Page 1
HI 4104
CALCIUM
COMBINATION
HI
4000-50
SENSOR HANDLE
Instruction Manual
HI 4004HI 4004
HI 4004
HI 4004HI 4004
HI 4104HI 4104
HI 4104
HI 4104HI 4104
Calcium Ion
Selective Electrode
Half-cell
Combination
1
Page 2

HI 4004 Calcium Half-cellHI 4004 Calcium Half-cell
HI 4004 Calcium Half-cell
HI 4004 Calcium Half-cellHI 4004 Calcium Half-cell
HI 4104 Calcium Combination ElectrodeHI 4104 Calcium Combination Electrode
HI 4104 Calcium Combination Electrode
HI 4104 Calcium Combination ElectrodeHI 4104 Calcium Combination Electrode
I. I.
Introduction:Introduction:
I.
Introduction:
I. I.
Introduction:Introduction:
The Hanna HI 4004 and HI 4104 are ion selective electrodes designed for the measurement of calcium ions in
aqueous solutions. They utilize a replaceable sensing module that contains an organic polymer membrane that is
sensitive to calcium ions. The HI 4004 is a half-cell electrode that requires a separate reference. The HI 4104 is a
combination ion selective electrode.
IIII
SpecificationsSpecifications
II.
Specifications
IIII
SpecificationsSpecifications
Type: PVC membrane with
organic ion exchanger
Ion measured: Calcium (Ca
2+
)
Measurement range: 1.0 M to 3 X 10
-6
M
40080 to 0.12 ppm
Interference:
Organic solvents and cationic detergents must be absent.
Ratio of interfering ion to Ca
2+
must be less than the
ratio indicated below:
15000 for Na+sodium
7000 for Mg3+magnesium
700 for Ni2+nickle
300 for Fe
2+
ferrous
250 for Al3+aluminum
2+
+
4
-
ammonium
lead
200 for NH
35 for Cu2+cupric
0.001 for Pb
Operating Temperature: 0-40°C
Operating pH: 4 to 10 pH (see Section
XIII)
Dimensions: 12 mm (OD) X 120 mm
nominal insertion
(0.47” X 4.72”)
Connection: BNC
2
Page 3

III. III.
Theory of OperationTheory of Operation
III.
Theory of Operation
III. III.
Theory of OperationTheory of Operation
::
:
::
The HI 4004 and HI 4104 calcium electrodes are potentiometric devices used for the rapid determination of free calcium ions in water, sea water and beverages. The electrode
functions as a sensor or ionic conductor. The HI 4004
requires a separate reference electrode to complete its electrolytic circuit. The HI 4104 is a combination electrode with
a Ag/AgCl reference electrode with gel stabilized Cl- electrolyte in it’s inner chamber. The external reference chamber
is refillable. The PVC membrane used on the sensor is
impregnated with the organic ion exchanger.
This organic ion exchanger is considered a carrier ionophore
in that it is capable of shielding and carrying the charged
calcium ion in it’s polar cage freely through the apolar
regions of the membrane. A charge imbalance developes
between the test solution and internal cell of the sensor.
This voltage changes in response to the sampleís ion activity. When the ionic strength of the sample is fixed, the
voltage is proportional to the concentration of nitrate ions in
solution. The sensor follows the Nernst Equation:
E = E
+ 2.3 RT/nF log A
a
ion
E = observed potential
Ea = Reference and fixed internal voltages
R = gas constant (8.314 J/K Mol)
n = Charge on ion (2+)
A
= ion activity in sample
ion
T = absolute temperature in K
F = Faraday constant (9.648 x 104 C/equivalent)
3
Page 4
IV. IV.
Design elements of the HI 4004 and HI 4104Design elements of the HI 4004 and HI 4104
IV.
Design elements of the HI 4004 and HI 4104
IV. IV.
Design elements of the HI 4004 and HI 4104Design elements of the HI 4004 and HI 4104
electrodeselectrodes
electrodes
electrodeselectrodes
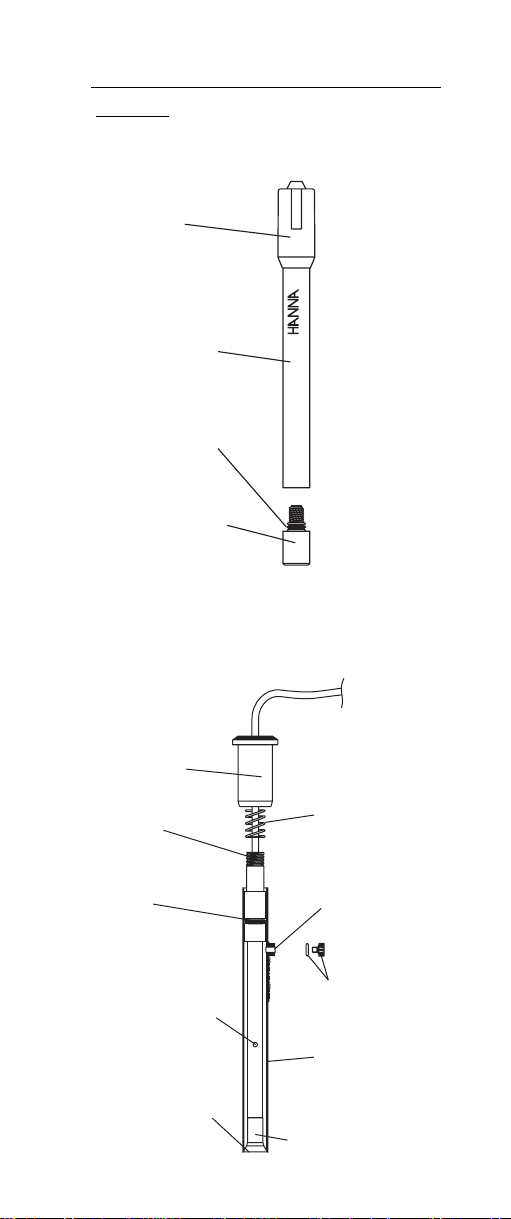
Cap
HI
4000-50
HANDLE
R
SENSO
Sensor
Handle
O-Ring
Sensing
module
Upper Cap
Upper
Threads
O-Ring
Ceramic Junction
on Inner Stem
Liquid junction
Spring
Fill Hole
O-Ring and
Plug
Outer Sleeve
Sensing Module
4
Page 5
V. V.
Equipment required:Equipment required:
V.
Equipment required:
V. V.
Equipment required:Equipment required:
• The HI 4004 requires the Hanna HI 5315 Double
junction reference electrode with HI 7082 as external
electrolyte.
• Hanna HI 4222 pH/ISE/mV meter or other suitable
ion or pH/mV meter (Note: log/linear graph paper is
useful if an ISE (ion) meter is not available).
• Hanna HI 180 magnetic stirrer or equivalent with
magnetic stirring bars (Note: Isolate beakers from stirrer motor heat by placing insulating material such as
foam or cork between them).
• Hanna HI 76404 electrode holder or equivalent.
• Plastic beakers (HI 740036P) or other suitable mea-
surement vessel.
VI. VI.
Solutions RequiredSolutions Required
VI.
Solutions Required
VI. VI.
Solutions RequiredSolutions Required
Standards for Calcium Measurements
0.1 M calcium standard,500 mL HI 4004-01
Ionic Strength Adjuster
ISA, 500 mL HI 4004-00
Conditioning and Storage Solution
Calcium storage solution, 500 mL HI 4004-45
Using volumetric pipettes and glassware make dilutions to
bracket the concentration of the samples. Store samples in
plastic bottles. Standards with concentrations < 10
-3
should be prepared daily.
Two mL of Hanna ISA HI 4004-00 should be added to
100 mL sample or standard.
M
VIIVII
General GuidelinesGeneral Guidelines
VII.
General Guidelines
VIIVII
General GuidelinesGeneral Guidelines
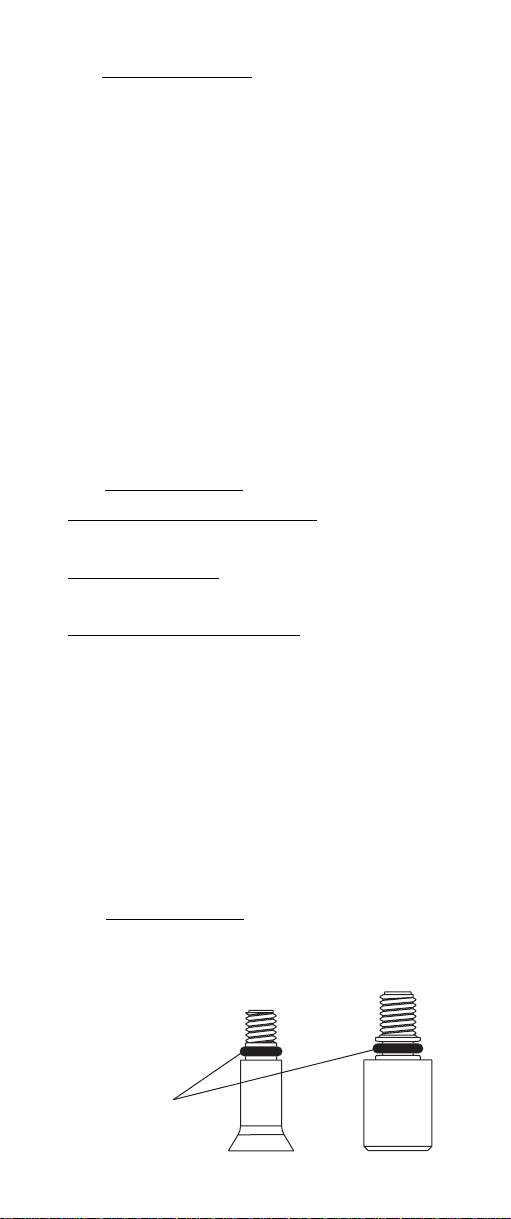
• Ensure the o-ring is installed on modules before
screwing into the sensor handle or inner stem.
O-Ring
5
Page 6

• Due to shipping or storage the internal solution inside
the PVC modules may have developed an air pocket
near the membrane. Gently shaking the sensor down
(like the old style mercury thermometer) will place the
internal solution next to the membrane.
• Presoaking the Calcium sensor in HI 4004-45
solution for at least half-hour before calibration will
help to optimize the sensor response.
• Do not leave your sensors in standard or samples
with ISA for long periods of time.
Note: The electrode membrane will develop a opaque
appearance when it becomes wetted. This is normal.
• Calibration standards and sample solutions should
have the same ionic strength. ISA should be added to
both samples and standards.
• Calibration standards and sample solutions should
be at same temperature.
• Thermally insulate solution vessel from magnetic stirrer.
• Calibration standards and sample solutions should
be stirred at the same rate using identical sized TFE
coated stir bars.
• Rinse electrodes with distilled or deionized water between samples and gently dab dry with lab wipe or
other soft disposable absorbent toweling. Do not rub
the sensing surface.
• Check for gas bubbles that may form near sensing
surface (due to solution temperature changes). Tap
off gently.
• Avoid large changes in temperature (thermal shock)
as it may damage the sensor.
6
Page 7

Additional HI 4104 guidelines
• Remove the protective plastic wrap that covers the
ceramic junction before assembling sensor for the first
time.
• Add reference HI 7082 fill solution to bottom of fill
hole or empty and refill fill solution daily before using.
• During measurement always operate electrode with
the fill hole open.
• During normal use, fill solution will slowly drain out
of the tapered cone junction at the lower portion of the
electrode. Excessive loss (>4 cm drop within 24 hours)
is not normal. If this occurs verify cap is tightened and
the interface between the internal cone and outer
body is free of debris.
• Add filling solution daily to maintain a good head
pressure. For optimum response, this level should be
maintained and not be allowed to drop more than 23 cm (1-inch) below fill hole.
• Do not use an electrode if crystallized salts are visible
inside the electrode. Drain electrode, disassemble and
rinse internal body with deionized water. Reassemble
and refill with fresh fill solution.
• If an erratic measurement occurs, check to see if foreign matter is seen trapped near the internal cone.
Drain by depressing the electrode cap then refill with
fresh fill solution.
VIII.VIII.
Electrode PreparationElectrode Preparation
VIII.
Electrode Preparation
VIII.VIII.
Electrode PreparationElectrode Preparation
HI 4004
The Hanna HI 4004 is a 2 piece design comprised of a
sensor handle (HI4000-50) and a sensing module (HI
4004-51).
1. Remove sensing module from shipping vial. Do not
touch the sensing membrane with the “H” hole pattern on it.
2. Screw the module into the sensor handle finger tight.
Do not overtighten.
7
Page 8

HI
4000-50
E
L
D
N
A
H
R
O
S
N
E
S
3. Holding the assembled electrode at the cable end,
shake the sensor to ensure internal fill solution that
may have separated during shipping is in contact
with inner membrane surface.
4. Prepare HI 5315 reference electrode by filling electrolyte reservoir with HI 7082 fill solution.
5. Place sensor and reference electrodes into electrode
holder and connect cable connectors to meter.
6. Soak the Calcium electrodes membrane in HI 4004-45
conditioning solution or a calcium standard (0.01M)
without ISA before calibration.
HI 4104
The Hanna HI 4104 is shipped disassembled. The
sensing module is found in the glass storage vial.
1. Unwrap Parafilm® seal found over ceramic junction
on inner stem and discard. This is only used for shipping or long term storage.
2. Remove sensing cone from shipping vial. Do not touch
the sensing membrane with the “H” hole pattern on
it.
3. Screw the cone into the inner stem finger tight. Do not
over tighten.
8
Page 9

Remove
Water
Deionized
Parafilm
Install
sensing
module
4. Rinse inner stem with deionized water making certain
to wet o-ring found on the inner stem.
5. Reassemble electrode by gently pushing the inner
assembly into the outer body, sliding spring down
cable, and screwing cap into place.
6. Remove fill hole cover and o-ring on fill hole spout.
Using the dropper pipette provided, add a few drops
HI 7082 fill solution to the electrode. Invert the electrode to wet the o-ring and rinse the fill solution chamber.
9
Page 10
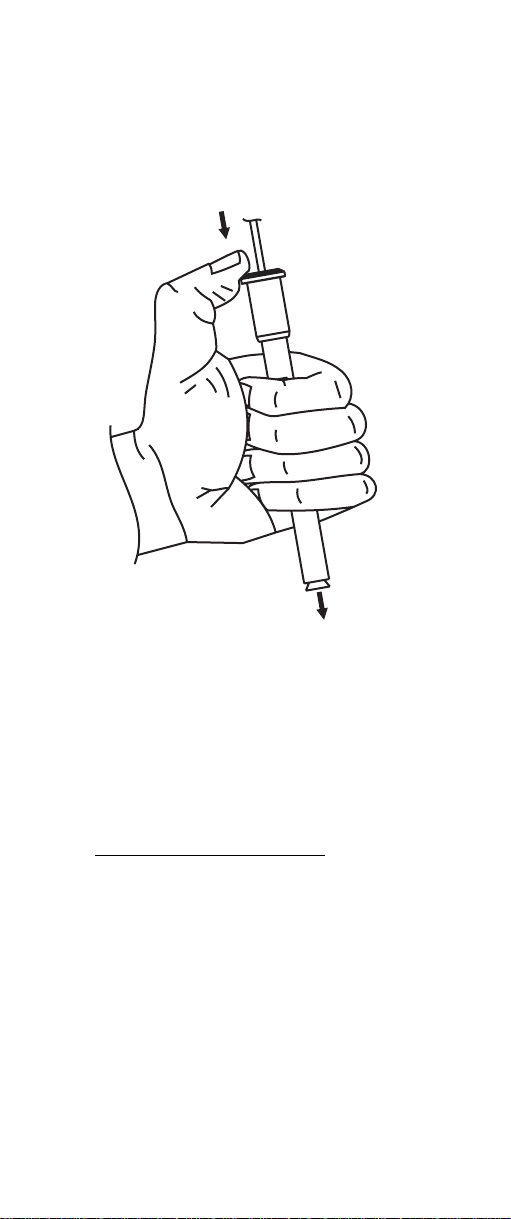
7. Holding the body of the electrode gently press upper
cap with your thumb. This permits the fill solution to
drain out of the body. Release cap and verify electrode returns to its original position. (You may need to
gently assist for this to occur).
COMBINATION
CALCIUM
HI 4104
8. Tighten the electrode cap onto the body and fill electrode body until fill solution volume is just below fill
hole.
9. Position electrode in a Hanna HI 76404 electrode
holder (or equivalent) and connect BNC connector to
meter.
IX. IX.
Quick Check of Electrode SlopeQuick Check of Electrode Slope
IX.
Quick Check of Electrode Slope
IX. IX.
Quick Check of Electrode SlopeQuick Check of Electrode Slope
• Connect electrode(s) to pH/mV/ISE meter
• Place meter in mV mode.
• Place 100 mL of deionized water into a beaker with
stir bar.
• Place reference and measuring half-cell or combination electrode into prepared sample.
• Add 1 mL of a standard to beaker. Record the mV
value when stable.
• Add an additional 10 mL of standard to the solution.
Record the mV when reading has stabilized. This
10
Page 11

value should be more positive than the previous
value noted.
• Determine the difference between the two mV values.
An acceptable value for this slope is
26 ± 4 mV (20-25°C).
X. X.
Corrective actionCorrective action
X.
Corrective action
X. X.
Corrective actionCorrective action
• Verify module has been screwed into sensor handle or
inner stem.
• Verify Parafilm® seal has been removed from ce-
ramic junction (HI 4104 or HI 5315 reference).
• Verify fill solution has been added to reference chamber.
• Verify electrodes are connected properly to meter and
meter is powered.
• Verify dilute standards are freshly made and stored.
Remake solutions if appropriate. Store in plastic
bottles.
• If the reading is jumpy or unstable, shake sensor
down (see section VII).
• If the sensor slope just misses the suggested slope
window, soaking the sensor in a standard solution
without ISA may solve the problem.
• If the membrane is damaged, the response becomes
extremely sluggish, or the slope of the electrode has
decreased significantly, and procedures above have
not helped, the module should be replaced.
For HI 4004
1. Dry off module and sensor handle.
2. Unscrew sensing module and replace with a new one.
(HI 4004-51).
3. Soak new module in calcium solution to condition it
before calibration.
For HI 4104
1. Drain the fill solution by depressing cap. Rinse electrode with distilled or deionized water. Drain.
11
Page 12

2. Unscrew upper cap and slide down cable toward connector.
3. Move spring and outer body down cable also.
4. Dry off inner stem and module with a soft tissue.
5. Hold inner stem and unscrew module and replace
with a new one. (HI 4104-51).
6. Reassemble electrode (see section VII), and refill with
electrolyte. Soak new membrane in calcium solution
without ISA to condition before calibration.
XI. XI.
Direct Calibration and MeasurementDirect Calibration and Measurement
XI.
Direct Calibration and Measurement
XI. XI.
Direct Calibration and MeasurementDirect Calibration and Measurement
This method is a simple procedure for measuring many
samples. A direct reading ISE meter (HI 4222 or equivalent) determines concentration of the unknown by a direct
reading after calibrating the meter with the standards. Add
HI 4004-00 to adjust ionic strength at a dose of 2 mL of per
100 mL sample or standard. The meter is calibrated using
freshly made standards that are in the measurement range
of the unknowns. Unknowns are read directly. In the region
where the electrode calibration becomes less linear, more
calibration points are needed, and calibration will need to
be repeated more frequently.
A pH/mV meter in mV mode and semi log graph paper
may also be used. Two or more freshly prepared standards
that are in the measurement range of the unknowns are
measured in mV mode on the meter.
These values are plotted on the semi-log paper and the
points are connected to form a straight-line curve. When
samples are measured, their mV values are converted to
concentration by following the mV to the concentration axis
on the semi-log plot.
12
Page 13

Procedure:
Follow sections VIII and IX to prepare electrodes for measurement.
1) Follow section VI to prepare standards/ solution. Standards should bracket and fall within the range of
interest. Standards and solutions should be at the
same temperature.
2) 2 mL of HI 4004-00 is added to 100 mL of both
samples and standards.
Add stir bar and mix before taking measurements.
3) Follow section VII; General Guidelines to optimize test
set-up.
4) During calibration it is best to start with lower concentration samples first. Wait for a stable reading before
reading/ recording values. Permit longer equilibration times at these levels (3 or 4 minutes).
To prevent carry over and contamination of samples, rinse
sensors with deionized water and remove moisture with
absorbant tissue between samples.
Typical HI 4004 and HI 4104 Linearity
100
80
60
40
20
mV
0
-20
-40
-60
-80
0123456
-Log of Molar Co ncentratio n
13
Page 14

XII. XII.
Other Measurement TOther Measurement T
XII.
Other Measurement T
XII. XII.
Other Measurement TOther Measurement T
Known additionKnown addition
Known addition
Known additionKnown addition
echniquesechniques
echniques
echniquesechniques
An unknown concentration can be determined by adding a
known volume and concentration of Ca2+ standard to the
sample. mV values are noted before and after the addition
of standard (∆E). An ideal sensor slope can be used in the
equation but actual determined slopes at the temperature
of measurement should be used if known (S). This method
is preprogrammed in the Hanna HI 4222 pH/ISE/mV meter,
which simplifies the method greatly.
Example:
Calcium ion determination with known addition.
1. A 50 mL sample of unknown (V
) is placed in a
SAMPLE
clean plastic beaker with an electrode (s). One mL of
ISA is added to the sample and permitted to mix. mV
1 is recorded.
2. 10 mL (V
STANDARD
) of 10
-1
M (C
) standard is added
STANDARD
to the beaker and the mV value increases. (Note: for
other concentration samples, add a known volume
and concentration of standard to produce approximately 30 mV change).
The unknown calcium concentration in the original
sample (C
) can then be determined by using the
SAMPLE
equation that follows.
3. The procedure can be repeated with second standard
addition to verify slope and operation of the method.
C
=
sample
(V
sample+Vstandard+VISA
(V
XIII.XIII.
pH and InterferentspH and Interferents
XIII.
pH and Interferents
XIII.XIII.
pH and InterferentspH and Interferents
C
standardVstandard
)10
T
∆E/S
(V
sample+VISA
- (VS’)
)= V
S’
)= V
V
V
T
S’
sample
HI 4004/ HI 4104 calcium electrodes can operate over a
14
Page 15

pH range of 4-10 . The electrode responds to free calcium
ions only. Precipitates with oxalate, carbonate, phosphate
and complexes with hydroxide, sulfate, and bicarbonate
reduce measureable calcium from solution. Using Known
Addition (section XII) with complexing reagents and pH
adjustments may permits total calcium to be measured.
Limiting the length of time of exposure to samples containing
interferences will prolong useful life of your electrode. If
sensor has been exposed to ions above recommended levels,
soaking in pure calcium solutions without ISA or HI 400445 will aid recovery of function.
XIV.XIV.
Storage and Care of the HI 4004 andStorage and Care of the HI 4004 and
XIV.
Storage and Care of the HI 4004 and
XIV.XIV.
Storage and Care of the HI 4004 andStorage and Care of the HI 4004 and
HI 4104 electrodesHI 4104 electrodes
HI 4104 electrodes
HI 4104 electrodesHI 4104 electrodes
The HI 4004 sensor can be stored in HI 4004-45 for short
time periods. For long term storage, unscrew sensing module from sensor handle and store dry in the shipping vial.
The model HI 4104 combination electrode can be left in
HI 4004-45 for short time periods. If the electrode will be
used frequently and needs to be ready for use, take measures to prevent evaporation of fill solution. Top off fill
solution, replace o-ring, fill hole cover on the fill hole opening, and place sensor in HI 4004-45 storage and conditioning solution. Store electrode upright. Prior to use, drain
electolyte chamber and refill with fresh HI 7082 fill solution.
For long term storage, the electrode should be drained,
disassembled and washed of salts with deionized water.
Wrap the ceramic junction in Parafilm® or other sealing
wrap. Unscrew the module and store dry in the shipping
vial. Refrigeration of module will extend its life. Store disassembled electrode in storage box provided with electrode.
XV. XV.
Conversion tablesConversion tables
XV.
Conversion tables
XV. XV.
Conversion tablesConversion tables
Multiply by Multiply by
Multiply by
Multiply by Multiply by
2+
(mg/L) 40080
Moles/L (M) Ca
2+
to ppm Ca
ppm (mg/L) to M (moles/L) 2.49 X 10
15
-5
Page 16

MAN4104 07/06R1
WARRANTY WARRANTY
WARRANTY
WARRANTY WARRANTY
Hanna Instruments Ion Selective Electrodes are warranted
to be free of defects in material and workmanship for 6
months from date of purchase when used for their intended
purpose and maintained according to instructions. If they
fail to work when first used contact your dealer immediately.
Damage due to accidents, misuse, misapplication, tampering
or lack of prescribed maintenance is not covered.
Hanna Instruments reserves the right to modify the design,
construction or appearance of its products without advance
notice.
16
 Loading...
Loading...