clamping Head Supports to table top or
Bucky.
Hand screw B: Clamping of head holder
Hand grips
The hand grips are slipped in the profile rails of the table. They may be fixed at any position and offer a reliable hold for the patient.
Grip screw (A): secures the grip in place
Important note:
The positioned patient may only hold on to the hand
grips. In no case may he put his hands around the
edge of the table top.
07/98 -15 von 18 - 0627
7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Maintenance:
Important note:
Like all technical equipment, this unit requires also a regular maintenance service
to increase the safety of the equipment.
Operator´s service and maintenance
The operator has to check the x-ray equipment for defects as listed below:
In case of functional defects or other deviations from the normal operation the
equipment has to be switched off at once and the service company has to be informed.
The equipment can not be used before all defects have been eliminated.
Daily routine checks
Check indicator light and operating elements for proper functioning.
Weekly checks
Check all cables and their connections for treaces of wear.
Periodic maintenance
For trouble-free operation of the ELEVATOR-2 as well as safety for patient and
user it is necessary to carry out a technical maintenance from the service company
every 12 months.
Please see „technical maintenance“ of the mounting instruction.
The steel rope of the column has to be replaced every three years.
Attention:
In case of failure from components, which can limit the safety of the equipment,
original spare parts have to be used.
We recommend that the service personnel is being asked to issue a certificate
specifying the kind and extend of work that was done. Also the certificate should
show the date of repair, the name of the service company and the signature of the
technician.
Cleaning:
The equipment must be switched off before cleaning. Plastic surface should only
be cleaned with mild soap. Do not use strong cleaners or solvents as they will
damage the finish or blur the lettering.
0627 7326 - 16 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

At least once a month external parts and exposed tracks on which rollers move
should be wiped to remove foreign material that may have accumulated.
DO NOT USE A DAMP CLOTH.
Wipe the tracks with a cloth lightly soaked with light machine oil or WD-40.
To protect the finish, polish the equipment with PURE liquid paste wax. Do not use
wax containing a cleaning substance. Polish all enamelled metal surfaces.
Disinfection:
The equipment has to be switched off before disinfection. Only disinfection methods can be used that correspond to the relevant regulations and rules as well as
the protection for explosion.
Spray disinfection is not recommended because it can get in the inside of the x-ray
equipment.
The Council Directive 93/42/EEC on Medical Devices Article 12
This document is revised at the moment by the council.
However the Article 12 must be followed by the company or the legal person who
put this X-ray unit into work.
The user is responsible for compliance and implementation of national deviationes
in the EU.
07/98 -17 von 18 - 0627
7326
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru

Name Plate Location:
Labeling:
Specifications are subject to change without notice. TV/Ru
0627 7326 - 18 von 18 - 07/98
Rev. 02 © 1998 Hans Pausch Röntgengerätebau Graf-Zeppelin-Str. 1 D-91056 Erlangen ALL RIGHTS RESERVED Ru
 Loading...
Loading...